Abstract
Introduction: Colorectal cancer (CRC) is the fourth most common malignancy in the Kaiser Permanente Northwest (KPNW) Region. The goals of CRC screening are early diagnosis of cancer in the preclinical state, down-staging of tumors, and increasing survival. This historical review summarizes the screening strategies since 1980 and their impact on early diagnosis, stage, and survival. During this period, the KPNW Tumor Registry documented the stage and survival, and screen-detection status of patients. We have observed that the percentage of screen-detected case measure has provided critical information that has contributed to the present success. CRC screening efforts by the end of 2010 had provided early diagnosis for one-third of patients.
Methods: KPNW membership has undergone more than 540,000 fecal blood tests, an estimated 130,000 flexible sigmoidoscopies (FS), and more than 100,000 colonoscopies. Since 1980 members older than age 50 years have increased from 48,627 to 137,617. This report represents a review of 5458 patients. Since 1980, 5 distinct periods of CRC screening have been compared. In 1980, the CRC screening practice was primarily office-based fecal occult blood testing (FOBT) and proctosigmoidoscopy. Data from the initial home-based FOBT testing initiative (1985), transitioning to an FS program (1995), adoption of colonoscopy (2005), and subsequent reintroduction of FOBT testing (2006) allows examination of results by period. After ever-increasing promotion of endoscopy, the goal of screening shifted from “screen detection to prevention by polypectomy.”
Results: By reexamining the outcomes of the CRC strategies from 1980–2005, the nature of the colonoscopy label of “gold standard” was questioned leading to a return to FOBT testing. Since then, the percentage of screen-detected patients exceeded expectations with a 6-fold increase (5% to 33%) allowing KPNW to reach its highest level of early detection.
Discussion: By examining the KPNW experience, we have come to better understand the significance of effectiveness measures: number of tests, stage of disease, percentage of screen-detected cancers and their relationship to survival. We examined the measures used to assess success and conclude that the current metrics—the number of examinations and disease stage—do not accurately reflect the effectiveness of screening efforts. Early detection of CRC saves lives when a program tests the most at-risk people. Using a good test (FOBT/fecal immunochemical test) that is able to reach more people, rather than the “perfect test” that reaches fewer people, transforms an ineffective program into a successful one. A critical element was the transition of the individual testing to population screening.
Introduction
Screening: The Public Face
In June 1985, President Ronald Reagan was diagnosed with colon cancer.1 This announcement stimulated public interest in early detection. The National Cancer Institute provided research support for early detection hoping “to reduce CRC deaths by 50% by the year 2000.”2 The Kaiser Permanente (KP) Northwest (KPNW) Center for Health Research,3 the KP Northern California (KPNC) Division of Research4 and the Group Health Organization Center for Health Promotion all received grants to examine their colorectal cancer (CRC) activities.
Again, in 1998, national attention refocused on colon cancer screening, when Katie Couric, a national TV commentator, was faced with her husband's illness and death from CRC. Her efforts led to the creation of the National Colon Cancer Research Alliance. She was then invited to provide Congressional testimony to the Senate Select Committee on Aging in 2000 about cancer screening.5 She also provided television coverage of her own colonoscopy, which boosted CRC awareness and galvanized support for colonoscopy as the primary screening tool.
Medicare reimbursement for colonoscopy, national consensus guidelines supporting colonoscopy, and specialty society endorsement of endoscopic screening all accelerated the shift from fecal occult blood testing (FOBT) and flexible sigmoidoscopy (FS) to colonoscopy as a primary tool. Medicare funding for colonoscopy in 2001 was seen as an entitlement, and colonoscopy rapidly became the new “gold standard.” I want my colonoscopy was heard loud and clear from patients.
Screening: the Clinician's Dilemma Nationally
In 1963, V Gilbertson, MD, at the Minnesota Cancer Detection Center, began reporting a reduction in the overall incidence of rectosigmoid cancers in the 25-year follow-up of 21,150 patients after removal of all polyps during 113,800 proctosigmoidoscopic exams.6,7 The Minnesota prospective randomized-controlled study of FOBT, from 1975–1978, randomized 46,551 patients into 3 FOBT screening arms: annual, biennial, and routine care. After 13 years, they reported a 33% and 6% (respectively) reduction in CRC deaths between the two screening groups as compared to the unscreened group. Of note, there was no overall difference in the death rate in the 3 groups during the report of the first 13 years of the study.8
In 1983, Hardcastle reported a United Kingdom prospective study of FOBT screening of 20,525 patients randomized to screening and control groups.9 The study findings supported the benefits of FOBT screening with earlier stages of disease at discovery and a presumed survival benefit. In 1996, he reported additional results of 152,000 patients with a survival benefit of 16%.10 Ongoing trials in Sweden, Denmark, France, and the US during the next 13 years provided more information about FOBT screening.11,12 The initial optimistic improvements in survival in the Minnesota study were not replicated in subsequent studies. Using statistical simulation programs, the large differences seen in screening benefits in different studies were reassessed, resulting in a reduction of the expected benefits.13,14
As complexity, costs, and time limited the possibility of future randomized studies, investigators focused on case-control studies. The series of studies undertaken at that time were designed to rapidly learn which initiatives would meet the National Cancer Institute goal of improving survival by 2000. In 1988, Selby examined the KPNC Multiphasic Evaluation program and the use of proctosigmoidoscopy within the population, demonstrating a reduction in CRC mortality.4 In the following year, the same group reported favorable results from FOBT testing.15 Studies continued over the next decade reflecting on the appropriate screening intervals for FS, colonoscopy, and FOBT testing.16 A Veterans Administration CRC screening project reported the feasibility of screening colonoscopy and their results.17,18 The National Polyp Study reported data that supported the effectiveness of polypectomy in the prevention of CRC.19
In 2009, the US Preventive Services Task Force (USPSTF) reexamined previous recommendations of FOBT screening for all individuals older than age 50 years (2002 (2008), and later limited screening beyond age 76 years.20,21 In 2009, a large series from the United Kingdom demonstrated the efficacy and benefits of FS screening.22 In the spring of 2010, the National Institutes of Health State-of-the-Science Conference on Colorectal Screening endorsed FOBT/fecal immunochemical test (FIT) testing as the primary outreach for early detection, noting efficacy for endoscopy modalities.23
For over a half-century of research, physicians and researchers had learned that CRC screening is effective in providing an early diagnosis, leading to a more favorable stage at presentation, and reducing CRC mortality. They had tested various population-based strategies that changed to accommodate presumed “best practices.” They embraced the belief that removal of all advanced neoplasms was a successful primary prevention strategy. They smeared, scoped, and hoped they were on the right path.
… convinced by medical experts that colonoscopy was the “gold standard” and confused by conflicting guidelines … redirection to FOBT/FIT testing was a profound dilemma for the clinician.
Kaiser Permanente Northwest Program: 1980–2010
From 1980 to early 1985, preventive care services were primarily delivered through the KPNW Health Appraisal Program, an Allied Health Practitioner-based clinic for routine physical examination. CRC screening was done using Hemoccult II kits (Beckman-Coulter; Brea, CA) with a 3-day dietary restriction. Though there were variations with instructions for diet, indications, collection, and laboratory processing, the most significant reason for the limited effectiveness was that most positive-FOBT patients did not receive a radiographic or endoscopic clearance of the colon. The lack of a standardized workup was reflected in the 1980 screen-detection rate of 5%.
By 1983, it was clear the CRC screening was ineffective, so a new comprehensive plan was proposed (David Clarke, MD; personal communication; 1984 Nov 7)a.24 The support of CRC testing required a standardized set of processes, an algorithm for the workup of a positive FOBT test, a Tumor Registry-based computerized tracking system, a 60-cm FS program, and outcome monitoring.
On May 15, 1985, the redesign was launched, followed a few weeks later by the announcement of President Reagan's diagnosis. After the launch, all positive-FOBT patients were evaluated by a protocol with a clinical resolution in almost all cases. As Tumor Registry staff had been trained to monitor cancer care, it was natural to monitor the workup of the positive-FOBT patients to ensure quality. To date, KPNW has tracked 13,631 patients with a positive FOBT, derived from 541,522 patient tests. Since 2009, the FIT test has been replacing the FOBT test and has >5% positivity rate. The FOBT-positive workup algorithm initially recommended clearing the colon by FS and barium enema, and was later modified in 2005 to employ colonoscopy.
From 1985 to 1995, there was skepticism about the efficacy of FOBT screening.25 The KPNW guideline changed to FS screening in 1995, with an active de-emphasis of FOBT testing. In 2001 (Craig Fleming, MD; personal communication; 2001)b, the FS strategy was re-reviewed concluding FS as the best overall option,26 even though there was clear evidence that FOBT was effective.27 Using simulation modeling, it was predicted that the cost savings from FS screening would accrue after 35 years, only after the program was in place for 30 years.28
Until 2005, colonoscopy was primarily reserved for symptomatic patients, though afterwards there was pressure to expand its screening indications. Colonoscopy screening was heavily promoted by the national media, specialty groups, and reimbursed by Medicare. The results of colonoscopy trials broadened the colonoscopy discussion by including secondary “prevention by polypectomy” with the removal of all polyps.29 Cost-effectiveness of this strategy was questioned at this time.30 The KPNW Region and the nation were unprepared for the rapid increase in demand for colonoscopy. Since 2005, even with extraordinary efforts to provide timely screening services, KPNW has been unable to meet the demand. Recommending any other screening strategy was felt to be a failure of current best practices, but we noted screen-detection performance was deteriorating.
KPNW leaders took the opportunity during the fall immunization campaign in 2006 to restart the FOBT testing program. The decade-long success of FOBT testing (1985–1995) produced an 18% (12–24%) screen-detection rate, compared with the FS period (1996–2005) at 9% (5–13%). By 2005, the screen-detection rate declined to 5%, which led to readoption of FOBT testing. On the basis of the flu campaign and internal performance data, FOBT testing was the primary screening recommendation in 2007. Subsequently, FIT replaced FOBT in 2009.31 The overall results of screen-detection are reflected in Figure 1.
Figure 1.
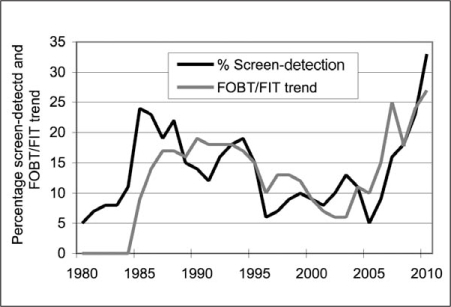
Percentage of screen detection relative to FOBT/FIT trend, 1980 to 2010.
FIT = fecal immunochemical test; FOBT = fecal occult blood testing
Patients and clinicians were slow to change to a new strategy, convinced by medical experts that colonoscopy was the “gold standard” and confused by conflicting guidelines. This redirection to FOBT/FIT testing was a profound dilemma for the clinician. One physician's description, overheard in the lunchroom, was, “It felt like downshifting to reverse.” They needed to trust the redirection, but were faced with the problem of explaining an old strategy that was so actively discouraged just a few years earlier.
Key Elements of Kaiser Permanente Northwest Screening Approach
“The greatest risk factor for a condition that has an effective screening test is the failure to be screened” (Tom Vogt, MD; personal communication; 1991).c
Following publication of large prospective-randomized studies, Northwest Permanente physicians increased cancer screening in their routine patient health appraisal during their office visits. Although well intentioned, much of the overall population at risk for CRC were never screened employing this strategy.
Over the years, there have been four key elements that have affected KPNW's present success:
-
Measuring the success of the screening efforts by the percentage of population reached, and the number of screen-detected cases found.
Since 1990, research has focused on the accuracy of the “best test,” rather than patient acceptance, resource availability, cost, and outcomes.
-
Shifting from episodic office-based screening to a population-based strategy.
Historically, prevention was linked to an episodic office-based testing model. In the search for quality and value in health care delivery, purchasers, insurers, policy makers, patients, and quality organizations have included cancer screening as a key indicator. In 2003, Healthcare Effectiveness Data and Information Set (HEDIS) adopted the percentage of the eligible population screened for CRC as a core quality measure.
-
The development and availability of innovative systems to reduce barriers, enhance tracking and follow-up, and measure outcomes.
These included the FOBT registry (1985) and disease management and follow-up systems using the computerized Tumor Registry databases.
-
Use of the Panel Support Tool (2006) to provide a platform for staff to eliminate care gaps in cancer screening.
KPNW inreach (office contacts) and outreach (non-office) efforts have contributed to continued improvement in CRC HEDIS measures during the last 6 years. KPNW is approaching HEDIS targets of >72% for commercial members, and >80% for Medicare members being screened by either laboratory or endoscopy testing.
KPNW has supported successive strategic improvements in early detection of CRC over time (Table 1).
Table 1.
Testing and screening strategies
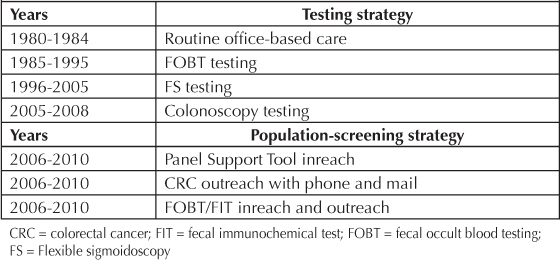
Since 1980, CRC screening goals have remained focused on early detection and prevention. As information from each time period was reviewed, shifts to the strategy noted above evolved. For example, in 2005, when the percentage of screen-detected cases fell to a 5% level again, a figure identical to the rate in 1980, the process was reexamined. Even after introducing more effective inreach and outreach patient contacts, increasing the number of endoscopies, and noting early stage shift, KPNW failed to find more screen-detected patients. The program realized that employing the most accurate screening method did not make a difference in screen-detection rate overall, if the availability of endoscopic resources and patient unwillingness to comply with the strategy did not support the program. Additionally, removal of adenomatous polyps as a method to prevent CRC could not demonstrate a beneficial effect on the entire population unless a much higher percentage of the membership could be reached by this strategy. Thus in 2006, a pivotal year, the CRC screening guideline was changed back to FOBT testing. An aggressive outreach campaign commenced, first with FOBT (2006) and subsequently with FIT (2009), by introducing interactive voice recognition phone outreach (2008), CRC mailings, birthday letters, and annual FIT testing reminders. Also beginning in 2006, the electronic medical record—HealthConnect—began providing clinicians with a gap analysis for each patient at every clinical contact using the Panel Support Tool. Enhancing the prevention mission with this combination of inreach and outreach activities increased the number of FOBT and FIT tests submitted.
Methods
The KPNW Region provides health and medical care to almost 500,000 members from SW Washington, the Portland metropolitan area and Salem, OR. Since 1980, members older than age 50 years have increased from 48,627 to 137,617. Unique characteristics made outcome assessments possible: a membership that is a valid statistical sample of the larger Pacific Northwest, an accredited Tumor Registry, a unique individual health record number for hospital and outpatient care, a unified electronic medical record, and supporting electronic databases.
Since 1960, the Tumor Registry has collected and tracked data on all new cancers originating in the KPNW membership. In 1976, the Tumor Registry began performing computerized tracking of abnormal test results that might represent malignancy. This system expanded in 1985 to include the positive-FOBT patients. From 1980 to 2010, 5999 patients were accessioned into the Tumor Registry database. This report represents a review of 5458 patients, including synchronous tumor (coded to the highest stage), and excluding all patients with metachronous tumors, unknown stage, and unknown screen-detection status. During this study period, there has been no organized outreach for the high-risk populations: patients with a history of polyps or intestinal cancer.
The Tumor Registry abstracts the age, sex, site, stage (See Sidebar: Surveillance and Epidemiology End Results [SEER]),32 treatment, recurrence, clinical status, survival, cause of death, and screen-detection status. The Tumor Registry has maintained a follow-up rate of over 95%.
In 1988, the Center for Health Research, Portland, OR, was awarded a National Cancer Institute grant to study CRC cases from 1980 to 1988, examining the reliability of intermediate variables as surrogate measures for survival.4 For this study, screen-detected CRC was defined as the diagnosis of an In-Situ or invasive cancer of the colon and rectum (excluding: anal, squamous cell, cloacogenic, carcinoid, lymphoma, melanoma, or appendiceal cancers) with no symptoms of bleeding, anemia (hemogram parameters were corrected for age and sex), new constipation or diarrhea, abdominal pain, abdominal mass, perforation, or significant nonintentional weight loss. All other patients were considered symptomatic or non-screen-detected. This definition of screen detection has remained unchanged for the duration of the review. The intestinal sites were defined as right colon (cecum to splenic flexure), left colon (descending, and sigmoid), and rectal (rectosigmoid and rectum). This site distribution permitted reliable assessments of the impact of flexible sigmoidoscopy on the early detection efforts. Since 1984, the CRC outcomes have been reviewed annually.
In 1989, a Tumor Registry data field of screen-detection was added to the abstraction process to facilitate the review of program performance. Whether a cancer patient diagnosis was made by screen detection, or by symptom, a searchable data field was created, updated, and crosschecked by the Tumor Registry staff. When paper charts were replaced in 1994 with HealthConnect, the review of laboratory tests, pathology, imaging reports, and operation and procedure notes became easier for all reviewers.
To further understand the characteristics of the screen-detected population, a companion database was created so that additional information about screening tests (laboratory, imaging, or endoscopy), personal characteristics (history of polyps or colon cancer, family history), nonscreened symptoms (anemia, bleeding, obstruction, perforation, mass, unexplained weight loss), and delays in care, would supplement the data from the Tumor Registry.
Surveillance and Epidemiology End Results (SEER) staging classification.
In Situ (IS):cancer limited to epithelium
Localized (LOC):cancer invading lamina propria
Regional Direct (REGD): cancer extending into peritoneum and adjacent tissue (ie, mesentery, adjacent organs)
Regional Lymph Node and Both (REGLB): cancer involving regional nodes, or regional nodes and adjacent tissue (ie, mesentery, adjacent organs)
Distant (DIST): cancer to distant nodes and organs.
Results
This study sought to determine the best measure of success in detecting CRC.
Screening periods
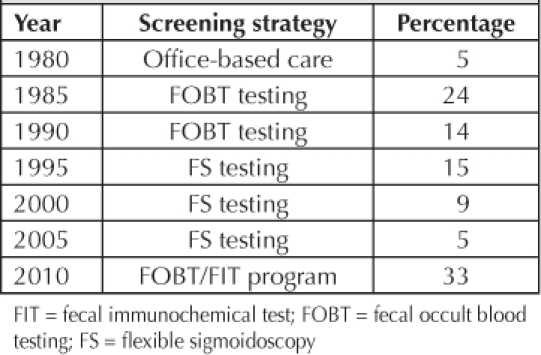
From 1980–2010, 5458 colorectal cancer patients represented KPNW members who may have been screened by 1 of 4 strategies: office-FOBT, home-FOBT/FIT, FS, or colonoscopy. It was not until 2006, that CRC screening transitioned from office-based testing to the present population-based focus, with active inreach and outreach strategies. Table 2 shows the initiatives that reflected best practice for the normal-risk population during 5-year intervals, with the percentage of screen-detected cases.
Table 2.
Screen-detection percentages by period
Screening tests
Variations in the percentage of cancers that were screen-detected are related to the frequency of FOBT/FIT testing. Screening peaks in 1985–1986 at 24% and 2009–2010 at 28% coincided with the increases in FOBT/FIT testing. The lowest screen-detection percentage periods were in 1980 and 2005 at 5%. The 1980 period represented office-based ambulatory care, whereas 2005 was a transitional period from FS to colonoscopy screening. When the nadir of screening reached 5% in 2005, the test that was predominately leading the screening effort was FS, even though more than 7000 colonoscopies were done that year.
In 2006, active outreach was introduced contributing to the remarkable increase, almost tripling the volume of FOBT/FIT tests from 13,362 (2005) to 37,916 (2010). Colonoscopies increased from 7123 (2005) to 18,255 (2010), predominately accommodating the colonoscopy workup of positive-fecal testing, rather than screening.
Risk
Average-risk patients, who have no history of colonic polyps, cancer, or high-risk family history, represent the majority of the study patients. There were 531 average-risk screen-detected patients: 436 by FOBT/FIT, 68 by FS, and 29 by colonoscopy. Figure 2 demonstrates the influence of each test on screen detection in the average-risk population.
Figure 2.
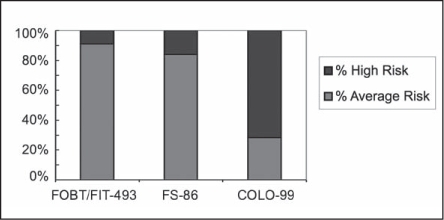
Screen-detected colorectal cancer and risk status.
COLO = colonoscopy; FIT = fecal immunochemical test; FOBT = fecal occult blood test; FS = flexible sigmoidoscopy
From 1995 to 2005, the impact of FS examinations identifying screen-detected cancers was small, finding only 68 average-risk patients. Similarly, from 1995 to 2010, the colonoscopy screening only marginally influenced the overall screen-detection percentages with only 29 average-risk patients diagnosed out of almost 100,000 colonoscopies. Most colonoscopy testing is done for positive-FOBT/FIT tests and high-risk patients (history of polyps, CRC, and positive family history of CRC). As the number of referrals for colonoscopy screening increased, there were difficulties in providing screening access for average-risk patients.
Stage
From 1980 to 2010, the screening-success calculation was converted to improved stage of disease based on the effectiveness of the screening percentage as noted in Figure 3. The main improvements in In-Situ and Localized staging are at the expense of the Regional-Direct stage. There is minimal change in the Regional-Nodal and Distant (DIST) disease categories. By comparing the stages between the least effective (5%), present state (33%), and best outcome (100%) scenarios, increasing the percentage of screen-detection provides the best survival. This provides a more realistic picture of the differences found between routine care, and an aggressive screening program. Table 3 illustrates 5-year survival rates for these stages in the KPNW population.
Figure 3.
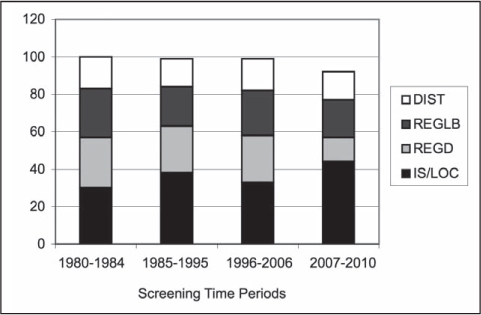
Colorectal cancer SEER stage trends, 1980 to 2010.
DIST = Distant; IS = In Situ; LOC = Localized; REGD = Regional Direct; REGLB = Regional Lymph Node and Both; SEER = Surveillance and Epidemiology End Results
Table 3.
Five-year survival rates for disease stages in the Kaiser Permanente Northwest population
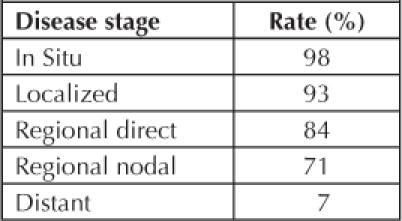
Variations in 5-year survival are based on age, location, and screen-detection status. The difference in survival between the screen-detected and symptomatic Regional Lymph Node and Both (REGLB) patient is 79% and 63% respectively (Figure 4). We note that even if all patients are screen-detected, some will die of disease. It is estimated that the silent phase for most CRC ranges up to 10 years. We have a 6- to 24-month lead time in patients who refuse an initial workup and subsequently become symptomatic.
Figure 4.
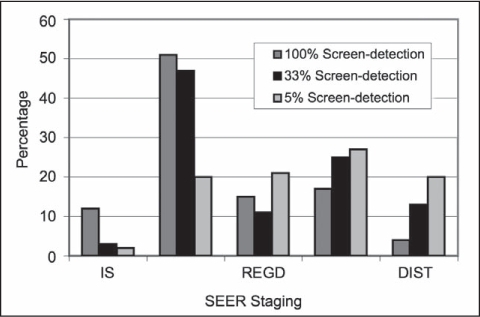
Relationship of colorectal cancer stage distribution and screen-detection effectiveness.
DIST = Distant; IS = In Situ; REGD = Regional Direct; SEER = Surveillance and Epidemiology End Results
Distribution
We have examined the percentage of screen detection during two time frames: 1980 to 1984 and 2007 to 2010, to see if there are significant differences when considering the location of the cancer in the colon. There has been an improvement in the stage of cancers when considering the location of cancer from the right and left colon, and the rectum. The impact of screening technique may influence the distribution of cancers as FS and colonoscopy have different ranges, though the influence of colonoscopy has been smaller than we may have expected.
We note that there is some shift to lower stages for right and left colon, and rectal tumors, but have to acknowledge the percentage of REGLB and DIST totals are about the same in Figure 5a. It should be kept in mind that the percentage of early detection is low, 5% to 13%. When the percentage of screen detection increased to 16% to 33% in Figure 5b, we have noted a more dramatic stage shift in almost all categories in all 3 locations, though there is minimal change in the percentage of REGLB. It is unclear what other influences are affecting stage distribution in the non-screened patients between the 2 time periods, because of their similarity.
Figusre 5a (left).
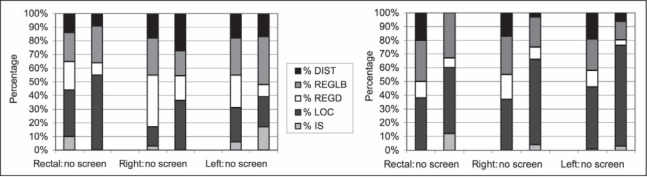
Relationship of colorectal cancer screen-detection status, 1980 to 1984 (5% to 13%), to site and stage. Figure 5b (right). Relationship of colorectal cancer screen-detection status, 2007–2010 (16% to 33%), to site and stage.
DIST = Distant; IS = In Situ; LOC = Localized; REGD = Regional Direct; REGLB = Regional Lymph Node and Both
Survival
Screening success from 1980 to 2010 has been linked to stage of disease. Early stage disease (In Situ, and Localized) is trended over time with improvements coming from the reduction of the Regional stage. There is negligible change in the percentage of cases with a DIST stage over the study period. However, the recent changes noted in Figure 4 suggest that increasing the number of screenings may generate a beneficial change in DIST stages as well.
Changes in early stage disease (In Situ and Localized) appear to mirror the percentage of screen-detection activities. The increasing slope of early stage disease from 1994 to 2005 is not consistent with the reduction of screen-detection percentage as noted as a gradual decline from 1994 to 2005. Table 4 presents the distribution of stage and screening status, comparing outcomes of most- and least-effective screening prospects.
Table 4.
Distribution for Screening at 5% and 100% Levels
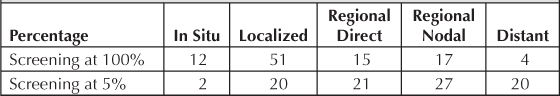
The KPNW five-year survival outcomes were derived directly from our population. Table 5 demonstrates the survival differences of an effective screening program compared with an unsuccessful testing program.
Table 5.
Survival at 5-Years of 100 Patients by Stage and Screening Levels
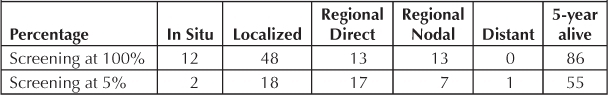
Incidence
The national SEER database has demonstrated a reduction in the incidence of CRC, but the KPNW incidence of colon and rectal cancer has not changed since 1991 for either men or women. The prospect that approximately 250,000 endoscopies (FS and colonoscopies) since 1991 have not influenced the incidence is disappointing. On further examination, there is a reduction in CRC in the left colon with a shift of lesions to the right colon.33 If this could be attributed to a polypectomy effect, then we would expect to see a reduction in cancers in the rectum, which did not occur.
Screen detection and stage as metrics
The goal of early detection is to discover the cancer in its earliest stage. It is common practice to assess the success of early detection activities by identifying the percentage of early stage disease: In Situ and Localized. The relationship between the percentage of screen-detected CRC and early stage disease is demonstrated by the gradual increase in In-Situ and Localized stage from 1995 to 2005, when the screen-detection was gradually declining to 5% (Figure 6).
Figure 6.
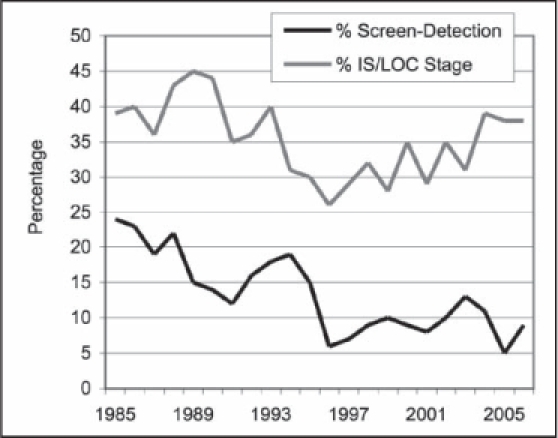
Relationship percentage of screen-detection and percentage of In-Situ and Localized stage.
IS = In Situ; LOC = Localized
Discussion
We have reviewed the last 30 years of KPNW experience in CRC screening, examining the present success by considering the key elements of the program. We have reviewed the influence of tests, population-based screening, systems of care, and inreach and outreach elements in the KPNW program. Achieving the best screening results in the last 2 years was a culmination of organizational initiatives.
When the KPNW Colon Cancer Task Force in 1984 proposed an improvement process for FOBT testing, we did not expect to continue to readjust the CRC strategy. We performed careful reviews of effectiveness over the years, and changed the strategies on the basis of presumed best practices. The early successes of FOBT testing from 1985 to 1995 were lost for over a decade, as the guidelines shifted to the “best test” and “prevention by polypectomy.” As we noted, a continuous decline in screening performance, the outcome data was contributory to a fortunate dramatic reversal of strategy.
In reviewing the overall effects of screening, we have attempted to examine the effective opportunities and benefits by comparing the lowest and highest periods of screening. If KPNW expects any greater benefits from future screening, we need to redouble efforts to the patients not tested, focusing on the demographics of the patients with advanced stages. This may not be as easy as increasing the number of tests performed. Additionally, we have evidence that screening also improves survival because of within-stage shifting.34
In summary, early detection of colon cancer saves lives when a program tests the most at-risk people. Using a good test (FOBT/FIT) that is able to reach more people, rather than the “perfect test” that reaches fewer people, transforms an ineffective program into a successful one when the strategy moves from individual testing to population-based screening. Rather than simply measure the number of tests, we identified the rate of screen-detected cases over time. Without high numbers of screening in the at-risk population, the stage shift from DIST and Regional Nodal disease will not occur and will prevent the best population outcomes from occurring. The organizational commitment to move from a testing strategy to a screening program was a key decision in its success. By organizing a screening program to test the largest number of average-risk individuals with an acceptable and deliverable test, the screening program has saved lives. We started a journey to change the care for patients with CRC in 1985, tried multiple strategies, and have managed to use our experience to establish a successful program.
The early successes of FOBT testing from 1985 to 1995 were lost for over a decade, as the guidelines shifted to the “best test” and “prevention by polypectomy.”
Disclosure statement
The author(s) have no conflicts of interest to disclose.
Acknowledgments
The authors would like to thank Beverly Battaglia; Judy Kimmey; David Clarke, MD; Christina Schwarz; Vickie Schindler; Micah Thorpe, DO, for their support of this work.
Footnotes
a Clinical Assistant Professor of Gastroenterology Emeritus and Senior Scholar at the Center for Ethics at Oregon Health & Science University (OHSU).
b Internist, Kaiser Permanente Northwest, Portland, OR.
c Epidemiologist, Fellow of the American Heart Association, and Director of the Kaiser Permanente Center for Health Research Hawaii program; Honolulu, HI.
References
- Brown ML, Potosky AL. The presidential effect: the public health response to media coverage about Ronald Reagan' s colon cancer episode. Public Opin Q. 1990 Fall;54(3):317–29. doi: 10.1086/269209. [DOI] [PubMed] [Google Scholar]
- Greenwald P, Sondik EJ. Cancer control objectives for the nation, 1985–2000. Bethesda, MD: National Cancer Institute; 1986. DHHS publication no. 86–2880, National Cancer Institute Monograph No. 2. [Google Scholar]
- Mullooly J. New York: 1990 Oct 4. Evaluation of a colorectal cancer screening program in an HMO. Presentation at the 1990 Annual Meeting of the American Public Health Association. [Google Scholar]
- Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992 Mar 5;325(10):653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- Sizing M. Katie Couric: a personal tragedy sparks a public campaign to prevent colon cancer. 2005 Mar. LE Magazine [serial on the Internet] [cited 2011 Nov 28];[about 3 p]. Available from: www.lef.org/magazine/mag2005/mar2005_cover_couric_01.htm?source=search&key=Colon%20Cancer.
- Gilbertsen VA. Proctosigmoidoscopy and polypectomy in reducing the incidence of rectal cancer. Cancer. 1974 Sep;34(3)(suppl):936–9. doi: 10.1002/1097-0142(197409)34:3+<936::aid-cncr2820340722>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Gilbertsen VA, McHugh R, Schuman L, Williams SE. The earlier detection of colorectal cancer: a preliminary report of the results of the Occult Blood Study. Cancer. 1980 Jun 1;45(11):2899–901. doi: 10.1002/1097-0142(19800601)45:11<2899::aid-cncr2820451132>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993 May 13;328(19):1365–71. doi: 10.1056/NEJM199305133281901. Erratum in: N Engl J Med 1993 Aug 26;329(9):672. [DOI] [PubMed] [Google Scholar]
- Hardcastle JD, Farrands PA, Balfour TW, Chamerlain J, Amar SS, Sheldon MG. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet. 1983 Jul 2;2(8340):1–4. doi: 10.1016/s0140-6736(83)90001-6. [DOI] [PubMed] [Google Scholar]
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecaloccult-blood test. Lancet. 1996 Nov 30;348(9040):1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- Kewenter J, Brevinge H, Engarås B, Haglind E, Ahrén C. Results of screening, rescreening, and follow-up in a prospective randomized study for detection of colorectal cancer by fecal occult blood testing. Results for 68,308 subjects. Scand J Gastroenterol. 1994 May;29(5):468–73. doi: 10.3109/00365529409096840. [DOI] [PubMed] [Google Scholar]
- Kronborg O, Fenger C, Olsen J, Bech K, Søndergaard O. Repeated screening for colorectal cancer fecal occult blood test. A prospective randomized study at Funen, Denmark. Scand J Gastroenterol. 1989 Jun;24(5):599–606. doi: 10.3109/00365528909093096. [DOI] [PubMed] [Google Scholar]
- Towler B, Irwig L, Glasziou P, Kewenter J, Weller D, Silagy C. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ. 1998 Aug 29;317(7158):559–65. doi: 10.1136/bmj.317.7158.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannsdorf-Vogelaar I, Wilschut J, van Ballegooijen M. Stage-specific survival of screen-detected versus clinically diagnosed colorectal cancer—evidence from the FOBT screening trials. 2010 Sep 20. Presentation at the Methods and Applications for Population-Based Survival. Frascati, Italy. Powerpoint presentation available from: www.irpps.cnr.it/it/system/files/S_Lansdorp.pdf.
- Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. Effect of fecal blood testing on mortality from colorectal cancer. A case-controlled study. Ann Intern Med. 1993 Jan 1;118(1):1–6. doi: 10.7326/0003-4819-118-1-199301010-00001. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997 Feb;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. Erratum in: Gastroenterology 1997 Mar;112(3):1060; Gastroenterology 1998 Mar;114(3):625. [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Smith FW. Screening for colon malignancy with colonoscopy. Am J Gastroenterol. 1991;86(8):946–51. [PubMed] [Google Scholar]
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343(3):162–8. doi: 10.1056/NEJM200007203430301. Erratum in: N Engl J Med 2000 Oct 19;343(16):1204. [DOI] [PubMed] [Google Scholar]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993 Dec 30;329(27):1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the US Preventive Services Task Force. Ann Intern Med. 2008 Nov 4;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Screening for colorectal cancer: recommendations and rationale. Ann Intern Med. 2002 Jul 16;137(2):129–31. doi: 10.7326/0003-4819-137-2-200207160-00003. [DOI] [PubMed] [Google Scholar]
- Atkin WS, Edwards R, Kralj-Hans I, et al. UK Flexible Sigmoidoscopy Trial Investigators Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomized controlled trial. Lancet. 2010 May;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- NIH State-of-the-Science Conference Statement on enhancing use and quality of colorectal cancer screening. 1. Vol. 27. Bethesda, MD: National Institutes of Health; 2010 Feb 2–4. [PubMed] [Google Scholar]
- Winchester DA, Shull JH, Scanlon EF. A mass screening program for colorectal cancer using chemical testing for occult blood in the stool. Cancer. 1980 Jun 15;45(12):2955–8. doi: 10.1002/1097-0142(19800615)45:12<2955::aid-cncr2820451211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Bandeau ML, Eddy DM. The workup of the asymptomatic patient with a positive fecal occult blood test. Med Decis Making. 1987 Jan–Mar;7(1):32–46. doi: 10.1177/0272989X8700700108. [DOI] [PubMed] [Google Scholar]
- Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000 Oct 18;284(15):1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000 Nov 30;343(22):1603–7. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- Loeve F, Brown ML, Voer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000 Apr 5;92(7):557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- Sonnenbreg A, Delcò F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med. 2000 Oct 17;133(8):573–84. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Song K. Projected national impact of colorectal cancer screening on clinical and economic outcomes and health services demand. Gastoenterology. 2005 Oct;129(4):1151–62. doi: 10.1053/j.gastro.2005.07.059. [DOI] [PubMed] [Google Scholar]
- Allison JE, Sakoda LC, Levin TR, et al. Screening for Colorectal Neoplasms with new Fecal Occult Blood Tests: Update on Performance Characteristics. J Natl Cancer Inst. 2007 Oct 3;99(19):1462–70. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- Young JL, Roffers SD, Ries LAG, Fritz AG, Hurlbut AA, editors. SEER summary staging manual—2000: codes and coding instructions. Bethesda, MD: National Cancer Institute; 2001. pp. 86–96. (eds) NIH Pub No. 01–4969. p. [Google Scholar]
- Gupta AK, Melton LJ, Jr, Peterson GM, et al. Changing trends in the incidence, stage, survival, and screen-detection of colorectal cancer: a population-based study. Clin Gastroenterol Hepatol. 2005 Feb;3(2):150–8. doi: 10.1016/s1542-3565(04)00664-0. [DOI] [PubMed] [Google Scholar]
- Kafadar K, Prorok PC. Effect of length biased sampling of unobserved sojourn times on the survival distribution when disease is screen detected. Stat Med. 2009 Jul 20;28(16):2116–46. doi: 10.1002/sim.3601. [DOI] [PubMed] [Google Scholar]


