Abstract
Melanopsin imparts an intrinsic photosensitivity to a subclass of retinal ganglion cells (ipRGCs). Generally thought of as irradiance detectors, ipRGCs target numerous brain regions involved in non-image-forming vision. ipRGCs integrate their intrinsic, melanopsin-mediated light information with rod/cone signals relayed via synaptic connections to influence light-dependent behaviors. Early observations indicated diversity among these cells and recently several specific subtypes have been identified. These subtypes differ in morphological and physiological form, controlling separate functions that range from biological rhythm via circadian photoentrainment, to protective behavioral responses including pupil constriction and light avoidance, and even image-forming vision. In this Mini-Symposium review, we will discuss some recent findings that highlight the diversity in both form and function of these recently discovered atypical photoreceptors.
Introduction
Melanopsin-containing intrinsically photosensitive retinal ganglion cell (ipRGCs) are atypical retinal photoreceptors separate from classical rod and cone photoreceptors. The discovery of this inner retinal photoreceptor (Provencio et al., 1998; Berson et al., 2002; Hattar et al., 2002) was motivated by attempts to explain circadian photoentrainment in otherwise blind individuals (Czeisler et al., 1995) and mice lacking rods and cones (Freedman et al., 1999; Lucas and Foster, 1999). Circadian rhythms should be independent of light input if rods and cones were the only retinal photoreceptors, however, another, unidentified photoreceptor in these blind individuals caused alignment of their circadian rhythms to the onset and offset of light/dark cycles (photoentrainment). ipRGCs were first identified in 2002 as this third class of circadian photoreceptors, and are now known to be critical for mediation of not only circadian photoentrainment, but also the pupillary light reflex (PLR) and sleep (Gooley et al., 2001; Berson et al., 2002; Göz et al., 2008; Güler et al., 2008; Hatori et al., 2008; Altimus et al., 2010). Previously cloned in the late 1990s (Provencio et al., 2000), melanopsin soon thereafter was demonstrated to be the photopigment for ipRGCs that convey their intrinsic light response (Gooley et al., 2001; Berson et al., 2002; Hattar et al., 2002; Panda et al., 2002; Ruby et al., 2002; Lucas et al., 2003) (for review, see Do and Yau, 2010). The confluence of modern molecular, transgenic, imaging, and electrophysiological techniques has allowed for rapid generation of tools necessary for studying ipRGCs, and as such, this field has seen exponential growth in the decade since these cells were first discovered.
Initially identified as a single retinal cell type, morphological and physiological classifications showed that ipRGCs comprise a far more complex population than originally thought. Early studies described the dendrites of ipRGCs as covering the retina, forming a “photoreceptive net” (Provencio et al., 2002), and gathering illuminance information from the entire retina. More recent characterization has shown that the cells identified by Provencio consist of two subclasses that form a mosaic covering the entire retina but that a third class of ipRGCs does not form a mosaic (Berson et al., 2010; Schmidt and Kofuji, 2010). In primates, ipRGCs are larger but display morphologies comparable to those of mice, with the additional feature that their dendrites have a minimal presence in the foveal pit, probably to avoid influencing high-acuity vision (Dacey et al., 2005; Berson et al., 2010). Research has now suggested that the ipRGC family has up to five distinct cell types (called M1 to M5) with differential dendritic morphology and axonal projections, as well as some unexpected intraretinal signaling (Hankins and Lucas, 2002; Sekaran et al., 2003; Tu et al., 2005; Barnard et al., 2006; Viney et al., 2007; Vugler et al., 2007; Zhang et al., 2008; Müller et al., 2010) (Figs. 1 and 2).
Figure 1.
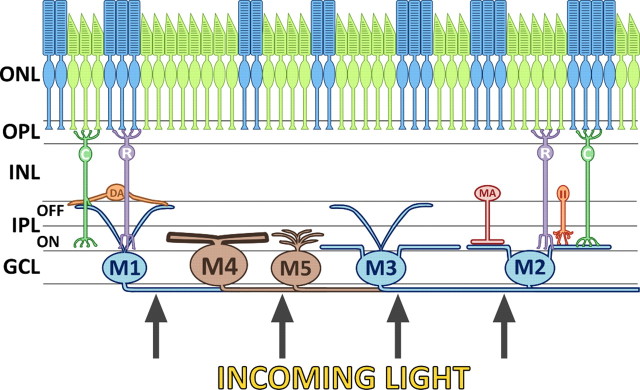
ipRGC subtypes differ in morphology and site of dendritic fields. The outer nuclear layer (ONL) of the mammalian retina consists of rod and cone photoreceptors, which have synaptic connections to their respective rod (R) or cone (C) bipolar cells in the outer plexiform layer (OPL). The soma of bipolar, amacrine (DA, MA, and II) and horizontal (not depicted) cells are localized to the inner nuclear layer (INL). The INL and GCL (ganglion cell layer) are separated by the IPL, another region of dendritic–axonal interactions. The GCL contains retinal ganglion cells (not depicted) and the melanopsin-positive class of retinal ganglion cells, the ipRGCs. The classification of ipRGCs (M1, M2, M3, M4, and M5) is based on morphology and somatic and dendritic localization. The figure is modified from Do and Yau (2010).
Figure 2.
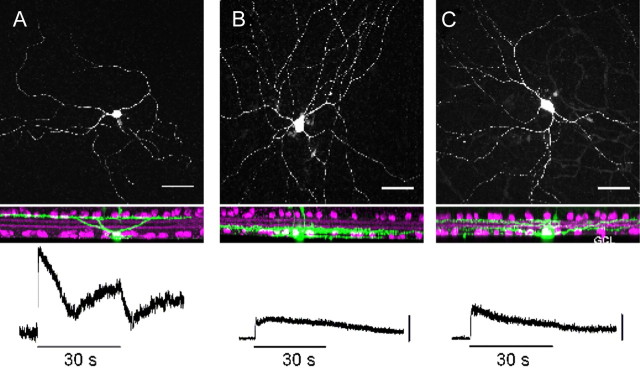
Morphology of M1, M2, and M3 ipRGCs and their physiological responses to light. Top, M1 (A), M2 (B), and M3 (C) ipRGCs are identified by their morphological characteristics and location of their dendritic arbors. M1 cells have dendritic ramifications in the OFF sublamina of the IPL, whereas M2 cells ramify in the ON sublamina. M3 cells have dendrites bistratifying in both the ON and OFF sublaminas of the IPL. Bottom, M1 cells have the largest intrinsic, melanopsin-mediated light-evoked response, while M2 and M3 cells have smaller light-evoked responses. Scale bars, 10 mV. The figure is modified from Schmidt and Kofuji (2011).
Light integration occurs at the level of ipRGCs as they combine input from the highly sensitive and fast rod and cone phototransduction pathways with their own sluggish melanopsin-mediated response. This integrated information is then transmitted to numerous discrete brain regions involved in both non-image-forming and image-forming vision (Gooley et al., 2003; Hattar et al., 2006; Brown et al., 2010; Ecker et al., 2010), including the suprachiasmatic nucleus (SCN) for circadian photoentrainment, the olivary pretectal nucleus (OPN) for the pupillary light reflex (PLR) in which bright light causes pupil constriction, and the dorsal lateral geniculate nucleus (dLGN) for image formation (Fig. 3). ipRGCs project to many additional brain regions, suggesting several other as yet unidentified light-related functions.
Figure 3.
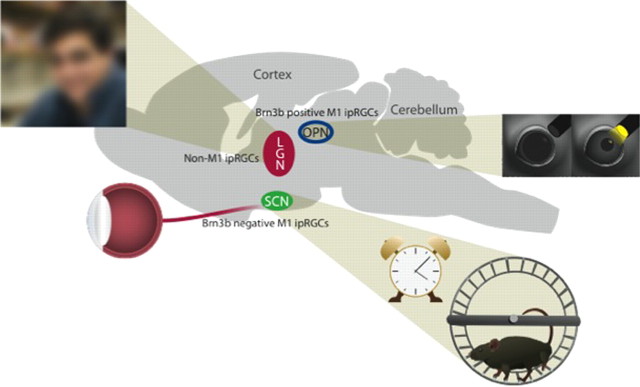
ipRGC project to numerous central regions and play a role in shaping behavior. Central projections of M1 cells include the OPN, controlling pupil constriction, and the SCN, controlling circadian photoentrainment. Non-M1 cells, which currently include M2–M5 cells, send projections to the LGN and are involved in a rudimentary, low-acuity visual function. Additional sites of axonal terminations are located throughout the brain, influencing activity, sleep/wake states, nociception, and areas implicating novel functions of these cells.
The range of visual responses to which ipRGCs are known to contribute has also expanded consistently over the 10 years since their discovery. There is now convincing experimental evidence that ipRGCs ultimately modulate a multiplicity of behaviors, including circadian photoentrainment, PLR, activity masking, sleep/arousal and neuroendocrine systems, anxiety, and light aversion, and even make a significant contribution to thalamocortical visual function (Güler et al., 2008; Hatori et al., 2008; Tsai et al., 2009; Brown et al., 2010; Ecker et al., 2010; Johnson et al., 2010; Thompson et al., 2010). Furthermore, these novel functions of ipRGCs have applications in clinical conditions and diagnoses of retinal degeneration and sleep disturbances (Kankipati et al., 2011; Kardon et al., 2011; Park et al., 2011) (for review, see La Morgia et al., 2011). This review will discuss recent advances in the field, describing the diversity of ipRGCs, their signaling properties, and previously unappreciated roles in image-forming and non-image-forming vision.
Morphological and physiological diversity defines classes of ipRGCs
The depth at which ipRGC dendrites ramify in the inner plexiform layer (IPL) of the retina gives us the first clue as to their diversity. Dendrites can ramify near the ganglion cell layer, in the ON-sublamina of the IPL, or ramify deeper in the IPL in the OFF-sublamina. ON-stratifying RGCs receive excitatory input from ON bipolar cells, which respond to light increments, while OFF-stratifying RGCs receive excitatory input from OFF bipolar cells, which respond to light decrements. Despite the initial view that ipRGCs consisted of a uniform population of cells, early staining with a sensitive anti-melanopsin antibody revealed two plexuses of melanopsin-immunoreactive dendrites (Provencio et al., 2002). To examine the morphological properties of ipRGCs in detail, Schmidt et al. (2008) used a mouse line expressing enhanced green fluorescent protein (eGFP) under the control of the melanopsin (Opn4) promoter. ipRGCs were identified under epifluorescent illumination and subsequent morphological and electrophysiological analyses were performed. These studies revealed three morphological subtypes: previously characterized M1 cells with dendrites stratifying in the OFF sublamina of the IPL, M2 cells with dendrites stratifying in the ON sublamina of the IPL, and M3 cells with surprising variability in the proportion of dendrites ramifying in the ON and OFF sublaminas of the IPL (Schmidt et al., 2008; Schmidt and Kofuji, 2011). These findings solidified earlier reports of two melanopsin-immunoreactive plexuses in the IPL as well as findings from retrograde viral labeling studies identifying three classes of ipRGCs (Provencio et al., 2002; Viney et al., 2007).
Morphologically, M2 cells had larger, more complex dendritic arbors and larger somas than M1 cells (Schmidt and Kofuji, 2009). Subsequent characterization of the rare M3 subtype revealed morphological characteristics similar to M2 cells (Schmidt and Kofuji, 2011). Research from the Hattar laboratory has since identified the ON-stratifying M4 cells with the largest somas and dendritic arbors of all ipRGC classes and ON-stratifying M5 cells with small, bushy dendritic arbors (Ecker et al., 2010).
When the functional properties of M1, M2, and M3 ipRGCs were examined, M1 cells were found to have large, sensitive intrinsic light responses in comparison to M2 cells. M1 cells were also found to have a higher input resistance and more depolarized resting membrane potential, and to spike at lower frequencies than M2 cells (Schmidt and Kofuji, 2009). Surprisingly, despite the variable morphology of M3 cells, this subtype had remarkably homogeneous physiological characteristics that most closely resembled those of M2 cells, arguing against M3 cells being a developmental anomaly or M1 and M2 “hybrid” somehow left undifferentiated (Schmidt and Kofuji, 2011).
ipRGCs, like conventional RGCs, receive rod and cone signals from the outer retina relayed via bipolar and amacrine cells. Given differences in the location of dendritic stratification and their intrinsic properties, one might predict that ipRGC subtypes receive very different types of outer-retinal inputs. When the synaptic light response was examined in detail for OFF-stratifying M1 and ON-stratifying M2 cells, it was found that both subtypes, perhaps surprisingly, received a predominantly ON pathway input. Recordings from melanopsin-null animals (Opn4−/−) revealed that this ON pathway input is both larger and more influential in shaping the light-evoked response of M2 cells compared to M1 cells, which rely primarily on melanopsin-mediated phototransduction (Schmidt and Kofuji, 2010). Collectively, these results indicate that the functional roles of the various ipRGC subtypes in behavior can be deduced by the relative influence of melanopsin, rod, and cone signaling on a given ipRGC-mediated behavior.
Unique characteristics of ipRGCs as light sensors
ipRGCs differ substantially from rods and cones in morphology and the molecular mechanism of phototransduction. They also appear to signal in brighter light and over longer durations than the rod and cone systems (Do and Yau, 2010). Despite these overt dissimilarities, common parameters govern photoreception by rods, cones, and ipRGCs, including the nature of the response to each captured photon, the effectiveness of photon capture, and the threshold for generating a signal that is communicated downstream. A recent study has defined these features for ipRGCs, suggesting how these ganglion-cell photoreceptors complement rods and cones to drive the full range of mammalian vision (Do et al., 2009).
All light responses represent the summed contributions of individual photopigment molecules with a linear range specific for each photopigment. Each photopigment is activated by absorption of one photon, producing the “single photon” or unitary response (Baylor et al., 1979), analogous to the current flowing through a single ion channel. Because the unitary response of rods is large, relatively few rods each absorbing one photon are sufficient to elicit visual perception (Field et al., 2005). Cones capture light with similar effectiveness to rods, but have a tiny unitary response and a correspondingly low sensitivity that allows them to function in daylight (Luo et al., 2008). To define the unitary response of ipRGCs, electrophysiological recordings were made from cells expressing the fluorescent reporter, tdTomato under the melanopsin promoter (Do et al., 2009). The smallest and brightest of these cells, which were targeted for study, were likely to be M1 cells (Do et al., 2009; Schmidt and Kofuji, 2009; Ecker et al., 2010). ipRGCs were repeatedly given a flash of light so dim that, in the majority of trials, photons passed through the cell without consequence. On some trials, however, one melanopsin molecule was activated, producing a unitary response. The unitary response of ipRGCs appears larger than that known for any vertebrate sensory neuron, including rod photoreceptors (Do et al., 2009). It is also extraordinarily prolonged, lasting nearly 10 s, or ∼20-fold longer than rods and 100-fold longer than cones. This prolonged kinetics improves sensitivity by conferring high temporal summation, while also smoothing the response to fluctuating light levels. Thus, even at its most elementary level, the intrinsic photosensitivity of ipRGCs operates over long time scales.
Despite this large and prolonged single-photon response, ipRGCs are even less sensitive than cones (Berson et al., 2002; Panda et al., 2002; Lucas et al., 2003; Do et al., 2009; Ecker et al., 2010). To determine whether this low sensitivity originates from a low probability of photon capture, the melanopsin content of ipRGCs was estimated. Since melanopsin is localized to the plasma membrane (Belenky et al., 2003), the number of melanopsin molecules per cell was divided by the cell's surface area, revealing a density of ∼3 molecules · μm−2 (Do et al., 2009). By contrast, rods and cones express their photopigments at a density of ∼25,000 molecules · μm−2, in specialized membranes that are stacked in the light path. The low melanopsin expression level may prevent interference with photon capture by rods and cones, which receive light after ipRGCs. In summary, the operation of ipRGCs in bright light is enabled by a low probability of photon capture and high amplification.
Unlike rods and cones, which signal within the retina with graded membrane voltages, ipRGCs signal to the brain using action potentials (spikes). Therefore, the number of unitary responses required to reach a spike threshold also determines their overall sensitivity. ipRGCs occasionally spike even in the absence of light, suggesting that they normally hover near spike threshold (for review, see Do and Yau, 2010). Because the single-photon response is so large and long lasting, just one captured photon can increase the probability of crossing spike threshold, driving the cell to increase its firing rate (Do et al., 2009). Thus, like rods, ipRGCs can signal single-photon responses to the brain. The cost of this low threshold for spike generation is the noise contributed by spontaneous firing. The impact of this spontaneous noise and any effects on downstream pathways remains to be determined.
It is presently unknown whether all ipRGC subtypes have a large single-photon response and low threshold for spike generation. The subtypes do appear to share phototransduction components downstream of melanopsin (Graham et al., 2008; Perez-Leighton et al., 2011) (for review, see Berson, 2007), potentially allowing for similar single-photon responses, and at least M2 cells readily fire spikes (Schmidt and Kofuji, 2009). Nevertheless, the M2 and M3 subtypes are less intrinsically photosensitive than M1 cells; M2 and Me cells by ∼10-fold and M4 cells by ∼100-fold (Schmidt and Kofuji, 2009, 2011; Ecker et al., 2010). Melanopsin density, however, appears to be lower in the M2 cells and even lower in the M4 cells (Schmidt and Kofuji, 2009; Berson et al., 2010; Ecker et al., 2010), consistent with their relative sensitivities to light (Ecker et al., 2010). Thus, ipRGCs as a class may tune their sensitivities by their level of melanopsin expression.
In summary, the intrinsic photosensitivity of ipRGCs complements that of rods and cones by virtue of operating at high light intensities and over long durations. ipRGCs establish these features by pairing sparse photon capture with a highly integrating single-photon response. How the unique light responses of ipRGCs influence behavior will likely depend on the subtype of ipRGC activated and signal processing in the brain regions specifically innervated.
Specialization of primate ipRGC-mediated visual function
The melanopsin-based retinal circuit appears fundamentally similar in mouse and primate, yet specializations that parallel the appearance of the unique primate fovea and trichromatic color vision suggest a more complex role in human visual processing. In primates, as in mice, the melanopsin photopigment shows a λmax of 483 nm and is expressed in two morphologically independent populations of ∼3000 ganglion cells with large, sparsely branched dendritic trees that stratify at the inner and outer borders of the IPL (Dacey et al., 2005). As in mouse, these ganglion cells combine input from rod and cone signaling pathways with the inherent melanopsin response and use these signals to generate an irradiance coding ON spiking response, and to drive the various components of the human and nonhuman primate PLR (Gamlin et al., 2007).
A unique specialization of the human visual system, however, is the ultra-high cone and ganglion cell density of the fovea that serves peak visual acuity and color and form vision via the topographic projection to the lateral geniculate nucleus (LGN) and in turn to a greatly expanded primary visual cortex. Amazingly, the melanopsin-expressing ganglion cells of human and macaque monkey retina are present in the central retina and form a photoreceptive network of dendrites throughout the foveal specialization but with only a few processes crossing the foveal pit. These ganglion cells also project to the LGN and receive input from the spectrally distinct short (S), middle (M), and long (L) wavelength-sensitive cone photoreceptor types unique to the trichromatic primate and show a rarely studied type of color sensitivity mediated by S cones (Dacey et al., 2005). Irradiance-detecting units have been recorded in both LGN and primary visual cortex and are implicated in the perception of surface luminance and brightness. The spectral tuning of such irradiance cells has not yet been measured. Recent evidence suggests, however, that humans lacking rods and cones can show a rudimentary conscious visual awareness (Zaidi et al., 2007).
In addition to the LGN, the primate melanopsin-expressing ganglion cells project widely to the superior colliculus and the pretectum. An important question that remains to be clarified is whether, as in mouse, the M1 and M2 types in primate show distinct response properties, central projections, and functional roles in the circadian, pupillary, and perceptual pathways. Indeed, recent reports suggest that projections from some melanopsin cells to the dorsal LGN in mice give rise to irradiance sensitivity in the thalamocortical pathway and could play a role in visual perception even in this species (Brown et al., 2010; Ecker et al., 2010).
The role of ipRGCs in vision
Convergent evidence from recent studies indicates that ipRGCs project to distinct brain regions involved in image-forming vision. Dacey et al. (2005) back labeled ipRGCs from the primate LGN, suggesting that the projection pattern of these photoreceptive ganglion cells extends to the visual thalamus. A discrepancy arose, however, when extensive analysis of the Opn4taulacZ knock-in mouse, in which histological stains can be used to label both cell bodies and axons of melanopsin-expressing cells, implied that any such input to thalamocortical projection neurons in the dorsal LGN (dLGN) would be extremely limited (Hattar et al., 2006). The subsequent discovery that the Opn4taulacZ reporter selectively labels the M1 class of ipRGC raised renewed uncertainty regarding the central projection pattern of ipRGCs. This controversy was resolved using Cre-recombinase-based reporter mouse lines, which more fully labeled the entire ipRGC population, and revealed extensive innervation of the dLGN (Brown et al., 2010; Ecker et al., 2010), showing species conservation of ipRGC projections.
Recent experiments from the Lucas laboratory directly assessed the contribution of melanopsin photoreceptors to visual activity in the mouse dLGN (Brown et al., 2010). Using a combination of extracellular recording electrodes and intrinsic optical imaging, widespread light-evoked activity was observed in both the dLGN and visual cortex of rodless/coneless mice. The thalamic light response in these mice recapitulated the low sensitivity and slow response kinetics reported for melanopsin phototransduction.
The contribution of melanopsin input to the mouse dLGN was not merely a feature of the retinally degenerate preparation. In mice with an intact visual system, 60 s stimuli at relatively high intensity elicited a persistent increase in firing in a large number of neurons in the dLGN. Two lines of evidence suggest that, under these conditions, melanopsin contributes to this persistent firing. First, it was recorded only in response to wavelengths within the melanopsin-sensitivity range. Second, it was much reduced in amplitude in melanopsin knock-out mice. Further study of melanopsin knock-out mice indicated that melanopsin functioned not only to sustain firing of these dLGN neurons under steady illumination, but was also critical in allowing this part of the brain to encode stimulus irradiance. Thus, in visually intact animals, the firing rate of many dLGN cells tracked irradiance over at least six orders of magnitude. This ability was substantially impaired in melanopsin knock-out mice.
These data imply that melanopsin plays an important role in providing information about spatial brightness to the thalamocortical visual projection. Experiments in visually intact, retinally degenerate and melanopsin knock-out mice all indicate that this signal appears in a large proportion of dLGN neurons, ∼40% of all light-responsive units detected (Brown et al., 2010). As all of those units also showed rod/cone-dependent transient responses to lights “on” and “off,” the melanopsin-dependent signal of illuminance is likely superimposed upon other visual codes at the level of the thalamus. Future work will be required to determine melanopsin's significance for brightness perception and, perhaps, other aspects of vision.
M1 and non-M1 specification of central function
Previous studies have indicated that rods, cones and melanopsin all influence circadian photoentrainment (Lall et al., 2010) and PLR (McDougal and Gamlin, 2010) via ipRGCs (Güler et al., 2008; Hatori et al., 2008). Rods have an unparalleled sensitivity while the kinetics of cone opsin and melanopsin signaling suggests that cones mediate an initial fast response across a range of light levels and that the sustained response of melanopsin mediates a longer-lasting physiological response (Brown et al., 2010). This simple model highlights complementary roles for each photoreceptive system in non-image-forming behaviors but an additional layer of complexity arises if differential synaptic input occurs. Research from the Hattar laboratory indicates that circadian photoentrainment is preferentially dependent on rod photoreceptors with cones providing little input (Altimus et al., 2010) (but also see Lall et al., 2010). To the contrary, cones seem to provide strong input to the PLR (Lall et al., 2010). These data provide the intriguing possibility that M1 ipRGCs projecting to the OPN are different from the M1 ipRGCs projecting to the SCN, hinting at the possibility of subpopulations within the M1 cells and adding another layer of complexity to this narrative.
To discriminate between OPN- and SCN-projecting M1 ipRGCs, identifying distinct markers for individual subtypes of ipRGCs was the critical next step. Functionally distinct subpopulations of M1 ipRGCs were molecularly defined by expression of the Brn3b transcription factor (Chen et al., 2011) with Brn3b expressed in all non-M1 ipRGCs but only in a fraction of M1 cells. Coincidentally, Brn3b-positive M1 ipRGCs project to all known M1 targets except the SCN. Consistent with this innervation pattern, ablation of Brn3b-positive ipRGCs severely impairs the PLR, but does not affect circadian photoentrainment. These data support the classification of the M1 subtype into two distinct subpopulations and provide a potential mechanistic explanation for the differential influence from rods and cones on circadian photoentrainment and the PLR, although the electrophysiological profile of M1 cells suggests a singular classification. The behavioral responses of these two M1 subpopulations could be controlled by integration of signals at their axonal targets but could also be explained by differential rod versus cone input at their dendritic arbors. Cone-mediated input predominates control of the PLR, presumably via Brn3b-positive M1 cells, suggesting that Brn3b-positive M1 cells either receive more input via cone signaling pathways or that their axonal targets weight this input more heavily. This raises the intriguing question of the interaction between the molecular identity, dendritic connectivity, and axonal targeting of ipRGCs. The transcription factor(s) responsible for specifying Brn3b-negative ipRGCs and differential targeting of M1 subpopulations to specific brain regions remain to be identified. Similar genetic strategies using cell-type-specific tools will eventually uncover the circuitry and specialized functions of all ipRGC subtypes.
It is now well established that M1 ipRGCs are predominantly responsible for circadian photoentrainment and PLR (Güler et al., 2008; Hatori et al., 2008). The M1 subclass is the best characterized and exemplifies how its design subserves its role. With the highest melanopsin levels and a resting membrane potential sitting near threshold for firing, it is the most light sensitive of the characterized subclasses, thereby contributing the most robust melanopsin component to their related behaviors. The non-M1 cells are likely to mediate behaviors that rely predominantly on rod and cone signaling, and less so on melanopsin photoreception. Thus understanding the relative influence of all three photoreceptive systems on ipRGC signaling is key to understanding how each subclass of ipRGC mediates specific behaviors. The non-M1 subclasses have only now begun to be studied for their functional role in behavior. These non-M1 ipRGCs may be responsible for image-forming behaviors via projections to the dLGN (Ecker et al., 2010) and projections to numerous other regions including the periaqueductal gray and amygdala, which suggests contributions to the pain, fear, and anxiety circuitry (Maren and Fanselow, 1996; Hattar et al., 2006).
Photo-allodynia (photophobia) is a condition in which even low levels of illumination result in mild discomfort to extreme ocular pain. Photo-allodynia affects up to 80% of migraineurs (Choi et al., 2009; Robbins and Lipton, 2010) and ∼50% of patients with mild traumatic brain injury (Craig et al., 2008), and also occurs from ocular inflammation or abrasion.
Similar to circadian photoentrainment, photo-allodynia is even experienced by patients who are visually blind (Amini et al., 2006). Furthermore, people with photo-allodynia are maximally sensitive to blue light and experience pain throughout the duration of light exposure, consistent with a role for ipRGCs in this condition (Newman et al., 2003; Amini et al., 2006; Noseda et al., 2010). To directly test this hypothesis, researchers used OPN4dta mice, in which all ipRGCs are ablated, in a light-aversion behavioral assay. Other groups had used naive mice in a light/dark box behavioral test that compounds light aversion with light-dependent anxiety (Recober et al., 2009; Semo et al., 2010; Thompson et al., 2010), an equally important consideration for photo-allodynia as pain and anxiety are interrelated. To assess light aversion separately from anxiety, mice were first habituated to a light/dark box chamber until their anxiety subsided. They were then tested for aversion to bright lights as an endophenotype for photo-allodynia. A. Matynia and M. B. Gorin (unpublished results) showed that mice with intact ipRGCs avoided bright lights, but mice lacking ipRGCs showed very little aversion to these bright lights, suggesting that ipRGCs are the primary circuit for light aversion and, by extension, photo-allodynia.
Under normal conditions, ipRGCs control light aversion behavior; however, rod/cone photoreceptors may also provide input in pathological states of light sensitivity. Following administration of a subanalgesic dose of morphine, mice normally show enhanced light aversive behavior that is μ opioid receptor dependent (Matynia and Gorin, unpublished observations). To test whether this behavior is also mediated by ipRGCs, researchers also gave subanalgesic doses of morphine to OPN4dta mice lacking ipRGCs. Interestingly, these mice exhibit the same level of opiate-induced light aversion, indicating that morphine-induced light aversion is mediated by the classical rod/cone photoreceptors through conventional RGCs (Matynia and Gorin, unpublished observations). These data implicate two distinct circuits for light avoidance behavior under normal (ipRGC-dependent) and pathological (ipRGC-independent) conditions. Future studies will help determine the specific brain regions that receive ipRGC projections to mediate light aversion in normal and pathological models, helping to define the neural circuitry associated with photo-allodynia in people.
Concluding remarks
The research discussed here demonstrates that understanding a complex neuronal system requires convergent molecular, physiological, genetic, circuitry, and behavioral approaches. The ipRGC population consists of several subtypes, each with distinct morphological features including dendritic field and soma size, as well as level of dendritic stratification. Each subtype has unique responses to light and other electrophysiological characteristics. Furthermore, the subtypes are distinguished by their axonal projection patterns. These distinct features suggest possible specific functional roles for each subtype in non-image-forming and image-forming behavior. However, given the lack of molecular markers for each ipRGC subtype, study of their individual roles in behavior is restricted. Molecular markers and development of additional genetic tools will be necessary to delineate their unique functions.
ipRGCs have transcended their earliest functional designations and their influence is now seen in image-forming vision, retinal development, migraine research, photo-allodynia, sleep disorders, anxiety, and depression. Furthermore, ipRGCs are now also implicated in numerous visually guided behaviors, suggesting that ipRGCs could one day be a therapeutic target in degenerative retinal diseases. It has been shown previously that ectopic melanopsin expression in retinal ganglion cells normally devoid of melanopsin improved vision in mice lacking classic photoreceptors (Lin et al., 2008). This research has also made its mark in various clinical settings, as assessment of retinal degeneration via examination of the relative contribution of rods, cones, and ipRGCs to pupil constriction is now being applied as a diagnostic tool (Kankipati et al., 2011; Kardon et al., 2011; Park et al., 2011). Initially classified as a single cell type regulating a small number of behaviors, ipRGCs have greatly exceeded expectations in the last decade, repeatedly pushing back the boundaries of retinal dogma. Future research will undoubtedly continue to illuminate the diverse characteristics, from form to function, of these atypical ganglion cell photoreceptors.
Footnotes
Work of the contributing laboratories was supported by NIH Grants EY06678 and EY09625 to D.D.; National Research Service Award fellowship and Visual Neuroscience Training Program training grant to M.T.H.D. and NIH Grant EY014596 and the Champalimaud Foundation to K.-W.Y. for M.T.H.D.; NIH Grant GM076430, David and Lucille Packard Foundation, and Alfred P. Sloan Foundation to S.H.; Wellcome Trust, Biotechnology and Biological Sciences Research Council, and European Research Council to R.L.; NIH Grants EY012949 and EY018885 to P.K. and the University of Minnesota for T.M.S.; and Stein/Oppenheimer Endowment Award to A.M. and M.B.G., and the Harold and Pauline Price Chair in Ophthalmology and the Jules Stein Eye Institute to M.B.G. Work by T.M.S. was done in the laboratory of Paulo Kofuji at the Department of Neuroscience, University of Minnesota, Minneapolis, MN. Work done by M.T.H.D. was done in the laboratory of King-Wai Yau in the Department of Neuroscience, School of Medicine, Johns Hopkins University, Baltimore, MD. Work by A.M. was done in the laboratory of Michael B. Gorin at the University of California, Los Angeles, Jules Stein Eye Institute, Department of Ophthalmology, Los Angeles, CA.
References
- Altimus CM, Güler AD, Alam NM, Arman AC, Prusky GT, Sampath AP, Hattar S. Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities. Nat Neurosci. 2010;13:1107–1112. doi: 10.1038/nn.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: an alternate visual pathway. Case report. J Neurosurg. 2006;105:765–768. doi: 10.3171/jns.2006.105.5.765. [DOI] [PubMed] [Google Scholar]
- Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM. Phototransduction in ganglion-cell photoreceptors. Pflugers Arch. 2007;454:849–855. doi: 10.1007/s00424-007-0242-2. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Berson DM, Castrucci AM, Provencio I. Morphology and mosaics of melanopsin-expressing retinal ganglion cell types in mice. J Comp Neurol. 2010;518:2405–2422. doi: 10.1002/cne.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Oh K, Kim BJ, Chung CS, Koh SB, Park KW. Usefulness of a photophobia questionnaire in patients with migraine. Cephalalgia. 2009;29:953–959. doi: 10.1111/j.1468-2982.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Craig S, Kapoor N, Ciuffreda KJ, Suchoff IB, Han ME, Rutner D. Profile of selected aspects of visually-symptomatic individuals with acquired brain injury—a retrospective study. J Behav Opt. 2008;19:7–10. [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., 3rd Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. [DOI] [PubMed] [Google Scholar]
- Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do MT, Kang SH, Xue T, Zhong H, Liao HW, Bergles DE, Yau KW. Photon capture and signalling by melanopsin retinal ganglion cells. Nature. 2009;457:281–287. doi: 10.1038/nature07682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field GD, Sampath AP, Rieke F. Retinal processing near absolute threshold: from behavior to mechanism. Annu Rev Physiol. 2005;67:491–514. doi: 10.1146/annurev.physiol.67.031103.151256. [DOI] [PubMed] [Google Scholar]
- Freedman MS, Lucas RJ, Soni B, von Schantz M, Muñoz M, David-Gray Z, Foster R. Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:502–504. doi: 10.1126/science.284.5413.502. [DOI] [PubMed] [Google Scholar]
- Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007;47:946–954. doi: 10.1016/j.visres.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PloS One. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DM, Wong KY, Shapiro P, Frederick C, Pattabiraman K, Berson DM. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99:2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins MW, Lucas RJ. The primary visual pathway in humans is regulated according to long-term light exposure through the action of a nonclassical photopigment. Curr Biol. 2002;12:191–198. doi: 10.1016/s0960-9822(02)00659-0. [DOI] [PubMed] [Google Scholar]
- Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PloS One. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Wu V, Donovan M, Majumdar S, Rentería RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankipati L, Girkin CA, Gamlin PD. The post-illumination pupil response is reduced in glaucoma patients. Invest Ophthalmol Vis Sci. 2011;52:2287–2292. doi: 10.1167/iovs.10-6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A. Chromatic pupillometry in patients with retinitis pigmentosa. Ophthalmology. 2011;118:376–381. doi: 10.1016/j.ophtha.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Lall GS, Revell VL, Momiji H, Al Enezi J, Altimus CM, Güler AD, Aguilar C, Cameron MA, Allender S, Hankins MW, Lucas RJ. Distinct contributions of rod, cone, and melanopsin photoreceptors to encoding irradiance. Neuron. 2010;66:417–428. doi: 10.1016/j.neuron.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Morgia C, Ross-Cisneros FN, Hannibal J, Montagna P, Sadun AA, Carelli V. Melanopsin-expressing retinal ganglion cells: implications for human diseases. Vision Res. 2011;51:296–302. doi: 10.1016/j.visres.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Lin B, Koizumi A, Tanaka N, Panda S, Masland RH. Restoration of visual function in retinal degeneration mice by ectopic expression of melanopsin. Proc Natl Acad Sci U S A. 2008;105:16009–16014. doi: 10.1073/pnas.0806114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas RJ, Foster RG. Neither functional rod photoreceptors nor rod or cone outer segments are required for the photic inhibition of pineal melatonin. Endocrinology. 1999;140:1520–1524. doi: 10.1210/endo.140.4.6672. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. [DOI] [PubMed] [Google Scholar]
- Luo D-G, Kefalov V, Yau K-W. Phototransduction in retinal rods and cones. In: Basbaun A., editor. The senses: a comprehensive reference. New York: Elsevier/Academic; 2008. pp. 269–301. [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- McDougal DH, Gamlin PD. The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex. Vision Res. 2010;50:72–87. doi: 10.1016/j.visres.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller LP, Do MT, Yau KW, He S, Baldridge WH. Tracer coupling of intrinsically photosensitive retinal ganglion cells to amacrine cells in the mouse retina. J Comp Neurol. 2010;518:4813–4824. doi: 10.1002/cne.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42:12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, DeGrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Park JC, Moura AL, Raza AS, Rhee DW, Kardon RH, Hood DC. Toward a clinical protocol for assessing rod, cone and melanopsin contributions to the human pupil response. Invest Ophthalmol Vis Sci. 2011;52:6624–6635. doi: 10.1167/iovs.11-7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Leighton CE, Schmidt TM, Abramowitz J, Birnbaumer L, Kofuji P. Intrinsic phototransduction persists in melanopsin-expressing ganglion cells lacking diacylglycerol-sensitive TRPC subunits. Eur J Neurosci. 2011;33:856–867. doi: 10.1111/j.1460-9568.2010.07583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Cooper HM, Foster RG. Retinal projections in mice with inherited retinal degeneration: implications for circadian photoentrainment. J Comp Neurol. 1998;395:417–439. doi: 10.1002/(sici)1096-9861(19980615)395:4<417::aid-cne1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Rollag MD, Castrucci AM. Photoreceptive net in the mammalian retina. This mesh of cells may explain how some blind mice can still tell day from night. Nature. 2002;415:493. doi: 10.1038/415493a. [DOI] [PubMed] [Google Scholar]
- Recober A, Kuburas A, Zhang Z, Wemmie JA, Anderson MG, Russo AF. Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J Neurosci. 2009;29:8798–8804. doi: 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30:107–119. doi: 10.1055/s-0030-1249220. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Functional and morphological differences among intrinsically photosensitive retinal ganglion cells. J Neurosci. 2009;29:476–482. doi: 10.1523/JNEUROSCI.4117-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Differential cone pathway influence on intrinsically photosensitive retinal ganglion cell subtypes. J Neurosci. 2010;30:16262–16271. doi: 10.1523/JNEUROSCI.3656-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Kofuji P. Structure and function of bistratified intrinsically photosensitive retinal ganglion cells in the mouse. J Comp Neurol. 2011;519:1492–1504. doi: 10.1002/cne.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TM, Taniguchi K, Kofuji P. Intrinsic and extrinsic light responses in melanopsin-expressing ganglion cells during mouse development. J Neurophysiol. 2008;100:371–384. doi: 10.1152/jn.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- Semo M, Gias C, Ahmado A, Sugano E, Allen AE, Lawrence JM, Tomita H, Coffey PJ, Vugler AA. Dissecting a role for melanopsin in behavioural light aversion reveals a response independent of conventional photoreception. PloS One. 2010;5:e15009. doi: 10.1371/journal.pone.0015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S, Recober A, Vogel TW, Kuburas A, Owens JA, Sheffield VC, Russo AF, Stone EM. Light aversion in mice depends on nonimage-forming irradiance detection. Behav Neurosci. 2010;124:821–827. doi: 10.1037/a0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JW, Hannibal J, Hagiwara G, Colas D, Ruppert E, Ruby NF, Heller HC, Franken P, Bourgin P. Melanopsin as a sleep modulator: circadian gating of the direct effects of light on sleep and altered sleep homeostasis in Opn4(-/-) mice. PLoS Biol. 2009;7:e1000125. doi: 10.1371/journal.pbio.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu DC, Zhang D, Demas J, Slutsky EB, Provencio I, Holy TE, Van Gelder RN. Physiologic diversity and development of intrinsically photosensitive retinal ganglion cells. Neuron. 2005;48:987–999. doi: 10.1016/j.neuron.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Viney TJ, Balint K, Hillier D, Siegert S, Boldogkoi Z, Enquist LW, Meister M, Cepko CL, Roska B. Local retinal circuits of melanopsin-containing ganglion cells identified by transsynaptic viral tracing. Curr Biol. 2007;17:981–988. doi: 10.1016/j.cub.2007.04.058. [DOI] [PubMed] [Google Scholar]
- Vugler AA, Redgrave P, Semo M, Lawrence J, Greenwood J, Coffey PJ. Dopamine neurones form a discrete plexus with melanopsin cells in normal and degenerating retina. Exp Neurol. 2007;205:26–35. doi: 10.1016/j.expneurol.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, 3rd, Czeisler CA, Foster RG, Moseley MJ, Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]