Abstract
The alpha-fetoprotein (afp) receptor (recaf) is an oncofetal antigen found in most types of cancer. Using a competitive radioimmunoassay, we measured the concentration of serum recaf in three sets of samples.
Set 1 was blind and consisted of 119 normal subjects, 43 breast cancer patients (stages i and ii), and 20 patients with benign breast conditions. In this set, the assay discriminated normal from cancer samples with a receiver operating characteristic for the area under the curve (ROCAUC) of 0.983; with 95% specificity and 93% sensitivity at a cut-off of 4.6 K (arbitrary) recaf units; and with 72% sensitivity and 100% specificity at a cut-off of 7.3 K units. At 7.3 K units, the specificity for benign breast conditions was 85%, and the sensitivity was 72% (ROCAUC was 0.773). Carcinoembryonic antigen and cancer antigen 15-3 respectively showed 39% and 41% sensitivity, with 95% specificity in comparisons of normal with cancer samples, and 34% and 44% sensitivity, with 85% specificity in comparisons of benign with cancer samples. Set 2 consisted of 353 normal, 30 benign, and 64 cancer samples (stages ii and iii). The recaf assay sensitivity in discriminating normal from cancer samples was 97%, with 97% specificity. Benign compared with cancer samples showed 87% sensitivity, with 97% specificity. Set 3 included only 40 normal and 40 cancer samples. The assay sensitivity was 89%, with 100% specificity. Sets 2 and 3 were not tested with carcinoembryonic antigen and cancer antigen 15-3.
These results strongly suggest that the recaf assay could be used for detecting breast cancer in its early stages.
Keywords: Alpha-fetoprotein, afp, afp receptor, recaf, ca15-3, cea, serum, tumour marker, biomarker, oncofetal antigen, sensitivity, specificity, radioimmunoassay, serum test, early-stage, breast cancer
1. INTRODUCTION
Alpha-fetoprotein (afp) is a major fetal serum protein 1–4 that is synthesized mainly by the yolk sac and liver 2,5. After birth, circulating levels of afp drop sharply, virtually disappearing from the blood of normal adult individuals 2.
Immature cells from most fetal structures internalize afp, and it has been suggested that the purpose of that internalization—and one of the functions of this protein—is to transport and deliver polyunsaturated fatty acids into fetal cells 6,7. The afp uptake ceases when embryonic cells and tissue structures approach a high degree of differentiation, even if the blood concentration of afp is still high or increasing 8–10.
In vitro and in vivo studies showed that malignant cells regain the ability to take up afp via a receptor that would be present in undifferentiated cells of either embryonic or tumour origin 10–15, but mostly absent in normal adult cells. The existence of such a receptor for afp (recaf) was then demonstrated and functionally characterized in several cell lines 16–18.
Because recaf is found in a variety of cancers and in fetal cells (but not in their mature counterparts), the receptor falls within the definition of a wide-spectrum oncofetal antigen with potential for cancer diagnosis, screening, and follow-up.
Previous immunohistology work using the same polyclonal antibodies described herein showed a clear difference in recaf staining between stomach cancer cells and nonmalignant cells 19, and recaf concentrations have been reported to be higher in serum from a variety of cancer patients than in serum from patients with benign proliferating diseases or from normal subjects 20.
In the present study, we describe a serum recaf radioimmunoassay that can detect the early stages of breast cancer. The test is capable of distinguishing cancer patients from healthy individuals—and from those with benign lesions—with a degree of accuracy so far unattainable with other cancer markers.
2. METHODS
2.1. Preparation of Mammary Cancer Cell Extracts
The MCF-7 human breast cancer cell line was obtained from the American Type Culture Collection (Manassas, VA, U.S.A.) and grown in rpmi medium containing 10% fetal calf serum. Before extraction, the cells were incubated for 1 hour at 37°C in serumfree rpmi medium to deplete them of bovine afp. The cells were then trypsinized and re-suspended at a concentration of 5×107 per millilitre of tbs medium (0.05 mol/L Tris–HCl plus 0.1 mol/L NaCl, pH 7.5). Next, the suspension was sonicated for 2 minutes in an ice bath using a Sonic Dismembrator (Thermo Fisher Scientific, Waltham, MA, U.S.A.) at 32 W, followed by centrifugation for 10 minutes at 14,000 rpm in an Eppendorf Microfuge (Eppendorf Canada, Mississauga, ON). The total protein concentration of the supernatants was 7–12 mg/mL as determined using a commercial protein assay (Total Protein: Bio-Rad Laboratory, Hercules, CA, U.S.A.). After 0.02% thimerosal was added, the cell extracts were stored at −20°C until used in the experiments.
2.2. RECAF Purification
Affinity chromatography on an afp-agarose column was used to purify the recaf. The afp was purified from the supernatant of HEP-2 culture medium by affinity chromatography using an anti-afp monoclonal antibody. One milliliter MCF-7 extract was incubated for 4 hours, at room temperature and under gentle agitation, with 10 mL afp-agarose in 0.05 mol/L Tris–HCl (pH 6.5). After thorough washing, the bound recaf was eluted using 0.8 mol/L KCl in the same buffer. The eluates were concentrated to 300–1000 μg/mL using a Centricom spin filter (Millipore Corporation, Billerica, MA, U.S.A.).
2.3. Preparation and Purification of Polyclonal Rabbit Antibody Against RECAF
Rabbits were immunized intradermally with a 50:50 emulsion of 1 mg purified recaf in 1 mL of phosphate-buffered saline (pbs) and 1 mL Freund’s complete adjuvant. Two boosts, each containing 0.5 mg recaf in incomplete Freund’s adjuvant, were administered at 2-week intervals. The rabbits were bled from the ear vein 1 week after the last boost. The blood was allowed to clot overnight at room temperature, and the serum was centrifuged and stored at −30°C until further use. The immunoglobulin G fraction of the anti-recaf rabbit antiserum was isolated on a protein A Sepharose column (Sigma–Aldrich, St. Louis, Missouri, U.S.A.) according to the manufacturer’s instructions.
2.4. Polyacrylamide Gel Electrophoresis and Western Blot
Polyacrylamide gels containing 10% sodium dodecylsulfate were used in a Bio-Rad polyacrylamide gel electrophoresis apparatus following the Laemmli procedure. The sample buffer consisted of 4 mL distilled water, 1 mL 0.5 mol/L Tris–HCl, 0.8 mL glycerol, 1.6 mL 10% sodium dodecylsulfate, 0.4 mL 2-mercaptoethanol, and 0.2 mL 0.05% (weight:volume) bromophenol blue. Gels were run for 2 hours at a constant current of 0.2 A (70–100 V) in a 1× Tris–glycine running buffer in a Bio-Rad system (Mini-Protein: catalog number 165-3301). Western blots followed standard procedures, using a Bio-Rad Mini-Transblot apparatus (catalog number 170-3930) and nitrocellulose membranes (0.45-μm pore: Bio-Rad, catalog number 162-0145). After blocking the membranes with 3% fish gelatin or 1% bovine serum albumin in tbs, the recaf bands were evidenced by incubating the membrane with a suitable concentration of anti-recaf antiserum or pure afp biotinylated with Sigma–Aldrich nhs-biotin according to the manufacturers’ instructions. Color development was obtained using diaminobenzidine and H2O2 after the membranes had been incubated with a commercial conjugate (Sigma–Aldrich) of either streptavidin (for the biotinylated afp) or anti-rabbit immunoglobulin labeled with horseradish peroxidase.
2.5. RECAF labeling with 125I
The recaf was radioiodinated with Na125I using the chloramine T method 21, with minor modifications. Briefly, 40 μg pure recaf in a volume of 435 μL was mixed with 127 μL 0.4 mol/L sodium phosphate buffer (pH 7.4), to which was then added 37 MBq of Na125I in a volume of 10 μL. Then, 20 μL chloramine T 1 mg/mL in H2O was added, and the mixture was incubated at room temperature for 60 seconds. The reaction was stopped with 20 μL sodium metabisulfite 2.5 mg/mL in phosphate buffer. After 60 seconds, 40 μL 1% Ovalbumin (Sigma–Aldrich) in pbs and 40 μL blue dextran solution were added. The 125I-recaf was then separated from free iodine on a Sephadex G-25 column (Pharmacia, Uppsala, Sweden) made with a disposable 5-mL pipette equilibrated with pbs, pH 7.4. The specific activity of the labeled 125I-recaf ranged from 74 kBq to 370 kBq per microgram of protein (3.7–11.1 kBq/μL).
2.6. Immunoassays
2.6.1. Carcinoembryonic Antigen and Cancer Antigen 15-3
Circulating levels of carcinoembryonic antigen (cea) and cancer antigen 15-3 (ca15-3) were measured at the Institut für Klinische Chemie, Munich, using the AxSYM system (Abbott Laboratories, Abbott Park, IL, U.S.A.) for cea and the Elecsys system (Hoffmann–La Roche, Nutley, NJ, U.S.A.) for ca15-3.
2.6.2. Radioimmunoassay Using 125I–RECAF
The recaf determinations were made at the Pacific Biosciences Research Centre facilities in Vancouver, British Columbia.
The test was designed as a solid-phase competitive immunoassay in which a constant amount of 125I-recaf competed with recaf in the serum sample for binding to the anti-recaf antibody immobilized on the plastic plate. The 96-well plates (LockWell MaxiSorp: Nalge Nunc International, Rochester, NY, U.S.A.) were coated overnight, at 4°C, with 100 μL/well of 20 μg/mL rabbit anti-recaf protein A purified immunoglobulin G in 0.1 mol/L carbonate buffer (pH 9.5). All subsequent steps were carried out at room temperature. After being washed 3 times with dH2O, the wells were blocked for 2 hours with 3% fish gelatin (Sigma–Aldrich) in tbs. A mixture of 50 μL serum and 50 μL 125I-recaf at 100 ng/mL was then transferred to the wells and incubated for 2 hours. The wells were washed 3 times with dH2O, each well was separated from the plastic frame, and radioactivity was measured on a gamma counter (ISOdata 20/10: Global Medical Instrumentation, Ramsey, MN, U.S.A.).
2.7. Human Sera
Blood was collected and processed according to approved standard protocols. The study included blind samples and open samples (in the latter, the diagnosis was previously known to the person conducting the assay). All samples were collected before treatment was administered; the diagnoses were histologically confirmed before the start of the present study.
In a routine clinical laboratory procedure, blood that had been drawn in sterile tubes without chemicals or in S-Monovette SST tubes (Sarstedt, Nümbrecht, Germany) was allowed to clot and was then centrifuged. Aliquots of serum were stored at −20°C. Before samples were tested for recaf, they were thawed, heated for 30 minutes at 56°C, and supplemented with 0.02% thimerosal.
2.8. Age and Circulating RECAF
Levels of recaf had previously been measured in 260 healthy girls and women 13–96 years of age, distributed as follows: <20 years (n = 2), 20–29 years (n = 22), 30–39 years (n = 41), 40–49 years (n = 70), 50–59 years (n = 64), 60–69 years (n = 30), 70–79 years (n = 18), and ≥80 years (n = 13). Linear regression analysis of recaf against age yielded R2 = 0.01 (F = 0.11), indicating that age does not influence the level of circulating recaf. Table i gives the age data for each group.
TABLE I.
Descriptive statisticsa for the receptor for alpha-fetoprotein (recaf) in normal, benign, and cancer samples
| Sample description | Samples (n) | Ageb(mean/median) | Mean | sd | Percentile | Min | Max | Median | |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 95 | ||||||||
| Set 1 (blind) | |||||||||
| Normal | 119 | Not available | 3.19 | 0.9 | 1.6 | 4.52 | 1.1 | 5.00 | 3.24 |
| Benign | 20 | 45.0/47.0 | 6.04 | 1.55 | 4.25 | 9.08 | 3.14 | 9.77 | 5.57 |
| Cancer | 43 | 59.2/58.0 | 7.83 | 1.88 | 4.25 | 10.5541 | 3.3 | 11.30 | 8.04 |
| Set 2 (open) | |||||||||
| Normal | 353 | 48.8/48.0 | 3.66 | 0.63 | 2.65 | 4.47 | 2.36 | 5.3 | 3.71 |
| Benign | 30 | 45.6/45.5 | 4.35 | 0.71 | 3.27 | 5.26 | 3.13 | 5.3 | 4.35 |
| Cancer | 64 | 55.1/55.0 | 6.67 | 1.35 | 4.92 | 10.11 | 4.45 | 10.66 | 6.49 |
| Set 3 (open) | |||||||||
| Normal | 40 | 60.7/62.0 | 3.07 | 0.63 | 1.94 | 4.08 | 1.73 | 4.16 | 3.04 |
| Cancer | 40 | 56.1/55.0 | 6.71 | 1.73 | 4.19 | 9.39 | 3.19 | 10.75 | 6.39 |
| All sets combined | |||||||||
| Normal | 504 | 50.0/49.0c | 3.51 | 0.74 | 2.36 | 4.47 | 1.10 | 5.30 | 3.59 |
| Benign | 50 | 45.4/46.0 | 5.03 | 1.39 | 3.27 | 7.44 | 3.13 | 9.77 | 5.00 |
| Cancer | 147 | 56.6/55.0 | 7.02 | 1.70 | 4.35 | 10.30 | 3.19 | 11.30 | 6.77 |
recaf values expressed in (arbitrary) K units.
Not related to circulating recaf (see text).
From Sets 2 and 3.
SD = standard deviation.
The three sample sets used in the study were:
-
Blind samples (Set 1)
The samples in Set 1 were collected and blinded at the Institut für Klinische Chemie in Munich before being sent to be tested for recaf. The 182 blinded serum samples included 43 samples from patients with stage i and ii breast cancer, 20 from patients with breast benign conditions (fibroadenoma, n = 12; galactorrhea, n = 1; nonmalignant suspicious mammography, n = 2; mastopathy, n = 1; papilloma of the lactiferous ducts, n = 1; cysts and abscesses of the breast, n = 3), and 119 from healthy control subjects.
-
Open samples (Sets 2 and 3)
To increase the number of cases tested with recaf, two additional sample sets were tested. Sets 2 and 3 were obtained at a site different from that where Set 1 had been collected. In Sets 2 and 3, neither cea nor ca15-3 was measured. Set 2 consisted of 447 serum samples: 64 from patients with breast cancer stages ii and iii, 30 from patients with various benign conditions (fibroadenoma, breast keratosis, chronic mastitis, cysts, papilloma of the lactiferous ducts, fibrocystic disease—henceforth called “benign” samples), and 353 from healthy control subjects (henceforth called “normal” samples). Set 3 consisted of 40 samples from healthy control subjects and 40 from breast cancer patients at unspecified stages. No benign samples were included in Set 3.
2.9. Standard Curve
Several dilutions of the MCF-7 cell extract were used to create a standard curve calibrated in arbitrary “recaf units” that allowed for the recaf measurements to be normalized from one experiment to another. The dilutions were carried out in 3% fish gelatin–tbs and were processed in the same manner as the serum samples. All sample readings were within the range of the standards. To extrapolate values from the standard curve, we used the Logit/Log function.
3. RESULTS
3.1. Purified RECAF and Anti-RECAF Polyclonal Antiserum Characterization
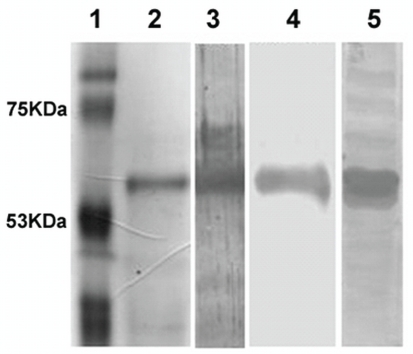
The radioimmunoassay design required pure recaf. Figure 1 shows that the recaf preparation used for rabbit immunizations and for radiolabelling has only 1 band with a molecular weight of approximately 62 kDa. That band is recognized by both biotinylated-afp and the rabbit anti-recaf antiserum. The monospecificity of the antiserum was verified against a total cell extract of MCF-7 as shown in lane 5 of Figure 1. The biotinylated-afp also detected a faint 67-kDa band that was absent from the Western blot done with the antiserum. (It is worth noting that the afp receptor has previously been described as a 62/67 kDa doublet found both in soluble form and associated to membranes 22,23.) The rabbit antiserum also inhibited the binding of biotinylated-afp to recaf (data not shown), which indicates that they both recognize the same recaf epitope.
FIGURE 1.
Sodium dodecylsulfate (sds) polyacrylamide gel electrophoresis and Western blot of rabbit antibody to receptor for alpha-fetoprotein (recaf). Lane 1: Molecular weight (MW) markers stained with Coomassie blue. Lane 2: Pure recaf preparation (MW: approximately 62 kDa) used for labeling. Lane 3: Western blot of the pure recaf preparation using biotinylated alpha-fetoprotein (afp). Lane 4: Same as lane 3, using the rabbit anti-recaf antibody instead of afp to detect recaf. Lane 5: Western blot on whole extract of the MCF-7 cell line, developed with the rabbit anti-recaf antibody, showing that the antiserum is monospecific (only 1 major stained band at approximately 62 kDa).
3.2. Data Analysis
3.2.1. Assay Reproducibility and Precision
Ten blind samples distributed within the range of measured recaf values were repeatedly tested to determine the intra- and inter-sample variability of the assay. The intra-sample coefficient of variation was ≤6% and the inter-assay coefficient of variation was <10%.
3.2.2. Serum RECAF Values and Cut-Off Value Determination
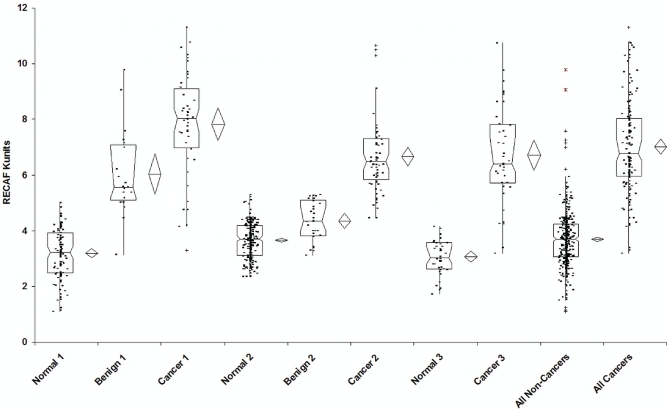
All 3 sample sets showed large differences in serum recaf when normal or benign samples were compared with samples from cancer patients (Table i and Figure 2).
FIGURE 2.
Comparative distribution of samples from normal subjects, patients with benign breast lesions, and patients with breast cancer for each set of values and for all values combined. The notched box shows the median, the lower and upper quartiles, and the confidence interval around the median. Whiskers extend to the furthest observations within ±1.5 interquartile ranges (iqrs) of the 1st or 3rd quartile. Observations outside 1.5 iqrs are marked (+) as near outliers; those outside 3.0 iqrs are marked (*) as far outliers.
Set 1 (Cancer Stages I and II, Blind Samples): The p value of an independent t-test (assuming unequal variances) comparing normal with cancer samples was 4.78×10−22. When benign and cancer samples were compared, the p value of the t-test was 1.3×10−4.
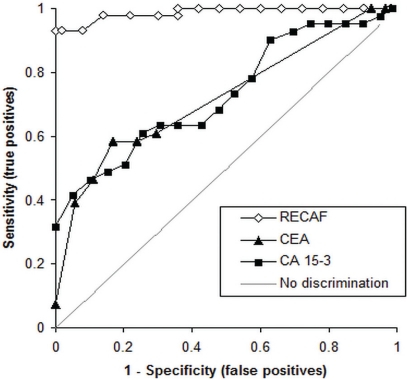
Figure 3 depicts the receiver operating characteristic (ROC) curves for cea, ca15-3, and recaf when normal samples were compared with samples from cancer patients. For recaf, the area under the curve (AUC) was 0.983. Using a cut-off value of 4.6 K recaf units, the sensitivity was 93%, with 95% specificity. At 95% specificity, the cea sensitivity was 39% (AUC: 0.723) and the ca15-3 sensitivity was 41% (AUC: 0.739).
FIGURE 3.
Receiver operating characteristic curves for the receptor for alpha-fetoprotein (recaf) in samples from cancer patients and from normal subjects [area under the curve (auc): 0.987], for carcinoembryonic antigen (cea, auc: 0.723), and for cancer antigen 15-3 (ca 15-3, auc: 0.739).
Increasing the recaf assay cut-off to 7.30 K units to attain 100% specificity, 72% detection of early breast cancers resulted. At 100% specificity, the cea and ca15-3 sensitivities were 32% and 7.3% respectively.
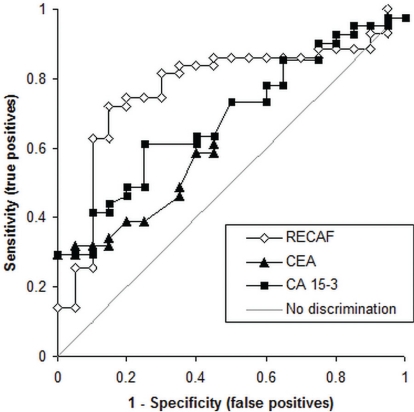
When benign samples were compared with samples from cancer patients (Figure 4), the higher recaf cut-off fared better. At 7.3 K units, the sensitivity was 72%, with 85% specificity (AUC: 0.773). On the same samples, at 85% specificity, the sensitivities of the cea and ca15-3 tests were 34% (AUC: 0.626) and 44% (AUC: 0.685) respectively.
FIGURE 4.
Receiver operating characteristic curves for the receptor for alpha-fetoprotein (recaf) in samples from cancer patients and from patients with benign lesions of the breast [area under the curve (auc): 0.77], for carcinoembryonic antigen (cea) (auc: 0.723), and for cancer antigen 15-3 (ca 15-3) (auc: 0.739).
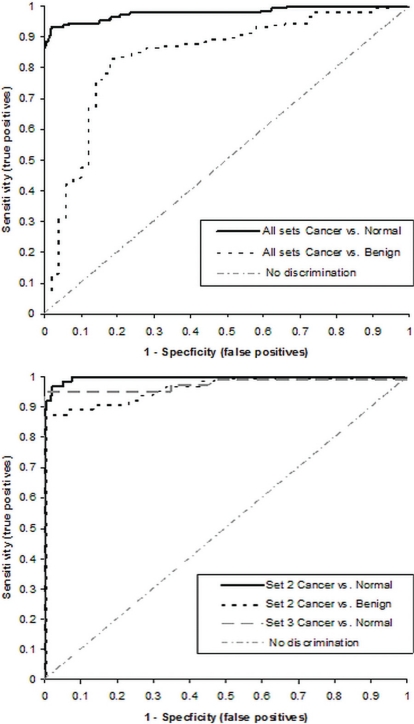
Set 2 (Cancer Stages II and III, Open Samples): Figure 5 shows the recaf ROC curves comparing control and benign samples with samples from cancer patients in Sets 2 and 3. At a cut-off value of 4.61 K recaf units, the sensitivity was 97% and the specificity was 97.5% (AUC: 0.99). When benign and cancer samples were compared using a cut-off of 5.3 K units, the sensitivity was 87%, with 97% specificity (AUC: 0.97).
FIGURE 5.
Receiver operating characteristic curves for the receptor for alpha-fetoprotein (recaf) for (A) Sets 2 and 3 (open samples), and (B) for all 3 sets combined.
Set 3 (Unspecified Cancer Stages, Open Samples): Set 3 included only control and cancer samples. In the ROC analysis, at 4.53 K recaf units, the sensitivity was 89%, with 100% specificity (Figure 5). The AUC was 0.98.
Figure 5 also shows the recaf ROC curves obtained after combining the normal, benign, and cancer samples from all sets.
4. DISCUSSION AND CONCLUSIONS
The results presented here show that a recaf-based serum immunoassay can discriminate, with high sensitivity and specificity, 504 normal subjects and 50 patients with benign breast lesions from 147 patients with breast cancer. More importantly, the assay can detect the early stages of breast cancer with a sensitivity and specificity unattainable to this point with other cancer markers.
Selecting a low cut-off value (4.6 K recaf units) maximizes cancer detection: sensitivity of 93%, with 95% specificity against normal subjects. In the blind group (Set 1), increasing the cut-off to 7.3 K units resulted in 100% specificity against normal subjects, and yet, against early cancers, the sensitivity remained relatively high (72%).
The sensitivity and specificity values in the blind samples (Set 1) were obtained when samples from stages i and ii breast cancer were tested. At those stages, the 5-year survival rates are 87% and 75% compared with 46% and just 13% for stages iii and iv 24. By contrast, the sensitivity reported by an expert panel from the American Society of Clinical Oncology for cea in stage i breast cancer was only 10%; for stage ii, it was 19% (both measured at 95% specificity 25 against normal subjects). In the same study, the sensitivity reported for ca15-3 was 9% in stage i cancer and 19% in stage ii, with a specificity of 95% against normal subjects and a specificity of 80% against benign breast lesions 26. In the present study, the sensitivity exhibited by the cea and ca15-3 tests was higher (39% and 41% respectively, both at 95% specificity). When sera from cancer patients were compared with sera from patients with benign breast lesions, the sensitivities for cea and ca15-3 were also higher than the published data already mentioned; and yet, the performance of the recaf assay was significantly better than either one of those two markers.
Using a cut-off value of 7.3 K units, the discrimination of positive cases among benign sera in the blind samples of Set 1 was 15%, which is slightly less than the 20% reported for ca15-3 25. In two thirds of benign samples, testing showed levels of less than 6 K recaf units.
The results from Sets 2 and 3 (open samples) were consistent with those from the blind group (Set 1), thus significantly expanding the number of samples.
It is unclear why some benign lesions are recaf-positive. It is common knowledge that, in general, cancer markers detect a certain percentage of benign lesions. Several explanations are possible:
Some benign lesions are considered premalignant, and the expression of cancer-associated markers might precede the morphology changes detected by a pathologist. Using TP53 as a prognostic marker, Rohan et al. concluded that p53 protein accumulation appears to be associated with an increased risk of progression to breast cancer in women with benign breast disease 27. The most recognizable premalignant lesion of the breast is atypical hyperplasia. However, unfolded lobules and ductal hyperplasia could be considered earlier premalignant epithelial abnormalities 28. The risk of a benign lesion evolving into cancer appears to be related to the degree of epithelial atypia 29, with fibroadenomas having the lowest risk. Interestingly, one of the patients in Set 1 had a histologic diagnosis of fibroadenoma, and yet, among the benign sera samples, hers had the highest amounts of circulating recaf. A year later, this patient was diagnosed with breast cancer. Removing her from the benign group in Set 1 would increase the specificity of the test from 85% to 89%.
Another reason that the discrimination between benign and cancer samples might be worse than the discrimination between normal and cancer samples is that healthy subjects do not normally undergo biopsy, but patients with benign lesions do. This difference introduces an additional source of error that can be measured by studies of agreement among pathologists examining the same breast lesion slides 30,31.
Future work on the biology of recaf might explain why some benign breast lesions test positive and might perhaps provide insight into how to better interpret the results of the recaf test.
Footnotes
5. CONFLICT OF INTEREST DISCLOSURES
RM and JGT receive remuneration from the company that owns the intellectual property rights for use of the recaf marker. PS has no financial conflicts of interest to disclose.
6. REFERENCES
- 1.Abelev GI, Perova SD, Khramkova NI, Postnikova ZA, Irlin IS. Production of embryonal alpha-globulin by transplantable mouse hepatomas. Transplantation. 1963;1:174–80. doi: 10.1097/00007890-196301020-00004. [DOI] [PubMed] [Google Scholar]
- 2.Deutsch HF. Chemistry and biology of alpha-fetoprotein. Adv Cancer Res. 1991;56:253–312. doi: 10.1016/S0065-230X(08)60483-2. [DOI] [PubMed] [Google Scholar]
- 3.Ruoslahti E, Seppälä M. α-Fetoprotein in cancer and fetal development. Adv Cancer Res. 1979;29:275–346. doi: 10.1016/S0065-230X(08)60849-0. [DOI] [PubMed] [Google Scholar]
- 4.Trojan J, Uriel J. Immunocytochemical localisation of alphafetoprotein (afp) and serum albumin (alb) in ecto-, meso- and endodermal tissue derivatives of the developing rat. Oncodev Biol Med. 1982;3:13–22. [PubMed] [Google Scholar]
- 5.Alpert E, Feller ER. Alpha-fetoprotein (afp) in benign liver disease. Evidence that normal liver regeneration does not induce afp synthesis. Gastroenterology. 1978;74(Pt 1):856–8. [PubMed] [Google Scholar]
- 6.Geuskens M, Naval J, Uriel J. Ultrastructural studies of the intracellular translocation of endocytosed alpha-foetoprotein (afp) by cytochemistry and of the uptake of 3H-arachidonic acid bound to afp by autoradiography in rat rhabdomyosarcoma cells. J Cell Physiol. 1986;128:389–96. doi: 10.1002/jcp.1041280307. [DOI] [PubMed] [Google Scholar]
- 7.Uriel J, Naval J, Laborda J. α-Fetoprotein–mediated transfer of arachidonic acid into cultured cloned cells derived from a rat rhabdomyosarcoma. J Biol Chem. 1987;262:3579–85. [PubMed] [Google Scholar]
- 8.Jacobsen M, Lassen LC, Møllgård K. Immunohistochemical evidence for intracellular localization of plasma proteins in cns and some neural crest derivatives in human embryos. Tumour Biol. 1984;5:53–60. [PubMed] [Google Scholar]
- 9.Mizejewski GJ. Alpha-fetoprotein binding proteins: implications for transmembrane passage and subcellular localization. Life Sci. 1995;56:1–9. doi: 10.1016/0024-3205(94)00401-D. [DOI] [PubMed] [Google Scholar]
- 10.Vidal RM. Selective localization of alpha-fetoprotein and serum albumin within the sensory ganglia cells of developing chicken. Neurosci Lett. 1983;41:253–7. doi: 10.1016/0304-3940(83)90459-7. [DOI] [PubMed] [Google Scholar]
- 11.Hajeri–Germond M, Naval J, Trojan J, Uriel J. The uptake of alpha-foetoprotein by C-1300 mouse neuroblastoma cells. Br J Cancer. 1985;51:791–7. doi: 10.1038/bjc.1985.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro R, Fielitz W, Esteves A, Grunberg J, Uriel J. In vivo uptake of heterologous alphafetoprotein and serum albumin by ependymal cells of developing chick embryos. Int J Dev Neurosci. 1984;2:143–8. doi: 10.1016/0736-5748(84)90005-4. [DOI] [PubMed] [Google Scholar]
- 13.Moro R, Heuguerot C, Vercelli–Retta J, Fielitz W, López JJ, Roca R. The use of radioiodinated alpha-fetoprotein for the scintigraphic detection of mouse mammary carcinomas. Nucl Med Commun. 1984;5:5–12. doi: 10.1097/00006231-198401000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Uriel J, Poupon MF, Geuskens M. Alphafoetoprotein uptake by cloned cell lines derived from a nickel-induced rat rhabdomyosarcoma. Br J Cancer. 1983;48:261–9. doi: 10.1038/bjc.1983.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uriel J, Villacampa MJ, Moro R, Naval J, Failly–Crépin C. Uptake of radiolabeled alpha-fetoprotein by mouse mammary carcinomas and its usefulness in tumor scintigraphy. Cancer Res. 1984;44:5314–19. [PubMed] [Google Scholar]
- 16.Naval J, Villacampa MJ, Goguel AF, Uriel J. Cell-type–specific receptors for alpha-fetoprotein in a mouse T-lymphoma cell line. Proc Natl Acad Sci U S A. 1985;82:3301–5. doi: 10.1073/pnas.82.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suzuki Y, Zeng CQ, Alpert E. Isolation and partial characterization of a specific alpha-fetoprotein receptor on human monocytes. J Clin Invest. 1992;90:1530–6. doi: 10.1172/JCI116021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villacampa MJ, Moro R, Naval J, Failly–Crepin C, Lampreave F, Uriel J. Alpha-fetoprotein receptors in a human breast cancer cell line. Biochem Biophys Res Commun. 1984;122:1322–7. doi: 10.1016/0006-291X(84)91236-1. [DOI] [PubMed] [Google Scholar]
- 19.Tsuboi S, Taketa K, Nouso K, et al. High level of expression of alpha-fetoprotein receptor in gastric cancers. Tumour Biol. 2006;27:283–8. doi: 10.1159/000096071. [DOI] [PubMed] [Google Scholar]
- 20.Moro R, Tcherkassova J, Song E, et al. A New Broad-Spectrum Cancer Marker. Fairfield, NJ: IVD Technology; 2005. [Available online at: http://www.ivdtechnology.com/article/new-broad-spectrum-cancer-marker; cited December 7, 2011] [Google Scholar]
- 21.Weir DM, editor. Handbook of Experimental Immunology. 3rd ed. Oxford, U.K: Blackwell Scientific Publications; 1978. [Google Scholar]
- 22.Moro R, Tamaoki T, Wegmann TG, Longenecker BM, Laderoute MP. Monoclonal antibodies directed against a widespread oncofetal antigen: the alpha-fetoprotein receptor. Tumour Biol. 1993;14:116–30. doi: 10.1159/000217864. [DOI] [PubMed] [Google Scholar]
- 23.Laderoute M, Willans D, Wegmann T, Longenecker M. The identification, isolation and characterization of a 67 kilodalton, pna-reactive autoantigen commonly expressed in human adenocarcinomas. Anticancer Res. 1994;14:1233–45. [PubMed] [Google Scholar]
- 24.Cotran RS, Robbins SL, Tucker C, Kumar V. Robbins Pathologic Basis of Disease. 6th ed. Philadelphia, PA: WB Saunders; 1999. [Google Scholar]
- 25.Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol. 1996;14:2843–77. doi: 10.1200/JCO.1996.14.10.2843. [DOI] [PubMed] [Google Scholar]
- 26.Hayes DF, Zurawski VR, Jr, Kufe DW. Comparison of circulating ca15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986;4:1542–50. doi: 10.1200/JCO.1986.4.10.1542. [DOI] [PubMed] [Google Scholar]
- 27.Rohan TE, Hartwick W, Miller AB, Kandel RA. Immunohistochemical detection of c-erbB-2 and p53 in benign breast disease and breast cancer risk. J Natl Cancer Inst. 1998;90:1262–9. doi: 10.1093/jnci/90.17.1262. [DOI] [PubMed] [Google Scholar]
- 28.Arpino G, Laucirica R, Elledge RM. Premalignant and in situ breast disease: biology and clinical implications. Ann Intern Med. 2005;143:446–57. doi: 10.7326/0003-4819-143-6-200509200-00009. [DOI] [PubMed] [Google Scholar]
- 29.Carter CL, Corle DK, Micozzi MS, Schatzkin A, Taylor PR. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128:467–77. doi: 10.1093/oxfordjournals.aje.a114995. [DOI] [PubMed] [Google Scholar]
- 30.Ellis IO, Coleman D, Wells C, et al. Impact of a national external quality assessment scheme for breast pathology in the UK. J Clin Pathol. 2006;59:138–45. doi: 10.1136/jcp.2004.025551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palli D, Galli M, Bianchi S, et al. Reproducibility of histological diagnosis of breast lesions: results of a panel in Italy. Eur J Cancer. 1996;32A:603–7. doi: 10.1016/0959-8049(95)00609-5. [DOI] [PubMed] [Google Scholar]