Abstract
Evidence for the involvement of the endocannabinoid system (ECS) in anxiety and fear has been accumulated, providing leads for novel therapeutic approaches. In anxiety, a bidirectional influence of the ECS has been reported, whereby anxiolytic and anxiogenic responses have been obtained after both increases and decreases of the endocannabinoid tone. The recently developed genetic tools have revealed different but complementary roles for the cannabinoid type 1 (CB1) receptor on GABAergic and glutamatergic neuronal populations. This dual functionality, together with the plasticity of CB1 receptor expression, particularly on GABAergic neurons, as induced by stressful and rewarding experiences, gives the ECS a unique regulatory capacity for maintaining emotional homeostasis. However, the promiscuity of the endogenous ligands of the CB1 receptor complicates the interpretation of experimental data concerning ECS and anxiety. In fear memory paradigms, the ECS is mostly involved in the two opposing processes of reconsolidation and extinction of the fear memory. Whereas ECS activation deteriorates reconsolidation, proper extinction depends on intact CB1 receptor signalling. Thus, both for anxiety and fear memory processing, endocannabinoid signalling may ensure an appropriate reaction to stressful events. Therefore, the ECS can be considered as a regulatory buffer system for emotional responses.
Keywords: Anxiety, cannabinoid CB1 receptor, classical conditioning, endocannabinoids, fear, gamma-aminobutyric acid, glutamate, habituation, neuronal plasticity, stress
Introduction
Negative emotions, such as anxiety and fear, alert the organism to potentially dangerous or harmful stimuli, and can hence promote survival. However, when anxiety and fear responses are disproportional in intensity, chronic, irreversible and/or not associated with any actual risk, they can impair physical and psychological functions. Such overreactions may be symptomatic of anxiety-related neuropsychiatric disorders such as generalized anxiety, phobia and post-traumatic stress disorder (Graham et al., 2011). Because not all patients respond to the currently available pharmacotherapeutical treatment options (Pillay and Stein, 2007), the search for novel therapeutic approaches deserves high priority. In recent years, mechanistic evidence for the involvement of the endocannabinoid system (ECS) in anxiety and fear has been accumulated. Elucidation of the role of the ECS in fear and anxiety may provide new therapeutic leads. One of the targets to study is the presynaptically located cannabinoid type 1 (CB1) receptor. The endogenous lipid ligands 2-arachidonoylglycerol (2-AG) and anandamide (AEA) are synthesized postsynaptically and travel retrogradely in order to activate CB1 receptors, which in turn reduce neurotransmitter release. Other constituents of the ECS are the degrading enzymes fatty acid amide hydrolase (FAAH) for AEA and monoacylglycerol lipase (MAGL) for 2-AG respectively (for extensive reviews, see Piomelli, 2003; Kano et al., 2009). The ECS can be manipulated by pharmacological means using exogenous drugs such as CB1 receptor agonists, CB1 receptor antagonists and endocannabinoid degradation inhibitors, as well as by genetic approaches. Recent studies have provided new insights into the molecular basis of the modulatory role of the ECS in anxiety (defined as innate fear for the purpose of this review) and its involvement in different phases of fear learning (defined as acquired fear for the purpose of this review).
Anxiety
ECS as a bimodal regulator of anxiety
Considering the well-known involvement of a plethora of neurotransmitter systems in the regulation of anxiety (Millan, 2003) and the function of the CB1 receptors in the suppression of neurotransmitter release in many different neuronal subtypes, the ECS can be seen as one of the key regulatory elements of anxiety behaviour. This wide influence of CB1 receptor signalling, together with the molecular promiscuity of endocannabinoids, has continuously complicated the interpretation of data. In this context, the paradox of AEA is illustrative. It has biphasic properties, acting as an anxiolytic agent on the CB1 receptor and as an anxiogenic agent on the transient receptor potential vanilloid type 1 channel (TRPV1) (Rubino et al., 2008b). This reveals a complex scenario where CB1 receptor-dependent processes are not the only regulators of anxiety responses by endocannabinoids.
However, pharmacological approaches using drugs that selectively target the major elements of the ECS and the use of genetically modified mice have led to new insights on the mechanisms underlying the bimodal actions of cannabinoids in anxiety.
CB1 receptor agonists are reported to induce biphasic effects, with lower doses being anxiolytic and higher doses being anxiogenic (Viveros et al., 2005). In addition, similar bimodal responses were found using CB1 receptor antagonists and other drugs interfering with the molecular machinery of the ECS (for review, see Lafenetre et al., 2007). The consistency of these results has been demonstrated in various anxiety paradigms, such as the Vogel conflict test, light/dark box and elevated plus-maze, where different components of the anxiety state can be measured. Despite the clear role of the ECS in anxiety, in detail experimental conditions, species differences and previous experiences of subjects among other parameters also affect the animals’ reactions, and consequently, the interpretation of the observations.
Several studies using CB1 receptor knockout mice have reported anxiogenic responses in classical anxiety paradigms such as elevated plus-maze (Haller et al., 2004b) and light/dark box (Martin et al., 2002). Nevertheless, contradictory data do also exist. Together with the susceptibility of the ECS to environmental variables (see below), the presence of CB1 receptors on glutamatergic and GABAergic neuronal subpopulations, could provide an explanation, at least in part, for these contradictory findings. Depending on whether the experimental conditions predominantly modulate excitatory or inhibitory transmission (i.e. glutamatergic or GABAergic), the effect of the absence of CB1 receptor signalling will lead to different behavioural outcomes.
Consequently, the recent development of new genetic models with CB1 receptor deletion in specific neuronal subpopulations is very useful in understanding the regulation of anxiety by the ECS. Therefore, we aim at discussing new insights in the interaction between the ECS and two of the most important neurotransmitters in the brain, i.e. glutamate and gamma-aminobutyric acid (GABA). In addition, the involvement of the monoaminergic system, the basal functionality of the ECS in terms of emotional processing and the cross-talk with other receptors activated by endocannabinoids will be discussed.
ECS susceptibility to environmental variables
Many factors are thought to influence behavioural reactivity, especially when referring to anxiety. Therefore, there are several parameters to consider when interpreting data from anxiety assays. The administration route of drugs in pharmacological approaches represents a relevant starting point in the analysis of the behavioural results, as anxiogenic and anxiolytic effects of ECS enhancement have been related to different brain areas (Rubino et al., 2008a). Thus, the same dose of delta 9-tetrahydrocannabinol (Δ9-THC) is able to promote anxiolytic responses when injected in the prefrontal cortex (PFC), whereas microinjections in the basolateral amygdala (BLA) lead to an anxiogenic response. Moreover, the appropriate selection of the studied species is fundamental. Haller et al. (2007) have proposed that contradictory anxiety-like results obtained in rats and mice can be explained by the different cannabinoid responsiveness of GABAergic and glutamatergic neurons in these two species. In addition, data from the analysis of CB1 receptor-deficient animals revealed that significant differences between CB1 receptor knockout animals and their wildtype littermates can only be found under aversive conditions (Haller et al., 2004a; Jacob et al., 2009). Therefore, the light conditions during the test and the housing and handling prior to the test are important parameters to be kept well-defined in experiments involving anxiety and the ECS. Regarding these variables, FAAH is of particular interest. A plethora of studies involving pharmacological blockade of FAAH by the specific inhibitor URB597 demonstrated anxiolytic behaviours in a variety of species using different anxiety paradigms (Scherma et al., 2008; Moreira et al., 2008; Patel and Hillard, 2006; Rubino et al., 2008b). Nevertheless, recent discrepant findings revealed that the anxiolytic effect of URB597 depends largely on the experimental conditions (Naidu et al., 2007; Trezza and Vanderschuren, 2008; Haller et al., 2009) and is only significant under stress conditions. In summary, dependent on the stress level, which is associated with the protocol used (unescapable vs escapable stressors, aversive conditions, previous exposure), the animal model used and the administration route in pharmacological protocols, a bimodal response will be seen as long as the experimental conditions do not exceed the buffering function of the ECS.
CB1 receptor-dependent regulation of biphasic responses in anxiety
Despite the difficulty to generate a physiological model that considers the vast amount of interactions associated with the ECS, two neurotransmitters have emerged as points of reference for the complexity in anxiety behaviour. Evidence from pharmacological and genetic alterations of GABAergic and glutamatergic transmission indicates that these neurotransmitters appear to exert their functions on anxiety in opposite ways (Millan, 2003). Due to the expression of CB1 receptors on axon terminals of both subpopulations, it is tempting to predict the relevance of the localization of this receptor as an explanation for the dual role of the ECS in the regulation of anxiety.
CB1 receptor influence on glutamatergic and GABAergic regulation of anxiety
Activation of the CB1 receptor by endocannabinoids leads to a reduction of neurotransmitter release by a retrograde mechanism (Wilson and Nicoll, 2002). The CB1 receptor agonist Δ9-THC was recently also shown to inhibit GABA release (Laaris et al., 2010). Considering the reproducibility of this result using the specific cannabinoid receptor agonist WIN55,212-2 (Laaris et al., 2010), the inhibition of GABA release by Δ9-THC is mediated by CB1 receptor activation. In addition, CB1 receptor-mediated inhibition of glutamate release was also demonstrated in rats (Hoffman et al., 2010) and mice (Kawamura et al., 2006).
The enhancement of GABAergic transmission via benzodiazepines (GABAA receptor positive allosteric modulators) has been used as an effective acute treatment for patients with anxiety disorders. Consequently, it could be assumed that a prominent increase in the endocannabinoid tone, and consequently CB1 receptor activation specifically on GABAergic neurons, would lead to an anxiogenic response via a decrease in GABAergic transmission (Roohbakhsh et al., 2009). However, two important characteristics of the ECS must be considered to implement this simplified view of the anxiogenic effect of cannabinoids. First, a different basal activation has been demonstrated for CB1 receptors expressed on glutamatergic neurons and GABAergic neurons, being higher on the latter (see below). The lower basal activation of the CB1 receptors on glutamatergic terminals suggests that their reactivity to an increase in the endocannabinoid tone would be higher than that of the CB1 receptor on GABAergic terminals (Katona and Freund, 2008). Second, the capacity of endocannabinoids to activate other receptors such as TRPV1 could underlie the anxiogenic effect in some cases (Rubino et al., 2008b).
On the other hand, excitatory neurotransmission, mediated mostly by glutamatergic transmission, is enhanced by stress, and stress is a key component regarding the vulnerability of developing mood and anxiety disorders (Simon and Gorman, 2006). In fact, inhibition of glutamate release in the periaqueductal gray (PAG) area by CB1 receptor activation was proposed as an explanation for the anxiolytic effect of AEA injections into this area (Lisboa et al., 2008). Likewise, experiments performed by Naderi et al. (2008) showed that ineffective doses of diazepam (a GABAA receptor positive allosteric modulator) and the FAAH inhibitor URB597 became effective when applied in combination. These results suggest a possible synergistic action on glutamatergic inhibition (by increase in AEA) and GABAergic enhancement (by the activation of GABAA receptors).
The development of new genetic models, using the Cre/lox-P recombination system, contributes to the understanding of the role of ECS in anxiety, via specific deletion of the CB1 receptor on different neuronal subpopulations, in particular on cortical and striatal GABAergic interneurons (GABA-CB1-KO) and cortical glutamatergic neurons (Glu-CB1-KO). The use of these two mutant mouse lines has revealed an ambivalent role for this receptor not only in anxiety, but also in feeding behaviour (Bellocchio et al., 2010) and impulsivity (Lafenetre et al., 2009). In the latter report, a classical impulsive trait, namely novelty-seeking behaviour, was shown to depend on proper functionality of the ECS. In this study, the lack of CB1 receptor on GABAergic neurons promoted a more impulsive response towards a novel object and palatable food, whereas Glu-CB1-KO mice were strongly inhibited in their approach behaviour. These responses can be related to anxiolytic and anxiogenic profiles respectively. However, it is important to keep in mind that impulsivity and anxiety behaviours are likely to be controlled by different neuronal mechanisms. In agreement with these results, a fundamental role to ensure constant levels of exploratory behaviour was related to the expression of the CB1 receptor on glutamatergic terminals in experiments involving exploration of novel object and novel juvenile conspecifics (Jacob et al., 2009). Notably, the great majority of the CB1 receptor are located on GABAergic neurons in the brain. However, the discrete location of CB1 receptors on glutamatergic neurons enables endocannabinoids to exert important functions (Monory et al., 2006). In summary, data from pharmacological and genetic experiments concerning CB1 receptor involvement in anxiety merge to the idea that the location of the CB1 receptor is one of the most important factors accounting for the biphasic effect of the ECS on this behaviour. Nevertheless, the presence of this receptor in these two relevant neuronal subtypes is not the only factor explaining the biphasic effect, and basal functionality should be considered as well.
ECS basal functionality in anxiety (tonic versus phasic activation)
In addition to the distinct abundance of the CB1 receptor on GABAergic and glutamatergic neurons, it is of central interest to define the basal activity of the CB1 receptor on both neuronal subpopulations. This property of the CB1 receptor will determine to what extent it can be additionally activated by a rise in endocannabinoid levels depending on the basal activation that is present under normal circumstances. Recent studies have shown a difference in basal activity between CB1 receptors localized on GABAergic and glutamatergic populations (Roberto et al., 2010; Slanina and Schweitzer, 2005). CB1 receptor antagonists such as AM251 and SR141716 have been shown to increase inhibitory transmission in the central nucleus of the amygdala (CeA), suggesting a tonic activation of CB1 receptors on GABAergic neurons under basal conditions (Roberto et al., 2010). This tonic activation was regulated by the postsynaptic neuron in response to elevated Ca2+ levels. Moreover, blockade of CB1 receptor moderately, but significantly augmented excitatory neurotransmission, indicating a tonic activity of the ECS also on this system (Slanina and Schweitzer, 2005). In this study, 2-AG was proposed as the endocannabinoid responsible for the tonic inhibition of excitatory neurotransmission, and interaction with the inhibitory network was excluded using GABAA and GABAB receptor antagonists. Nevertheless, increases of glutamate release induced by AM251 were never higher than 110% of the tonic release (Slanina and Schweitzer, 2005), whereas augmentation of GABAergic release after treatment with the same CB1 receptor antagonist reached levels of 130–140% (Roberto et al., 2010). Hence, the tonic inhibition mediated by the CB1 receptor is much more relevant on GABAergic synapses than on glutamatergic synapses. Consequently, the CB1 receptor on GABAergic terminals is thought to be a general suppressor of GABA release (predominantly relevant in tonic activation), whereas the CB1 receptor on glutamatergic terminals has a different physiological role, being responsible for the on-demand inhibition only after excessive glutamate release (predominantly relevant in phasic activation) (Katona and Freund, 2008).
Neuronal subpopulations classified by CB1 receptor agonist sensitivities
Keeping in mind the relevance of the differences between GABAergic and glutamatergic neurons in terms of CB1 receptor basal activity and its abundance, there is another fundamental aspect to consider; the relation between CB1 receptor localization and sensitivity to CB1 receptor agonists. Depolarization-induced suppression of inhibition (DSI) and excitation (DSE) are processes that can be mediated by the ECS due to the presynaptic localization of the CB1 receptor and their property to inhibit neurotransmitter release after activation (Wilson and Nicoll, 2002). Therefore, the term sensitivity refers to the capacity of CB1 receptors to be activated by a CB1 receptor agonist and consequently to mediate DSI or DSE. In fact, DSI and DSE are not equally executed, as excitatory transmission has been estimated to be around 30-fold less sensitive to cannabinoids than inhibitory transmission (Ohno-Shosaku et al., 2002). Accordingly, biochemical experiments using blockers of enzymes involved in synthesis and degradation of 2-AG and AEA confirm that the more abundant 2-AG is the most probable endocannabinoid implicated in DSE (Hashimotodani et al., 2007). These studies also propound the existence of three different types of synapses classified by their sensitivity to the CB1 receptor agonist WIN55,212-2. Whereas excitatory synapses are homogeneous and have moderate sensitivities, inhibitory synapses are dichotomized into two distinct populations, one with a high sensitivity and one that is not sensitive to WIN55,212-2. Recent experiments have demonstrated that inhibitory synapses that are sensitive to cannabinoid-induced DSI can even be further subdivided into two different groups. The group with higher sensitivity is formed by perisomatically projecting basket cells (BC), whereas the dendritically projecting Schaffer-collateral associated cells (SCA) belong to the group with lower sensitivity to WIN55,212-2 (Lee et al., 2010). Given the different neuroanatomical features of these two different neuronal subtypes, the action of a different endocannabinoid ligand cannot be excluded, due to the fact that the 2-AG synthesizing molecular pathways are located in dendritic spines (Katona et al., 2006), and consequently, SCA synapses should have a higher sensitivity than BC synapses, which is certainly not the case. Nevertheless, it is worth mentioning that the relative densities of CB1 receptors on GABAergic and glutamatergic neurons are not fully reliable indicators per se of their respective strengths in regulating neurotransmitter release. As a matter of fact, the presence of presynaptic functional differences downstream from the CB1 receptor, involving coupling to G proteins and/or Ca2+ channels, has been shown between GABAergic and glutamatergic neurons. A recent study revealed that the capacity to recruit G proteins is more prominent in CB1 receptors located on glutamatergic terminals than on GABAergic terminals (Steindel et al., 2008), suggesting a compensatory effect to the lower abundance of CB1 receptors on glutamatergic terminals.
Taken together, there are three important characteristics (localization, basal activation and sensitivity) associated with CB1 receptor-mediated regulation of anxiety, accounting for the biphasic effect commonly described. In this scenario, GABAergic localization, together with high basal activation and high sensitivity would confer an important role in the development of anxiogenic responses to cannabinoids to the CB1 receptor. On the other hand, glutamatergic localization, low basal activation and low sensitivity are factors that give the CB1 receptor an essential function in the development of anxiolytic responses to cannabinoids.
CB1 receptor influence on monoaminergic regulation of anxiety
Monoaminergic transmission has also been related to the cannabinoid-dependent regulation of emotional homeostasis. Local (Page et al., 2008) and systemic (Page et al., 2007) administration of the CB1 receptor agonist WIN55,212-2 causes an increase in extracellular norepinephrine (NE) in the PFC. In the latter study, the increase in NE release was accompanied by enhanced expression of tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of catecholamine neurotransmitters (Boundy et al., 1998). Notably, these molecular responses were also associated with anxiety-like behaviours (Page et al., 2007). Further experiments have demonstrated that chronic treatment with WIN55,212-2 induced downregulation of the adrenergic receptor alpha 2A (α2A-AR) in the nucleus accumbens (Carvalho et al., 2010). Considering that the α2A-AR seems to function as an autoreceptor by inhibiting NE release from the presynaptic terminal (Kable et al., 2000), this downregulation provides a molecular mechanism underlying the increase in NE release. In addition, the strong CB1 receptor agonist HU-210 was shown to reproduce stress-mediated activation of the hypothalamic-pituitary-adrenal (HPA) axis (McLaughlin et al., 2009). In this study, the increase in corticosterone secretion following HU-210 administration was significantly reduced by pretreatment with antagonists to the α1-adrenoceptor (prazosin) and β-adrenoceptor (propranolol). Taken together, these findings reveal an important role for NE transmission in the development of anxiogenic responses, supporting an alternative explanation for the anxiogenic properties of CB1 receptor agonists at high doses. Additionally, antagonists of the serotonergic type 1A (5-HT1A; WAY100635) and 5-HT2A/2C (ketanserin) receptors attenuated the CB1 receptor-dependent increase of corticosterone secretion. Thus, the anxiogenic action of the ECS through monoaminergic transmission appears to be related not only to NE but also to 5-HT. On the other hand, antidepressant-like behaviour has also been associated with noradrenergic (locus coeruleus) and serotonergic (dorsal raphe nucleus) enhancement following treatment with the FAAH inhibitor URB597 (Gobbi et al., 2005). Along this line, FAAH knockout mice present a clear emotional phenotype characterized by reduced anxiety and increased sociability when compared to wildtypes (Cassano et al., 2011). Interestingly, this emotional differentiation was accompanied by increased serotonergic tone in the PFC. In addition, Bambico et al. (2007) have reported antidepressant responses after treatment with the CB1 receptor agonist WIN55,212-2 comparable to those produced by the clinically used selective serotonin reuptake inhibitor (SSRI) antidepressant citalopram. Again, this antidepressant effect was characterized by stimulation of serotonergic transmission in the PFC (Bambico et al., 2007). Taken together, it can be hypothesized that cannabinoid enhancement of NE transmission is commonly associated with anxiogenic responses, whereas cannabinoid-mediated stimulation of serotonergic signalling is associated with anxiolytic responses after mild activation (FAAH inhibition, low doses of WIN55,212-2) and to anxiogenic-like reactions after stronger activation (HU-210) (McLaughlin et al., 2009). Therefore, cannabinoid regulation of monoaminergic transmission should be considered as an important mechanism in the modulation of emotional homeostasis.
Non-CB1 receptor-dependent regulation of biphasic responses in anxiety
Due to the receptor promiscuity frequently reported for endocannabinoids, the implication of receptors different from CB1 has been proposed to explain certain aspects of the anxiety behaviour after cannabinoid exposure. Notably, anxiety responses in both directions have been associated with different non-cannabinoid receptors as well, indicating prominent interaction of the ECS with other signalling systems.
CB2 receptor
The presence of cannabinoid type 2 (CB2) receptor in brain tissue has recently been related to feeding behaviour (Ishiguro et al., 2010), pain processing (Bingham et al., 2007), drug abuse and depression (Onaivi et al., 2008). However, the role of this receptor in anxiety has never been fully investigated, although some evidence demands special attention to this traditionally ignored receptor when referring to brain-dependent behaviours.
In mice tested in the marble-burying test, only the highest dose of the CB2 receptor agonist GW405833 produced an anxiolytic-like effect, accompanied by a clear reduction in locomotor activity and an increase in cataleptic response (Valenzano et al., 2005). Due to the alterations in locomotion, it cannot be clearly concluded that the CB2 receptor agonist GW405833 modulated anxiety behaviour. In contrast, the other CB2 receptor agonist JWH015, tested in mice in the two-compartment black and white box paradigm, reduced the time spent in the white compartment, while increasing the time in the black compartment, revealing a clear anxiogenic-like effect (Onaivi et al., 2008). Interestingly, 2-AG-mediated suppression of inhibitory transmission was not blocked by the CB1 receptor antagonist LY320135, but with the CB2 receptor antagonist AM630 (Morgan et al., 2009), revealing an interesting role for the CB2 receptor in (endo)cannabinoid signalling and, consequently, a possible influence of the ECS on anxiety via CB2 receptor activation.
Owing to the disputed expression of the CB2 receptor in neuronal tissue, CB2 receptor-deficient mice have been used traditionally for purposes different from studying the involvement in anxiety regulation. However, in the study by Onaivi et al. (2008), blocking CB2 receptor expression by an antisense oligonucleotide induced an anxiolytic-like effect in the elevated plus-maze test. Taken together, these results suggest that the CB2 receptor could play an important role in the anxiogenic effects of cannabinoids. Nevertheless, its postsynaptic localization (Brusco et al., 2008), and the marked lower expression of this receptor in neurons, as compared with the CB1 receptor, add a new level of complexity to the analysis of CB1/CB2 receptor-mediated regulation of anxiety (Atwood and Mackie, 2010).
TRPV1
In the central nervous system, TRPV1 is expressed in various brain areas such as basal ganglia, hippocampus and cortex (Cristino et al., 2006). Its effects on anxiety have recently been linked to the ECS due to the fact that AEA acts also as a TRPV1 ligand.
Regarding the pharmacology of this receptor, a biphasic effect was found in the elevated plus-maze after prefrontal microinjections of methanandamide (mAEA) at low (anxiolytic) and high doses (anxiogenic) (Rubino et al., 2008b). In this study, these effects were counteracted by CB1 receptor and TRPV1 antagonists respectively. Moreover, overexpression of FAAH with lentiviral vectors microinjected into the same area corroborated the anxiogenic profile expected after blocking the tonic activation of CB1 receptors by AM251. Complementary studies targeting the PAG showed a similar pattern, where TRPV1 was required for the anxiogenic effect of high doses of the CB1 receptor agonist WIN55,212-2 (Campos and Guimaraes, 2009). Finally, the development of new drugs with a dual blocking profile over FAAH and TRPV1 provide clear evidence that the CB1 receptor and TRPV1 perform their actions on anxiety regulation in opposite ways, being activated and antagonized, respectively, to produce anxiolytic-like responses in mice (Micale et al., 2009).
In addition, one of the clearest pieces of evidence regarding the involvement of this receptor in anxiety behaviour came from the analysis of TRPV1-deficient mice. In the study of Marsch et al. (2007), an anxiolytic phenotype was observed in TRPV1 knockout mice tested in the elevated plus-maze and the light/dark box when compared with their wildtype littermates. Finally, based on the capacity to bind AEA, TRPV1 could be a complementary molecular mechanism for the anxiogenic effect produced by endocannabinoids, and consequently, also be a promising target for the development of new drugs with anxiolytic properties.
5-HT1A receptor
SSRIs have been largely used for the treatment of anxiety disorders and depression. The main effect of SSRIs is achieved by the enhancement of serotonin activity, which involves the desensitization of 5-HT1A inhibitory autoreceptors (Nutt and Stein, 2006). Concerning the implication of the ECS, certain compounds of the plant Cannabis sativa such as cannabidiol (CBD) have a very low affinity for CB1 and CB2 receptors. However, they also exert agonistic effects on 5-HT1A receptors (Russo et al., 2005). Furthermore, CBD-mediated anxiolytic responses were reported in different studies using elevated plus-maze and Vogel conflict paradigms (Moreira et al., 2009). Therefore, 5-HT1A receptors are possibly involved in the anxiolytic effects of CBD, as demonstrated by microinjections of low doses of CBD in the PAG area, which caused anxiolytic-like effects that are counteracted by the 5-HT1A receptor antagonist WAY-100635, but not by the CB1 receptor antagonist AM251 (Campos and Guimaraes, 2008). In addition, ineffective doses of 8-OH-DPAT (a selective 5-HT1A receptor agonist) or Δ9-THC promoted an anxiolytic response in the elevated plus-maze when administered together in rats (Braida et al., 2007), emphasizing the involvement of the serotonergic system in the regulation of anxiety by the ECS.
CCK
Cholecystokinin (CCK) is widely distributed throughout the brain and is acting as a neurotransmitter in the cortex and limbic regions. Because of its colocalization with many other neurotransmitters that are involved in emotional homeostasis (such as GABA, dopamine, serotonin and opioids), CCK has classically been implicated in the development of anxiety (Rotzinger et al., 2010). Studies published to date support a role of CCK receptor 2 (CCK2) in the acute modulation of anxiety and suggest that the BLA is an important site for this effect. CCK2 agonists are anxiogenic, and CCK2 antagonists reduce potentiated states of anxiety but do not appear to affect baseline anxiety responses (Rotzinger and Vaccarino, 2003). Recently, microdialysis experiments have revealed an increase in GABA efflux underlying the anxiogenic-like effect produced by the CCK2 agonist CCK-8S (Antonelli et al., 2009). Paradoxically, the use of benzodiazepines is an established treatment for anxiety disorders. Muscimol, a potent GABAA receptor agonist, was able to increase the percentage of open arm time and entries in the elevated plus-maze, when injected into the CA1 area of rat hippocampus (Rezayat et al., 2005). A possible explanation for this paradox could be that CCK and GABA operate on different steps in a sequence of neuronal events that initiates and maintains the anxiolytic reaction (Antonelli et al., 2009). Strikingly, in the same study, sub-threshold concentrations of the CB1 receptor agonist WIN55,212-2 and CCK-8S, induced an enhancement of GABA efflux when injected in combination, suggesting the intriguing possibility of a CB1 receptor-CCK2 interaction at the membrane level (Fuxe et al., 2008). Nevertheless, the complexity of the relation between the ECS and the CCK system increases with the fact that CCK has opposite actions on inhibitory neurotransmission, which originates from distinct interneurons (Karson et al., 2008).
Stress/reward induction of ECS plasticity
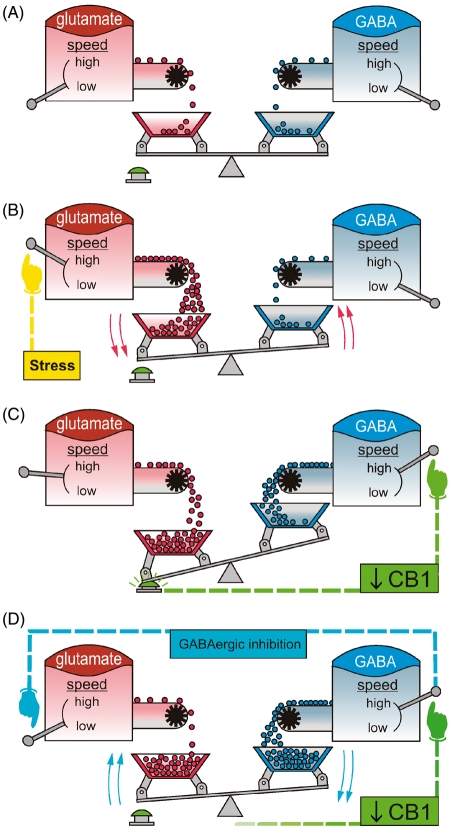
For proper ECS-dependent regulation of anxiety, it is necessary that every part of the ECS functions optimally. Therefore, experiences which alter one of its parts (e.g. CB1 receptor or endocannabinoid synthesizing and degrading enzymes), would lead to an impairment of the physiological reaction to (endo)cannabinoids. Recent evidence suggests that stress alters endocannabinoid content in limbic areas and PFC (Rademacher et al., 2008). Further experiments have confirmed that chronic psychoemotional stress (viz. social defeat) blocks the normal reduction of inhibitory postsynaptic potentials (IPSPs) produced after application of the CB1 receptor agonist HU-210 to corticostriatal slices of C57BL/6 mouse brains (Rossi et al., 2008). In addition, this blockade was counteracted by pretreatment with CB1 and glucocorticoid receptor antagonists, revealing the importance of HPA-axis reactivity in this process. CB1 receptor downregulation on GABAergic neurons is likely to be the cause for this absence of reduction due to the fact that basal properties and sensitivity of these synaptic transmission processes were not affected. Interestingly, stress did not affect the normal CB1 receptor-mediated reduction of excitatory postsynaptic potentials (EPSPs), suggesting a more static profile of the CB1 receptor on glutamatergic terminals. In agreement with the data on IPSPs, an increase of AEA levels by genetic or pharmacological inactivation of its degrading enzyme was able to reverse the stress-induced effect, and this response was also mediated by the CB1 receptor (Rossi et al., 2010). Analyzing the implication of this stress-dependent downregulation of the CB1 receptor, at the behavioural level, Campos et al. (2010) concluded that an anxiogenic increase of AEA signalling, via injections of the AEA reuptake inhibitor AM404 into the ventral hippocampus, turned out to be anxiolytic (tested on the elevated plus-maze), when the animals were pre-exposed to restraint stress. Consequently, it can be hypothesized that the anxiogenic properties of the enhancement of AEA signalling (via AM404) rely on the activation of CB1 receptors on GABAergic terminals. In addition, the stress-mediated downregulation of these CB1 receptors results in a stronger activation of CB1 receptors on glutamatergic terminals, leading to an anxiolytic response. These findings corroborate a model of endocannabinoid adaptation (explained in Figure 1), involving a stress-mediated re-orientation of endocannabinoid signalling (via CB1 receptor downregulation on GABAergic terminals) towards GABAergic disinhibition in order to prevent overexcitation and restore the excitatory–inhibitory equilibrium required for appropriate emotional reactivity.
Figure 1.
Proposed model for the stress induction of cannabinoid type 1 (CB1) receptor-mediated adaptation, associated with the emotional regulation exerted by the endocannabinoid system (ECS). Among the plethora of neurotransmitters implicated in processing of emotions, glutamatergic (left) and GABAergic (right) systems play a central role in the regulation of anxiety. (A), Under basal conditions, the equilibrium between excitatory and inhibitory transmission provides an appropriate emotional reactivity. (B), Stressful experiences are characterized by enhancement of the glutamatergic tone, which leads to an unbalance between excitatory and inhibitory transmission. (C), The CB1 receptors on GABAergic terminals represents a dynamic element of the ECS that can be expressed at a higher or lower level, depending on the characteristics of the stimuli. The overexcitation induced by stressful stimuli triggers CB1 receptor downregulation exclusively on GABAergic terminals, which eventually modifies the balance between GABAergic and glutamatergic CB1 receptor activation by endocannabinoids. (D), This long-lasting CB1 receptor downregulation on GABAergic terminals leads to a persistent increase in the strength of GABAergic inhibition of the glutamatergic transmission. This model represents a precise buffer system for the homeostasis of emotions.
In contrast, stimuli known to be activators of the reward system, such as voluntary exercise or sucrose consumption in rodents, were responsible for a larger reduction of the IPSPs by the CB1 receptor agonist HU-210 (De Chiara et al., 2010). Notably, this increase in CB1 receptor-dependent inhibition of IPSPs was not shown on EPSPs, and it was prevented by previous exposure to psychoemotional stress. In agreement with the previous data, chronic cocaine administration was shown to induce sensitization of GABAergic synapses (unlike glutamatergic) to the stimulation of the CB1 receptor (Centonze et al., 2007). Interestingly, the enhancement of CB1 receptor-mediated inhibition of IPSPs triggered by the rewarding stimuli mentioned above (voluntary exercise, sucrose consumption and chronic cocaine administration), was presumably caused by CB1 receptor upregulation on GABAergic neurons, due to the absence of alterations on the basal properties and sensitivity. However, the reward-mediated re-orientation of endocannabinoid signalling (via CB1 receptor upregulation on GABAergic terminals) towards GABAergic inhibition should not be considered as a compensatory effect like the stress-mediated ECS plasticity. In fact, CB1 receptor upregulation on GABAergic synapses is thought to facilitate cocaine-dependent corticostriatal synaptic plasticity (Centonze et al., 2007).
Taken together, these findings provide a new view of the ECS plasticity where the CB1 receptor on GABAergic terminals plays a major role. In this context, there are three important aspects of the susceptibility of CB1 receptors to be upregulated (stress-mediated) or downregulated (reward-mediated) that should be considered. First of all, alterations regarding CB1 receptor-mediated inhibition of neurotransmission are only detectable on GABAergic synapses. Therefore, this differential susceptibility is a new factor that helps to understand the different functionality of CB1 receptors on GABAergic and glutamatergic terminals. Second, stimuli that modify endocannabinoid transmission represent a possible alteration of the ECS reactivity. Consequently, the modulation of the behavioural outcome by the ECS depends on the susceptibility of the CB1 receptor on GABAergic terminals to be up- and downregulated. Third, the consequences associated with these differential susceptibilities should be interpreted in the context of the analyzed behaviour. As a matter of fact, alterations of the CB1 receptor on GABAergic terminals have been considered as a compensatory mechanism to counteract stress stimuli (GABAergic CB1 receptor downregulation) and also as a facilitator to maintain the rewarding properties of cocaine (GABAergic CB1 receptor upregulation).
Fear
Besides its involvement in the modulation of anxiety behaviours, the ECS also regulates acquired fear induced by specific cues. Natural stimuli that signal danger (e.g. pain, predators and loud tones) elicit an innate fear response. This reaction is used in the fear-conditioning paradigm, where an initially neutral stimulus (called conditioned stimulus (CS), e.g. an acoustic, visual or olfactory cue) is presented together with a fear-inducing stimulus (unconditioned stimulus (US), e.g. a mild electric shock delivered to the paws). After one or more pairings of the US with the CS, the subject associates the two stimuli and the presentation of the CS alone is able to evoke a fear response (LeDoux, 2000). Instead of a discrete cue such as a tone and/or light, which are often used as CS, stimuli present in the environment where the US is presented may also acquire aversive properties and elicit conditioned emotional responses (called context conditioning) (Radulovic and Tronson, 2010). In rodents, freezing (Fanselow, 1980) or a startle response to a sudden strong stimulus other than the CS (e.g. Chhatwal et al., 2005; Lin et al., 2006) are often used as an indicator of fear.
After conditioning, the acquired short-term fear memory is consolidated in a more stable long-term memory, a process involving new gene expression and protein synthesis (for review, see Pape and Pare, 2010). Fear expression in a long-term test (re-exposure to CS-only after at least 24 hours) measures the combined effects of acquisition, consolidation and retrieval of the fear memory.
With each CS-only exposure, two opposite processes are initiated. Short exposure triggers a second round of memory consolidation, so that new information can be integrated into the original memory. This process of reconsolidation stabilizes the original memory and requires protein synthesis. When protein synthesis is pharmacologically blocked, the fear memory can be lost (for review, see Tronson and Taylor, 2007). Prolonged or repeated exposure to the CS triggers extinction, resulting in a decline of CS-evoked fear response (for review, see Myers and Davis, 2007). Three different mechanisms have been proposed to explain extinction. First, its behavioural properties indicate that a new inhibitory memory is formed that competes with the initial fear memory. Second, the original fear memory is weakened by changes in synaptic efficacy induced during fear conditioning. Different from these two mechanisms that involve associative learning, non-associative processes play a role in the third mechanism of extinction. There, the responsiveness to the presentation of the non-reinforced CS is decreased by a process called habituation (for review see Herry et al., 2010; Pape and Pare, 2010). The reduced response to the CS seen during and shortly after the extinction session is not stable, because the original memory reappears with the passage of time (spontaneous recovery), in a new context (renewal) and upon unpredictable US presentations (re-instatement) (Myers and Davis, 2007).
Various brain regions have been implicated in the different phases of conditioned fear learning, with a major role being attributed to the amygdala (Pape and Pare, 2010). The BLA is important for acquisition and consolidation of fear memories, although the actual memory does not seem to be stored in this structure (McGaugh, 2002; Pare, 2003). For the reconsolidation and extinction of fear memories, the amygdala has also been implicated. Protein synthesis in this area is required for proper reconsolidation (Nader et al., 2000). The hippocampus stores spatial components (i.e. contextual) of the fear memory (see Pare, 2003). In extinction, the hippocampus and medial PFC (mPFC), especially the infralimbic (IL) area, are involved besides the amygdala (Herry et al., 2010; Pape and Pare, 2010; Sotres-Bayon and Quirk, 2010; Lin et al., 2010). As spontaneous recovery is impaired in rats with a lesion in the mPFC, this area is also involved in the reappearance of fear responses after extinction (Zelinski et al., 2010). Importantly, limited studies in humans hint at a considerable similarity of these processes and pathways between species (Pare, 2003; Delgado et al., 2008).
Components of the ECS, including the CB1 receptor and the synthesizing and degrading enzymes of the lipid ligands, are present in many brain areas that were implicated in the different phases of fear conditioning (Kano et al., 2009). In recent years, research efforts have focused on elucidating the role of the ECS in fear memory processing.
Fear responses during acquisition
The effects of (endo)cannabinoid signalling on behaviour during the initial acquisition phase of conditioned fear have not been studied extensively. As the ECS is also implicated in pain processing (Guindon and Hohmann, 2009; Sagar et al., 2009), studies have mainly attempted to choose conditions that do not change nociception, a response that was monitored by freezing behaviour during the conditioning session (Arenos et al., 2006; Tan et al., 2010; Sink et al., 2010), shock reactivity (Lin et al., 2009) or pain threshold (Marsicano et al., 2002; Marsch et al., 2007). These precautionary measures reduce the risk of confounding during the association process and improve the validity of the investigation of subsequent phases of fear memory processing. The pain threshold of the complete CB1 receptor knockout mouse was not different from that of wildtypes upon first exposure (Marsicano et al., 2002). However, these animals showed a decreased reaction upon repeated shock presentations (Azad et al., 2004). Like the results that were discussed above for anxiety (ECS susceptibility to environmental variables), this emphasizes the importance of a careful choice of experimental conditions. In wildtype mice, one study reported increased freezing during the conditioning session after injection of the CB1 receptor antagonist AM251 (Reich et al., 2008). This effect seemed to be specific for paired CS–US presentations, as it was not seen when the mice received presentations of the CS only (tone) or the US only (1 s, 0.8 mA footshocks) (Reich et al., 2008), which appears to exclude an effect on pain processing. The possible involvement of the ECS in early phases of fear memory processing was also suggested by the finding of a 20% increase in CB1 receptor mRNA expression in the IL cortex 48 hours after contextual fear conditioning (Lisboa et al., 2010).
Fear expression
The fear response to the first CS/context exposure after the conditioning session, also called fear expression, is a result of acquisition, consolidation and retrieval of the fear memory. The involvement of the ECS in fear expression has been studied using a variety of conditioning protocols, with injections of CB1 receptor agonists and antagonists, as well as other manipulators of the ECS at different time points in the phases of the fear conditioning paradigm (pre-/post-conditioning or pre-exposure/test) in different brain regions or systemically administered in both mice and rats. Not surprisingly, mixed outcomes were reported. We will focus our discussion on some important parameters that have been identified in these studies.
First, as with other neurotransmitters (Calandreau et al., 2010; Raybuck and Gould, 2010), there seems to be a difference in the role of the ECS in cued and contextual learning. Intraperitoneal injections of the CB1 receptor antagonist AM251 before the (cued) conditioning session were shown to have opposite effects on fear expression of rats in subsequent context and cue tests (Arenos et al., 2006; Sink et al., 2010). Whereas Arenos et al. found reduced freezing to the context and increased freezing to the tone after AM251 treatment, the work by Sink et al. showed the exact opposite effects with similar doses of AM251. Interestingly, the latter work included adaptation sessions in several contexts before and between the training and test sessions. This experience may alter the basal aversiveness of and hence the stress response to the contexts. Such subtle differences in the initial state of the animals may invert the effect of ECS manipulations through alterations in the brain’s responsiveness to (endo)cannabinoids. Differential effects were also reported after pre-training injections of the CB1 receptor agonist WIN55,212-2, with a reduced fear response to the context, but no effect on freezing to the tone (Pamplona and Takahashi, 2006). The effects in this study (reduced freezing to the context after agonist treatment) resemble those reported by Sink et al. (2010) (increased freezing to the context after antagonist treatment), although the authors do not report the use of adaptation sessions. The conditioning protocol seems to be weaker in this study than in the first two studies. Therefore, as described for anxiety (see above), the effects of ECS manipulations on cued and contextual fear expression seem to depend strongly on subtle external factors. These external factors include, but are not restricted to, previous experiences in different contexts.
Second, the time of injections will determine which learning phases can be affected. Pre-training injections are thought to influence mostly acquisition, whereas post-training injections predominantly influence consolidation. Pre-test injections can only interfere with retrieval (and reconsolidation and extinction, which are discussed below). In auditory fear conditioning, systemic AM251 injections increased freezing to the tone upon re-exposure when given prior to conditioning (acquisition) or prior to re-exposure (retrieval) (Arenos et al., 2006; Reich et al., 2008). However, upon injection immediately after training (consolidation), AM251 did not affect freezing in the test 24 hours later (Reich et al., 2008). A study in which the CB1 receptor agonist WIN55,212-2 was injected systemically either before training or before the 24-hour test, confirmed the involvement of the ECS in acquisition (reduced freezing to the context), but found no effect when the agonist was injected before retrieval (Pamplona and Takahashi, 2006). In studies of inhibitory avoidance, time-dependent effects were also observed. Upon pre-training injection, AEA and the endocannabinoid uptake inhibitor AM404 had no effect on the subsequent latency to step onto the grid floor, whereas the CB1 receptor antagonist AM251 either had no effect or increased this latency at this time point (de Oliveira Alvares et al., 2008b; Ganon-Elazar and Akirav, 2009). With pre-test injections, however, AM251 increased the latency in both reports, whereas AEA or AM404 either had no effect or reduced the latency (de Oliveira Alvares et al., 2008b; Ganon-Elazar and Akirav, 2009). When injected immediately post training, AM251 reduced the latency to step onto the grid floor in the subsequent test (opposite to its effect on retrieval), whereas a low dose of AEA increased this latency (de Oliveira Alvares et al., 2008b). Therefore, reduced ECS activity during acquisition and retrieval seems to increase the subsequent fear response, whereas reduced ECS activity during consolidation may reduce the subsequent fear response.
The divergent results from the latter two studies may reflect a third important source of variation: the site of injection. Because different brain regions are proposed to play different roles in the processing of fear and fear learning, manipulations of the ECS in these regions can be expected to exert differential effects on the expression of fear. In this case, almost identical doses of AM251 seemed to be more effective when injected into the BLA (Ganon-Elazar and Akirav, 2009) than in the hippocampus (de Oliveira Alvares et al., 2008b), but the general tendency of the observed effects was similar. In another study, local pre-test injections of CBD, a plant-derived cannabinoid with multiple endogenous targets (Mechoulam et al., 2007), revealed opposite roles for the IL and the prelimbic (PL) cortex in the retrieval of contextual fear: whereas systemic and PL injections reduced freezing in the context, IL injection increased the fear response (Lemos et al., 2010).
As summarized in Table 1, the results are rather heterogeneous and divergent, although possible factors, such as the influence of cued versus contextual fear conditioning, the time point and site of injections and previous experience (e.g. pre-conditioning adaptation sessions), have to be considered for interpretation of the data and the understanding of the underlying mechanisms. In cued fear conditioning, the exact effect of pre-training (acquisition) ECS manipulations seems to depend crucially on such adaptation sessions, whereas pre-test (retrieval) activation of the ECS generally seems to decrease fear expression. In more spatial paradigms (e.g. contextual fear conditioning), adaptation sessions do not seem to alter the effects, but a clear effect of ECS manipulation was also lacking. Post-training (consolidation) and pre-test (retrieval) manipulations seem to have opposite effects, with the former increasing fear response when in the BLA or hippocampus and reducing fear upon systemic injections; whereas ECS activation during retrieval seems to reduce fear when in the BLA, hippocampus, IL or PAG and to enhance fear expression when systemic or in the PL.
Table 1.
Effects of endocannabinoid system (ECS) manipulations on fear expression (the fear response to the first conditioned stimulus (CS)/context-exposure after conditioning)
| Type | Timing | Site | Paradigm | Presessions | Fear expression after manipulation |
Reference | |
|---|---|---|---|---|---|---|---|
| Observed effect of injection/knockout | Postulated effect of ECS activation | ||||||
| Cued | Pre-train | Systemic | Auditory FC | Different contextse | AM251, AM4113 reduce | Increase | Sink et al. (2010) |
| Systemic | Olfactory FC | Both contexts | AM251 reduces | Increase | Tan et al.(2010) | ||
| BLA & PL | Olfactory FC | Both contexts | AM251 reducesa | Increase | Tan et al. (2010) | ||
| mPFC | Olfactory FC | Both contexts | WIN55,212-2 increases; AM251 reduces | Increase | Laviolette and Grace (2006) | ||
| Systemic | Auditory FC | No | AM251 increases | Reduction | Arenos et al. (2006) | ||
| Systemic | Auditory FC | No | AM251 increases | Reduction | Reich et al. (2008) | ||
| Pre-test | Systemic | Auditory FC | No | AM251 increases | Reduction | Arenos et al. (2006) | |
| Systemic | Auditory FC | No | AM251 increases | Reduction | Reich et al. (2008) | ||
| CeA | Auditory FC | No | AM251 increases | Reduction | Kamprath et al. (2011) | ||
| IL | Visual FC | Conditioning context | WIN55,212-2, AM404, URB597, HU-210 reduce | Reduction | Lin et al. (2009) | ||
| Knockout | Complete | Auditory FC | No | TRPV1 ko reduces | Reduction | Marsch et al. (2007) | |
| Spatial | Pre-train | Systemic | Contextual FC | No | AM251 reduces | Increase | Arenos et al. (2006) |
| Systemic | Contextual FC | Different contextse | AM251 increases | Reduction | Sink et al. (2010) | ||
| Systemic | Contextual FC | No | WIN55,212-2 reduces | Reduction | Pamplona and Takahashi (2006) | ||
| BLA | Inhibitory avoidance | No | AM251 increases | Reduction | Ganon-Elazar and Akirav (2009) | ||
| Post-train | BLA | Inhibitory avoidance | No | WIN55,212-2 increases; AM251 reduces | Increase | Campolongo et al. (2009) | |
| Hippocampus | Inhibitory avoidance | No | AEA increases, AM251 reduces | Increase | de Oliveira Alvares et al. (2008b) | ||
| Hippocampus | Contextual FC | No | AM251 reducesb | Increase | de Oliveira Alvares et al. (2010) | ||
| Systemic | Contextual FC | No | HU-210 reduces | Reduction | Mackowiak et al. (2009) | ||
| Pre-test | Systemic | Contextual FC | No | AM251 reduces | Increase | Arenos et al. (2006) | |
| Systemic | Contextual FC | No | URB597 increases (in presence of pain) | Increase | Butler et al. (2008) | ||
| Systemic | Contextual FC | No | WIN55,212-2 increases; AM251 reduces | Increase | Mikics et al. (2006) | ||
| Systemic | Contextual FC | No | WIN55,212-2 reduces/increasesc | Reductiond | Pamplona et al. (2006) | ||
| BLA | Contextual FC | No | SR141716 increases (in presence of pain) | Reduction | Roche et al. (2007) | ||
| BLA | Inhibitory avoidance | No | AM404 lower; AM251 increases | Reduction | Ganon-Elazar and Akirav (2009) | ||
| Hippocampus | Inhibitory avoidance | No | AM251 increases | Reduction | de Oliveira Alvares et al. (2008b) | ||
| IL | Contextual FC | Conditioning context | AEA, AM404 reduces; AM251 increases | Reduction | Lisboa et al. (2010) | ||
| PAG | Contextual FC | Conditioning context | AEA, AM404 reduces | Reduction | Resstel et al. (2008) | ||
| Knockout | Complete | Contextual FC | No | CB1 receptor ko reduces | Increase | Mikics et al. (2006) | |
| Complete | Barnes maze | No | FAAH ko increases, blocked by SR141716 | Increase | Wise et al. (2009) | ||
In paradigms that test fear expression after ECS manipulation, the type of paradigm (cued vs spatial), time point of manipulation (chronic (knockout), pre-training, post-training, pre-test), site of injection (systemic, various brain areas) and the use or lack of pre-conditioning adaptation sessions can explain part, but not all, of the variation in outcomes. The postulated effect of ECS activation on fear expression (column 7) is derived from the effects of ECS manipulations; studies that reported no effect are not included in this table.
Half the dose in PL-only or BLA-only produced no effect; bwith a strong shocking protocol, or with a weaker shocking protocol only after previous stress; creduction after a low dose, increase after a high dose; dat a low dose; eincluding neither the conditioning context nor the extinction context.
BLA: basolateral amygdale, CeA: central amygdale, FC: fear conditioning, IL: infralimbic cortex, ko: knockout, mPFC: medial prefrontal cortex, PAG: periaqueductal gray, PL: prelimbic cortex.
Fear reconsolidation
It is proposed that upon each re-exposure to the CS or the shock context, the original fear memory is retrieved and thereby destabilized so that the memory can be strengthened and updated with new relevant information before reconsolidation (Tronson and Taylor, 2007). Some studies have reported the ECS to be involved in this process of reconsolidation. These studies have employed various strategies to distinguish reconsolidation from extinction: intensified training (Kobilo et al., 2007), shorter re-exposure (Suzuki et al., 2004; de Oliveira Alvares et al., 2008a; Suzuki et al., 2008) or post-re-exposure injection of the substance to be studied (Lin et al., 2006).
Injections of the CB1 receptor antagonist SR141716 did not affect reconsolidation of the fear memory (Suzuki et al., 2004; Kobilo et al., 2007; Suzuki et al., 2008), but abolished the amnesia induced by anisomycin injections (Suzuki et al., 2008). In addition, hypo-activation of the ECS by hippocampal AM251 injections improved reconsolidation (de Oliveira Alvares et al., 2008a), and hyperactivation by AEA, WIN55,212-2 or HU-210 injections in rats all reduced reconsolidation of the fear memory. These effects were independent of injection site (hippocampus, amygdala or insular cortex), paradigm (cued or contextual fear conditioning, or conditioned taste aversion) and time of measurement (at re-exposure, after re-instatement or spontaneous recovery after 7 days) (Lin et al., 2006; Kobilo et al., 2007; de Oliveira Alvares et al., 2008a). These studies all point to an amnesic role of endocannabinoid signalling. Therefore, it seems likely that activation of the ECS reduces the reconsolidation of fear memories, whereas hypo-activation of the ECS promotes their reconsolidation, and thereby leads to enduring fear responses.
Fear extinction
The ECS plays a major role in extinction in the classical fear conditioning paradigm (Marsicano et al., 2002) as well as in fear-potentiated startle (Chhatwal et al., 2005) and in the more hippocampus-dependent trace (Reich et al., 2008) and context conditioning paradigms (Suzuki et al., 2004). As several groups showed that endocannabinoid signalling is dispensable for extinction of appetitive memories (e.g. Niyuhire et al., 2007; Manwell et al., 2009), the role of the endocannabinoids in extinction seems to be specific for aversive memories. In recent years, many studies focused on uncovering the mechanism of CB1 receptor signalling in fear extinction and on its function in the different brain regions involved in extinction learning. For this part of the review, we selected studies that reported similar initial freezing to CS presentation, so that extinction behaviour could be compared from a similar starting point.
CB1 receptor knockout mice are impaired in short-term freezing reduction over a 200-s tone presentation (within-session), as well as in long-term extinction (between-session) after cued conditioning (Marsicano et al., 2002). In the same study, similar results were obtained in wildtype mice treated with the CB1 receptor antagonist SR141716 before extinction training. Thus, CB1 receptor signalling did not seem to be involved in consolidation of the extinction memory, as the authors found no effect of pharmacological blockade immediately after extinction training (Marsicano et al., 2002). CB1 receptor knockout mice also showed a sustained freezing response in a sensitization paradigm in which fear response to a new, potentially harmful stimulus was measured after experiencing an inescapable footshock (Kamprath et al., 2006). This lack of habituation to neutral as well as conditioned stimuli suggests that CB1 receptor signalling is critically involved in this non-associative learning process (Kamprath et al., 2006). This is consistent with the findings that endocannabinoids mediate habituation to homotypic stressors (Patel et al., 2005). To dissect the role of the ECS in short- versus long-term habituation in extinction of acquired fear to a cue, Plendl and Wotjak (2010) compared CB1 receptor knockout mice and wildtype littermates in different exposure modalities. Freezing behaviour was analyzed in two different protocols, one with shorter tone presentations (10 × 20 s) with variable intervals and the other with constant tone presentation (over 200 s). These experiments showed that CB1 receptor signalling is dispensable for between-session extinction, whereas within-session extinction was strongly dependent on intact CB1 receptor signalling. These recent findings underline the involvement of the ECS in non-associative habituation learning. Another study suggested that endocannabinoids reduce the basal state of responsiveness during an aversive encounter (Reich et al., 2008). In this study, systemic injections of the CB1 receptor antagonist AM251 prior to extinction training sessions in a trace conditioning paradigm (footshock not directly after CS offset, but after a short delay), impaired freezing reduction. The strength of this impairment depended on the current state of the ECS, rather than on the history of AM251 versus vehicle injections. Additionally, the fact that the observed levels of baseline freezing (before the CS) in the extinction context were also dependent on the current state of the ECS, was interpreted to indicate an involvement of CB1 receptor signalling in the generalization of the fear response (Reich et al., 2008). An explanation for the high levels of generalized freezing in the setup used by Reich et al. might be the strong conditioning protocol (eight CS–US pairings), as the fear-reducing effect of the ECS highly depends on the strength of the harmful stimulus encountered (Kamprath et al., 2009). In the latter study, several conditional CB1 receptor knockout mice with deletion of the CB1 receptor only in specific neuronal subpopulations were tested to uncover the neurotransmitter systems that are involved in CB1 receptor-controlled fear adaptation. The fear-alleviating effect of endocannabinoids depended on endocannabinoid-driven modulation of glutamatergic transmission (Kamprath et al., 2009). This is consistent with the finding that stress habituation mediated by the ECS also crucially involves the modulation of glutamatergic neurotransmission (Patel and Hillard, 2008). In the conditional CB1 receptor knockout mice used by Kamprath et al. (2009), the CB1 receptor is deleted on all forebrain glutamatergic neurons, including some of the main brain regions involved in fear extinction, i.e. in hippocampus, PFC and BLA. It remains to be investigated which of these regions with altered ECS-driven modulation of glutamatergic neurotransmission is involved in the emergence of the phenotypes observed.
In cued fear conditioning, (endo)cannabinoids are thought to exert one of their main effects in the BLA, as presentation of the CS during the extinction trial increases endocannabinoid levels selectively in the BLA (Marsicano et al., 2002). In several recent studies, the relevance of amygdala-specific endocannabinoid signalling was addressed. BLA-targeted infusion of CB1 receptor antagonist was found to impair extinction both in fear conditioning (unilateral SR141716) (Roche et al., 2007) and in inhibitory avoidance (bilateral AM251) (Ganon-Elazar and Akirav, 2009). There is recent evidence that the CB1 receptor is not only present in the BLA, but also on axon terminals of glutamatergic projection neurons from BLA to the medial part of the CeA and on GABAergic neurons projecting from the lateral to the medial CeA (Kamprath et al., 2011). With local infusion of CB1 receptor antagonist into the BLA or the CeA, before the extinction training in a cued conditioning paradigm, the authors demonstrated that CB1 receptors in the BLA are involved in long-term extinction processes, whereas CB1 receptors in the CeA are more important for acute fear expression and within-session reduction of freezing.
As the hippocampus is known to process contextual information and thus transfers the contextual representation to the amygdala, where it is associated with the US (Phillips and LeDoux, 1992), contextual fear conditioning is often used to investigate the function of hippocampal endocannabinoid signalling in fear conditioning. Bilateral infusion of the CB1 receptor antagonist AM251 into the dorsal hippocampus of rats after an extinction session blocked extinction consolidation as there was no extinction retention detectable in the test on the following day (de Oliveira Alvares et al., 2008a).
The PFC is also thought to be involved in extinction, as extinction training induced synaptic plasticity in this area (Herry and Garcia, 2002). Two recent studies addressed PFC function in extinction. Local infusion of SR141716 in the insular cortex of rats blocks the extinction of conditioned taste aversion (Kobilo et al., 2007). IL infusion of the CB1 receptor antagonist AM251 blocked cue-alone-induced reduction of fear-potentiated startle in rats (Lin et al., 2009). Thus, cortical blockade of CB1 receptor signalling seems to decrease the inhibitory output of the amygdala, which is necessary to reduce the fear output (Quirk and Mueller, 2008).
These pharmacological studies with either systemic or brain region-specific blockade of endocannabinoid signalling in mice and rats together with the investigations that used complete or conditional CB1 receptor knockout mice showed that the ECS is crucial for efficient extinction learning. Therefore, it is tempting to hypothesize that the activation of the CB1 receptor would improve extinction, but the available data reveal a more complicated picture.
Recent studies that found a significant effect of treatment with CB1 receptor agonist on extinction were mostly performed with rats, suggesting that the agonist treatment might have species-specific effects (analogous to the data of Haller et al. (2007) discussed in the section on ECS susceptibility to environmental variables). Several studies with rats have shown that systemic treatment with AM404, an inhibitor of endocannabinoid uptake and/or metabolism as well as with URB597, an inhibitor of the AEA-degrading enzyme FAAH, enhanced extinction in several paradigms (Chhatwal et al., 2005; Bitencourt et al., 2008; Pamplona et al., 2008; Manwell et al., 2009) and that this extinction is more resistant to re-instatement (Chhatwal et al., 2005). When CB1 receptor agonists were administered locally, similar facilitation of extinction was demonstrated for several brain regions that modulate and process learned fear. Thus, injection of AEA into the hippocampus of rats directly after an extinction session led to improved extinction retention on the following day (de Oliveira Alvares et al., 2008a). Microinjection of WIN55,212-2 into the BLA of rats before exposure to elevated platform stress, reversed the effect of impaired extinction usually induced by the stressor (Ganon-Elazar and Akirav, 2009). WIN55,212-2 also facilitated extinction of fear memory when it was infused into the IL cortex of rats in a dose that had no effect on startle potentiation alone (Lin et al., 2009). Interestingly, intra-IL administration of WIN55,212-2 decreased startle potentiation irrespective of whether rats received CS+ or CS− alone trials, again strongly pointing to an involvement of (endo) cannabinoid signalling in long-term adaptation to aversive situations (Kamprath et al., 2006).
Systemic treatment with CB1 receptor agonist also appears to influence extinction, but in a dose-dependent manner, resembling the biphasic effect found for anxiety-related behaviours. Low doses of WIN55,212-2 were shown to reduce within-session extinction in a context conditioning paradigm and even resulted in long-term reduction in a drug-free test 1 week later (Pamplona et al., 2008). However, treatment with high doses of this agonist disrupted extinction in context conditioning (Pamplona et al., 2006) and had no extinction-improving effect in fear-potentiated startle (Chhatwal et al., 2005). Chronic treatment with a CB1 receptor agonist leads to impaired extinction as shown in a study by Ashton et al. (2008), demonstrating that treatment with a high dose of Δ9-THC over 6 days strongly delayed cued extinction. Seven days of chronic treatment with WIN55,212-2 made rats resistant to the reduction of fear-potentiated startle, which was induced by extinction training in non-treated rats (Lin et al., 2008). Also, local infusion of WIN55,212-2 into the IL, which was previously shown to improve extinction, did not have any effect after 7 days of chronic treatment. The authors showed that the pretreated rats had significantly lower levels of CB1 receptor in synaptoneurosome preparations from the IL and that the inhibition of GABA release in the IL, which would produce the startle-reducing effect of WIN55,212-2 was attenuated by 7 days of chronic WIN55,212-2 treatment (Lin et al., 2008). Thus, treatment with CB1 receptor agonist improves extinction in rats when administered in a low dose, whereas it has no effect or even delays extinction when a high dose or chronic treatment is used. This effect of high dose or chronic agonist treatment might be due to internalization/desensitization of the CB1 receptor upon strong or continuous activation (Wu et al., 2008), leading to a resistance to exogenous as well as endogenous cannabinoids and therefore having the same effect as blocking CB1 receptor signalling. Thus, the clinical use of CB1 receptor agonists over a longer time does not seem to be a promising therapeutic approach.
Conclusions
The ECS is centrally involved in the regulation of anxiety and fear responses. The recent elucidations of the underlying mechanisms appear promising in respect to the development of new therapeutic strategies for the treatment of anxiety disorders.
It has been commonly accepted that the anxiety state can be altered by cannabinoids in a bimodal manner, implying an activation of different subsets of neurons dependent on the conditions and the doses. Concerning CB1 receptor signalling pathways, the presynaptic release of two major neurotransmitters, namely GABA and glutamate, is affected by CB1 receptor activation (Laaris et al., 2010; Hoffman et al., 2010). Based on pharmacological data, CB1 receptors on GABAergic and glutamatergic terminals can be distinguished by differences in at least two important characteristics: basal activity and sensitivity. Consequently, the influence of this receptor on GABA release seems to be required to develop anxiogenic-like responses to a high dose of cannabinoids. On the other hand, activation of the CB1 receptor on glutamatergic terminals apparently mediates the anxiolytic-like reaction commonly observed after exposure to a low dose of cannabinoids.
The results obtained from the analysis of genetically modified mice in anxiety are in agreement with the idea of opposite effects being exerted by the same receptor on different neuronal subpopulations in impulsivity and feeding behaviour (Lafenetre et al., 2009; Bellocchio et al., 2010). In this context, the idea previously proposed by Katona and Freund (2008) about GABAergic CB1 receptors as members of a regular buffer of inhibitory transmission, in opposition to the glutamatergic CB1 receptors, which have the task to reduce excitatory transmission only after excessive glutamate release, would fit perfectly into this model of CB1 receptor-dependent regulation of anxiety by cannabinoids. Considering the predominant action of stress/reward-mediated plasticity on GABAergic transmission, the CB1 receptors on GABAergic terminals could be considered as a dynamic element that can be altered in order to continuously maintain an appropriate and optimal emotional reactivity. The CB1 receptors on glutamatergic terminals, on the other hand, would be required only in situations of more prominent GABA-glutamate unbalance, emphasizing the ideal profile of the CB1 receptor to be a pharmacological target in the treatment of anxiety disorders.
However, the regulation of GABAergic and glutamatergic systems is not the only mechanism by which the ECS controls anxiety-related behaviours. Monoaminergic signalling has been shown to be induced after treatment with both synthetic CB1 receptor agonists (WIN55,212-2, HU-210) and the FAAH inhibitor URB597 (Page et al., 2007; Gobbi et al., 2005). This enhancement of monoaminergic transmission has been related to anxiolytic and anxiogenic responses, depending on the specific neurotransmitter affected. In this context, increases of NE release in the locus coeruleus and PFC commonly led to anxiogenic responses, whereas serotonergic enhancement in the dorsal raphe nucleus and PFC induced anxiolytic responses accompanied by increased sociability and antidepressant-like behaviours. Thus, monoaminergic transmission must be considered as an important mechanism in the modulation of emotional homeostasis by the ECS.
Furthermore, the promiscuity of (endo)cannabinoids entails also non-CB1 receptor-dependent processes, which can then account for certain aspects of the anxiety behaviour after cannabinoid exposure. Regarding the anxiolytic response, the serotonin receptor 5-HT1A has been shown to be involved in the effect of CBD. On the other hand, TRPV1 seems to be required for the anxiogenic effect of AEA.
Fear conditioning, the most widely used paradigm to investigate fear behaviour, involves various phases of learning, introducing a new level of complexity as compared to anxiety tests. The ECS has been shown to be differentially involved in the various phases of fear learning.
A proper interpretation of the participation of the ECS in the phase of fear memory acquisition can be compromised by the fact that the ECS regulates pain processing. However, exogenous cannabinoids hardly affect the fear response during the training session, as long as the dose is carefully chosen outside of the range in which the ECS modulates nociception. A lack of effect of ECS manipulation on the phase of acquisition provides a well-comparable starting point of the fear response between the different (treatment) groups, assuring the validity of the analysis of ECS involvement in subsequent phases of fear learning.
The role of the ECS becomes increasingly prominent in the later phases of the fear-conditioning paradigm. Fear expression (the result of acquisition, consolidation and retrieval) seems to be affected more strongly by (endo)cannabinoids than the fear acquisition phase. However, this effect is not robust and seems to be highly dependent on the multiple factors described in Table 1.
The role of the ECS is clearer in the next two phases of the paradigm, i.e. in reconsolidation and extinction, which are triggered by CS-only exposures. Interestingly, activation of the ECS, achieved by different substances, in various paradigms and conditions, deteriorates reconsolidation. This emphasizes the amnesic properties of endocannabinoid signalling. In contrast, intact CB1 receptor signalling appears to be essential for proper extinction of aversive memories. Therefore, it may be postulated that the ECS tone determines the balance between the processes of maintaining or strengthening the original memory (reconsolidation) and the establishment of a new memory or habituation to the situation (extinction). In this way, the ECS may protect the organism from over-reaction to aversive events. This suggests that endocannabinoid-mediated plasticity may play a similar role in fear memory-processing as in anxiety, namely to ensure an appropriate reaction to negative events. Thus, the ECS may be considered as a regulatory buffer system for emotional responses.
Furthermore, the effects of exogenous cannabinoid administration depend on the pre-existing activity of the ECS, which in turn depends on the initial state of the system as influenced by the life history of the animal (e.g. stress) (de Oliveira Alvares et al., 2008a). This may offer an explanation for the strong dependence of reported effects on subtle differences in the experimental conditions used. It also emphasizes the importance of determining the appropriate physiological range for these conditions (e.g. dose, shock intensity, strength of extinction protocol). The importance of administration of the right dose can be deduced from the contrasting data on extinction obtained with low and high doses of CB1 receptor agonists (Pamplona et al., 2006; 2008).
To further elucidate the underlying mechanisms through which the ECS modulates fear behaviour, it is essential to characterize its downstream signalling pathways. In particular, it should be established whether ECS signalling in fear processing has any differential effects on GABAergic and glutamatergic signalling, as found in anxiety regulation.
Finding new molecular mechanisms that underlie ECS modulation of aversive memories and anxiety will be helpful in the search for novel therapeutic approaches. Side effects associated with current CB1 receptor-based therapies are related to the ubiquitous presence of the CB1 receptor on different neuronal subpopulations. Therefore, new approaches may wish to focus specifically on targeting particular neuronal subpopulations of interest.
Acknowledgments
The authors would like to thank Michael Plenikowski for help with the design of the figure and Dr Robin White for critical reading of the manuscript.
Funding
We would like to acknowledge the grants to BL from the German Research Foundation (Priority Program SPP1226 on Nicotine, Research Unit FOR926 on Cannabinoids, and Collaborative Research Centre SFB/TRR 58 on Fear and Anxiety).
Conflict of interest
None declared.
References
- Antonelli T, Tomasini MC, Mazza R, Fuxe K, Gaetani S, Cuomo V, et al. (2009) Cannabinoid CB1 and cholecystokinin CCK2 receptors modulate, in an opposing way, electrically evoked [3H]GABA efflux from rat cerebral cortex cell cultures: possible relevance for cortical GABA transmission and anxiety. J Pharmacol Exp Ther 329: 708–717 [DOI] [PubMed] [Google Scholar]
- Arenos JD, Musty RE, Bucci DJ. (2006) Blockade of cannabinoid CB1 receptors alters contextual learning and memory. Eur J Pharmacol 539: 177–183 [DOI] [PubMed] [Google Scholar]
- Ashton JC, Smith PF, Darlington CL. (2008) The effect of delta 9-tetrahydrocannabinol on the extinction of an adverse associative memory. Pharmacology 81: 18–20 [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. (2010) CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160: 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad SC, Huge V, Schoeps P, Hilf C, Beyer A, Dodt HU, et al. (2004) Endogenes Cannabinoidsystem: Einfluss auf neuronale Plastizität und Schmerzgedächtnis. Schmerz 19: 521–527 [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. (2007) Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci 27: 11700–11711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, Grandes P, et al. (2010) Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci 13: 281–283 [DOI] [PubMed] [Google Scholar]
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, et al. (2007) Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol 151: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. (2008) Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 18: 849–859 [DOI] [PubMed] [Google Scholar]
- Boundy VA, Gold SJ, Messer CJ, Chen J, Son JH, Joh TH, Nestler EJ. (1998) Regulation of tyrosine hydroxylase promoter activity by chronic morphine in TH9. 0-LacZ transgenic mice. J Neurosci 18: 9989–9995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Limonta V, Malabarba L, Zani A, Sala M. (2007) 5-HT1A receptors are involved in the anxiolytic effect of Delta9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague-Dawley rats. Eur J Pharmacol 555: 156–163 [DOI] [PubMed] [Google Scholar]
- Brusco A, Tagliaferro PA, Saez T, Onaivi ES. (2008) Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann N Y Acad Sci 1139: 450–457 [DOI] [PubMed] [Google Scholar]
- Butler RK, Rea K, Lang Y, Gavin AM, Finn DP. (2008) Endocannabinoid-mediated enhancement of fear-conditioned analgesia in rats: opioid receptor dependency and molecular correlates. Pain 140: 491–500 [DOI] [PubMed] [Google Scholar]
- Calandreau L, Desgranges B, Jaffard R, Desmedt A. (2010) Switching from contextual to tone fear conditioning and vice versa: the key role of the glutamatergic hippocampal-lateral septal neurotransmission. Learn Mem 17: 440–443 [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. (2009) Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A 106: 4888–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AC, Ferreira FR, Guimaraes FS, Lemos JI. (2010) Facilitation of endocannabinoid effects in the ventral hippocampus modulates anxiety-like behaviors depending on previous stress experience. Neuroscience 167: 238–246 [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. (2008) Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 199: 223–230 [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimaraes FS. (2009) Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog Neuropsychopharmacol Biol Psychiatry 33: 1517–1521 [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Mackie K, Van Bockstaele EJ. (2010) Cannabinoid modulation of limbic forebrain noradrenergic circuitry. Eur J Neurosci 31: 286–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano T, Gaetani S, Macheda T, Laconca L, Romano A, Morgese MG, et al. (2011) Evaluation of the emotional phenotype and serotonergic neurotransmission of fatty acid amide hydrolase-deficient mice. Psychopharmacology (Berl) 214: 465–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Rossi S, De Chiara V, Prosperetti C, Battista N, Bernardi G, et al. (2007) Chronic cocaine sensitizes striatal GABAergic synapses to the stimulation of cannabinoid CB1 receptors. Eur J Neurosci 25: 1631–1640 [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, Ressler KJ. (2005) Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology 30: 516–524 [DOI] [PubMed] [Google Scholar]
- Cristino L, De Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. (2006) Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139: 1405–1415 [DOI] [PubMed] [Google Scholar]
- De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, et al. (2010) Voluntary exercise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the striatum. Neuropsychopharmacology 35: 374–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Engelke DS, Diehl F, Scheffer-Teixeira R, Haubrich J, de Freitas Cassini L, et al. (2010) Stress response recruits the hippocampal endocannabinoid system for the modulation of fear memory. Learn Mem 17: 202–209 [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Molina VA, Quillfeldt JA. (2008a) Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience 154: 1648–1655 [DOI] [PubMed] [Google Scholar]
- de Oliveira Alvares L, Pasqualini Genro B, Diehl F, Quillfeldt JA. (2008b) Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem 90: 1–9 [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. (2008) Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron 59: 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. (1980) Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 15: 177–182 [DOI] [PubMed] [Google Scholar]
- Fuxe K, Marcellino D, Rivera A, Diaz-Cabiale Z, Filip M, Gago B, et al. (2008) Receptor-receptor interactions within receptor mosaics. Impact on neuropsychopharmacology. Brain Res Rev 58: 415–452 [DOI] [PubMed] [Google Scholar]
- Ganon-Elazar E, Akirav I. (2009) Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J Neurosci 29: 11078–11088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. (2005) Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA 102: 18620–18625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Langton JM, Richardson R. (2011) Pharmacological enhancement of fear reduction: preclinical models. Br J Pharmacol 164: 1230–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. (2009) The endocannabinoid system and pain. CNS Neurol Disord Drug Targets 8: 403–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Matyas F, Soproni K, Varga B, Barsy B, Nemeth B, et al. (2007) Correlated species differences in the effects of cannabinoid ligands on anxiety and on GABAergic and glutamatergic synaptic transmission. Eur J Neurosci 25: 2445–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Barna I, Freund TF. (2004a) Context-dependent effects of CB1 cannabinoid gene disruption on anxiety-like and social behaviour in mice. Eur J Neurosci 19: 1906–1912 [DOI] [PubMed] [Google Scholar]
- Haller J, Varga B, Ledent C, Freund TF. (2004b) CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav Pharmacol 15: 299–304 [DOI] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Gyimesi Pelczer K, Yasar S, Panlilio LV, Goldberg S. (2009) Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology 204: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. (2007) Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci 27: 1211–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. (2010) Neuronal circuits of fear extinction. Eur J Neurosci 31: 599–612 [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. (2002) Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J Neurosci 22: 577–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. (2010) Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci 30: 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, et al. (2010) A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 64: 92–96 [DOI] [PubMed] [Google Scholar]
- Jacob W, Yassouridis A, Marsicano G, Monory K, Lutz B, Wotjak CT. (2009) Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav 8: 685–698 [DOI] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. (2000) In vivo gene modification elucidates subtype-specific functions of alpha(2)-adrenergic receptors. J Pharmacol Exp Ther 293: 1–7 [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, et al. (2006) Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci 26: 6677–6686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Plendl W, Marsicano G, Deussing JM, Wurst W, Lutz B, Wotjak CT. (2009) Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav 8: 203–211 [DOI] [PubMed] [Google Scholar]
- Kamprath K, Romo-Parra H, Haering M, Doengi M, Lutz B, Pape HC. (2011) Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 36: 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. (2009) Endocannabinoid-mediated control of synaptic transmission. Physiol Rev 89: 309–380 [DOI] [PubMed] [Google Scholar]
- Karson MA, Whittington KC, Alger BE. (2008) Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology 54: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF. (2008) Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med 14: 923–930 [DOI] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, Piomelli D, et al. (2006) Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci 26: 5628–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, et al. (2006) The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci 26: 2991–3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Hazvi S, Dudai Y. (2007) Role of cortical cannabinoid CB1 receptor in conditioned taste aversion memory. Eur J Neurosci 25: 3417–3421 [DOI] [PubMed] [Google Scholar]
- Laaris N, Good CH, Lupica CR. (2010) Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology 59: 121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. (2009) Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology 57: 715–721 [DOI] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. (2007) The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol Res 56: 367–381 [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. (2006) Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26: 6458–6468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. (2000) Emotion circuits in the brain. Annu Rev Neurosci 23: 155–184 [DOI] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. (2010) Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci 30: 7993–8000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, Guimaraes FS. (2010) Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res 207: 105–111 [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Chen PS, Gean PW. (2008) Chronic cannabinoid administration in vivo compromises extinction of fear memory. Learn Mem 15: 876–884 [DOI] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Gean PW. (2006) Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem 13: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. (2009) The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb Cortex 19: 165–175 [DOI] [PubMed] [Google Scholar]
- Lin PY, Wang SP, Tai MY, Tsai YF. (2010) Differential involvement of medial prefrontal cortex and basolateral amygdala erk in extinction of conditioned taste aversion is dependent on different intervals of extinction following conditioning. Neuroscience 171: 125–133 [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Reis DG, da Silva AL, Correa FM, Guimaraes FS, Resstel LB. (2010) Cannabinoid CB1 receptors in the medial prefrontal cortex modulate the expression of contextual fear conditioning. Int J Neuropsychopharmacol 13: 1163–1173 [DOI] [PubMed] [Google Scholar]
- Lisboa SF, Resstel LB, Aguiar DC, Guimaraes FS. (2008) Activation of cannabinoid CB1 receptors in the dorsolateral periaqueductal gray induces anxiolytic effects in rats submitted to the Vogel conflict test. Eur J Pharmacol 593: 73–78 [DOI] [PubMed] [Google Scholar]
- Mackowiak M, Chocyk A, Dudys D, Wedzony K. (2009) Activation of CB1 cannabinoid receptors impairs memory consolidation and hippocampal polysialylated neural cell adhesion molecule expression in contextual fear conditioning. Neuroscience 158: 1708–1716 [DOI] [PubMed] [Google Scholar]
- Manwell LA, Satvat E, Lang ST, Allen CP, Leri F, Parker LA. (2009) FAAH inhibitor, URB-597, promotes extinction and CB(1) antagonist, SR141716, inhibits extinction of conditioned aversion produced by naloxone-precipitated morphine withdrawal, but not extinction of conditioned preference produced by morphine in rats. Pharmacol Biochem Behav 94: 154–162 [DOI] [PubMed] [Google Scholar]
- Marsch R, Foeller E, Rammes G, Bunck M, Kossl M, Holsboer F, et al. (2007) Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J Neurosci 27: 832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. (2002) The endogenous cannabinoid system controls extinction of aversive memories. Nature 418: 530–534 [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. (2002) Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 159: 379–387 [DOI] [PubMed] [Google Scholar]
- McGaugh JL. (2002) Memory consolidation and the amygdala: a systems perspective. Trends Neurosci 25: 456–461 [DOI] [PubMed] [Google Scholar]
- McLaughlin RJ, Hill MN, Gorzalka BB. (2009) Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol 624: 71–76 [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. (2007) Cannabidiol–recent advances. Chem Biodivers 4: 1678–1692 [DOI] [PubMed] [Google Scholar]
- Micale V, Cristino L, Tamburella A, Petrosino S, Leggio GM, Drago F, Di Marzo V. (2009) Anxiolytic effects in mice of a dual blocker of fatty acid amide hydrolase and transient receptor potential vanilloid type-1 channels. Neuropsychopharmacology 34: 593–606 [DOI] [PubMed] [Google Scholar]
- Mikics E, Dombi T, Barsvari B, Varga B, Ledent C, Freund TF, Haller J. (2006) The effects of cannabinoids on contextual conditioned fear in CB1 knockout and CD1 mice. Behav Pharmacol 17: 223–230 [DOI] [PubMed] [Google Scholar]
- Millan MJ. (2003) The neurobiology and control of anxious states. Prog Neurobiol 70: 83–244 [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, et al. (2006) The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron 51: 455–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, Guimaraes FS. (2008) Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1. Neuropharmacology 54: 141–150 [DOI] [PubMed] [Google Scholar]
- Moreira FA, Aguiar DC, Campos AC, Lisboa SF, Terzian AL, Resstel LB, Guimaraes FS. (2009) Antiaversive effects of cannabinoids: is the periaqueductal gray involved. Neural Plast 2009: 625469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NH, Stanford IM, Woodhall GL. (2009) Functional CB2 type cannabinoid receptors at CNS synapses. Neuropharmacology 57: 356–368 [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. (2007) Mechanisms of fear extinction. Mol Psychiatry 12: 120–150 [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406: 722–726 [DOI] [PubMed] [Google Scholar]
- Naderi N, Haghparast A, Saber-Tehrani A, Rezaii N, Alizadeh AM, Khani A, Motamedi F. (2008) Interaction between cannabinoid compounds and diazepam on anxiety-like behaviour of mice. Pharmacol Biochem Behav 89: 64–75 [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. (2007) Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology 192: 61–70 [DOI] [PubMed] [Google Scholar]
- Niyuhire F, Varvel SA, Thorpe AJ, Stokes RJ, Wiley JL, Lichtman AH. (2007) The disruptive effects of the CB1 receptor antagonist rimonabant on extinction learning in mice are task-specific. Psychopharmacology (Berl) 191: 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ, Stein DJ. (2006) Understanding the neurobiology of comorbidity in anxiety disorders. CNS Spectr 11: 13–20 [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Tsubokawa H, Mizushima I, Yoneda N, Zimmer A, Kano M. (2002) Presynaptic cannabinoid sensitivity is a major determinant of depolarization-induced retrograde suppression at hippocampal synapses. J Neurosci 22: 3864–3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong JP, Patel S, Meozzi PA, Myers L, et al. (2008) Functional expression of brain neuronal CB2 cannabinoid receptors are involved in the effects of drugs of abuse and in depression. Ann N Y Acad Sci 1139: 434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Bitencourt RM, Takahashi RN. (2008) Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem 90: 290–293 [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, Takahashi RN. (2006) The cannabinoid receptor agonist WIN 55,212–2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 188: 641–649 [DOI] [PubMed] [Google Scholar]
- Pamplona FA, Takahashi RN. (2006) WIN 55212–2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci Lett 397: 88–92 [DOI] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. (2008) Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett 431: 1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. (2007) Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav 86: 162–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. (2010) Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90: 419–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare D. (2003) Role of the basolateral amygdala in memory consolidation. Prog Neurobiol 70: 409–420 [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. (2006) Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther 318: 304–311 [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. (2008) Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur J Neurosci 27: 2821–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. (2005) Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci 21: 1057–1069 [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. (1992) Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106: 274–285 [DOI] [PubMed] [Google Scholar]
- Pillay NS, Stein DJ. (2007) Emerging anxiolytics. Expert Opin Emerg Drugs 12: 541–554 [DOI] [PubMed] [Google Scholar]
- Piomelli D. (2003) The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884 [DOI] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. (2010) Dissociation of within- and between-session extinction of conditioned fear. J Neurosci 30: 4990–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. (2008) Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33: 56–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher DJ, Meier SE, Shi L, Ho WS, Jarrahian A, Hillard CJ. (2008) Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology 54: 108–116 [DOI] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. (2010) Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 21: 1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. (2010) The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol Learn Mem 94: 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich CG, Mohammadi MH, Alger BE. (2008) Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol 22: 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Lisboa SF, Aguiar DC, Correa FM, Guimaraes FS. (2008) Activation of CB1 cannabinoid receptors in the dorsolateral periaqueductal gray reduces the expression of contextual fear conditioning in rats. Psychopharmacology (Berl) 198: 405–411 [DOI] [PubMed] [Google Scholar]
- Rezayat M, Roohbakhsh A, Zarrindast MR, Massoudi R, Djahanguiri B. (2005) Cholecystokinin and GABA interaction in the dorsal hippocampus of rats in the elevated plus-maze test of anxiety. Physiol Behav 84: 775–782 [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz M, Bajo M, Siggins GR, Parsons LH, Schweitzer P. (2010) The endocannabinoid system tonically regulates inhibitory transmission and depresses the effect of ethanol in central amygdala. Neuropsychopharmacology 35: 1962–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M, O’Connor E, Diskin C, Finn DP. (2007) The effect of CB(1) receptor antagonism in the right basolateral amygdala on conditioned fear and associated analgesia in rats. Eur J Neurosci 26: 2643–2653 [DOI] [PubMed] [Google Scholar]
- Roohbakhsh A, Keshavarz S, Hasanein P, Rezvani ME, Moghaddam AH. (2009) Role of endocannabinoid system in the ventral hippocampus of rats in the modulation of anxiety-like behaviours. Basic Clin Pharmacol Toxicol 105: 333–338 [DOI] [PubMed] [Google Scholar]
- Rossi S, De C V, Musella A, Kusayanagi H, Mataluni G, Bernardi G, et al. (2008) Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J Neurosci 28: 7284–7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, De C V, Musella A, Sacchetti L, Cantarella C, Castelli M, et al. (2010) Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol 78: 260–268 [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Lovejoy DA, Tan LA. (2010) Behavioral effects of neuropeptides in rodent models of depression and anxiety. Peptides 31: 736–756 [DOI] [PubMed] [Google Scholar]
- Rotzinger S, Vaccarino FJ. (2003) Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. J Psychiatry Neurosci 28: 171–181 [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Guidali C, Vigano D, Realini N, Valenti M, Massi P, Parolaro D. (2008a) CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 54: 151–160 [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Castiglioni C, Guidali C, Vigano D, Marras E, et al. (2008b) Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex 18: 1292–1301 [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. (2005) Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30: 1037–1043 [DOI] [PubMed] [Google Scholar]
- Sagar DR, Gaw AG, Okine BN, Woodhams SG, Wong A, Kendall DA, Chapman V. (2009) Dynamic regulation of the endocannabinoid system: implications for analgesia. Mol Pain 5: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherma M, Medalie J, Fratta W, Vadivel SK, Makriyannis A, Piomelli D, et al. (2008) The endogenous cannabinoid anandamide has effects on motivation and anxiety that are revealed by fatty acid amide hydrolase (FAAH) inhibition. Neuropharmacology 54: 129–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon AB, Gorman JM. (2006) Advances in the treatment of anxiety: targeting glutamate. NeuroRx 3: 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Segovia KN, Collins LE, Markus EJ, Vemuri VK, Makriyannis A, et al. (2010) The CB1 inverse agonist AM251, but not the CB1 antagonist AM4113, enhances retention of contextual fear conditioning in rats. Pharmacol Biochem Behav 95: 479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P. (2005) Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology 49: 653–659 [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. (2010) Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol 20: 231–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steindel F, Haering M, Marsicano G, Lutz B, Monory K. (2008) Differential coupling of G proteins to CB1 receptors in hippocampal glutamatergic and GABAergic neurons, 18th Annual Symposium on the Cannabinoids, Burlington, Vermont, International Cannabinoid Research Society p. 119 [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24: 4787–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Mukawa T, Tsukagoshi A, Frankland PW, Kida S. (2008) Activation of LVGCCs and CB1 receptors required for destabilization of reactivated contextual fear memories. Learn Mem 15: 426–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. (2010) Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb Cortex 20: 1486–1496 [DOI] [PubMed] [Google Scholar]
- Trezza V, Vanderschuren LJ. (2008) Cannabinoid and opioid modulation of social play behavior in adolescent rats: differential behavioral mechanisms. Eur Neuropsychopharmacol 18: 519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8: 262–275 [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, et al. (2005) Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology 48: 658–672 [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. (2005) Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav 81: 331–342 [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. (2002) Endocannabinoid signaling in the brain. Science 296: 678–682 [DOI] [PubMed] [Google Scholar]
- Wise LE, Harloe JP, Lichtman AH. (2009) Fatty acid amide hydrolase (FAAH) knockout mice exhibit enhanced acquisition of an aversive, but not of an appetitive, Barnes maze task. Neurobiol Learn Mem 92: 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, et al. (2008) Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem 104: 1132–1143 [DOI] [PubMed] [Google Scholar]
- Zelinski EL, Hong NS, Tyndall AV, Halsall B, McDonald RJ. (2010) Prefrontal cortical contributions during discriminative fear conditioning, extinction, and spontaneous recovery in rats. Exp Brain Res 203: 285–297 [DOI] [PubMed] [Google Scholar]