Abstract
Eosinophils are the predominant inflammatory cells recruited to allergic airways. Here we demonstrate that human and murine eosinophils express SWAP-70, an intracellular RAC-binding signaling protein, and examine its role in mediating eosinophil trafficking and pulmonary recruitment in a murine model of allergic airway inflammation. Compared to WT eosinophils, SWAP-70 deficient (Swap-70−/−) eosinophils revealed altered adhesive interactions within inflamed post capillary venules under conditions of blood flow by intravital microscopy exhibiting enhanced slow rolling but decreased firm adhesion. In static adhesion assays, Swap-70−/− eosinophils adhered poorly to VCAM-1 and ICAM-1 and exhibited inefficient leading edge and uropod formation. Adherent Swap-70−/− eosinophils failed to translocate RAC1 to leading edges and displayed aberrant cell surface localization/distribution of α4 and Mac-1. Chemokine-induced migration of Swap-70−/− eosinophils was significantly decreased correlating with reduced intracellular calcium levels, defective actin polymerization/depolymerization and altered cytoskeletal rearrangement. In vivo, compared to WT mice, recruitment of eosinophils to the lungs of allergen-challenged Swap-70−/− mice was significantly reduced along with considerable attenuation of airway inflammation indicated by diminished IL-5, IL-13 and TNFα levels, reduced mucus secretion and improved airway function. These findings suggest that regulation of eosinophil trafficking and migration by SWAP-70 is important for the development of eosinophilic inflammation after allergen exposure.
Introduction
Eosinophil infiltration to sites of inflammation is long recognized as one of the hallmarks of allergic inflammation (1). Eosinophils contain highly specific basic granule mediators such as eosinophil peroxidase, major basic protein (MBP)3, and eosinophil cationic protein, which combined with various eosinophil-derived cytokines, chemokines and growth factors contribute to the development and maintenance of inflammation and injury at sites of allergic inflammation (2). Chemoattractants such as eotaxins, C5a, C3a, MCP-2, MCP-3, MCP-4, RANTES released by other immune cells such as T cells, macrophages, mast cells (MC), and in some cases eosinophils themselves, play an important role in recruitment and microlocalization of eosinophils (3). Additionally, the specific and sequential engagement of cell surface adhesion receptors with vascular counter ligands in inflamed blood vessels (4, 5) initiates a cascade of events regulated by various signaling molecules resulting in F-actin rearrangement and cytoskeletal reorganization leading to directed cell shape changes as a prelude to cell adhesion and migration into inflamed tissues (6–10).
SWAP-70 is a RAC-binding, F-actin binding intracellular signaling molecule first identified in the nucleus of activated B cells as part of a complex involved in immunoglobulin class switching (11–13). Subsequently, its expression has been demonstrated in MC (14), primary embryonic fibroblasts (15), DC (16), trophoblasts (17) and gliomas (18). In B cells, SWAP-70 is involved not only in nuclear events but also in signaling events in the cytoplasm during cell activation (19) and in efficient homing to the lymph nodes (20). SWAP-70-deficient B cells exhibit reduced migration into lymph nodes in vivo due to altered adhesive interactions with the vascular endothelium of lymphoid organs. Studies with immature BM-derived MC from SWAP-70 deficient (Swap-70−/−) mice have demonstrated that SWAP-70 is required for Fcε receptor-mediated reactions as well as for signal transduction from the stem cell factor receptor c-Kit, regulating events such as degranulation, adhesion, migration, differentiation and survival of MC (14, 21). Further, IgE-mediated passive cutaneous and systemic anaphylactic responses are strongly reduced in Swap-70−/− mice (22). SWAP-70 also regulates surface localization of MHCII molecules and is therefore important in DC-dependent immune responses (23). Upon cell stimulation, SWAP-70 has been shown to regulate the F-actin cytoskeleton by binding to non-muscle F-actin (24, 25) and promoting membrane ruffling (25). Overall, the cytoskeleton-modulating effects of SWAP-70 is probably due to its ability to interact with and regulate levels of activated RAC, a member of the Rho family of GTPases (15, 25), which acts as a central molecular switch in a number of signal transduction pathways relevant to leukocyte recruitment (8, 26). To date, there are no studies describing the expression of this signaling molecule by eosinophils and the potential role this molecule may play in eosinophil recruitment, particularly in the context of allergic inflammation. In the current study, we have identified an important and novel role for SWAP-70 in mediating eosinophil trafficking.
Materials and Methods
Mice
Mice (male and female) on C57BL/6 background that were rendered deficient for SWAP-70 (Swap-70−/−) by removing the first exon and part of the 5′ UTR in both alleles as described previously (13) were bred together yielding Swap-70−/−offspring. Age and gender matched C57BL/6 mice were used as WT controls. All studies involving mice were performed following standards and procedures approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Murine and human eosinophils
Murine eosinophils were cultured from BM of WT and Swap-70−/− mice as previously described (27), but with minor modifications. Briefly, BM cells collected from the femurs of naïve Swap-70−/− and WT mice were cultured in RPMI 1640 supplemented with 20% FBS, 2 mM glutamine, 25 mM HEPES, nonessential amino acids, 1mM sodium pyruvate and 50 μM 2-ME (all reagents from Invitrogen, Carlsbad, CA). Stem cell factor and FLT3 ligand (PeproTech, Rocky Hill, NJ) were added to the medium at 80 ng/ml during initial culture. On day 4, cells were transferred to medium containing 10 ng/ml recombinant murine (rm) IL-5 (PeproTech) and sub-cultured every other day up to day 14, maintaining starting cell density at 106/ml each time. Eosinophil differentiation was assessed starting from day 8 by staining cytocentrifuged preparations of BM cultures with Hema 3 System (Thermo Fisher Scientific Co., Pittsburgh, PA) and evaluating cells for expression of MBP by confocal microscopy with rat mAb against murine MBP (2.5 μg/ml) followed by FITC-conjugated goat anti-rat IgG and of Siglec-F by flow cytometry using PE-conjugated rat anti mouse Siglec-F (5 μg/ml, BD Biosciences, San Diego, CA). Differentiated cells between day 12–14 of culture that were 99% Hema 3 positive and expressed both MBP and Siglec-F were used in studies.
Human eosinophils were isolated from peripheral blood of allergic donors. Granulocytes were first collected from peripheral blood by centrifugation over Ficoll-Paque (GE Healthcare, Piscataway, NJ) and red blood cells were removed by lysis with ammonium chloride solution (150 mM ammonium Chloride, 10 mM potassium bicarbonate, 0.5 M EDTA, pH 8.0). Eosinophils were then enriched based on negative selection using EasySep® eosinophil enrichment kits according to the manufacturer’s protocol (Stemcell Technologies, Vancouver, BC, Canada). Viability and purity were routinely found to be >95%.
Expression of SWAP-70 by murine and human eosinophils
BM-cultured eosinophils were immunostained with affinity purified rabbit anti-mouse SWAP-70 antibodies (10 μg/ml)(11) or with rat mAb against murine MBP (described above) and the bound antibodies were detected using FITC-conjugated goat anti-rabbit or anti-rat IgG as described previously (28). Rabbit IgG (for SWAP-70) and rat IgG (for MBP) were used as isotype controls. Slides were observed at ambient temperature using a FLUOVIEW FV1000/BX61 - Confocal Laser Scanning Biological Microscope equipped with an UPlanSApo lens (20 ×/0.85 [oil]) and a PlanApo N lens (60 ×/1.42 [oil]). FV10-ASW 2.0 software was used for image acquisition (Olympus, Melville, NY). Western blot analysis of murine and human eosinophil lysates (40 μg protein/lane/sample on 5–10% Tricine gels under reduced conditions) was performed using antibodies against mouse SWAP-70 (500 ng/ml) followed by horse radish peroxidase-conjugated goat anti-rabbit IgG (1:10000, Cell Signaling Technology, Danvers, MA). Protein bands were detected with ECL Western Blotting Substrate (Pierce Biotechnology, Rockford, IL) and visualized on X-ray films.
Expression of SWAP-70 by human and murine eosinophils at the mRNA level was assessed by RT-PCR. Total RNA (1 μg) extracted with TRIzol® Reagent (Invitrogen) was used for RT-PCR with SuperScript® III Reverse Transcriptase (Invitrogen) according to manufacturer’s instructions. Primers for murine and human eosinophils specific for the first exon of the SWAP-70 gene (5′-GCTGACCAGGTCTGTTAGGG-3′ and 5′-GACTTGCGACTTGGAGACCT-3′ for murine and 5′-TCCACCATCCATCTGTTGAA-3′ and 5′-GCGTTTCTTTTCCTCGTCTG-3′ for human) were designed using Primer3 (v. 0.4.0) (available online at http://frodo.wi.mit.edu/primer3/). Expression of β-actin (for mouse) (29) and GAPDH (for human, primers derived at http://www.rtprimerdb.org) were used as internal controls. PCR amplification was performed using GoTaq® Green Master Mix (Promega, Madison, WI) in a DNA Engine® PTC-0200 cycler (Bio-Rad Laboratories, Hercules, CA) under the following conditions: initial denaturation at 95°C for 2 min followed by 38 cycles of amplification (95°C for 20 s [denature], 60°C for 1 min [anneal], and 72°C for 30 s [extension]) and a final extension at 72°C for 5 min. PCR products were analyzed by 1.5 % agarose gel electrophoresis
Migration assay
Eosinophils from BM of WT and Swap-70−/− mice were suspended in DMEM and added to the upper wells (5 × 104/well in 50 μl) of 96-well Transwell® Chambers. Murine eotaxin-1 (CCL11, 100 nM, PeproTech) or media alone was added to the lower wells and the plates were incubated at 37°C for 3 h. The number of cells that migrated in each case was evaluated under an Olympus CK2 inverted microscope using a 40× objective and cells in three different fields of each well were counted. The assay was performed three times in duplicate. The average number of cells/field/well was determined and results were expressed as fold increase over background (media alone) which was in the range of 12 – 21 cells/field/well for WT and 24 – 31 cells/field/well for Swap-70−/− eosinophils.
Actin polymerization and cytoskeletal changes
Actin polymerization was assessed based on phalloidin binding using rhodamine-phalloidin (Invitrogen) followed by flow cytometry as previously described (21), but with minor modifications. Eosinophils (2 × 105 in 1% BSA in HEPES buffer) were stimulated for various time points with 100 nM eotaxin-1. Subsequently, cells were fixed and permeabilized with 3.6% formaldehyde and 0.5% Saponin in PBS for 15 min. Total cellular filamentous actin (F-actin) was stained by using rhodamine-conjugated phalloidin (2.5 U/ml) and analyzed by flow cytometry. A total of 10,000 events were acquired, and results were expressed as percent increases in cell fluorescence relative to untreated cells.
To assess the effect of eotaxin-1 on cytoskeletal changes in WT and Swap-70−/− eosinophils, cells were allowed to attach to chamber slides (Nalgene Nunc International, Rochester, NY) coated with poly-L-Lysine (Sigma Chemical Co., St Louis, MO, 10 μg/ml in PBS, 100 μl/chamber) and incubated with 100 nM eotaxin-1 for different durations (10 s to 10 min). Cells were permeabilized and actin filament rearrangement was evaluated by confocal microscopy using rhodamine-phalloidin as previously described (30).
Calcium (Ca2+) flux measurements
Calcium flux in response to stimulation with eotaxin-1 was measured by flow cytometry using Indo-1, AM (Invitrogen), a cell-permeant, ratiometric Ca2+ binding dye, as described previously (31). WT and Swap-70−/− eosinophils (1 × 106) in RPMI containing 10% FBS were loaded with 3 μM Indo-1, AM (concentration identified after initial optimization), for 30 min at 37°C and 5% CO2. Following incubation, cells were washed once and resuspended in medium. Cells were equilibrated at 37°C for 5 min prior to acquisition on a BD LSR II flow cytometer (BD Biosciences). Indo-1, AM is excited in the UV range and fluoresces at different wavelengths depending on whether it is bound to Ca2+ (~405nm) or free (~530nm). The ratio of these two wavelengths, which is indicative of changes in intracellular Ca2+ concentration, was measured up to 3 min after the addition of eotaxin-1 (100 nM) or ionomycin (2 μM), an ionophore as a positive control, at the 30 s time point.
Assessment of eosinophil trafficking in vivo by IVM
Trafficking of WT and Swap-70−/− eosinophils in inflamed post capillary venules of the mouse cremaster muscle microcirculation was evaluated as described previously (32). WT mice were first administered TNFα (250 ng, intrascrotal) and surgery to expose the cremaster muscle microcirculation was initiated approximately 4–5 h later. Eosinophils (1 × 107) labeled with 5 μM carboxyfluorescein succinimidyl ester (CFSE, Invitrogen) were infused via the carotid artery to the prepared animal (approximately 5–6 h after TNFα) placed on the stage of an intravital microscope (Leica Microsystems Inc., Bannockburn, IL). Interaction of fluorescently labeled eosinophils with the vascular endothelium of the cremaster muscle microvessels was made visible by stroboscopic epi-illumination using a Xenon lamp and all images recorded using StreamPix digital video recording software (NorPix, Inc., Montreal, Quebec, Canada). This was followed by off-line analysis of recorded digital images to determine the number of rolling and adherent cells as described in our previous studies (33). On average, rolling and adhesion in four to six microvessels was analyzed per mouse. In addition, velocity of rolling eosinophils in 3–5 venules/mouse was analyzed (34).
Expression of cell surface receptors by flow cytometry
Baseline (non-activated) expression of α4 integrin (CD49d), LFA-1 (CD11a), Mac-1 (CD11b), L-selectin, PSGL-1 and CCR3 by WT and Swap-70−/− eosinophils (3 × 105 cells) was evaluated by flow cytometry with a FACScan flow cytometer (BD Biosciences) and analyzed with FlowJo software (version 7.1, Tree Star, Ashland, OR). Expression of α4 and LFA-1 was evaluated using rat anti-mouse α4 (clone PS/2) and rat anti-mouse LFA-1 (eBiosciences, San Diego, CA), respectively, followed by FITC-conjugated anti-rat antibodies (Jackson ImmunoResearch, Westgrove, PA) with rat IgG2a as the isotyope-matched control. Expression of L-selectin was assessed with FITC conjugated rat anti mouse L-selectin (clone Mel-14, BD Biosciences). PSGL-1 and Mac-1 expression were evaluated using PE-conjugated rat anti-mouse PSGL-1 and Mac-1 (BD Biosciences), respectively, while expression of CCR3 was evaluated using FITC-conjugated rat anti-mouse CCR3 (R & D Systems, Minneapolis, MN). PE- or FITC-conjugated rat IgG2a (BD Bioscience) or unconjugated rat IgG2b (eBiosciences) were used as isotype-matched control antibodies. All antibodies were used at a final concentration of 5 μg/ml.
Adhesion assay
Flat bottom 96-well plates were pre-coated with recombinant murine (rm) ICAM-1 (R & D Systems, 10 μg/ml) or VCAM-1 (5 μg/ml)(28) in PBS with Ca2+ and Mg2+ for 2 h at 37°C. BM-cultured murine eosinophils were labeled with 1 μM Calcein-AM (Invitrogen) (30) and added to the wells (5 × 104 in 100 μl PBS/well). After incubation at 37°C for 5 or 30 min, plates were gently washed with PBS 4–5 times to remove non-adherent cells. Adherent eosinophils were quantitated using a FLUOStar Optima microplate reader (BMG Labtech, Durham, NC) against a generated standard curve of Calcein-AM-labeled eosinophils.
To assess cytoskeletal rearrangement, cell morphology, RAC1 and adhesion molecule expression after attachment, WT and Swap-70−/− eosinophils (5 × 105) were first allowed to adhere to cover-slips coated with rm ICAM-1 or VCAM-1 for 5 or 30 min at 37°C. Non-adherent cells were removed by washing as described above and adhered cells were fixed with 4% paraformaldehyde. To evaluate the F-actin cytoskeleton adherent cells were permeabilized, stained with FITC-phalloidin (2.5 U/ml, Invitrogen) as previously described (30) and visualized by confocal microscopy. To assess differences in cell morphology, adherent cells after 30 min in five different fields of the cover-slips were counted and the number of polarized cells, defined as cells with a distinct leading edge and uropod compartment, was identified and expressed as a percentage of the total number of adhered cells in the field. To examine RAC1 expression, cells were permeabilized and incubated overnight with polyclonal antibodies against RAC1 (20 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit IgG as the isotype control, followed by rhodamine-conjugated secondary antibodies (5 μg/ml, Jackson ImmunoResearch) after washing. Cells were then stained with FITC-phalloidin and visualized by confocal microscopy. To evaluate α4 expression, cells adhered to VCAM-1 were fixed and first incubated with donkey IgG in PBS as blocking buffer for 30 min at room temperature. This was followed by overnight incubation with goat anti-mouse α4 (5 μg/ml, Santa Cruz Biotechnology) or isotype control and then with rhodamine-conjugated donkey anti-goat IgG (5 μg/ml, Jackson ImmunoResearch). Cells were finally stained with FITC-phalloidin in buffer containing 0.5% saponin and visualized by confocal microscopy. For Mac-1 expression, cells adhered to ICAM-1 were fixed, incubated overnight with rat anti-mouse Mac-1 (10 μg/ml, eBiosciences) or isotype control (rat IgG2b) in 0.1% BSA in PBS and then with rhodamine-conjugated goat anti rat IgG (5 μg/ml, Jackson ImmunoResearch). Cells were finally stained with FITC-phalloidin as described above for α4 before visualization under a confocal microscope.
Murine model of acute allergic airway inflammation
WT and Swap-70−/− mice (8–10 wk old) were sensitized with 50 μg OVA (Grade V, Sigma Chemical Co.) in 0.5 mg aluminum hydroxide by s.c. injections on days 0, 7, 14 and 21 and then challenged with OVA (20 μg/mouse) intranasally on days 23, 25, 28 as previously established (35). Control mice were administered saline instead of OVA for sensitization and challenge.
Measurement of airway responsiveness
AHR was assessed in control and allergen-challenged WT and Swap-70−/− mice using the FinePointe™ RC invasive plethysmography system (Buxco, Wilmington, NC) equipped with a nebulizer, ventilator and heated bed as per the RC system application manual and according to previously published protocols (36, 37) This system is capable of directly measuring pulmonary resistance (RL) and dynamic compliance (Cdyn). Anesthetized mice were ventilated at a tidal volume of 230 μl and a respiratory frequency of 120 breaths per minute. After monitoring baseline RL and Cdyn, changes in airway resistance and compliance were monitored continuously in response to saline followed by increasing concentrations of inhaled methacholine (3–25 mg/ml) nebulized for 18–20 seconds. To measure the degree of airway responsiveness, mean RL and Cdyn values following each dose of methacholine were expressed as percent change from the baseline value.
Sample collection and analysis
Mice were sacrificed 24 h after the last allergen challenge. Total and differential cell counts were determined in BM, blood and BALF based on morphologic and histologic criteria after staining with Hema 3 System (Thermo Fisher Scientific Co.). BALF supernatants were stored at −70°C till further evaluation. Cells recovered from the BALF of WT mice by cytocentrifugation were dual stained with affinity purified rabbit antibodies against murine SWAP-70 (10 μg/ml)(11) and rat mAb against murine MBP as described earlier in this section an evaluation of SWAP-70 expression by confocal microscopy. Right lungs were snap-frozen and left lungs were perfused with 4% paraformaldehyde to preserve pulmonary structure, fixed in 4% paraformadehyde and paraffin-embedded.
Lung histology
Paraffin-embedded tissue sections (4 μm thick) were stained with Harris Modified Hematoxylin and Shandon Instant Eosin (Thermo Fisher Scientific Co.) to determine cellular infiltration. For all immunohistological analysis, tissue sections were subjected to antigen retrieval followed by quenching of endogenous peroxidase activity prior to staining with specific antibodies and sections were briefly counterstained (5 sec) with hematoxylin at the end of the procedure. Appropriate VECTASTAIN ABC kits with biotinylated secondary antibodies and avidin-biotin horseradish peroxidase complex (Vector Laboratories, Burlingame, CA) and the Peroxidase AEC (3-amino-9-ethylcarbazole) substrate kit (Vector Laboratories) were used for detection. Stained slides were analyzed using a Nikon Microphot EPI-FL microscope (magnification of ×100 or ×400) and images were captured with an Olympus DP71 camera. Expression of SWAP-70 in the lungs of allergen-challenged WT mice was assessed using rabbit anti-mouse SWAP-70 antibodies (10 μg/ml). Evaluation and quantitation of eosinophil infiltration was performed by immunohistochemical staining for eosinophil-specific major basic protein (MBP) with rat mAb against murine MBP as described earlier (33). Rat IgG (for MBP) and rabbit IgG (for SWAP-70) were used as control antibodies. To detect airway mucus production, deparaffinized lung sections were stained with PAS reagent (Sigma Chemical Co.) and PAS-positive areas in horizontally sectioned airways were quantitated using ImageJ (38). Results are expressed as μm2 PAS positive area/100 μm basement membrane length (BML)
Measurement of BALF cytokines and chemokines
Cytokine levels in BALF were measured using Flex Set kits (BD Biosciences) for Th1 (IL-2, IFN-γ)/Th2 (IL-4, IL-5) cytokines as well as IL-13 according to the manufacturer with a FACScan flow cytometer and FCAP Array™ Software (BD Biosciences) using the same volume of BALF for each sample and results were expressed as pg/ml of BALF. Eotaxin-1 levels were measured using ELISA kit (R & D Systems) as recommended by the manufacturer.
Statistical Analysis
Results are expressed as the mean ± SEM. Statistical significance was determined by unpaired Student’s t-test using Microsoft Excel. A two-tailed test was used to first establish statistical significance between WT and Swap-70−/− eosinophils in cellular trafficking and recruitment in vivo (Figs. 2 and 6C). A one-tailed test was used for all other analyses. A p value <0.05 was considered as significant.
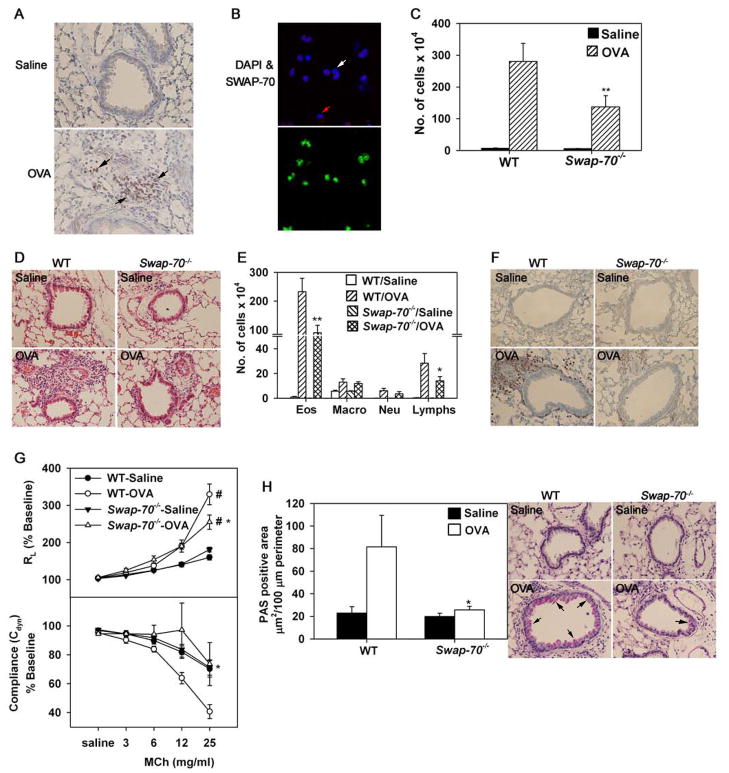
Fig 2. Swap-70−/− eosinophils exhibit altered rolling and decreased adhesion within inflamed blood vessels.
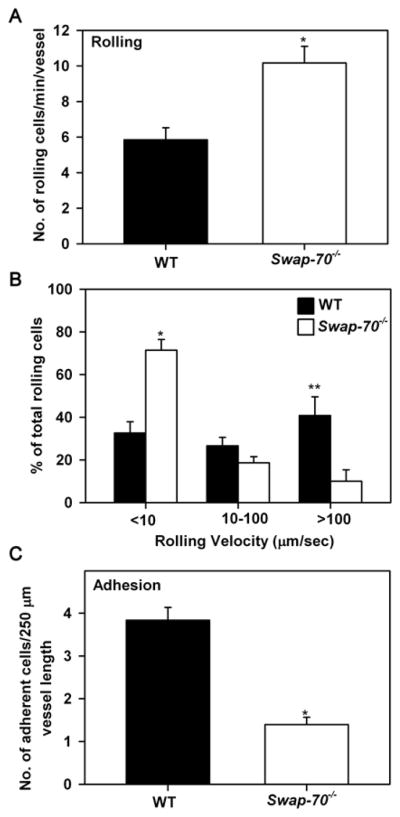
(A) Rolling of infused WT and Swap-70−/− eosinophils in inflamed (TNFα-stimulated) cremaster muscle microvessels of anesthetized mice by IVM. (B) Velocities of rolling WT and Swap-70−/− eosinophils under conditions of blood flow. Combined data from 55 Swap-70−/− and 51 WT rolling eosinophils in 3–5 vessels/mouse is shown. (C) Adhesion of infused WT and Swap-70−/− eosinophils in cremaster muscle microvessels. Combined data from 4–6 vessels per mouse is shown for A and C. Data represent mean ± SEM in A-C. n = 3 mice/group in A-C. *p < 0.01 and ** p < 0.05 for comparison of WT versus Swap-70−/− eosinophils.
Fig 6. Allergen-induced airway inflammation is attenuated in Swap-70−/− mice.
(A) Immunohistology of paraffin embedded lung sections from saline and allergen (OVA)-challenged WT mice with polyclonal antibodies against murine SWAP-70. Black arrows in lung sections of allergen-challenged mice indicate cells positive for SWAP-70. Magnification ×200. (B) Immunofluorescence staining of BALF cells from allergen-challenged WT mice with polyclonal antibodies against SWAP-70 (upper panel) and rat mAb against murine MBP (lower panel) followed by rhodamine-conjugated goat anti-rabbit IgG and FITC-conjugated goat anti-rat IgG, respectively. Images of the same field with and without DAPI staining are shown. White arrows indicate cells in BALF that are negative for both SWAP-70 and MBP expression, while red arrows indicate cells that are positive for SWAP-70 but negative for MBP expression. Magnification ×600. Representative images are shown for A and B. (C) Total cell counts in BALF from saline and OVA-challenged WT and Swap-70−/− mice. (D) Cellular infiltration of lungs after H&E staining of paraffin embedded lung sections. Representative images (magnification ×100) are shown. (E) Differential cell counts in BALF from saline and OVA-challenged WT and Swap-70−/− mice. Combined data from experiments repeated at least three times is shown for C and E. n = 11 mice for saline groups and 15 mice for OVA-challenged groups. (F) Infiltration of lung tissue by eosinophils after staining with rat mAb against murine MBP. Representative images (magnification ×200) are shown for saline and allergen-challenged groups. (F) Pulmonary resistance and compliance measured in saline and OVA-challenged WT and Swap-70−/− mice by invasive plethysmography after exposure to increasing concentrations of aerosolized methacholine (MCh). Combined data of three experiments is shown. n = 7–9 per group. (G) Mucus production in airways after PAS staining. PAS-positive area in airways of relatively similar size (3–12 airways/slide) was quantitated by ImageJ analysis of captured images (left). Representative images (magnification ×200) are shown for saline and allergen-challenged groups (right). For A and B, n = 5 mice per group and for D, F and H, n = 9 mice per group. Data represent mean ± SEM in C, E, G and H (left panel). *p < 0.05 and **p < 0.02 for allergen-challenged Swap-70−/− versus WT mice. #p < 0.01 for allergen-challenged group versus respective saline group.
Results
Human and murine eosinophils express SWAP-70
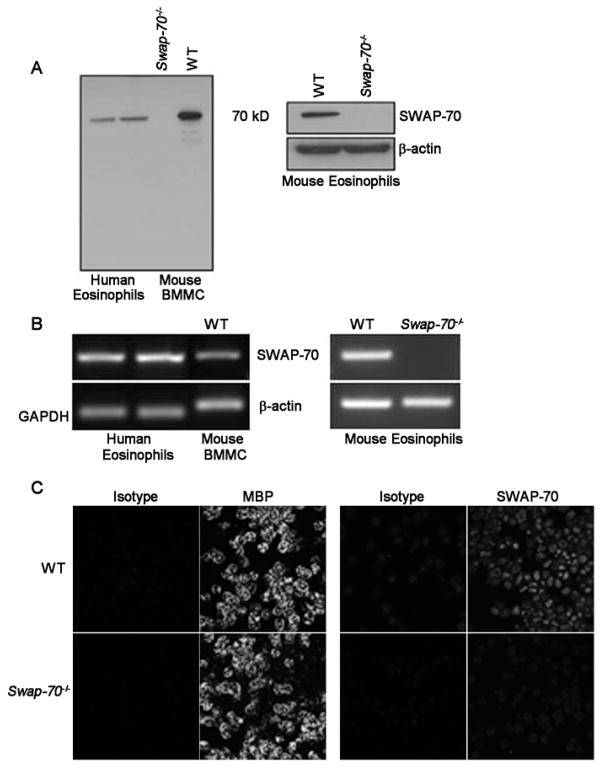
Western blots of lysates of human eosinophils isolated from peripheral blood of allergic donors (n=5) with antibodies against murine SWAP-70 demonstrated a band of 70 kD corresponding to the reported molecular weight of SWAP-70 (Fig. 1A, left panel, two representative donors shown). In addition, a band corresponding to 70 kD was observed in lysates of WT BM-cultured murine eosinophils, but not eosinophils from Swap-70−/− mice (Fig. 1A, right panel). Lysates of BM MC from WT mice known to strongly express SWAP-70 (14) were used as a positive control and demonstrated a band of similar molecular weight, while lysates of BM MC from Swap-70−/− mice that served as the negative control did not demonstrate any bands in this region (Fig. 1A, left panel). Further, human eosinophils (Fig. 1B, left panel) and WT, but not Swap-70−/−, murine eosinophils (Fig. 1B, right panels) were found to express SWAP-70 mRNA by RT-PCR. Expression of SWAP-70 by murine eosinophils was also confirmed by confocal microscopy (Fig. 1C), where WT eosinophils were positive for expression of eosinophil-specific MBP (Fig. 1C, upper left panels) as well as SWAP-70 (Fig. 1C, upper right panels), and Swap-70−/− eosinophils were positive only for expression of MBP (Fig. 1C, lower left panels). Together, these studies clearly demonstrate that human and murine eosinophils express SWAP-70.
Fig 1. Expression of SWAP-70 by murine and human eosinophils.
(A) Western blot of human eosinophil lysates (two representative allergic donors) as well as lysates of BMMC and eosinophils from WT and Swap-70−/− mice with affinity purified polyclonal antibodies against murine SWAP-70. Lysates of Swap-70−/− and WT BMMC served as the negative and positive control, respectively. (B) RT-PCR with total RNA from human eosinophils (two representative allergic donors) as well as eosinophils from WT and Swap-70−/−mice using species specific primers for the first exon of the SWAP-70 gene. Total RNA from WT BMMC was used as the positive control. Expression of GAPDH (for human eosinophils) and β-actin (for murine BMMC and eosinophils) served as internal controls. (C) Immunofluorescence staining for eosinophil-specific MBP and SWAP-70 expression by WT and Swap-70−/− eosinophils by confocal microscopy with rat anti-mouse MBP antibodies and affinity purified rabbit anti-mouse SWAP-70 antibodies followed by FITC-conjugated goat anti-rat or anti-rabbit IgG, respectively. Rat IgG1 (for MBP) and rabbit IgG (for SWAP-70) were used as isotype controls. Data shown in C is representative of three independent experiments with eosinophils from three different mice for each group.
Altered adhesive interactions of Swap-70−/− eosinophils under conditions of flow in vivo
To evaluate whether SWAP-70 is involved in promoting adhesive interactions of eosinophils with the inflamed vascular endothelium, trafficking of fluorescently-labeled WT and Swap-70−/− eosinophils was examined in vivo within TNFα-treated post capillary venules under conditions of blood flow by intravital microscopy (IVM). Compared to WT eosinophils, an increased number of Swap-70−/− eosinophils were found to roll along the vessel wall (Fig. 2A). Interestingly, however, a large fraction (~ 70%) of these rolling cells were slow rollers that demonstrated extremely slow rolling with velocities < 10 μm/sec without adhering while the remaining eosinophils (~ 30%) were intermediate and fast rollers with velocities in the range of 10–100 μm/sec and > 100 μm/sec, respectively (Fig. 2B). In contrast, only 35% of WT eosinophils exhibited slow rolling with near equal proportions of intermediate and fast rollers. Further, a significantly larger number of WT eosinophils were found to adhere to the vascular endothelium compared to Swap-70−/− eosinophils (p < 0.01) (Fig. 2C). Overall, these findings suggest that SWAP-70 plays a role in regulating adhesive interactions (rolling and adhesion) of eosinophils with the vascular endothelium under conditions of blood flow in vivo.
Reduced adhesion of Swap-70−/− eosinophils to VCAM-1 and ICAM-1
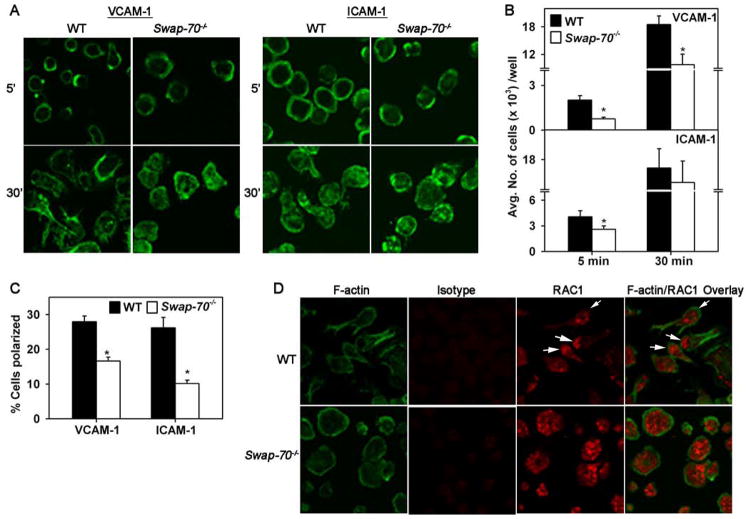
Since Swap-70−/− eosinophils exhibited considerably decreased adhesion to the vascular endothelium in post capillary venules, adhesion to specific vascular adhesion molecules was evaluated by confocal microscopy (Fig. 3A). Evaluation of the actin cytoskeleton in ICAM-1- and VCAM-1-adhered eosinophils following phalloidin binding demonstrated subtle differences after 5 min. For example, adherent WT eosinophils displayed phalloidin binding mostly localized to the cell periphery/margin while Swap-70−/− eosinophils exhibited more dispersed phalloidin binding, particularly in cells adhered to ICAM-1, although no major difference in cell shape was visible between the two cell types (Fig. 3A, upper panels). At 30 min, the actin cytoskeleton of eosinophils of both groups was similar with cells exhibiting dispersed phalloidin-binding all over the cell (Fig. A B, lower panels). However, at this later time point, many WT eosinophils adhered to VCAM-1 and ICAM-1 exhibited distinct cell polarization, i.e leading edge formation and development of uropodia and filopodia, while Swap-70−/− eosinophils exhibited less polarization and more irregular spreading with no distinct leading edges or filopodia. This correlated with the number of adherent cells in static adhesion assays (Fig. 3B). At 5 min, Swap-70−/− eosinophils showed significantly reduced adhesion to VCAM-1 (p < 0.01) or to ICAM-1 (p < 0.05) compared to WT eosinophils. By 30 min, Swap-70−/− eosinophils continued to exhibit significantly decreased adhesion to VCAM-1 (p < 0.01) compared to WT eosinophils although adhesion to ICAM-1 was similar in both groups. Quantitation of the number of polarized cells, defined as cells with a distinct leading edge and uropod compartment, revealed that a significantly larger percentage of WT eosinophils adhered to VCAM-1 and ICAM-1 were polarized compared to Swap-70−/− eosinophils (p < 0.01) (Fig. 3C). Immunofluorescence studies were performed to evaluate expression of RAC1, which plays a key role in regulating the actin cytoskeleton and participates in the formation of lamellipodia (39). In Swap-70−/− eosinophils adhered to VCAM-1, RAC1 expression was dispersed over the entire cell (Fig. 3D, lower panels), while in polarized WT eosinophils, RAC1 expression translocated to the leading edge of the cells with less expression observed in other areas of the cell (Fig. 3D, upper panels). The few non-polarized WT eosinophils exhibited dispersed RAC1 staining similar to Swap-70−/− eosinophils. However, in both genotypes, there was no overlapping of RAC1 with F-actin expression in contrast to previous observations with both MC (21) and B cells (20).
Fig 3. Decreased adhesion associated with altered cytoskeletal rearrangement and impaired RAC1 translocation in Swap-70−/− eosinophils.
(A) Staining of WT and Swap-70−/− eosinophils adhered to ICAM-1 or VCAM-1 with FITC-phalloidin followed by confocal microscopy. Magnification × 600. (B) Adhesion of fluorescently labeled WT and Swap-70−/− eosinophils to wells coated with rm VCAM-1 (5 μg/ml) or rm ICAM-1 (10 μg/ml) in static adhesion assays. Adherent cells were quantitated in a microplate reader against a generated standard curve of fluorescently-labeled eosinophils. (C) Polarization of adherent WT and Swap-70−/− eosinophils. The number of polarized cells, defined as cells with a distinct leading edge and uropod compartment, after adhesion to VCAM-1 and ICAM-1 for 30 min were assessed and expressed as a percentage of the total number of cells in a given field. (D) Immunofluorescence staining for RAC1 expression in WT and Swap-70−/− eosinophils adhered to VCAM-1. Adhered cells were stained for F-actin with FITC-phalloidin and for RAC1 using polyclonal antibodies against RAC1 (20 μg/ml) followed by rhodamine-conjugated secondary antibodies. Rabbit IgG was used as the isotype control. Magnification × 600. Combined data (mean ± SEM) of two (for VCAM-1) or three (for ICAM-1) independent experiments in triplicate for B and of five fields/slide from two independent experiments for C is shown. Data in B and D is representative of two-three independent experiments. *p value for comparison of WT versus Swap-70−/− eosinophils < 0.01 for VCAM-1 and <0.05 for ICAM-1 in A and <0.01 for VCAM-1 and ICAM-1 in C.
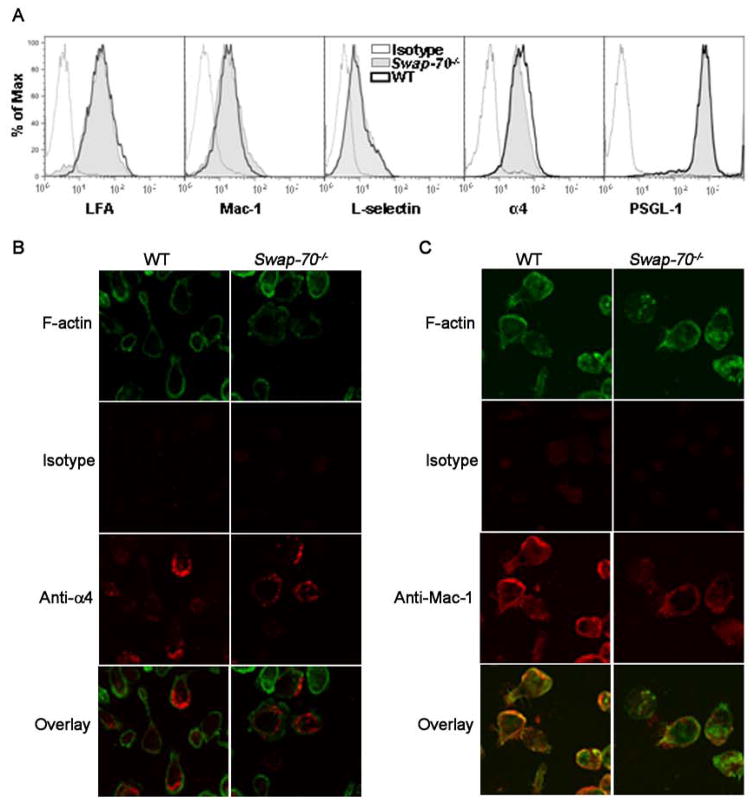
To determine whether this decreased adhesion of Swap-70−/− eosinophils was due to baseline differences in cell surface adhesion molecule expression, LFA-1, Mac-1, L-selectin, PSGL-1 and α4 expression was assessed by flow cytometry and indicated no significant differences between WT and Swap-70−/− eosinophils (Fig. 4A). We next investigated whether the expression and distribution of these receptors on the cell surface during adhesion may be altered in Swap-70−/− eosinophils compared to WT cells by confocal microscopy. Interestingly, distinct differences were observed in the distribution of α4 (Fig. 4B). WT eosinophils adhered to VCAM-1 exhibited intense α4 expression at the leading edge of polarized cells with less expression in the main body and uropod compartment, while expression of this receptor by Swap-70−/− eosinophils was observed around the main body of the cell. Expression of α4 did not colocalize with F-actin. With Mac-1, not only distribution but even expression was altered in SWAP-70−/− eosinophils (Fig. 4C). Firstly, WT eosinophils adhered to ICAM-1 demonstrated increased Mac-1 expression compared to Swap-70−/− eosinophils. While eosinophils express Mac-1 constitutively, studies have shown that it can be increased by exposure to various stimuli (cytokines, chemokines, interaction with endothelial cells)(40). Secondly, in WT eosinophils, Mac-1 expression was predominantly observed at the cell periphery with polarized cells exhibiting more intense Mac-1 expression at leading edges. In contrast to WT eosinophils, Mac-1 expression in Swap-70−/− eosinophils was dispersed all over the cell. F-actin was variably expressed in both cell types, however, F-actin expression colocalized with Mac-1 at the cell periphery in WT eosinophils but not in Swap-70−/− eosinophils. These data suggest that the distribution, but not the level of expression, of α4 on the cell surface of adherent eosinophils is regulated by SWAP-70, while for Mac-1, both distribution and level of expression are regulated by this molecule. Expression and spatial distribution of cell surface adhesion receptors is critical for efficient cellular rolling and/or firm adhesion. Therefore, although no significant differences in baseline α4 and Mac-1 expression were observed between WT and Swap-70−/− eosinophils by flow cytometry (Fig. 4A), aberrations in distribution and level of expression observed with adherent eosinophils may account for the continued slow rolling of Swap-70−/− eosinophils with failure to adhere efficiently in vivo under conditions of blood flow.
Fig 4. Adhesion molecule distribution is altered in Swap-70−/− eosinophils.
(A) Expression of adhesion molecules by WT and Swap-70−/− eosinophils by flow cytometry. Expression of LFA-1 and α4 was evaluated using rat mAbs against LFA-1 and α4, respectively, followed by FITC-conjugated anti-rat antibodies with rat IgG2b as the isotype control. PE-conjugated rat mAbs against Mac-1 and PSGL-1, and FITC-conjugated rat mAbs against L-selectin were used to evaluate expression of Mac-1, PSGL-1 and L-selectin, respectively. PE- or FITC-conjugated rat IgG2a was used as the isotype control. All antibodies were used at a concentration of 5 μg/ml. (B and C) Immunofluorescence staining to evaluate distribution of α4 and Mac-1 on the cell surface of WT and Swap-70−/− eosinophils adhered to VCAM-1 and ICAM-1, respectively. Adhered cells were first stained with goat anti-mouse α4 (5 μg/ml) or rat anti-mouse Mac-1 (10 μg/ml) followed by rhodamine-conjugated secondary antibodies and then with FITC-phalloidin in buffer containing 0.5% saponin. Goat IgG (for anti-α4) or rat IgG2b (for anti-Mac-1) was used as the isotype control. Magnification × 600. Data shown in A and B is representative of three independent experiments with eosinophils from three different mice for each group.
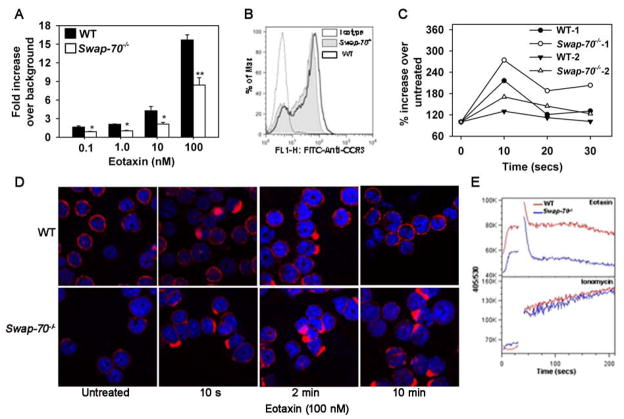
Diminished chemokine-induced migration by Swap-70−/− eosinophils
Previous studies with MC and B cells have indicated a role for SWAP-70 in cell migration (20, 21, 41). In the current study, migration of WT and Swap-70−/− eosinophils in response to eotaxin-1 at concentrations ranging from 0.1 to 100 nM was evaluated in vitro. Relative to WT eosinophils, Swap-70−/− eosinophils exhibited significantly decreased migration (p<0.01 at 100 nM) (Fig. 5A), although no difference in cell surface expression of the eotaxin-1 receptor CCR3 was noted between Swap-70−/− and WT eosinophils by flow cytometry (Fig. 5B). Since actin polymerization and cytoskeletal reorganization in response to activation is critical for directional movement and migration, we evaluated F-actin levels and examined the actin cytoskeleton in WT and Swap-70−/− eosinophils before and after exposure to eotaxin-1. WT and Swap-70−/− eosinophils exhibited a similar pattern of increase and decrease in F-actin levels up to 30 s. However, based on rhodamine-phalloidin binding, the increase in baseline fluorescence (untreated) after treatment with eotaxin-1, which is indicative of total F-actin levels, was higher in Swap-70−/− eosinophils at every time point than in WT cells. While experimental variation was observed in absolute cell fluorescence, the trends were identical and two representative experiments out of a total of five are shown (Fig. 5C). Difference in actin polymerization was also assessed by confocal microscopy of WT and Swap-70−/− eosinophils adhered to poly-L-Lysine-coated surfaces (Fig. 5D). Untreated WT and Swap-70−/− eosinophils (without eotaxin-1) demonstrated a defined pattern of membrane associated F-actin by confocal microscopy. After exposure to eotaxin-1 for 10 s, both WT and Swap-70−/− eosinophils exhibited increased phalloidin binding mostly localized at one end of the cells. By 2 min, more Swap-70−/− eosinophils showed increased phalloidin binding compared to WT eosinophils. Interestingly, by 10 min, the actin cytoskeleton in WT eosinophils reverted back to that observed in untreated cells while Swap-70−/− eosinophils continued to demonstrate increased phalloidin binding. Overall, deficiency of SWAP-70 appears to result in irregular F-actin polymerization/depolymerization causing persistent elevated F-actin levels and altered cytoskeletal changes upon stimulation with eotaxin-1.
Fig 5. Eotaxin-1-induced migration is impaired in Swap-70−/− eosinophils.
(A) Migration of WT and Swap-70−/− eosinophils towards increasing concentrations of murine eotaxin-1 or media alone (background) in Transwell® plates. Combined data (mean ± SEM) from three experiments in duplicate is shown. (B) Expression of CCR3, the receptor for eotaxin-1, by WT and Swap-70−/− eosinophils by flow cytometry using FITC-conjugated anti-mouse CCR3 (5 μg/ml). Rat IgG2a was used as the isotype control. (C) Total F-actin in WT and Swap-70−/− eosinophils by flow cytometry after rhodamine-phalloidin staining of cells before and after treatment with eotaxin-1 (100 nM) for the indicated time points. Data of two representative experiments from a total of five independent experiments is shown. (D) Staining of WT and Swap-70−/− eosinophils adhered to poly-L-Lysine with rhodamine-phalloidin after treatment with PBS (untreated) or eotaxin-1 (100 nM) for the indicated time points. Magnification × 600. (E) Ca2+ flux in WT and Swap-70−/− eosinophils based on Indo-1, AM binding before and after stimulation with 100 nM eotaxin-1 (upper panel) or 2 μM ionomycin (lower panel) evaluated by flow cytometry. Eotaxin was added at 30 s. Data is shown as wavelengths 405/530 which reflects the ratio of bound to free Ca2+. Data shown in B, D and E is representative of three independent experiments with eosinophils from three different mice. *p < 0.05 and **p < 0.01 for comparison of WT versus Swap-70−/− eosinophils in A.
Eotaxin-1 is known to induce calcium flux in eosinophils (42). Calcium flux in response to eotaxin-1 (100 nM) in WT and Swap-70−/− eosinophils was investigated by flow cytometry (Fig. 5E, upper panel). Baseline (unstimulated) intracellular Ca2+ levels in WT eosinophils were higher compared to Swap-70−/− cells. Further, although both WT and Swap-70−/− eosinophils demonstrated a similar pattern of an initial surge in free intracellular Ca2+ in response to eotaxin-1 within few seconds followed by a decline, intracellular Ca2+ levels remained elevated in WT eosinophils compared to Swap-70−/− eosinophils up to 3 min. In contrast, both WT and Swap-70−/− eosinophils demonstrated a similar response to 2 μM ionomycin (Fig. 5E, lower panel) indicating that Ca2+ release as such is not affected and that it is the eotaxin-1-induced Ca2+ responses that are altered in Swap-70−/− eosinophils. These data suggest that eotaxin-1-induced migration of eosinophils is dependent upon intracellular SWAP-70 signaling.
Allergic airway inflammation results in recruitment of SWAP-70-expressing inflammatory cells, including eosinophils, to the lungs
The identification of a role for SWAP-70 in regulating trafficking and migration of eosinophils, the predominant cells recruited during allergic inflammation, prompted us to examine whether allergen-exposed lungs demonstrate increased presence of SWAP-70-positive cells in vivo in a murine model of acute allergic airway inflammation. Expression of SWAP-70 in the lungs of WT mice in response to allergen (OVA) challenge was examined by immunohistology using affinity purified rabbit polyclonal antibodies against murine SWAP-70 (Fig. 6A). While no positive staining was detected in sections from control mice sensitized and challenged with saline instead of the allergen, expression of SWAP-70 by inflammatory cells infiltrating the lung tissue after allergen challenge was clearly evident. Further, we investigated specifically whether eosinophils recruited to the airways in response to allergen challenge express SWAP-70. In these studies, cells recovered from the bronchoalveolar lavage fluid (BALF) of acute allergen-challenged mice were dual stained with antibodies against eosinophil-specific MBP and SWAP-70 and examined by confocal microscopy (Fig. 6B). As expected, BALF from allergen-challenged mice was found to contain predominantly cells that expressed eosinophil-specific MBP. All MBP-positive cells were also positive for expression of SWAP-70. Few cells that were positive for SWAP-70 but negative for MBP (Fig. 6B, red arrows) or negative for both MBP and SWAP-70 (Fig. 6B, white arrows) were observed.
Swap-70−/− mice exhibit decreased airway inflammation after allergen-challenge
Since SWAP-70 promotes eosinophil adhesion (Fig. 3B) as well as migration (Fig. 5A) and SWAP-70-expressing eosinophils are recruited to allergen-exposed lungs (Fig. 6B), the role of SWAP-70 in the development of allergic airway inflammation was investigated in allergen-challenged Swap-70−/− and WT mice. While both groups demonstrated an increased number of inflammatory cells in the BALF and lung tissue by H&E staining compared to respective control mice, allergen-challenged Swap-70−/− mice had significantly fewer cells in the BALF (p<0.02) and lung tissue compared to WT counterparts (Fig. 6C and D). Differential cell counts in the BALF indicated a marked reduction in eosinophil recruitment (p<0.02) to the airways of allergen-challenged Swap-70−/− mice relative to WT mice (Fig. 6E). Further, a significant reduction in recruitment of lymphocytes (p < 0.05) but not of macrophages or neutrophils was noted. Immunohistology to detect lung tissue eosinophils also indicated a decreased number of eosinophil-specific MBP-positive cells in lung sections of allergen-challenged Swap-70−/− mice compared to WT mice (Fig 6F), while very few to no MBP-positive cells were detected in control mice of both groups. No difference was observed between WT and Swap-70−/− mice with respect to the number of eosinophils in the BM after allergen challenge (16.22 ± 2.76 % for WT mice and 12.65 ± 1.56 % for Swap-70−/− mice, p = 0.156) indicating that eosinophil generation is not impaired in SWAP-70 deficient mice. Further, blood eosinophils in naïve WT and Swap-70−/− mice before sensitization and challenge (2.16 ± 0.32 % for WT and 2.05 ± and 0.4 for Swap-70−/− mice) and their increase following allergen challenge were similar (3.98 ± 0.56 % for WT mice and 5.62 ± 0.65 % for Swap-70−/− mice, p = 0.065), indicating that the role of SWAP-70 in regulating eosinophil recruitment is more likely at the level of cell adhesion and migration during inflammation.
Associated with the increased airway eosinophilia, allergen-challenged WT mice displayed significantly elevated airway reactivity with increased pulmonary resistance (RL) and decreased compliance [Cdyn] compared to control mice (p < 0.01) in response to aerosolized methacholine by invasive plethysmography (Fig. 6G). Allergen-challenged Swap-70−/− mice demonstrated considerable improvement in airway function with a significant reduction in pulmonary resistance and improved compliance compared to allergen-challenged WT mice (p < 0.05 at 25 mg/ml methacholine), although airway reactivity was higher compared to the respective control group. Next, airway mucus production by goblet cells in lung sections from control and allergen-challenged WT and Swap-70−/− mice was assessed after periodic acid-Schiff (PAS) staining (Fig. 6H). Negligible PAS-positive staining was observed in lung sections of control mice from both groups. As expected, the bronchial epithelium of allergen-challenged WT mice exhibited an increased number of PAS-positive mucus-producing cells and was found to be significantly higher than in allergen-challenged Swap-70−/− mice (p < 0.05).
Decreased Th2 cytokine levels in lungs of allergen-challenged Swap-70−/− mice
Th1 (IL-2, INF-γ)/Th2 (IL-4, IL-5, IL-13) cytokine, TNFα and eotaxin-1 levels in the BALF of control and allergen-challenged WT and Swap-70−/− mice were quantitated (Table 1). In WT mice, levels of IL-5, IL-13 and TNFα significantly increased relative to control mice in response to allergen challenge with no change in IL-2 and IFN-γ levels. While IL-4 and eotaxin-1 levels in allergen-challenged WT mice also tended to be higher than in control mice, the increase was not statistically significant. More importantly, although levels of IL-5, IL-13 and TNFα were also found to be higher in allergen-challenged Swap-70−/− mice compared to corresponding controls, they were significantly lower than in WT counterparts (p < 0.05). IL-4 and eotaxin-1 levels in allergen-challenged Swap-70−/− mice were similar to levels in corresponding control mice.
Table I.
BALF cytokines in allergen-challenged WT and Swap-70−/− mice
| Cytokine | WT mice Mean ± SEM pg/ml BALF | Swap-70−/− mice Mean ± SEM pg/ml BALF | ||
|---|---|---|---|---|
| Saline | OVA | Saline | OVA | |
| IL-4 | 3.09 ± 0.43 | 24.77 ± 19.53 | 2.25 ± 0.32 | 4.06 ± 0.93 |
| IL-5 | 7.37 ± 1.37 | 62.22 ± 24.77* | 5.8 ± 0.97 | 14.32 ± 4.11*,# |
| IL-13 | <0.01 | 16.59 ± 6.03* | <0.01 | 1.9 ± 1.0*,# |
| IFNγ | 4.45 ± 0.4 | 3.98 ± 0.9 | 2.59 ± 0.45 | 3.38 0.48 |
| IL-2 | 3.54 ± 0.53 | 3.51 ± 0.69 | 2.64 ± 0.35 | 3.02 ± 0.32 |
| TNFα | 10.27 ± 1.66 | 24.18 ± 6.0* | 5.98 ± 1.15 | 11.15 ± 2.46*,# |
| Eotaxin-1 | 13.27 ± 2.41 | 27.05 ± 8.38 | 11.34 ± 2.85 | 13.43 ± 4.61 |
n = 8 mice for saline groups and 9 mice for OVA-challenged groups
p < 0.05 Saline versus OVA-challenged groups for WT and Swap-70−/− mice
p < 0.05 OVA-challenged Swap-70−/− mice versus OVA-challenged WT mice
Discussion
Eosinophils are central effector cells in an ongoing allergic inflammation and are recruited to the site of inflammation from the peripheral blood. This process is driven by a cascade of events involving the specific and sequential engagement of cell surface adhesion receptors with vascular counter ligands in inflamed blood vessels followed by a series of signaling events resulting in actin rearrangement and cytoskeletal reorganization leading to directed cell shape changes that facilitate cell adhesion and migration into inflamed tissues (5, 8, 9, 43). SWAP-70 is an intracellular signaling protein previously shown to play a role in regulating B cell adhesion and migration (20, 41) and to participate in events such as degranulation, adhesion, migration, differentiation and survival during MC activation (14, 21).
In the current study, we show for the first time that both human and murine eosinophils express SWAP-70 and demonstrate a role for this molecule in eosinophil trafficking and migration as well as its impact on the development of allergic airway inflammation in mice. Swap-70−/− eosinophils exhibited altered trafficking in inflamed blood vessels under physiologic conditions of blood flow with a large fraction of cells demonstrating slow rolling with velocities < 10 μm/sec as opposed to majority of the WT eosinophils that rolled at higher velocities which is suggestive of aberrant interactions with the vascular endothelium. Interestingly, most of the slow rolling Swap-70−/− eosinophils failed to adhere to the vascular endothelium, while WT eosinophils exhibited significant adhesion. This finding is in contrast to previous studies with B cells where Swap-70−/− cells rolled and adhered as well as WT cells, but accumulated in high endothelial venules of lymph nodes and failed to migrate (20). Consistent with failure to adhere efficiently to the vascular endothelium under conditions of flow, Swap-70−/− eosinophils demonstrated reduced attachment to ICAM-1 and even more profoundly to VCAM-1. Based on phalloidin staining, Swap-70−/− eosinophils exhibited decreased cell polarization with poor formation of leading edges, filopodia and uropods causing more cells to retain an irregular morphology and appear spread out compared to WT eosinophils. In this respect, Swap-70−/− eosinophils appear to behave similar to CCL19-stimulated SWAP-70 deficient B cells which also failed to form uropods or stable lamellipodia after attachment to anti-CD44 coated surfaces and exhibited decreased polarization on ICAM-1 and MadCAM (20, 41). Leukocytes are known to undergo distinct shape change from a round free-flowing form to a polarized morphology with formation of a leading edge and uropod upon activation (44). Therefore, although Swap-70−/− eosinophils roll slowly and can potentially become activated by encountering endothelial ligands or immobilized chemokines, lack of formation of a leading edge allowing directional movement of the cell may perhaps be responsible for their continued slow rolling with decreased ability to adhere under conditions of flow in vivo.
RAC1 plays a central role in cell motility by regulating the actin cytoskeleton and participating in the formation of lamellipodia and filopodia (39). In the present study, while WT eosinophils attached to VCAM-1 exhibited localization of F-actin to the cell periphery and translocation of RAC1 to the leading edge, in Swap-70−/− eosinophils F-actin and RAC1 expression remained dispersed all over the cell and did not localize to the periphery. Similar findings have been reported for B cells and MC, where RAC1 and F-actin localize to the cell periphery upon activation (attachment or chemokine stimulation) in a SWAP-70-dependent manner (20, 21), except that in WT and Swap-70−/− eosinophils RAC1 and F-actin do not colocalize as in MC and B cells. Studies have also shown that SWAP-70 interacts with and regulates RAC1 (15) and also binds to F-actin and promotes membrane ruffling (25). Therefore absence of SWAP-70 may lead to aberrant RAC1 translocation and F-actin localization resulting in failure of Swap-70−/− eosinophils to efficiently form leading edges and undergo polarization.
α4β1-VCAM-1 and β2 integrin-ICAM-1 interactions together drive eosinophil rolling, tethering and arrest (5). As observed with B cells (20), absence of SWAP-70 in eosinophils did not appear to affect the basal (non-activated) level of expression of α4, Mac-1 and LFA-1. However, SWAP-70 was found to be essential for regulating the distribution of α4 and Mac-1 on adherent eosinophils. In addition to the conformational state, receptor and ligand density, the spatial distribution of integrins on the cell surface determines their adhesiveness (45). Accordingly, in the current study, the more adhesive WT eosinophils express α4 localized to the leading edge of polarized cells, while in the less adhesive Swap-70−/− eosinophils, perhaps due to the failure to form leading edges, α4 expression does not localize to specific structures and remains dispersed all over the cell. Likewise, Mac-1 expression colocalized with F-actin at the cell periphery in WT eosinophils but remained diffused in Swap-70−/− eosinophils without colocalizing with F-actin. α4β1 integrins mediate rolling and initial attachment of eosinophils on VCAM-1 (46) and TNFα-stimulated HUVEC (47), while β2 integrins are thought to act later and mediate eosinophil arrest/firm adhesion(48). Therefore, based on their role in adhesive events promoting cell trafficking, it is conceivable that α4 is presented at the leading edge to initiate attachment with endothelial counter receptors and Mac-1 is presented at the periphery of the entire cell to establish firm adhesion along a wide surface area under conditions of blood flow. Interestingly, using transfected cells, integrins such as α4β1 have previously been shown to localize at surface projections such as membrane ruffles and microvilli, whereas β2 integrins localized to the cell body (49). Along similar lines, absence of SWAP-70 in MC and B cells was found to alter regulation of LFA-1 resulting in homotypic hyperaggregation of these cells in vitro without affecting its level of expression (20, 21).
Along with the impaired adhesion, Swap-70−/− eosinophils also demonstrate significantly reduced migration in response to eotaxin-1 associated with decreased intracellular Ca2+ levels and altered kinetics of actin polymerization/depolymerization compared to WT cells. Eotaxin-1-induced F-actin levels were higher in Swap-70−/− eosinophils and persisted for a longer time in adherent (poly L-Lysine) Swap-70−/− eosinophils compared to WT eosinophils. Further, unlike WT eosinophils, Swap-70−/− eosinophils demonstrated increased F-actin localized at one end of the cells without returning to basal (untreated) levels even at 10 min. This is suggestive of defective F-actin depolymerization rather than impaired polymerization in the absence of SWAP-70. Remodeling of the actin cytoskeleton, often mediated by the Rho family of GTPases in response to extracellular signals, is critical for cellular activities such as cell motility and active processes including cell adhesion and migration and requires the coordinated polymerization and depolymerization of F-actin. The eotaxin-1 receptor CCR3 belongs to the family of G protein-coupled receptors (50), which activate small RAC GTPases upon stimulation (51, 52). Since the level of expression of this receptor is not affected by SWAP-70 deficiency, it is more likely that regulation of signaling through CCR3 is impaired as indicated by the reduced intracellular Ca2+ levels and delayed F-actin depolymerization upon stimulation resulting in aberrant cytoskeletal rearrangement in Swap-70−/− eosinophils that can impact cell polarization towards a chemokine gradient. Absence of SWAP-70 in MC results in a similar pattern of sustained elevated F-actin-positivity and formation of multiple elongated filopodia-like F-actin protrusions in response to SCF associated with decreased migration towards this chemokine (21).
Since SWAP-70 is required for normal eosinophil trafficking and migration, we investigated its contribution to the development of allergic airway inflammation, which is largely eosinophil-associated, in a murine model. In contrast to allergen-challenged WT mice that develop a classical asthmatic response characterized by airway eosinophil infiltration, airway hyperresponsiveness (AHR), elevated eotaxin-1 and Th2 cytokines (IL-5, IL-4 and IL-13) as well as airway mucus secretion, the allergic inflammatory response in Swap-70−/− mice was attenuated. Most striking was the significant decrease in airway recruitment of eosinophils. This decrease in airway eosinophil recruitment in Swap-70−/− mice is most likely due to impaired trafficking (rolling and adhesion) and chemokine-induced migration of Swap-70−/− eosinophils. Although eotaxin-1 levels in the BALF of allergen-challenged WT mice tended to be higher than in Swap-70−/− counterparts, the increase was not statistically significant and moreover the in vitro studies indicate that Swap-70−/− eosinophils migrate less efficiently than WT eosinophils (despite similar levels of eotaxin-1 and CCR3 expression). In addition to eosinophils, Swap-70−/− allergen-challenged mice demonstrated a reduction in the number of total lymphocytes (T and B cells) recruited to the airways compared to WT mice. B cell migration (20) and IgE class switching (13) is known to be impaired in SWAP-70−/− mice and since non-activated T cells do not express SWAP-70 (11), decreased T cell recruitment to the airways may be an indirect effect due to inhibition of other factors that are known to promote T cell recruitment including MC-derived mediators (histamine) released during degranulation (53). Indeed, both migration and FcεRI-mediated degranulation of MC are impaired in the absence of SWAP-70 (14, 21). Alternatively, while not well established, it is conceivable that activated T cells may express SWAP-70 and would thus be affected by its deficiency.
Associated with the decreased eosinophilia, allergen-challenged Swap-70−/− mice displayed significantly reduced AHR, mucus secretion, IL-5, IL-13 and TNFα compared to WT mice. Eosinophils, through the release of mediators (leukotrienes) and cytokines (IL-13), are crucial for mucus secretion in the airways and in the development of AHR (54–56). Decreased levels of IL-13 in allergen-challenged Swap-70−/− mice may be responsible for the attenuated AHR and mucus secretion. While reduced IL-13 may also contribute to overall decreased cellular recruitment by virtue of its ability to support leukocyte trafficking via induction of VCAM-1 on endothelial cells (57), altered trafficking of Swap-70−/− eosinophils within inflamed blood vessels of SWAP-70 competent mice (slow rolling and decreased adhesion) combined with decreased migration is indicative of an important role for SWAP-70 in eosinophil recruitment during inflammation irrespective of the potential contribution of the vascular endothelium. The decreased recruitment of lymphocytes to the airways may also in part contribute to the decreased BALF cytokines, especially IL-5, which in turn can contribute to the reduced eosinophilia. Finally, eosinophils and MC are a source of TNFα (58, 59) and while we have not evaluated TNFα release by Swap-70−/− eosinophils, Swap-70−/− MC demonstrate impaired TNFα release (14). Nonetheless, reduced TNFα levels in allergen-challenged Swap-70−/− mice due to decreased airway eosinophil recruitment may further attenuate airway inflammation.
In summary, eosinophils deficient in SWAP-70 exhibit altered trafficking (slow rolling with decreased firm adhesion) in inflamed blood vessels attributable to reduced polarization, failure of RAC1 translocation and aberrant adhesion molecule localization/distribution along with impaired calcium flux and defective actin depolymeriztion upon chemokine activation resulting in reduced ability to migrate in vitro. Further, in a model of allergic airway inflammation, Swap-70−/− mice show significantly reduced airway eosinophil recruitment and eosinophil-associated inflammatory responses. Since eosinophils are important contributors to the pathogenesis of allergen-induced airway inflammation, our findings, together with the previously identified role of SWAP-70 in B cell and MC recruitment as well as in immunoglobulin class switching, suggest that blockade of SWAP-70 may help in preventing allergic airway inflammation including asthma.
Acknowledgments
The authors wish to thank Yana Greenberg for excellent technical assistance.
Footnotes
This work was supported by National Institutes of Health grants AI35796, U19-AI70535 and HL0793041.
Abbreviations: AHR, airway hyperresponsiveness; BALF, bronchoalveolar lavage fluid; BM, bone marrow; Cdyn, dynamic compliance; IVM, intravital microscopy; MBP, major basic protein; MC, mast cells; PAS, periodic acid-Schiff; rm, recombinant murine; RL, pulmonary resistance
References
- 1.Boyce JA, Broide D, Matsumoto K, Bochner BS. Advances in mechanisms of asthma, allergy, and immunology in 2008. J Allergy Clin Immunol. 2009;123:569–574. doi: 10.1016/j.jaci.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Minai-Fleminger Y, Levi-Schaffer F. Mast cells and eosinophils: the two key effector cells in allergic inflammation. Inflamm Res. 2009;58:631–638. doi: 10.1007/s00011-009-0042-6. [DOI] [PubMed] [Google Scholar]
- 3.Palmqvist C, Wardlaw AJ, Bradding P. Chemokines and their receptors as potential targets for the treatment of asthma. Br J Pharmacol. 2007;151:725–736. doi: 10.1038/sj.bjp.0707263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Broide D, Sriramarao P. Cellular Adhesion in Inflammation. In: Adkinson NF, et al., editors. Middleton’s Allergy Principles and Practice. Vol. 1. 2008. pp. 149–165. [Google Scholar]
- 6.Critchley DR. Focal adhesions - the cytoskeletal connection. Curr Opin Cell Biol. 2000;12:133–139. doi: 10.1016/s0955-0674(99)00067-8. [DOI] [PubMed] [Google Scholar]
- 7.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 8.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 9.Huveneers S, Danen EHJ. Adhesion signaling - crosstalk between integrins, Src and Rho. J Cell Sci. 2009;122:1059–1069. doi: 10.1242/jcs.039446. [DOI] [PubMed] [Google Scholar]
- 10.Abram CL, Lowell CA. The Ins and Outs of Leukocyte Integrin Signaling. Ann Rev Immunol. 2009;27:339–362. doi: 10.1146/annurev.immunol.021908.132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borggrefe T, Wabl M, Akhmedov AT, Jessberger R. A B-cell-specific DNA Recombination Complex. J Biol Chem. 1998;273:17025–17035. doi: 10.1074/jbc.273.27.17025. [DOI] [PubMed] [Google Scholar]
- 12.Borggrefe T, Masat L, Wabl M, Riwar B, Cattoretti G, Jessberger R. Cellular, intracellular, and developmental expression patterns of murine SWAP-70. Eur J Immunol. 1999;29:1812–1822. doi: 10.1002/(SICI)1521-4141(199906)29:06<1812::AID-IMMU1812>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 13.Borggrefe T, Keshavarzi S, Gross B, Wabl M, Jessberger R. Impaired IgE response in SWAP-70-deficient mice. Eur J Immunol. 2001;31:2467–2475. doi: 10.1002/1521-4141(200108)31:8<2467::aid-immu2467>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Gross B, Borggrefe T, Wabl M, Sivalenka RR, Bennett M, Rossi AB, Jessberger R. SWAP-70-deficient mast cells are impaired in development and IgE-mediated degranulation. Eur J Immunol. 2002;32:1121–1128. doi: 10.1002/1521-4141(200204)32:4<1121::AID-IMMU1121>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 15.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, Fukui Y, Jessberger R. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 16.Oberbanscheidt P, Balkow S, Kühnl J, Grabbe S, Bähler M. SWAP-70 associates transiently with macropinosomes. Eur J Cell Biol. 2007;86:13–24. doi: 10.1016/j.ejcb.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Li D, Cao B, Li Y-x, Herva R, Piao Y-s, Wang Y-l. Expression and Localization of SWAP-70 in Human Fetomaternal Interface and Placenta During Tubal Pregnancy and Normal Placentation. J Histochem Cytochem. 2007;55:701–708. doi: 10.1369/jhc.6A7151.2007. [DOI] [PubMed] [Google Scholar]
- 18.Seol HJ, Smith CA, Salhia B, Rutka JT. The guanine nucleotide exchange factor SWAP-70 modulates the migration and invasiveness of human malignant glioma cells. Transl Oncol. 2009;2:300–309. doi: 10.1593/tlo.09172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masat L, Caldwell J, Armstrong R, Khoshnevisan H, Jessberger R, Herndier B, Wabl M, Ferrick D. Association of SWAP-70 with the B cell antigen receptor complex. Proc Natl Acad Sci U S A. 2000;97:2180–2184. doi: 10.1073/pnas.040374497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearce G, Angeli V, Randolph GJ, Junt T, von Andrian U, Schnittler H-J, Jessberger R. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nat Immunol. 2006;7:827–834. doi: 10.1038/ni1365. [DOI] [PubMed] [Google Scholar]
- 21.Sivalenka RR, Jessberger R. SWAP-70 Regulates c-kit-induced mast cell activation, cell-cell adhesion, and migration. Mol Cell Biol. 2004;24:10277–10288. doi: 10.1128/MCB.24.23.10277-10288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sivalenka RR, Sinha M, Jessberger R. SWAP-70 regulates mast cell FcepsilonRI-mediated signaling and anaphylaxis. Eur J Immunol. 2008;38:841–854. doi: 10.1002/eji.200737597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ocana-Morgner C, Wahren C, Jessberger R. SWAP-70 regulates RhoA/RhoB-dependent MHCII surface localization in dendritic cells. Blood. 2009;113:1474–1482. doi: 10.1182/blood-2008-04-152587. [DOI] [PubMed] [Google Scholar]
- 24.Hilpela P, Oberbanscheidt P, Hahne P, Hund M, Kalhammer G, Small JV, Bahler M. SWAP-70 Identifies a Transitional Subset of Actin Filaments in Motile Cells. Mol Biol Cell. 2003;14:3242–3253. doi: 10.1091/mbc.E03-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihara S, Oka T, Fukui Y. Direct binding of SWAP-70 to non-muscle actin is required for membrane ruffling. J Cell Sci. 2006;119:500–507. doi: 10.1242/jcs.02767. [DOI] [PubMed] [Google Scholar]
- 26.Wittchen ES, van Buul JD, Burridge K, Worthylake RA. Trading spaces: Rap, Rac, and Rho as architects of transendothelial migration. Curr Opin Hematol. 2005;12:14–21. doi: 10.1097/01.moh.0000147892.83713.a7. [DOI] [PubMed] [Google Scholar]
- 27.Dyer KD, Moser JM, Czapiga M, Siegel SJ, Percopo CM, Rosenberg HF. Functionally Competent Eosinophils Differentiated Ex Vivo in High Purity from Normal Mouse Bone Marrow. J Immunol. 2008;181:4004–4009. doi: 10.4049/jimmunol.181.6.4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ge XN, Bahaie NS, Kang BN, Hosseinkhani RM, Ha SG, Frenzel EM, Liu FT, Rao SP, Sriramarao P. Allergen-induced airway remodeling is impaired in galectin-3 deficient mice. J Immunol. 2010;185:1205–1214. doi: 10.4049/jimmunol.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, Aronica M, Li X. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kushnir-Sukhov NM, Gilfillan AM, Coleman JW, Brown JM, Bruening S, Toth M, Metcalfe DD. 5-Hydroxytryptamine Induces Mast Cell Adhesion and Migration. J Immunol. 2006;177:6422–6432. doi: 10.4049/jimmunol.177.9.6422. [DOI] [PubMed] [Google Scholar]
- 31.June CH, Moore JS. Measurement of intracellular ions by flow cytometry. Curr Protoc Immunol. 2004;Chapter 5(Unit 5.5) doi: 10.1002/0471142735.im0505s64. [DOI] [PubMed] [Google Scholar]
- 32.Zanardo RCO, Bonder CS, Hwang JM, Andonegui G, Liu L, Vestweber D, Zbytnuik L, Kubes P. A down-regulatable E-selectin ligand is functionally important for PSGL-1-independent leukocyte-endothelial cell interactions. Blood. 2004;104:3766–3773. doi: 10.1182/blood-2004-02-0578. [DOI] [PubMed] [Google Scholar]
- 33.Zuberi RI, Ge X, Jiang S, Bahaie NS, Kang BN, Hosseinkhani RM, Frenzel EM, Fuster MM, Esko JD, Rao SP, Sriramarao P. Deficiency of endothelial heparan sulfates attenuates allergic airway inflammation. J Immunol. 2009;183:3971–3979. doi: 10.4049/jimmunol.0901604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sikora L, Rao SP, Sriramarao P. Selectin-dependent rolling and adhesion of leukocytes in nicotine-exposed microvessels of lung allografts. Am J Physiol Lung Cell Mol Biol. 2003;285:L654–663. doi: 10.1152/ajplung.00448.2002. [DOI] [PubMed] [Google Scholar]
- 35.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen-induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichavant M, Goya S, Hamelmann E, Gelfand EW, Umetsu DT. Curr Protoc Immunol. Unit 15.18. Chapter 15. 2007. Animal models of airway sensitization. (c) 2007 by John Wiley & Sons, Inc. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Lai K, Xie J, Chen G, Zhong N. Does unrestrained single-chamber plethysmography provide a valid assessment of airway responsiveness in allergic BALB/c mice? Respir Res. 2009;10:61. doi: 10.1186/1465-9921-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 39.Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- 40.Walker C, Rihs S, Braun RK, Betz S, Bruijnzeel PL. Increased expression of CD11b and functional changes in eosinophils after migration across endothelial cell monolayers. J Immunol. 1993;150:4061–4071. [PubMed] [Google Scholar]
- 41.Pearce G, Audzevich T, Jessberger R. SYK regulates B-cell migration by phosphorylation of the F-actin interacting protein SWAP-70. Blood. 2011;117:1574–1584. doi: 10.1182/blood-2010-07-295659. [DOI] [PubMed] [Google Scholar]
- 42.Rothenberg ME, Ownbey R, Mehlhop PD, Loiselle PM, van de Rijn M, Bonventre JV, Oettgen HC, Leder P, Luster AD. Eotaxin triggers eosinophil-selective chemotaxis and calcium flux via a distinct receptor and induces pulmonary eosinophilia in the presence of interleukin 5 in mice. Mol Med. 1996;2:334–348. [PMC free article] [PubMed] [Google Scholar]
- 43.Grailer JJ, Kodera M, Steeber DA. L-selectin: Role in regulating homeostasis and cutaneous inflammation. J Dermatol Sci. 2009;56:141–147. doi: 10.1016/j.jdermsci.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation. 2003;10:297–311. doi: 10.1038/sj.mn.7800195. [DOI] [PubMed] [Google Scholar]
- 45.Barreiro O, de la Fuente H, Mittelbrunn M, Sánchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 46.Sriramarao P, DiScipio RG, Cobb RR, Cybulsky M, Stachnick G, Castenada D, Elices M, Broide DH. VCAM-1 is more effective than MAdCAM-1 in supporting eosinophil rolling under conditions of flow. Blood. 2000;95:592–601. [PubMed] [Google Scholar]
- 47.Ulfman LH, Kuijper PHM, van der Linden JAM, Lammers JWJ, Zwaginga JJ, Koenderman L. Characterization of eosinophil adhesion to TNF-α-activated endothelium under flow conditions: α4 integrins mediate initial attachment, and E-selectin mediates rolling. J Immunol. 1999;163:343–350. [PubMed] [Google Scholar]
- 48.Kitayama J, Fuhlbrigge RC, Puri KD, Springer TA. P-selectin, L-selectin, and alpha 4 integrin have distinct roles in eosinophil tethering and arrest on vascular endothelial cells under physiological flow conditions. J Immunol. 1997;159:3929–3939. [PubMed] [Google Scholar]
- 49.Abitorabi MA, Pachynski RK, Ferrando RE, Tidswell M, Erle DJ. Presentation of Integrins on Leukocyte Microvilli: A Role for the Extracellular Domain in Determining Membrane Localization. J Cell Biol. 1997;139:563–571. doi: 10.1083/jcb.139.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rothenberg ME. Eotaxin. An Essential Mediator of Eosinophil Trafficking into Mucosal Tissues. Am J Respir Cell Mol Biol. 1999;21:291–295. doi: 10.1165/ajrcmb.21.3.f160. [DOI] [PubMed] [Google Scholar]
- 51.Woo C-H, Tak Jeong D, Yoon S-B, Kim K-S, Yup Chung I, Saeki T, Kim J-H. Eotaxin induces migration of RBL-2H3 mast cells via a Rac-ERK-dependent pathway. Biochem Biophys Res Comm. 2002;298:392–397. doi: 10.1016/s0006-291x(02)02432-4. [DOI] [PubMed] [Google Scholar]
- 52.Cancelas JA, Jansen M, Williams DA. The role of chemokine activation of Rac GTPases in hematopoietic stem cell marrow homing, retention, and peripheral mobilization. Exp Hematol. 2006;34:976–985. doi: 10.1016/j.exphem.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Amaral MM, Alvarez C, Langellotti C, Geffner J, Vermeulen M. Histamine-treated dendritic cells improve recruitment of type 2 CD8 T cells in the lungs of allergic mice. Immunology. 2010;130:589–596. doi: 10.1111/j.1365-2567.2010.03262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O’Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, Lenkiewicz E, Colbert D, Rinaldi L, Ackerman SJ, Irvin CG, Lee NA. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 2004;305:1773–1776. doi: 10.1126/science.1099472. [DOI] [PubMed] [Google Scholar]
- 55.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118:789–798. doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 56.Mattes J, Yang M, Siqueira A, Clark K, MacKenzie J, McKenzie ANJ, Webb DC, Matthaei KI, Foster PS. IL-13 Induces Airways Hyperreactivity Independently of the IL-4Ralpha Chain in the Allergic Lung. J Immunol. 2001;167:1683–1692. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 57.Bochner BS, Klunk DA, Sterbinsky SA, Coffman RL, Schleimer RP. IL-13 selectively induces vascular cell adhesion molecule-1 expression in human endothelial cells. J Immunol. 1995;154:799–803. [PubMed] [Google Scholar]
- 58.Costa JJ, Matossian K, Resnick MB, Beil WJ, Wong DT, Gordon JR, Dvorak AM, Weller PF, Galli SJ. Human eosinophils can express the cytokines tumor necrosis factor-alpha and macrophage inflammatory protein-1 alpha. J Clin Invest. 1993;91:2673–2684. doi: 10.1172/JCI116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shakoory B, Fitzgeraldm SM, Lee SA, Chi DS, Krishnaswamy G. The role of human mast cell-derived cytokines in eosinophil biology. J Interferon Cytokine Res. 2004;24:271–281. doi: 10.1089/107999004323065057. [DOI] [PubMed] [Google Scholar]