Abstract
Background
Lung cancer leads cancer-related mortality in the world. The objective of the present systematic review was to compare fine-needle aspiration biopsy (fnab) with core-needle biopsy (cnb) for diagnostic characteristics and yields for diagnosing lung cancer in patients with lung lesions.
Methods
The medline and embase databases (from January 1, 1990, to September 14, 2009), the Cochrane Library (to Issue 4, 2009), and selected guideline Web sites were searched for relevant articles.
Results
For overall diagnostic characteristics (benign vs. malignant) of fnab and cnb, the ranges of sensitivity were 81.3%–90.8% and 85.7–97.4% respectively; of specificity, 75.4%–100.0% and 88.6%–100.0%; and of accuracy, 79.7%–91.8% and 89.0%–96.9%. For specific diagnostic characteristics of fnab and cnb (identifying the histologic subtype of malignancies or the specific benign diagnoses), the ranges of sensitivity were 56.3%–86.5% and 56.5–88.7% respectively; of specificity, 6.7%–57.1% and 52.4%–100.0%; and of accuracy, 40.4%–81.2% and 66.7%–93.2%. Compared with fnab, cnb did not result in a higher complication rate (pneumothorax or hemoptysis). No study has yet compared the diagnostic yields of fnab and of cnb for molecular predictive-marker studies in patients with lung lesions.
Discussion and Conclusions
The evidence is currently insufficient to support a difference between fnab and cnb in identifying lung malignancies in patients with lung lesions. Compared with fnab, cnb might have a higher specificity to diagnose specific benign lesions. Well-designed, good-quality studies comparing fnab with cnb for diagnostic characteristics and yields in diagnosing lung cancer should be encouraged.
Keywords: Fine-needle aspiration biopsy, core-needle biopsy, diagnostic characteristics, diagnostic yields, lung cancer, systematic review
1. BACKGROUND
Cancer is a leading cause of death, and lung cancer is the most common cause of cancer-related mortality in the world 1. In Canada, the estimated percentage of cancer-related death for lung cancer was 27% in 2011 2. Early and accurate diagnosis is the key for the optimal treatment of lung cancer patients. New treatment strategies are becoming more complex, with certain novel therapeutics being restricted to specific histologic or molecular subtypes of lung cancer, thus requiring more precise classification and performance of molecular testing such as that for epidermal growth factor receptor mutations 3,4.
For patients with a lung nodule or mass on chest radiography or computed tomography (ct), a histologic or cytologic confirmation of malignancy is required before treatment. Flexible bronchoscopy has high sensitivity for the diagnosis of central lesions and low sensitivity for the diagnosis of peripheral lesions 5. Transthoracic needle biopsy is usually performed under imaging guidance for patients with peripheral lesions or in whom flexible bronchoscopy is not possible 6. The two transthoracic biopsy techniques currently being used are fine-needle aspiration biopsy (fnab) and core-needle biopsy (cnb). The sensitivity and specificity of both techniques for diagnosing lung cancer have been reported to be high, with acceptable complication rates 7,8; however, a number of questions about these two procedures remain unanswered. The present systematic literature review addressed these questions:
Is one technique superior to the other for diagnosing lung cancer?
Is there a difference in complication rates between the two techniques?
Is one technique better than the other in obtaining samples for molecular marker studies such as mutation analysis or fluorescence in situ hybridization?
2. METHODS
2.1. Search Strategy
A literature search through Ovid of the medline and embase databases for the period January 1, 1990, to September 14, 2009, used various alternative terms for “fine-needle aspiration biopsy,” “core-needle biopsy,” and “lung cancer,” and then used the “and” operator to combine the results of the searches (specific details available from the corresponding author). A check for existing systematic reviews and practice guidelines was made using the Cochrane Library (to Issue 4, 2009), the U.S. National Guideline Clearinghouse, the U.K. National Institute for Health and Clinical Excellence, and Scottish Intercollegiate Guidelines Network (to August 28, 2009), the American Society of Clinical Oncology guidelines, Australia’s National Health and Medical Research Council, the New Zealand Guidelines Group, the Canadian Medical Association Infobase (to August 31, 2009), and the U.S. National Cancer Institute’s PDQ database (to September 8, 2009).
2.2. Study Selection Criteria
Studies were included if they
had been published in full text between January 1, 1990, and September 14, 2009.
were systematic reviews, meta-analyses, clinical practice guidelines, randomized trials, or comparative cohort studies.
reported or provided sufficient data to calculate, for both fnab and cnb in lung cancer, at least 1 diagnostic characteristic (that is, sensitivity, specificity, positive or negative likelihood ratio, or accuracy), complication rates, or diagnostic yields 9 for molecular predictive-marker studies.
included patients with an undiagnosed lung nodule or mass demonstrated on imaging.
stated that the reference standard for final diagnosis was histologic confirmation from wedge biopsy, surgical resection, metastases, or autopsy, or from clinical follow-up.
Studies were excluded if they
had recruited patients with a previous or current diagnosis of lung cancer at baseline 10.
regarded the biopsy results from fnab or cnb (or both) as a part of the reference standard 10.
performed fnab and cnb on different patient populations (for example, technique chosen according to the size of the lesion).
were published in a language other than English.
2.3. Data Abstraction
One author scanned the retrieved citation titles and abstracts from the search sources to identify potentially relevant articles, which were then retrieved for full-text review. Three authors independently assessed the articles for possible inclusion. Differences in assessment were resolved by discussion. A standardized data extraction sheet was used. All the authors contributed to reviewing and revising the draft document.
2.4. Study Quality Assessment
Study quality was assessed using the 11-item checklist from Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy 11. Each item was rated “yes” (meaning high quality), “unclear,” or “no” (meaning low quality).
2.5. Data Analysis
For each study, if the data were reported, we constructed a 2×2 contingency table for fnab and cnb. Meta-analyses of the eligible studies for diagnostic characteristics and complication rates were considered, but were not feasible because of clinical heterogeneity. The Stata statistical software application (version 9.0: StataCorp LP, College Station, TX, U.S.A.) was used to compare fnab with cnb for diagnostic characteristics, diagnostic yields, and procedure complications. Significance was assumed at a two-sided α of 0.05.
3. RESULTS
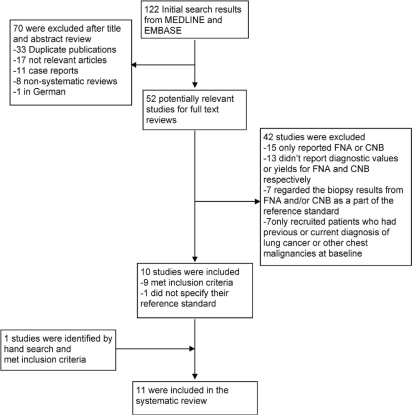
No locatable systematic reviews or practice guidelines focused on comparing fnab with cnb for diagnosing lung cancer in patients with a lung lesion. The electronic search identified one hundred twenty-two citations (Figure 1). After titles and abstracts had been reviewed, seventy articles were excluded. Another forty-two papers were disqualified after review of the full texts, leaving ten potentially eligible articles 12–21. Of those ten articles, one (Lourenço et al. 2006 20) did not state the reference standard used to make the final diagnoses. The original author was contacted, but no feedback was received. That article was therefore analyzed separately from other included studies. One additional study was identified from the reference sections of the eligible articles 22. The present systematic review included eleven studies in total.
FIGURE 1.
Flow of studies considered for this systematic review.
3.1. Study Details and Quality
Table i shows detailed information for the included studies. In five studies 13,15,16,19,22, fnab and cnb were performed on the same patient. One study recruited children less than 13 years of age 13, and ten studies included seniors more than 80 years of age 12–14,16–22. In six studies, lesion diameters ranged from 3 mm to 150 mm 12,13,15,16,18,21. In six studies 14,16–18,20,21, the lesions were located exclusively in the lung; in the other five studies 12,13,15,19,22, they were located in lung, mediastinum, pleura, or chest wall.
TABLE I.
Study and patient information from eligible studies
| Reference | Study design | Pts (n) | Age in years [range (mean)] |
Lesion |
Distance from skin to lesion (mm) | |
|---|---|---|---|---|---|---|
| Location | Diameter (mm) | |||||
| Cheong et al., 199217 | Prospectivea | 128 | 19–85 (61.4) | Lung | Mean: 37 | nr |
| Moulton et al., 199322,b | Prospective | 114 | 22–92 (64.7)c | Lung (76% of patients), mediastinum, pleura | nr | nr |
| Arakawa et al., 199612 | Retrospective | 107 | 20–85d (62.7) | Lung (84% of patients), mediastinum, pleura | 5–100 | nr |
| Staroselsky et al., 199813,b | Retrospective | 182 | 10–84 (62) | Lung (82% of patients), chest wall, mediastinum, pleura | 10–100 | nr |
| Laurent et al., 200018 | Prospectivee | 220 | 24–84 (61.9 for fnab, 65.4 for cnb) | Lung | 8–150 (Mean: 35.4) | nr |
| Sagar et al., 200015,b | nr | 30 | 14–66 (43.5) | Lung (43% of patients), mediastinum, pleura | 30–100 | nr |
| Anderson et al., 200321 | Retrospective | 182 | 29–87 (67.5) | Lung | 8–100 (Mean: 41) | 5–70 (Mean: 24) |
| Yamagami et al., 200316,b | nr | 134 | 16–92 (67.1) | Lung | 3–100 (Mean: 22.1) | 0–63 (Mean: 13.8) |
| Ohno et al., 200414 | nr | 390 | 16–86 (63.3) | Lung | 71.0% of lesions > 10 | nr |
| Schubert et al., 200519,b | Prospective | 85f | 27–84 (56) | Lung (91% of patients) mediastinum, paravertrebral and supraclavicular lesions | nr | nr |
| Lourenco et al., 200620,g | Retrospective | 92 | 28–87 (64.4) | Lung | nr | nr |
Choice of needles was randomized, but no detail of the randomization procedure was provided.
Fine-needle aspiration biopsy and core-needle biopsy performed on the same patient.
Procedures for thoracic masses numbered 114; age is for 267 patients who had thoracic, hepatic, renal, pancreatic, adrenal, splenic, retroperitoneal, or musculoskeletal soft-tissue masses.
The study recruited 122 patients, but original authors reported results only for 107 who had a definitive final diagnosis; age is for 122 patients.
Quasi-random allocation: 125 consecutive patients underwent fine-needle aspiration biopsy in the first 21 months of the study; 98 consecutive patients underwent core-needle biopsy in the final 15 months.
The study recruited 97 patients, but original authors reported results only for 85 who underwent both procedures; age is for 97 patients.
Study did not specify the reference standard.
Pts = patients; nr = not reported; fnab = fine-needle aspiration biopsy; cnb = core-needle biopsy.
Table ii summarizes study quality.
TABLE II.
Study quality assessment
| Study |
Item 1(a) Right patient groupa |
Item 1(b) Method of samplingb |
Item 2 Acceptable reference standard |
Item 3 Acceptable delay between testsc |
Item 4 Partial verification avoided |
Item 5 Differential verification avoidedd |
Item 6 Incorporation avoided |
Item 7(a) Index test results blinded |
Item 7(b) Index tests blinded to each other iffnabandcnbperformed on same patients |
Item 8 Reference standard results blinded |
Item 9 Relevant clinical information |
Item 10 Uninterpretable results reported |
Item 11 Withdrawals explained |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheong et al., 199217 | Yes | Yes | Yes | na | Yes | No | Yes | Yes | na | Unclear | Yes | Yes | No |
| Moulton et al., 199322 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Unclear | Yes | Yes | Yes |
| Arakawa et al., 199612 | No | No | Yes | na | Yes | No | Yes | Unclear | na | Unclear | Yes | Yes | No |
| Staroselsky et al., 199813 | No | No | Yes | Yes | Yes | No | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Laurent et al., 200018 | Yes | Yes | Yes | na | Yes | No | Yes | Yes | na | Unclear | Yes | Yes | Yes |
| Sagar et al., 200015 | No | nr | Yes | Yes | Yes | No | Yes | Unclear | Unclear | Unclear | Yes | Yes | Yes |
| Anderson et al., 200321 | Yes | No | Yes | na | Yes | No | Yes | Unclear | na | Unclear | Yes | Yes | Yes |
| Yamagami et al., 200316 | Yes | nr | Yes | Yes | Yes | No | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes |
| Ohno et al., 200414 | Yes | nr | Yes | na | Yes | No | Yes | Unclear | na | Unclear | Yes | Yes | Yes |
| Schubert et al., 200519 | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Lourenco et al., 200620,e | Yes | No | Unclear | na | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | No |
“Yes” was arbitrarily defined as a study recruiting patients all of whom had lung lesions.
“Yes” indicates a prospective study; “No” indicates a retrospective study.
Delay between fnab and cnb was assessed if fnab and cnb were performed on the same patients. Both procedures were performed at the same visit on each patient in all five studies.
Reference standard was histologic confirmation from wedge biopsy, surgical resection, metastases, or autopsy or from clinical follow-up in all studies except for the Lourenco et al. study.
Study did not specify the reference standard.
fnab = fine-needle aspiration biopsy; cnb = core-needle biopsy; na = not available (fnab and cnb were not performed on the same patients), nr = not reported; Yes = high quality; No = low quality.
3.2. Diagnostic Characteristics
In nine reports, the data provided were sufficient to allow for the calculation of at least 1 diagnostic characteristic for fnab and cnb used to identify malignancies in patients with a lung lesion 12–16,18,19,21,22. The prevalence of malignant lesions was 67.3%–85.7%, and one paper did not report that information 19.
Diagnosis in the included studies was defined in two possible ways based on the data as originally reported: overall diagnosis and specific diagnosis (Tables iii and iv). In overall diagnosis, the purpose of lung biopsy was to differentiate malignant from benign lesions without specific cytologic or histologic subtype diagnoses. In specific diagnosis, the purpose of biopsy was to determine the specific cytologic or histologic subtype of the malignancy or the specific benign diagnosis; hence, the true positive and true negative results of fnab or cnb were exactly the same as the final histologic diagnoses for patients listed in the full text. In brief, five studies reported overall diagnostic characteristics, with a total sample size of 1033 (Table iii) 12–14,16,18; seven studies reported specific diagnostic characteristics, with a total sample size of 834 (Table iv) 12,13,15,16,19,21,22.
TABLE III.
Overall diagnostic characteristicsa
| Reference | Prevalence of malignant lesions (%) | Procedure (p Value) | Sensitivity [% (95%ci)] | Specificity [% (95%ci)] | Likelihood ratio | Accuracy [% (95%ci)] | |
|---|---|---|---|---|---|---|---|
| Positive (95%ci) | Negative (95%ci) | ||||||
| Arakawa et al., 199612,b | 67.3 | fnab | 81.3 (63.6 to 92.8) | 46.7 (21.3 to 73.4) | 1.52 (0.92 to 2.52) | 0.40 (0.16 to 0.99) | 70.2 (55.1 to 82.7) |
| cnb | 85.7 (71.5 to 94.6) | nr | nr | nr | nr | ||
| p=0.611 | na | na | na | na | |||
| Staroselsky et al., 199813,c | 77.5 | fnab | 90.8 (84.7 to 95.0) | 95.1 (83.5 to 99.4) | 18.61 (4.81 to 71.98) | 0.10 (0.06 to 0.16) | 91.8 (86.8 to 95.3) |
| cnb | 91.5 (85.6 to 95.5) | 100.0d | 75.94 (4.83 to 1194.4) | 0.09 (0.05 to 0.15) | 93.4 (88.8 to 96.5) | ||
| p=0.836 | p=0.151 | p=0.368 | p=0.766 | p=0.560 | |||
| Combinede | nr | nr | nr | nr | nr | ||
| Laurent et al., 200018,f | 80.5 | fnab | 82.7 (73.7 to 89.6) | 100.0d | 45.46 (2.91 to 709.68) | 0.18 (0.11 to 0.27) | 86.4 (79.1 to 91.9) |
| cnb | 97.4 (90.9 to 99.7) | 95.0 (75.1 to 99.9) | 19.48 (2.88 to 131.64) | 0.03 (0.01 to 0.11) | 96.9 (91.2 to 99.4) | ||
| p=0.002 | p=0.240 | p=0.620 | p=0.010 | p=0.007 | |||
| Yamagami et al., 200316,g | 68.1–71.0 | fnab | nr | nr | nr | nr | 79.7 (72.0 to 86.1) |
| cnb | nr | nr | nr | nr | 89.1 (82.7 to 93.8) | ||
| nr | nr | na | na | p=0.031 | |||
| Combinede | nr | nr | nr | nr | 94.2 (88.9 to 97.5) | ||
| Ohno et al., 200414,g | 74.7 | fnab | 90.4 (85.1 to 94.3) | 75.4 (63.1 to 85.2) | 3.67 (2.39 to 5.64) | 0.13 (0.08 to 0.20) | 86.4 (81.4 to 90.4) |
| cnb | 89.1 (82.0 to 94.1) | 88.6 (73.3 to 96.8) | 7.79 (3.09 to 19.65) | 0.12 (0.07 to 0.21) | 89.0 (82.9 to 93.4) | ||
| p=0.716 | p=0.115 | p=0.148 | p=0.824 | p=0.447 | |||
Meant to differentiate malignant from benign lesions without specific cytologic or histologic subtype diagnoses.
Reported definitive diagnosis per biopsy procedure.
Reported definitive diagnosis per patient.
Provided from data in article; other numbers calculated from data in article.
Combined fnab and cnb (2 procedures performed on each patient).
Patient number inconsistent at 5 areas in article; reported definitive diagnosis per patient in fnab group, but reporting basis unclear in cnb group.
Reported definitive diagnosis per lesion.
ci = confidence interval; fnab = fine-needle aspiration biopsy; cnb = core-needle biopsy; ct = computed tomography; nr = not reported; na = not available.
TABLE IV.
Specific diagnostic characteristicsa
| Reference | Prevalence of malignant lesions (%) | Procedure (p Value) | Sensitivity [% (95%ci)] | Specificity [% (95%ci)] | Likelihood ratio | Accuracy [% (95%ci)] | |
|---|---|---|---|---|---|---|---|
| Positive (95%ci) | Negative (95%ci) | ||||||
| Moulton et al., 199322,b | 85.1 | fnab | 83.5 (74.6 to 90.3) | 41.2 (18.4 to 67.1) | 1.42 (0.95 to 2.13) | 0.40 (0.19 to 0.83) | 77.2 (68.4 to 84.5) |
| cnb | 88.7 (80.6 to 94.2) | 94.1 (71.3 to 99.9) | 15.07 (2.25 to 101.05) | 0.12 (0.07 to 0.21) | 89.5 (82.3 to 94.4) | ||
| p=0.295 | p=0.001 | p=0.018 | p=0.010 | p=0.013 | |||
| Combinedc | 92.8 (85.7 to 97.0) | 94.1 (71.3 to 99.9) | 15.77 (2.35 to 105.70) | 0.08 (0.04 to 0.16) | 93.0 (86.6 to 96.9) | ||
| Arakawa et al., 199612,b | 67.3 | fnab | 56.3 (37.7 to 73.6) | 6.7 (0.2 to 31.9) | 0.60 (0.43 to 0.84) | 6.56 (0.95 to 45.39) | 40.4 (26.4 to 55.7) |
| cnb | 73.8 (58.0 to 86.1) | 52.4 (29.8 to 74.3) | 1.55 (0.96 to 2.51) | 0.50 (0.26 to 0.96) | 66.7 (53.7 to 78.0) | ||
| p=0.115 | p=0.004 | p=0.002 | p=0.014 | p=0.006 | |||
| Staroselsky et al., 199813,c | 77.5 | fnab | 86.5 (79.8 to 91.7) | 31.7 (18.1 to 48.1) | 1.27 (1.02 to 1.58) | 0.43 (0.23 to 0.79) | 74.2 (67.2 to 80.4) |
| cnb | 78.0 (70.3 to 84.5) | 87.8 (73.8 to 95.9) | 6.40 (2.80 to 14.61) | 0.25 (0.18 to 0.35) | 80.2 (73.7 to 85.7) | ||
| p=0.062 | p<0.001 | p<0.001 | p=0.126 | p=0.173 | |||
| Combinedc | nr | nr | nr | nr | nr | ||
| Sagar et al., 200015,d | 76.7 | fnab | 82.6 (61.2 to 95.0) | 57.1 (18.4 to 90.1) | 1.93 (0.80 to 4.63) | 0.30 (0.10 to 0.91) | 76.7 (57.7 to 90.1) |
| cnb | 56.5 (34.5 to 76.8) | 100.0e | 8.48 (0.57 to 126.37) | 0.47 (0.28 to 0.77) | 66.7 (47.2 to 82.7) | ||
| p=0.054 | p=0.051 | p=0.308 | p=0.470 | p=0.390 | |||
| Combinedc | 91.3 (72.0 to 98.9) | 100.0e | 13.70 (93.8 to 199.91) | 0.09 (0.02 to 0.39) | 93.3 (77.9 to 99.2) | ||
| Anderson et al., 200321,b | 85.7 | fnab | nr | nr | nr | nr | 78.1 (70.7 to 84.5) |
| cnb | nr | nr | nr | nr | 93.2 (81.3 to 98.6) | ||
| p<0.005 in favour of cnbe | nr | na | na | p=0.023 | |||
| Yamagami et al., 200316,f | 68.1–71.0 | fnab | nr | nr | nr | nr | 58.7 (50.0 to 67.0) |
| cnb | nr | nr | nr | nr | 83.3 (76.0 to 89.1) | ||
| nr | nr | na | na | p<0.001 | |||
| Combinedc | nr | nr | nr | nr | 86.2 (79.3 to 91.5) | ||
| Schubert et al., 200519,d | nr | fnab | nr | nr | nr | nr | 81.2 (71.2 to 88.8) |
| cnb | nr | nr | nr | nr | 80.0 (69.9 to 87.9) | ||
| nr | nr | na | na | p=0.843 | |||
| Combinedc | nr | nr | nr | nr | 89.4 (80.8 to 95.0) | ||
Meant to determine the specific subtype of cancer or the specific benign diagnosis.
Reported definitive diagnosis per biopsy procedure.
Combined fnab and cnb (2 procedures performed on each patient).
Reported definitive diagnosis per patient.
Provided from data in article; other numbers calculated from data in article.
Reported definitive diagnosis per lesion.
ci = confidence interval; fnab = fine-needle aspiration biopsy; cnb = core-needle biopsy; nr = not reported; na = not available.
3.3. Overall Diagnostic Characteristics
Three of the five studies in Table iii provided sufficient data to calculate sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and accuracy for distinguishing malignant from benign lesions 13,14,18. The range of sensitivity was 81.3%–90.8% for fnab and 85.7%–97.4% for cnb; of specificity, 75.4%–100.0% and 88.6%–100.0%; and of accuracy, 79.7%–91.8% and 89.0%–96.9%. The range of the positive likelihood ratio was 3.67–45.46 for fnab and 7.79–75.94 for cnb; the range of the negative likelihood ratio was 0.10–0.18 for fnab and 0.03–0.12 for cnb. To reduce bias, we did not analyze the data from studies that reported characteristics for only one procedure—for example, the Arakawa et al. study 12, in which specificity was available only for fnab. Among 17 comparisons in Table iii, only 4 p values were less than 0.05, and all of them favored cnb over fnab: sensitivity, negative likelihood ratio, and accuracy in the Laurent et al. study 18, and accuracy in the Yamagami et al. study 16. It was noted that the report by Laurent et al. had 5 areas in which patient numbers were inconsistent, raising questions about the reliability of the results.
3.4. Specific Diagnostic Characteristics
Four studies in Table iv provided sufficient data to calculate diagnostic characteristics 12,13,15,22. The range of sensitivity was 56.3%–86.5% for fnab and 56.5%–88.7% for cnb. The range of specificity was 6.7%–57.1% for fnab and 52.4%–100.0% for cnb. Specificity was significantly or marginally higher for cnb than for fnab in all four studies. It appears that cnb may be superior to fnab for classifying benign disease. The range of the positive likelihood ratio was 0.60–1.93 for fnab and 1.55–15.07 for cnb. Three of four studies supported cnb against fnab 12,13,22. The range of the negative likelihood ratio was 0.30–6.56 for fnab and 0.12–0.50 for cnb. Two of four studies favoured cnb 12,22. Accuracy was available in seven studies. The range of accuracy was 40.4%–81.2% for fnab and 66.7%–93.2% for cnb. Four papers showed statistically significantly higher accuracy for cnb than for fnab 12,16,21,22.
3.5. Image Guidance
In current practice, ct imaging has largely replaced fluoroscopy alone or ultrasonography to guide fnab or cnb for thoracic lesions. Thus, a separate subgroup analysis of ct-guided fnab and cnb, with or without other forms of guidance, is relevant.
Seven studies used ct or ct plus fluoroscopy or ct plus multiplanar reconstruction images in more than 90% of patients 12,14,16,18,21,22. That total rose to eight studies if the one study 20 that did not specify a reference standard was included (Table v).
TABLE V.
Procedure information and complications
| Reference |
Procedures (n) |
Needle gauge |
Image guidance | Performed by | On-sitea |
Needle pass |
Complication |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fnab | cnb | fnab | cnb | fnab | cnb | Pneumothorax (fnabvs.cnb) | Pulmonary hemorrhage (fnabvs.cnb) | ||||
| Cheong et al., 199217 | 56 | 75 | 22 | 18, 19.5, or 21.5 | Single-plane fluoroscopy | Radiologist | No | Usually 1–2 (Mean: 1.9) | Usually 1–2 (Mean: 1.9) | 35.8% vs. 35.5%b | 4.5% vs. 12.7%b |
| Moulton et al., 199322,c | 114 | 114 | 22 | 18 | 105 by ct 9 by fluoroscopy |
Radiologist | No | 2–5 | 2–5 | 11.4% | Self-limiting hemoptysis or perilesional hemorrhage, or both, in 2.6% |
| Arakawa et al., 199612,d | 47 | 63 | 20 | 18 | ct | Radiologist or resident under supervision | No | Mean: 1.6 | Mean: 2 | 34.6% vs. 24.3%b (3.8% vs. 4.1%b needed drainage) | 5.8% vs. 4.1% for mild hemoptysisb |
| Staroselsky et al., 199813,c | 182 | 182 | 25 | 18 | ct | Respirologist | Yes | Mean: 2 | Mean: 1.3 | 24.7% (2.7% needed drainage) | Moderate hemoptysis in 1.1% |
| Laurent et al., 200018,e | 125 | 98 | 20 or 22 | 19.5 | ct | Radiologist | Yes | Repeated samples until satisfactory | 2–5 | 20.0% vs. 15.3%b (2.4% vs. 2.0%b needed drainage) | 13.6% vs. 28.6%f (2.4% vs. 4.1%b for mild hemoptysis) |
| Sagar et al., 200015,c | 30 | 30 | 21 | 18 | Ultrasonography | Radiologist | Yes | 1–3 | 2 | No | No |
| Anderson et al., 200321 | 151 | 44 | 20 or 22 | 18 or 20 | ct | Radiologist or resident under supervision | No | 1–4 (Mean: 1.8) | 1–4 (Mean: 1.8) | 35.1% vs. 15.9%g (2% needed drainage) | Self-limiting hemoptysis in 6 patients |
| Yamagami et al., 200316,c | 138 | 138 | 21 | 18 or 20 | ct + fluoroscopy | Radiologist | No | 1–2 | 1–4 | 32.6% (3.6% needed drainage) | Hemorrhage in 25.4%, Hemoptysis in 6.5%, Subcutaneous hematoma in 0.7% |
| Ohno et al., 200414 | 242 | 154 | 22 | 18 | 250 by ct 81 by ct + fluoroscopy 65 by ct + multiplanar reconstruction image |
Radiologist | No | nr | nr | 22.7% vs. 28.6%b | nr |
| Schubert et al., 200519,c | 85 | 85 | 22 | 14 | Ultrasonography | Respirologist | Yes | Up to 3 | Up to 5 | No major complications | No major complications |
| Lourenco et al., 200620,d,h | 89 | 13 | 22 | 18 | ct | Radiologist | No | nr | nr | 7.9% vs. 0%b | 4.5% vs. 0%b (hemothorax needing drainage in 1.1%) |
Assumed no on-site cytopathologist if article did not mention.
Nonsignificant between fnab and cnb.
Each patient underwent both fnab and cnb.
Patient numbers inconsistent at 1 area in the article.
Patient numbers inconsistent at 5 areas in the article.
Significant in favour of fnab.
Significant in favour of cnb.
Study did not specify the reference standard.
fnab = fine-needle aspiration biopsy; cnb = core-needle biopsy; On-site = on-site cytopathologist; ct = computed tomography; nr = not reported.
For overall diagnosis, all studies in Table iii used ct imaging. Hence, the analyses were the same as described earlier.
For specific diagnosis (Table iv), five of seven studies used ct imaging in most patients 12,13,16,21,22. Three studies provided sufficient data to calculate diagnostic characteristics 12,13,22. The range of sensitivity was 56.3%–86.5% for fnab and 73.8%–88.7% for cnb. The range of specificity was 6.7%–41.2% for fnab and 52.4%–94.1% for cnb. Specificity was significantly higher for cnb than for fnab in all three studies. The range of the positive likelihood ratio was 0.60–1.42 for fnab and 1.55–15.07 for cnb. All three studies supported cnb against fnab. The range of the negative likelihood ratio was 0.40–6.56 for fnab and 0.12–0.50 for cnb. Two of three studies favoured cnb 12,22. Accuracy was available in five studies 12,13,16,21,22. The range of accuracy was 40.4%–78.1% for fnab and 66.7%–93.2% for cnb. In four of five papers, accuracy was significantly higher for cnb than for fnab 12,16,21,22.
An interesting finding is that, for specific diagnosis (Table iv), the Sagar et al. study 15, which used ultrasonography guidance, had the highest specificity for fnab and cnb, and the Schubert et al. study 19, which also used ultrasonography guidance, had the highest accuracy for fnab. However, both studies had an on-site cytopathologist, and because they used ultrasonography guidance, the lung lesions included in the studies might have been limited to those abutting the chest wall.
3.6. Lung Biopsy
Four papers included patients with lung lesions only, the sample size being 926 14,16,18,21. In all four studies, fnab and cnb were guided by ct. Among three studies reporting overall diagnostic characteristics (Table iii), two studies provided all diagnostic characteristics 14,18. Ohno et al. 14 found no statistical difference between fnab and cnb. Laurent et al. 18 reported that, compared with fnab, cnb showed significantly higher sensitivity, accuracy, and negative likelihood ratio, but as noted earlier, the data as reported contained inconsistencies. Yamagami et al. 16 reported only accuracy values, which favoured cnb over fnab.
For specific diagnosis (Table iv), an accuracy value was available in two studies 16,21, and one also reported the p value for a comparison of sensitivity between fnab and cnb 21; all three values favoured cnb.
3.7. On-Site Cytopathologist
Four of seven studies stated that a cytopathologist was present on site in their centres to assess whether the specimens from fnab were adequate for cytologic analysis, and needle passes were repeated until the samples were satisfactory. Those four studies included 517 patients 13,15,18,19. Two of four reported overall diagnostic characteristics 13,18 (Table iii). Laurent et al. 18 showed that cnb was significantly better than fnab for sensitivity, negative likelihood ratio, and accuracy. However, as discussed earlier, the reliability of the data in that study is questionable. The study by Staroselsky et al. 13 observed no significant difference between fnab and cnb for any diagnostic characteristic.
Specific diagnostic characteristics were available in three studies (Table iv). Sagar et al. 15 and Staroselsky et al. 13 found that fnab might have greater sensitivity than cnb, with marginally significant p values (0.054 and 0.062 respectively), but that cnb had better specificity than fnab. Schubert et al. 19 reported only accuracy and observed no difference between the two procedures.
3.8. FNAB and CNB Performed on the Same Patient
Five studies performed fnab and cnb at the same biopsy session in each patient 13,15,16,19,22. For specific diagnosis, all five studies reported accuracy, which in two studies was significantly higher for cnb than for fnab 16,22 (Table iv). The range of accuracy was 58.7%–81.2% for fnab, 66.7%–89.5% for cnb, and 86.2%–93.3% for the combination. The combined diagnostic characteristics of fnab and cnb were apparently higher than the diagnostic characteristics of either fnab or cnb alone in the four available studies 15,16,19,22. Valid statistical comparisons could not be performed because the data were not independent in the studies.
Two of four studies reported overall diagnostic characteristics (Table iii). Staroselsky et al. 13 found no statistical difference between fnab and cnb; Yamagami et al. 16, who reported only accuracy, observed a higher value for cnb than for fnab.
3.9. Diagnostic Yields
No eligible articles reported the diagnostic yield for molecular predictive-marker studies by mutation analysis or fluorescence in situ hybridization. None of the eligible studies quantified the amount of tumor in cores, the cellularity of smears or other cytologic preparations, or the availability of a cell block. Immunohistochemistry was not used as a standard of practice in any of the studies.
3.10. Complications
All eleven eligible papers reported complication rates for the two procedures (Table v). The main complications of fnab and cnb were pneumothorax and pulmonary hemorrhage. The needle sizes were 20G–22G for fnab and 14G–21.5G for cnb in the four prospective studies 17–19,22. In two prospective studies that compared fnab and cnb, no statistical difference was found for pneumothorax rates between the two procedures 17,18. The pulmonary hemorrhage rate was significantly higher in cnb than in fnab in the study by Laurent et al. (28.6% vs. 13.6%) 18, but not in the study by Cheong et al. 17. A very low rate of mild hemoptysis occurred in the Laurent et al. study, with no statistically significant difference between fnab and cnb (2.4% vs. 4.1%) 18. As noted earlier, inconsistencies were apparent in the data reported in that study. In the remaining two prospective studies, fnab and cnb were performed at the same visit in each patient, and so procedures that resulted in complications could not be separately identified 19,22. Schubert et al. 19 reported that no major complications occurred during or after the two procedures in 85 patients. Moulton et al. 22 reported pneumothorax in 13 patients (11.4%) and self-limiting hemoptysis or perilesional hemorrhage (or both) in 2.6%. Based on that information, complication rates did not appear to be higher when two procedures (compared with a single procedure) were performed on an individual; however, no independent statistical comparison could be done.
In seven non-prospective studies, needle sizes were 20G–25G for fnab and 18G–20G for cnb 12–16,20,21. The pneumothorax rates were 0.0%–35.1% for fnab and 0.0%–28.6% for cnb. Anderson et al. 21 and Lourenco et al. 20 reported that, compared with fnab, cnb had a lower pneumothorax rate. No study showed a significant difference for rates of pulmonary hemorrhage and hemoptysis between the two procedures. The highest pulmonary hemorrhage rate was 25.4% (35 patients), which occurred in one study in which fnab and cnb were performed on the same patients 16. In addition to the most common complications of pneumothorax, pulmonary hemorrhage, hemoptysis, and subcutaneous hematoma reported in the eligible studies, Staroselsky et al. 13 also reported 5 patients (2.7%) with chest pain, successfully treated with analgesics.
4. DISCUSSION
The data identified in this systematic review are limited and inconsistent. As described in Tables i and v and discussed in the Results section, the studies differed considerably in terms of study design, patient population, lesion sizes, method of procedure guidance, and exact procedure technique. Those differences complicate interpretation and comparison of the reported data. Meta-analyses could not therefore be performed.
Overall, the quality of the eligible studies in this systematic review was poor, both in design and reporting (Table ii):
Five of eleven studies recruited some patients with chest wall, mediastinal, or pleural lesions. Inclusion of these patients could have the effect of widening the quoted range of sensitivity for fnab because, when compared with lung cancer, some thoracic lesions are less amenable to diagnosis by fnab.
A properly designed comparative-accuracy systematic review of diagnostic studies should be based on a fully paired design (that is, fnab and cnb are both performed in each patient) or a randomized design (that is, patients are assigned randomly to fnab or cnb) 10. However, in this systematic review, a fully paired study is not a good design. If cnb is performed just after fnab, fnab may be used to determine whether the outer needle is within the lesional tissue, thus influencing diagnostic yields and overestimating the diagnostic characteristics of cnb. Such a pairing happened in four of five studies 13,15,16,19 (Table i). In the fifth study, fnab was usually done first, but not always 22. A randomized design should be the best for the current research questions. In the Cheong et al. study 17, the choice of needles was randomized. Laurent et al. 18 assigned 125 consecutive patients into the fnab group during the first 21 months and 96 patients into the cnb group during the next 15 months of the study 18 (quasi-random allocation). In the other four studies 12,14,20,21, the diagnostic characteristics of fnab were estimated in one set of patients, and the diagnostic characteristics of cnb were estimated in a different set of non-overlapping or only partially overlapping patients. Indirect comparisons of this kind are prone to selection bias 10.
The assessors of the fnab and cnb outcomes should not know the final diagnosis from the reference standard 23. In the present review, we regarded histologic confirmation or clinical follow-up as the reference standard, and thus, the fnab and cnb assessors were blinded to the reference standard by the very nature of the four prospective studies 17–19,22. The other seven studies did not address this blinding issue.
The assessors of fnab and cnb outcomes should also be blinded to each other 23, but of five studies in which the two procedures were performed on the same patient, only one clearly stated that the cytologic and histologic evaluations were performed separately by different pathologists 16.
Ideally, the reference standard should be interpreted not knowing the index test results 23. However, none of the studies included in the present systematic review discussed that issue.
All patients received verification, but in some patients who lacked histologic confirmation, the diagnosis was confirmed by clinical follow-up. It is impossible to confirm a specific histologic diagnosis by clinical follow-up.
The data suggest that fnab and cnb have similar overall diagnostic values and that, compared with fnab, cnb might have higher specificity (to diagnose benign lesions) and accuracy for specific diagnosis (probably because of the higher specificity). If an on-site cytopathologist is available, fnab might be marginally more sensitive than cnb in diagnosing lung malignancy. These are preliminary data that, to be validated, require further study.
No available evidence suggests that, compared with fnab, cnb leads to a higher rate of pneumothorax or hemoptysis, even though the needle used for cnb is typically larger. The combination of fnab and cnb performed in an individual patient may improve the diagnostic parameters without increasing the rate of complications, but statistical support for that hypothesis is lacking. The fnab and cnb procedures both appear to be safe: no serious complications were reported in the eleven studies included in this analysis. However, in four studies in which patients were not randomized and not subject to both procedures, the selection criteria for the use of cnb or fnab were not mentioned 12,14,20,21. The lack of randomization might have created a selection bias, because some characteristics of patients or lesions (comorbidities, lesion size, or distance to pleura) influence the likelihood of complications.
Among the eleven eligible studies, only two were published after 2005 19,20, and one of them did not report a reference standard 20. The techniques both for performing and for analyzing fnab and cnb samples improve over time. Hence, the evidence from the medical literature presented here may not accurately reflect current clinical practice.
5. CONCLUSIONS
The evidence is insufficient to determine whether fnab, cnb, or some combination thereof should be the standard of care for diagnosing lung malignancies in patients with a lung lesion. The best technique in a given diagnostic centre may in part be determined by the local availability of resources and expertise in biopsy technique and sample interpretation. Given that new diagnostic information derived from immunohistochemistry and molecular biology are necessary for optimal treatment in lung cancer patients, and given that both techniques have evolved since 2000, well-designed, good-quality studies to compare fnab with cnb should be encouraged.
6. ACKNOWLEDGMENTS
The present work was supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario. The authors thank Dr. Dan Mozeg for comments on an early draft of this manuscript.
Footnotes
7. CONFLICT OF INTEREST DISCLOSURES
No author has a financial conflict of interest with respect to this project.
8. REFERENCES
- 1.Egleston BL, Meireles SI, Flieder DB, Clapper ML. Population-based trends in lung cancer incidence in women. Semin Oncol. 2009;36:506–15. doi: 10.1053/j.seminoncol.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society’s Steering Committee on Cancer Statistics . Canadian Cancer Statistics 2011: Featuring Colorectal Cancer. Toronto, ON: Canadian Cancer Society; 2011. [Available online at: http://www.cancer.ca/Canada-wide/About%20cancer/~/media/CCS/Canada%20wide/Files%20List/English%20files%20heading/PDF%20-%20Policy%20-%20Canadian%20Cancer%20Statistics%20-%20English/Canadian%20Cancer%20Statistics%202011%20-%20English.ashx; cited December 12, 2011] [Google Scholar]
- 3.Reck M, von Pawel J, Zatloukal P, et al. Phase iii trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: avail. J Clin Oncol. 2009;27:1227–34. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 4.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer—molecular and clinical predictors of outcome. N Engl J Med. 2005;353:133–44. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest. 2003;123(suppl 1):115S–28S. doi: 10.1378/chest.123.1_suppl.115S. [DOI] [PubMed] [Google Scholar]
- 6.Detterbeck FC, Rivera MP. Reliability of needle biopsy of pulmonary nodules to assess the presence of cancer. In: Detterbeck FC, Rivera MP, Socinski MA, Rosenman JG, editors. Diagnosis and Treatment of Lung Cancer: An Evidence-Based Guide for the Practicing Clinician. Philadelphia, PA: WB Saunders; 2001. p. 57. [Google Scholar]
- 7.Wallace MJ, Krishnamurthy S, Broemeling LD, et al. ct-guided percutaneous fine-needle aspiration biopsy of small (< or =1-cm) pulmonary lesions. Radiology. 2002;225:823–8. doi: 10.1148/radiol.2253011465. [DOI] [PubMed] [Google Scholar]
- 8.Milman N. Percutaneous lung biopsy with a fine bore cutting needle (Vacu-Cut): improved results using drill technique. Thorax. 1995;50:560–2. doi: 10.1136/thx.50.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schauer DA, Linton OW. NCRP Report No. 160, Ionizing Radiation Exposure of the Population of the United States, medical exposure—are we doing less with more, and is there a role for health physicists? Health Phys. 2009;97:1–5. doi: 10.1097/01.HP.0000356672.44380.b7. [DOI] [PubMed] [Google Scholar]
- 10.Reitsma JB, Rutjes AWS, Whiting P, Vlassov VV, Leeflang MMG, Deeks JJ. Chapter 9. Assessing methodological quality. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The Cochrane Collaboration; 2009. Ver. 1.0.0. [Available online at: http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/ch09_Oct09.pdf; cited December 9, 2011] [Google Scholar]
- 11.Bossuyt PM, Leeflang MM. Chapter 6. Developing criteria for including studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy. The Cochrane Collaboration; Sep, 2008. Ver. 0.4. [Available online at: http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/Chapter06-Including-Studies%20%28September-2008%29.pdf; cited December 9, 2011] [Google Scholar]
- 12.Arakawa H, Nakajima Y, Kurihara Y, Niimi H, Ishikawa T. ct-guided transthoracic needle biopsy: a comparison between automated biopsy gun and fine needle aspiration. Clin Radiol. 1996;51:503–6. doi: 10.1016/S0009-9260(96)80191-7. [DOI] [PubMed] [Google Scholar]
- 13.Staroselsky AN, Schwarz Y, Man A, Marmur S, Greif J. Additional information from percutaneous cutting needle biopsy following fine-needle aspiration in the diagnosis of chest lesions. Chest. 1998;113:1522–5. doi: 10.1378/chest.113.6.1522. [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Hatabu H, Takenaka D, Imai M, Ohbayashi C, Sugimura K. Transthoracic ct-guided biopsy with multiplanar reconstruction image improves diagnostic accuracy of solitary pulmonary nodules. Eur J Radiol. 2004;51:160–8. doi: 10.1016/S0720-048X(03)00216-X. [DOI] [PubMed] [Google Scholar]
- 15.Sagar P, Gulati M, Gupta SK, et al. Ultrasound-guided transthoracic co-axial biopsy of thoracic mass lesions. Acta Radiol. 2000;41:529–32. doi: 10.1080/028418500127346117. [DOI] [PubMed] [Google Scholar]
- 16.Yamagami T, Iida S, Kato T, Tanaka O, Nishimura T. Combining fine-needle aspiration and core biopsy under ct fluoroscopy guidance: a better way to treat patients with lung nodules? Am J Roentgenol. 2003;180:811–15. doi: 10.2214/ajr.180.3.1800811. [DOI] [PubMed] [Google Scholar]
- 17.Cheong WY, Thomas A, Chee SG, Tan KP. Percutaneous lung aspiration biopsy: a comparison between two fine needles. Australas Radiol. 1992;36:112–14. doi: 10.1111/j.1440-1673.1992.tb03093.x. [DOI] [PubMed] [Google Scholar]
- 18.Laurent F, Latrabe V, Vergier B, Michel P. Percutaneous ct-guided biopsy of the lung: comparison between aspiration and automated cutting needles using a coaxial technique. Cardiovasc Intervent Radiol. 2000;23:266–72. doi: 10.1007/s002700010067. [DOI] [PubMed] [Google Scholar]
- 19.Schubert P, Wright CA, Louw M, et al. Ultrasound-assisted transthoracic biopsy: cells or sections? Diagn Cytopathol. 2005;33:233–7. doi: 10.1002/dc.20342. [DOI] [PubMed] [Google Scholar]
- 20.Lourenço R, Camacho R, Barata MJ, Canário D, Gaspar A, Cyrne C. ct-guided percutaneous transthoracic biopsy in the evaluation of undetermined pulmonary lesions [English and Portuguese] Rev Port Pneumol. 2006;12:503–24. [PubMed] [Google Scholar]
- 21.Anderson JM, Murchison J, Patel D. ct-guided lung biopsy: factors influencing diagnostic yield and complication rate. Clin Radiol. 2003;58:791–7. doi: 10.1016/S0009-9260(03)00221-6. [DOI] [PubMed] [Google Scholar]
- 22.Moulton JS, Moore PT. Coaxial percutaneous biopsy technique with automated biopsy devices: value in improving accuracy and negative predictive value. Radiology. 1993;186:515–22. doi: 10.1148/radiology.186.2.8421758. [DOI] [PubMed] [Google Scholar]
- 23.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149:889–97. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]