Abstract
Background
This open-label phase i study with an accelerated titration design was performed to determine the maximum tolerated dose of BI 2536, a potent, highly selective small-molecule polo-like kinase 1 (Plk1) inhibitor.
Methods
Patients with advanced solid tumours received a single 60-minute intravenous infusion of BI 2536 (50–70 mg) on days 1–3 of each 21-day treatment course. Recipients without disease progression or untenable toxicity could receive additional treatment courses. The maximum tolerated dose was determined based on dose-limiting toxicities. Other assessments included safety, pharmacokinetic profile, and antitumour activity according to the Response Evaluation Criteria in Solid Tumors.
Results
The study enrolled 21 patients. The maximum tolerated dose for BI 2536 was determined to be 60 mg for the study schedule. Dose-limiting toxicities included hematologic events, hypertension, elevated liver enzymes, and fatigue. The most frequently reported drug-related adverse events were mild-to-moderate fatigue, leukopenia, constipation, nausea, mucosal inflammation, anorexia, and alopecia. The pharmacokinetics of BI 2536 were linear within the dose range tested. Plasma concentration profiles exhibited multi-compartmental pharmacokinetic behaviour, with a terminal elimination half-life of 20–30 hours.
Conclusions
In the present study, BI 2536 showed an acceptable safety profile warranting further investigation of Plk1 inhibitors in this patient population.
Keywords: Polo-like kinase, Plk1 inhibitor, BI 2536, phase i, dose escalation, solid tumours
1. INTRODUCTION
New therapies targeting the cell cycle offer an attractive potential cancer-treatment option. Polo-like kinases (Plks) control the mitotic entry of proliferating cells and are important regulators of mitotic progression 1,2. Plk1, the most extensively characterized mammalian Plk, has specific functions attributed to mitosis, ensuring that mitotic entry, centrosome maturation and separation, formation of the bipolar spindle, metaphase-to-anaphase transition, and initiation of cytokinesis progress in an orderly fashion 3,4.
Plk1 represents an attractive selective target for drug development because it is specifically active during mitosis and appears to have no activity in nondividing cells 5. Furthermore, Plk1 is overexpressed in various human cancers, including non-small-cell lung cancer (nsclc) 6 and colorectal cancer 7, and it is associated with poor prognosis in those patient populations 1. A number of Plk1 inhibitors or inhibitors of modulators of the Plk1 pathway are currently in early clinical development 8–10.
The dihydropteridinone BI 2536 is a potent and highly selective small-molecule Plk1 inhibitor, with selectivity at a factor of more than 1000 against a large panel of other kinases and a half-maximal inhibitory concentration of 0.83 nmol/L 11. Compared with established antimitotic agents, such as vinca alkaloids or taxanes, which bind directly to structural components of the spindle, BI 2536 has a very different mode of action 5. Preclinical studies showed that depletion of Plk1 by small interfering rna is associated with mitotic arrest, typified by dumbbell-shaped chromatin organization, and apoptosis; this phenotype is known as a “polo arrest” 12. Similarly, in preclinical studies, tumour cells treated with BI 2536 arrested in prometaphase, contained aberrant mitotic spindles, and subsequently entered apoptosis 11,13. Although efficacy was demonstrated in various murine models 11, local tolerability prohibited comprehensive scheduling experiments in vivo. Thus, target ranges for plasma concentration and duration of Plk1 inhibition required for antitumour activity could not be optimized in that setting.
A two-part, first-in-humans study was conducted to determine the maximum tolerated dose (mtd) and the safety profile of BI 2536 in humans. Because optimal plasma levels and the area under the curve for target inhibition and antitumour efficacy had not been established in mouse models, the trial also investigated various dosing schedules of BI 2536. Results from the first part of the study, in which the mtd of BI 2536 administered as a 1-hour intravenous infusion on day 1 of each 3-week treatment cycle was determined to be 200 mg, have shown some antitumour activity. The relatively limited side effects shown by BI 2536 can be largely attributed to the effect of BI 2536 on highly proliferating cells such as hematopoietic precursors 14.
We hypothesized that increasing the number of administrations of BI 2536 at lower single doses would allow for an increase in the total dose given per course, resulting in improved tolerability and antitumour efficacy compared with the 3-week treatment schedule used in the first part of the trial. Here, we report the findings of the second part of the first-in-humans, phase i, open-label, dose-escalation study in which the schedule of BI 2536, with 1-hour infusions at 50–70 mg on 3 consecutive days every 3 weeks, was investigated in patients with advanced solid tumours.
2. OBJECTIVES
The primary objective of the present study was to determine the mtd of BI 2536 when administered as 1-hour infusions on 3 consecutive days in patients with advanced solid tumours. Secondary objectives were evaluations of the safety, efficacy, and pharmacokinetics of BI 2536.
2.1. Methods
2.1.1. Study Design
The 21 patients in the part of the study reported here were enrolled between July 28, 2005, and April 21, 2006, at one clinical site in Germany. An accelerated-titration dose-escalation design was used, with 100% dose increments until the first reports of grade 2 drug-related toxicity according to the Common Terminology Criteria for Adverse Events (ctcae), with smaller dose increments thereafter. This trial design is a modification of the method described by Eisenhauer et al. 15. Each treatment cohort consisted of 3–6 patients. The mtd was defined as the highest dose at which not more than 1 of 6 patients experienced dose-limiting toxicities (dlt) in the first treatment course.
The two parts of this study investigated two different BI 2536 dosing schedules. The first part, already reported 14, was a dose escalation study of BI 2536 administered as a 1-hour infusion on day 1 of each treatment course, with a starting single intravenous dose of 25 mg BI 2536. The second part, reported here, aimed to determine whether BI 2536 administration on consecutive days could improve tolerability and efficacy. The starting dose for this second schedule was chosen based on the mtd of 200 mg observed in the first treatment schedule of a single dose delivered by 1-hour infusion and by taking into account the pharmacokinetic profile observed in patients who had received that schedule. Patients received single doses of BI 2536, starting at 50 mg, on days 1, 2, and 3 of a treatment course until the mtd was determined as previously described. For both dosing schedules, each treatment course spanned 21 days. Patients who reached day 21 without experiencing disease progression or excessive toxicity were eligible to receive additional courses of BI 2536 treatment. Patients who had experienced a dlt could be retreated at a lower dose if they experienced benefit from therapy and had recovered from the adverse event or events.
2.1.2. Study Population
Adult patients with a confirmed diagnosis of advanced nonresectable or metastatic solid tumours (or both), evaluable tumour deposits, and an Eastern Cooperative Oncology Group performance score of 0–2 were included in the study. Patients with known brain metastases, active infectious disease, or a serious illness thought to interfere with the protocol, were excluded from the study. Full patient eligibility criteria were previously reported 14.
The trial was carried out in compliance with the study protocol and the principles laid down in the Declaration of Helsinki (1996 version), and in accordance with the International Conference on Harmonisation tripartite Guideline for Good Clinical Practice and with applicable regulatory requirements. Written informed consent was obtained from each patient before participation in the study.
2.2. Concomitant Medications
Concomitant medications were given as clinically necessary. Symptomatic treatment of adverse events or tumour-associated symptoms were allowed. Additional chemotherapy, immunotherapy, and hormone or radiation therapy were not permitted during the study.
2.3. Efficacy Assessments
Objective response was assessed by tumour measurements evaluated using the Response Evaluation Criteria in Solid Tumors 16. Patients were assessed at screening and then at the end of every other treatment course.
2.4. Safety and Tolerability Assessments
Adverse events according to the ctcae (version 3.0), laboratory evaluations, electrocardiography, patient performance, physical examination, and vital signs were all used to determine safety. Adverse events whose onset fell within 21 days after the start of BI 2536 administration were considered to have occurred on treatment. A dlt was defined as a drug-related ctcae grade 3 or 4 nonhematologic toxicity (except for reversible emesis or diarrhea) or a drug-related ctcae grade 4 neutropenia for 7 or more days or a complicated infection or a grade 4 hematologic toxicity other than neutropenia.
2.5. Pharmacokinetic Sampling and Data Analysis
Blood samples for the evaluation of pharmacokinetic parameters were collected during and after the intravenous infusion at several time points up to 216 hours after the first drug administration. Plasma concentrations of BI 2536 were determined by high-performance liquid chromatography coupled to tandem mass spectrometry at Boehringer Ingelheim Pharma, Biberach, Germany.
2.6. Statistical Analyses
Safety, efficacy, and pharmacokinetic characteristics were analyzed in an exploratory and descriptive manner. Non-compartmental pharmacokinetic parameters were determined using WinNonlin (Pharsight, Sunnyvale, CA, U.S.A.) or another validated software program in all patients who received at least 1 dose of BI 2536 (“treated set”).
3. RESULTS
3.1. Patient Population
A total of 21 patients received BI 2536 on days 1–3 of a 3-week treatment schedule. Table i summarizes patient demographics and clinical characteristics. Patients had received a median of 2 prior chemotherapy regimens (range: 0–8 regimens). All but 1 patient received at least 1 treatment course; Table ii presents the disposition of the study patients.
TABLE I.
Patient demographics and characteristics, treated set
| Characteristic | Value |
|---|---|
| Patients (n) | 21 |
| Sex (n) | |
| Men | 12 |
| Women | 9 |
| Age (years) | |
| Median | 61 |
| Range | 33–75 |
| ecog performance status (n) | |
| 0 | 15 |
| 1 | 4 |
| 2 | 2 |
| Cancer type (n) | |
| Colorectal | 6 |
| Melanoma | 3 |
| Liver and biliary tree | 2 |
| Ovary/fallopian tube | 2 |
| Other | 8 |
| Prior anticancer therapy (n) | |
| Surgery | 21 |
| Curative | 20 |
| Non-curative | 4 |
| Radiotherapy | 11 |
| Chemotherapy | 16 |
| Lines of prior chemotherapy (n) | |
| Hormone therapy | 1 |
| Immunotherapy | 8 |
ecog = Eastern Cooperative Oncology Group.
TABLE II.
Disposition of patients
| Variable |
BI 2536 dose group |
|||
|---|---|---|---|---|
| 50 mg | 60 mg | 70 mg | Overall | |
| Patients (n) | ||||
| Enrolled | 7 | 12 | 2 | 21 |
| Treated | 7 | 12 | 2 | 21 |
| Completed first course | 6 | 12 | 2 | 20 |
| Courses initiated (n) | ||||
| Median | 3 | 2 | 2 | 2 |
| Range | 1–8 | 1–10 | 1–2 | 1–10 |
| Reason for trial termination (n) | ||||
| Worsening disease | 6 | 8 | 2 | 16 |
| Adverse eventa | 0 | 3 | 0 | 3 |
| Consent withdrawn | 1 | 1 | 0 | 2 |
Including drug-related.
3.2. Safety and Tolerability
Although the trial protocol allowed for dose escalation in 100% increments, smaller increases in dose were agreed upon by both the investigator and the sponsor for safety reasons; in the previous part of the trial, hematologic side effects increased rapidly once a cumulative dose of 200 mg had been reached. The median number of completed courses was 2 (range: 1–10 courses), and the median period of exposure was 48 days (range: 24–220 days).
Table iii lists the dlts that occurred during treatment course 1. After 50 mg was demonstrated to be tolerable, both patients treated at the next higher BI 2536 dose of 70 mg experienced dlts during the first treatment course. Of 6 patients who were then treated at the intermediate BI 2536 dose of 60 mg, none experienced a dlt during the first treatment course. Thus, the mtd for BI 2536 was determined to be 60 mg when administered as a 1-hour infusion on days 1–3 of a 3-week treatment schedule. Additional patients were enrolled to ensure that 12 patients were treated at the mtd. One patient experienced grade 3 fatigue during treatment course 1.
TABLE III.
Patients with dose-limiting toxicities, treatment course 1
| Dose group |
Adverse event |
|
|---|---|---|
| Type | Grade | |
| 50 mg | ↑ Alanine aminotransferase | 3 |
| 60 mg | Aggravated fatigue | 3 |
| 70 mg | Thrombocytopenia | 4 |
| Neutropenic fever | 4 | |
| 70 mg | Hematochezia | 3 |
| Thrombocytopenia | 4 | |
| Anemia | 4 | |
| Enterocolitis | 3 | |
Two dlts not taking place during treatment course 1 were reported. One patient experienced grade 3 exacerbated hypertension during treatment course 2, and one patient experienced grade 3 exacerbated hypertension during treatment course 10. Both of these patients were receiving BI 2536 at the 60-mg dose.
In summary, 6 patients experienced dlts during the course of the study. Hematologic dlts occurred in 2 patients; hypertension, in 2 patients; reversible elevated liver enzymes, in 1 patient; and fatigue that qualified as a dlt, in 1 patient.
During the study, all patients experienced at least 1 adverse event. Table iv summarizes the most frequently reported drug-related adverse events. Of the 21 treated patients, 19 (90%) experienced a drug-related adverse event. The most frequently reported drug-related adverse events were fatigue (62%), leukopenia (38%), constipation (29%), nausea (29%), mucosal inflammation (29%), anorexia (29%), neutropenia (29%), and alopecia (33%). With regard to hematologic adverse events, drug-related neutropenia was reported in 6 patients (29%); drug-related leukopenia, in 8 patients (38%); and drug-related anemia, in 3 patients (14%).
TABLE IV.
Most frequently reported drug-related adverse events (>10% of patients) during the study, treated set
| Variable |
BI 2536 dose group |
|||
|---|---|---|---|---|
| 50 mg | 60 mg | 70 mg | Overall | |
| Patients [n (%)] | ||||
| Enrolled | 7 (100) | 12 (100) | 2 (100) | 21 (100) |
| With related adverse events | 5 (71.4) | 12 (100) | 2 (100) | 19 (90.5) |
| Disorder [n (%)] | ||||
| Blood and lymphatic system | 0 | 7 (58.3) | 2 (100) | 9 (42.9) |
| Anemia | 0 | 1 (8.3) | 2 (100) | 3 (14.3) |
| Leukopenia | 0 | 6 (50) | 2 (100) | 8 (38.1) |
| Neutropenia | 0 | 6 (50) | 0 | 6 (28.6) |
| Gastrointestinal | 4 (57.1) | 7 (58.3) | 1 (50.0) | 12 (57.1) |
| Constipation | 1 (14.3) | 5 (41.7) | 0 | 6 (28.6) |
| Nausea | 2 (28.6) | 4 (33.3) | 0 | 6 (28.6) |
| Vomiting | 2 (28.6) | 1 (8.3) | 0 | 3 (14.3) |
| General and administration site | 3 (42.9) | 9 (75.0) | 1 (50.0) | 13 (61.9) |
| Fatigue | 3 (42.9) | 9 (75.0) | 1 (50.0) | 13 (61.9) |
| Mucosal inflammation | 2 (28.6) | 4 (33.3) | 0 | 6 (28.6) |
| Investigationsa | 3 (42.9) | 3 (25.0) | 1 (50.0) | 7 (33.3) |
| ↓ Hemoglobin | 2 (28.6) | 3 (25.0) | 0 | 5 (23.8) |
| ↑ Alanine aminotransferase | 1 (14.3) | 0 | 0 | 1 (4.8) |
| ↓ Weight | 0 | 0 | 1 (50.0) | 1 (4.8) |
| Metabolism and nutrition | 3 (42.9) | 3 (25.0) | 0 | 6 (28.6) |
| Anorexia | 3 (42.9) | 3 (25.0) | 0 | 6 (28.6) |
| Skin and subcutaneous tissue | 4 (57.1) | 4 (33.3) | 0 | 8 (38.1) |
| Alopecia | 3 (42.9) | 4 (33.3) | 0 | 7 (33.3) |
| Vascular | 1 (14.3) | 3 (25.0) | 0 | 4 (19.0) |
| Phlebitis | 1 (14.3) | 2 (16.7) | 0 | 3 (14.3) |
As defined by the Medical Dictionary for Regulatory Activities (MedDRA MSSO, Chantilly, VA, U.S.A.).
Table v lists the number of patients with drug-related ctcae grade 3 or 4 adverse events. Those events included grade 3 or 4 leukopenia in 8 patients, grade 3 or 4 neutropenia in 6 patients, and grade 3 or 4 febrile neutropenia in 2 patients. The listed grades 3 and 4 events include those that occurred in 2 patients who reported dlts when treated with BI 2536 70 mg.
TABLE V.
Patients with drug-related Common Terminology Criteria for Adverse Events grade 3 or 4 adverse events
| Variable |
Adverse event grade |
|
|---|---|---|
| 3 | 4 | |
| Patients [n (%)] | ||
| Enrolled | 21 (100.0) | 21 (100.0) |
| With related adverse events | 2 (9.5) | 8 (38.1) |
| Disorder [n (%)] | ||
| Leukopenia | 5 (23.8) | 3 (14.3) |
| Neutropenia | 0 (0.0) | 6 (28.6) |
| Anemia | 1 (4.8) | 1 (4.8) |
| Febrile neutropenia | 1 (4.8) | 1 (4.8) |
| Thrombocytopenia | 0 (0.0) | 2 (9.5) |
| Hypertension | 2 (9.5) | 0 (0.0) |
| Enterocolitis | 1 (4.8) | 0 (0.0) |
| Hematochezia | 1 (4.8) | 0 (0.0) |
| Fatigue | 1 (4.8) | 0 (0.0) |
| ↑ Alanine aminotransferase | 1 (4.8) | 0 (0.0) |
One case (4.8%) of a grade 3 increase in alanine aminotransferase and one case (4.8%) of a grade 2 increase in aspartate aminotransferase were reported; both were reported as drug-related. No grade 4 abnormalities were reported.
Adverse events leading to treatment discontinuation were reported in 3 patients. BI 2536 was discontinued in 2 patients because of treatment-related adverse events (grade 2 fatigue, grade 2 anemia, and grade 3 fatigue) and in 1 patient because of adverse events that were deemed not to be treatment-related (grade 3 nausea and vomiting). Serious adverse events were reported during the on-treatment period in 12 patients (57%), and 3 patients experienced treatment-related serious adverse events—specifically, 1 case of constipation requiring hospitalization (at the 60-mg dose) and 2 cases of hematologic serious adverse events (both at the 70-mg dose) with anemia, febrile neutropenia, and thrombocytopenia requiring hospitalization, one with hematochezia. During the on-treatment period, 2 patients died because of adverse events; in both of those patients, disease progression was reported as an adverse event.
3.3. Efficacy
No objective responses (partial or complete) were reported. Stable disease was recorded in 8 patients (38%) who received the day 1–3 dosing schedule; of those 8 patients, 6 remained progression-free for more than 3 months. Among the 12 patients treated at the mtd (60 mg), 4 (33%) experienced stable disease.
3.4. Pharmacokinetics
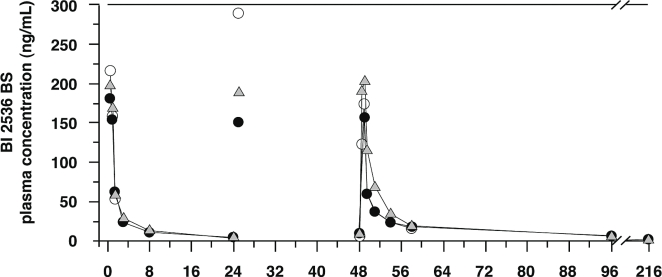
Table vi shows the pharmacokinetic parameters of BI 2536 for days 1–3. Figure 1 depicts individual geometric mean, dose-normalized drug plasma concentration–time profiles.
TABLE VI.
Geometric mean (gMean) and geometric coefficient of variation (gCV%) non-compartmental pharmacokinetic parameters of BI 2536 after 1-hour intravenous infusion, course 1
| Variable |
Dose groups, day 1 |
Dose groups, day 3 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
50 mg |
60 mg |
70 mg |
50 mg |
60 mg |
70 mg |
|||||||
| gMean | gCV% | gMean | gCV% | gMean | gCV% | gMean | gCV% | gMean | gCV% | gMean | gCV% | |
| Patients (n) | 7 | 11 | 2 | 6 | 9 | 2 | ||||||
| auc0–24 (ng·h/mL) | 467 | 29.6 | 509 | 30.1 | 459 | 21.4 | 669 | 51.0 | 921 | 42.9 | 637 | 11.9 |
| auc0–24,norm [(ng·h/mL)/mg] | 9.34 | 29.6 | 8.48 | 30.1 | 6.56 | 21.4 | 13.4 | 51.0 | 15.3 | 42.9 | 9.10 | 11.9 |
| Cmax (ng/mL) | 231 | 48.8 | 215 | 28.4 | 182 | 27.6 | 234 | 19.6 | 288 | 51.7 | 163 | 10.9 |
| Cmax,norm [(ng/mL)/mg] | 4.63 | 48.8 | 3.58 | 28.4 | 2.60 | 27.6 | 4.69 | 19.6 | 4.81 | 51.7 | 2.33 | 10.9 |
| t1/2 (h) | 8.79 | 32.8 | 9.17 | 30.3 | 10.3 | 25.7 | 25.2 | 36.6 | 38.9 | 23.9 | 32.5 | 33.7 |
| Clearance (mL/min) | 1590 | 27.8 | 1720 | 27.9 | 2180 | 27.4 | 747 | 51.3 | 640 | 32.2 | 956 | 27.1 |
| Vss (L) | 751 | 52.4 | 922 | 47.8 | 1310 | 4.91 | 1260 | 61.9 | 1350 | 53.5 | 2200 | 5.93 |
auc = area under the curve; norm = dose-normalized; Vss = volume of distribution.
FIGURE 1.
Concentration–time profiles of geometric mean plasma drug levels on days 1 and 3 after 1-hour intravenous infusions of BI 2536, course 1. Open circles = 50 mg, day 1, n = 7, day 2, n = 7, day 3, n = 6; shaded triangle = 60 mg, day 1, n =11, day 2, n = 9, day 3, n = 9; filled circles = 70 mg, day 1, n = 2, day 2, n = 2, day 3, n = 2.
BI 2536 exhibited multi-compartmental pharmacokinetic behaviour: after infusion, a fast disposition phase was followed by a slower elimination phase. BI 2536 has a high volume of distribution (>750 L), suggesting extensive distribution into deeper compartments, resulting in a terminal elimination half-life of 20–30 hours. In addition, BI 2536 can be considered a high-clearance drug, having clearance values parallelling the hepatic blood flow. The pharmacokinetics of BI 2536 increased in a linear fashion with increasing doses. Comparison of the pharmacokinetic parameters on days 1 and 3 showed a slight increase in the 24-hour exposure on day 3 compared with day 1. By contrast, the maximum plasma concentration was in the same range on both days. Because of the limited number of patients in each dose group, no statistical evaluation was performed.
4. DISCUSSION
This phase i open-label dose-escalation study was designed to determine the mtd, safety, efficacy, and pharmacokinetics of two BI 2536 dosing schedules in patients with advanced solid tumours. The mtd of BI 2536 when administered on day 1 of a 21-day treatment course was 200 mg, and full data are published elsewhere 14. The results from the day 1–3 treatment schedule administered over a 21-day treatment course are discussed here. The mtd of BI 2536 administered according to the latter schedule was found to be 60 mg (total dose: 180 mg per course). Splitting the total dose into smaller portions (as reported here) does not allow for an increase in the total dose determined for the day 1 schedule.
With regard to safety profile, BI 2536 was generally tolerable and could be administered without cumulative toxicity or neurotoxicity. The frequency and types of adverse events observed with the schedule reported here were similar to those observed in the previously investigated schedule 14, with the most frequently reported adverse events being neutropenia and gastrointestinal events. Only hematologic adverse events were thought to be mechanism-related. The most relevant treatment-related effect, neutropenia, can be attributed to the transient inhibition of bone marrow precursor cell proliferation. Given the pharmacologic profile of BI 2536, those adverse events were expected, and they were fully reversible. Clinical investigation of other available antimitotic therapies, such as docetaxel or vinorelbine, shows that neurologic and hematologic side effects are the principal adverse events 17–19. Data from the present study suggest that BI 2536 is not associated with relevant neurotoxicity, possibly because Plk1 is active during mitosis and may therefore be specific to dividing cells only.
No objective response or substantial tumour regression was observed in this patient population (Table i). Pharmacokinetic evaluation showed that BI 2536 exhibits multi-compartmental pharmacokinetic behaviour. Because BI 2536 distributes into a very large volume, exceeding the total body water, and clearance parallels liver blood flow, the terminal elimination half-life of BI 2536 represents re-distribution from deeper tissue compartments rather than clearance by drug-metabolizing enzymes.
In parallel with the present study, BI 2536 was also investigated in another phase i repeated dose-escalation study in patients with advanced solid tumours 20. In that study, patients received intravenous infusions of BI 2536 on days 1 and 8 of a 3-week treatment course 20. The mtd for the latter dosing schedule was defined as 200 mg (100 mg on day 1 and 100 mg on day 8). Notably, the toxicity profile for that schedule was similar to the profiles observed for the day 1 and the day 1–3 dosing schedules. Given that the cumulative dose, overall safety, and presumed pharmacodynamic effects (hematotoxicity) were similar for all three schedules, safety appears to be a consequence of the total dose administered [which results in similar total drug exposures (area under the curve)], rather than of the maximum plasma concentration.
Although a number of other compounds that target the Plk pathway are under investigation 8–10, BI 2536 is the first selective member of the Plk1 inhibitor class to have begun clinical trials, wherein it has been evaluated in the treatment of patients with solid tumours, including metastatic or advanced pancreatic cancer 21, prostate cancer 22, and nsclc [as monotherapy 23 and in combination with pemetrexed 24]. Although antitumour efficacy has been noted in individual patients in these clinical trials, overall antitumour activity—in terms of response rate, duration of response, clinical benefit, and progression-free survival—was disappointing. Given those data, further development of the compound in those tumour types was not considered warranted.
BI 2536 has also been investigated in patients with acute myeloid leukemia (aml) 25,26. Analyses of hematopoietic precursor cells taken from patients with aml provide evidence for target inhibition in vivo. In a study in aml patients, BI 2536 was shown to induce mitotic arrest and apoptosis in bone marrow precursors from treated patients. That finding indicates that the levels of neutropenia seen in patients receiving treatment with BI 2536 may be associated with, and be an indirect marker for, target inhibition 25.
There is a substantial need for validated biomarkers to identify patients most likely to benefit from Plk1 inhibition. Work to identify suitable biomarkers is ongoing, but no candidates have yet been validated. In a genome-wide rna interference screen to identify synthetic lethal interactions with the KRAS oncogene, it was observed that Ras-mutant cells were particularly susceptible to BI 2536 inhibition of Plk1 27. However, in another preclinical study, BI 2536 was found to block the proliferation of Ras-mutant cell lines from a variety of tissue origins in a way comparable to that in which it blocked proliferation of wild-type cell lines 11. Given those contrasting observations, patients in clinical studies of Plk1 inhibitors have not so far been selected for treatment based on Ras mutation status.
Volasertib (BI 6727), a dihydropteridinone derivative and the current focus of Plk1 inhibitor clinical development, has an improved pharmacokinetic profile compared with that for BI 2536 28,29. Volasertib exhibits increased tissue penetration and a correspondingly prolonged terminal half-life; it may therefore exert a greater effect on proliferating tumour cells than BI 2536 does. Volasertib has been investigated in a phase i study 30 and is currently undergoing phase ii investigation in nsclc and other tumour types.
5. CONCLUSIONS
The data reported here demonstrate an acceptable safety profile without cumulative toxicity for BI 2536 administered on 3 consecutive days in patients with advanced solid tumours.
6. ACKNOWLEDGMENTS
This study was supported by Boehringer Ingelheim. The authors acknowledge the editorial assistance of Ogilvy Healthworld. Boehringer Ingelheim provided financial support for that assistance. The authors thank Renate Angerer and Brigitte Häring (Klinik für Tumorbiologie, Freiburg) for support with administration and documentation.
Footnotes
7. CONFLICT OF INTEREST DISCLOSURES
RK, DT, and GM are employees of Boehringer Ingelheim.
8. REFERENCES
- 1.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–30. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 2.Barr FA, Silljé HH, Nigg EA. Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol. 2004;5:429–40. doi: 10.1038/nrm1401. [DOI] [PubMed] [Google Scholar]
- 3.Eckerdt F, Yuan J, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene. 2005;24:267–76. doi: 10.1038/sj.onc.1208273. [DOI] [PubMed] [Google Scholar]
- 4.Yap TA, Molife LR, Blagden SP, de Bono S. Targeting cell cycle kinases and kinesins in anticancer drug development. Expert Opin Drug Discov. 2007;2:539–60. doi: 10.1517/17460441.2.4.539. [DOI] [PubMed] [Google Scholar]
- 5.Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted antimitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. doi: 10.1038/nrc2049. [DOI] [PubMed] [Google Scholar]
- 6.Wolf G, Elez R, Doermer A, et al. Prognostic significance of polo-like kinase (Plk) expression in non-small cell lung cancer. Oncogene. 1997;14:543–9. doi: 10.1038/sj.onc.1200862. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi T, Sano B, Nagata T, et al. Polo-like kinase 1 (Plk1) is overexpressed in primary colorectal cancers. Cancer Sci. 2003;94:148–52. doi: 10.1111/j.1349-7006.2003.tb01411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland LL, Taylor C, Pilkington DL, Cohen JL, Von Hoff DD. A phase i pharmacokinetic study of HMN-214, a novel oral stilbene derivative with polo-like kinase-1–interacting properties, in patients with advanced solid tumors. Clin Cancer Res. 2006;12:5182–9. doi: 10.1158/1078-0432.CCR-06-0214. [DOI] [PubMed] [Google Scholar]
- 9.Jimeno A, Li J, Messersmith WA, et al. Phase i study of ON 01910.Na, a novel modulator of the polo-like kinase 1 pathway, in adult patients with solid tumors. J Clin Oncol. 2008;26:5504–10. doi: 10.1200/JCO.2008.17.9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olmos D, Allred A, Sharma R, et al. Phase i first-in-human study of the polo-like kinase-1 selective inhibitor, GSK461364, in patients with advanced solid tumors [abstract 3536] J Clin Oncol. 2009. p. 27. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=31639; cited December 6, 2011]
- 11.Steegmaier M, Hoffmann M, Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–22. doi: 10.1016/j.cub.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Erikson RL. Polo-like kinase (Plk) 1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A. 2003;100:5789–94. doi: 10.1073/pnas.1031523100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lénárt P, Petronczki M, Steegmaier M, et al. The small-molecule inhibitor BI 2536 reveals novel insights into mitotic roles of polo-like kinase 1. Curr Biol. 2007;17:304–15. doi: 10.1016/j.cub.2006.12.046. [DOI] [PubMed] [Google Scholar]
- 14.Mross K, Frost A, Steinbild S, et al. Phase i dose escalation and pharmacokinetic study of BI 2536, a novel polo-like kinase 1 inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2008;26:5511–17. doi: 10.1200/JCO.2008.16.1547. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase i clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–92. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase iii trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000;18:2354–62. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt M, Bastians H. Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updat. 2007;10:162–81. doi: 10.1016/j.drup.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 20.Hofheinz RD, Al-Batran SE, Hochhaus A, et al. An open-label, phase i study of the polo-like kinase-1 inhibitor, BI 2536, in patients with advanced solid tumors. Clin Cancer Res. 2010;16:4666–74. doi: 10.1158/1078-0432.CCR-10-0318. [DOI] [PubMed] [Google Scholar]
- 21.Mross K, Dittrich C, Aulitzky W, et al. A randomized phase ii trial of the novel polo-like kinase 1 inhibitor BI 2536 in chemonaïve patients with unresectable advanced pancreatic cancer—a study in cooperation with the cesar network of investigators [abstract 493P and poster] Ann Oncol. 2008;19(suppl 8):viii163. [Google Scholar]
- 22.Pandha HS, Protheroe A, Wylie J, et al. An open label phase ii trial of BI 2536, a novel Plk1 inhibitor, in patients with metastatic hormone refractory prostate cancer (hrpc) [abstract 14547] J Clin Oncol. 2008;26 [ http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=35269; cited December 6, 2011] [Google Scholar]
- 23.Sebastian M, Reck M, Waller CF, et al. The efficacy and safety of BI 2536, a novel Plk-1 inhibitor, in patients with stage iiib/iv non-small cell lung cancer who had relapsed after, or failed, chemotherapy: results from an open-label, randomized phase ii clinical trial. J Thorac Oncol. 2010;5:1060–7. doi: 10.1097/JTO.0b013e3181d95dd4. [DOI] [PubMed] [Google Scholar]
- 24.Ellis PM, Chu QS, Leighl NB, et al. A phase i dose escalation trial of BI 2536, a novel Plk1 inhibitor, with standard dose pemetrexed in previously treated advanced or metastatic non-small cell lung cancer (nsclc) [abstract 8115] J Clin Oncol. 2008;26 [ http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=55&abstractID=34557; cited December 6, 2011] [Google Scholar]
- 25.Lee KH, Schlenk RF, Bug G, et al. Polo-like kinase-1 (Plk-1) inhibitor BI 2536 induces mitotic arrest and apoptosis in vivo: first demonstration of target inhibition in the bone marrow of aml patients [abstract 2641] Blood. 2008;112 doi: 10.1182/blood-2008-02-141689. [Available online at: http://ash.confex.com/ash/2008/webprogram/Paper12116.html; cited December 6, 2011] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Müller–Tidow C, Bug G, Schlenk R, et al. Phase i/ii study of BI 2536, an intravenous polo-like kinase-1 (Plk-1) inhibitor, in elderly patients with relapsed or refractory acute myeloid leukemia (aml): first results of a multi-center trial [abstract 2973] Blood. 2008;112 [Available online at: http://ash.confex.com/ash/2008/webprogram/Paper11727.html; cited December 6, 2011] [Google Scholar]
- 27.Luo J, Emanuele MJ, Li D, et al. A genome-wide rnai screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137:835–48. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolph D, Steegmaier M, Hoffmann M, et al. BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–102. doi: 10.1158/1078-0432.CCR-08-2445. [DOI] [PubMed] [Google Scholar]
- 29.Schöffski P. Polo-like kinase (Plk) inhibitors in preclinical and early clinical development in oncology. Oncologist. 2009;14:559–70. doi: 10.1634/theoncologist.2009-0010. [DOI] [PubMed] [Google Scholar]
- 30.Gil T, Schöffski P, Awada A, et al. Final analysis of a phase i single dose-escalation study of the novel polo-like kinase 1 inhibitor BI 6727 in patients with advanced solid tumors [abstract 3061] J Clin Oncol. 2010;28 [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=74&abstractID=52463; cited December 6, 2011] [Google Scholar]