Abstract
Purpose
Platinum-based regimens represent the standard first-line treatment for non-small-cell lung cancer (nsclc). However, newer data have established a role for pemetrexed in the treatment of this disease. Such data suggest that histology represents a determining factor in the selection of treatment.
Methods
We undertook a systematic review of the literature for randomized controlled trials that compared the efficacy of pemetrexed with that of other treatments in advanced nsclc. Data and study quality were assessed according to published guidelines.
Results
We identified five trials that compared pemetrexed with other treatments or with placebo. Overall survival for patients treated with pemetrexed was superior to that with other treatments: hazard ratio (hr): 0.89; 95% confidence interval (ci): 0.80 to 0.99. The survival benefit was limited to patients with non-squamous histology: hr: 0.82; 95% ci: 0.73 to 0.91. Pemetrexed was inferior to other chemotherapy options in patients with squamous histology: hr: 1.19; 95% ci: 0.99 to 1.43.
Conclusions
Compared with other chemotherapy agents, pemetrexed is more effective for the treatment of nsclc in patients with non-squamous histology.
Keywords: nsclc, meta-analysis, pemetrexed
1. INTRODUCTION
For both men and women with cancer, lung cancer is the leading cause of mortality 1, and non-small-cell lung cancer (nsclc) represents more than 80% of all lung cancer cases. Although nsclc includes many histologic subtypes, those subtypes can be broadly categorized as squamous and non-squamous 2.
A large proportion of patients with lung cancer present with locally advanced or metastatic disease. Modest improvements in the survival of patients with advanced or metastatic nsclc have been achieved though the use of chemotherapy compared with best supportive care. A platinum-based doublet is considered the standard first-line treatment in advanced nsclc 2. Available data suggest that several platinum-based regimens are all similarly efficacious 3. Several options—including docetaxel 4, pemetrexed 5, and erlotinib 6—are available for subsequent lines of therapy, with no one treatment being considered superior.
Pemetrexed is structurally similar to other antifolates such as methotrexate, but unlike methotrexate, which is chiefly a dihydrofolate reductase inhibitor, pemetrexed chiefly targets thymidylate synthase (ts), which gives it a different spectrum of activity 7. Pemetrexed has favorable pharmacokinetic profile, and its major toxicity has been myelosuppression, which has been abrogated with dietary folate supplementation and additional vitamin B12.
Traditionally, the histologic subtype of nsclc has not influenced the choice of chemotherapy. However, recent data demonstrate that expression of ts, the primary target for pemetrexed, varies with the histologic subtype of nsclc 8. Expression of ts is reported to be highest in squamous cancers, and clinical data suggest a differential response to pemetrexed based on histologic subtype, supporting the latter biologic finding. We therefore undertook a systematic review and meta-analysis of the available evidence on the efficacy of pemetrexed compared with other chemotherapeutic agents as first- or second-line treatment in advanced nsclc. The meta-analysis included a planned subgroup analysis to examine the differential effect of pemetrexed according to histologic subtype of nsclc.
2. METHODS
Our systematic review of the literature included a number of electronic databases—amed, Cochrane Database of Systematic Reviews, American College of Physicians Journal Club, Database of Abstracts of Reviews of Effectiveness, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Health Technology Assessment Database (Cochrane Library), Economic Evaluation Database (U.K. National Health Service), embase, Global Health, Ovid medline—and selected electronic proceedings of international conferences. Reference lists of articles were reviewed for completeness.
The literature search used the terms “exp carcinoma, non-small-cell lung,” “folic acid antagonists,” “pemetrexed,” “Alimta” (pemetrexed from Eli Lilly and Company, Indianapolis, IN, U.S.A.), “non small cell lung,” and “multitargeted antifolate.” We combined that search with a method developed to identify, with high sensitivity, citations of randomized control trials 9. The latter method used the terms “effect:,” “trial,” “random,” “control,” “investigat,” “experimental,” “double blind,” “compar,” “matched,” “blind,” “examine,” “study,” “comparative study,” “randomized controlled trial,” and “phase III”. No language restriction was imposed. The Ovid search engine (http://www.ovid.com) was used to conduct the search in the selected databases. The search was completed in the fourth week of January 2010. We also contacted Eli Lilly Canada and determined that no unpublished data relevant to the present review were available. We contacted the primary authors of the studies located in the literature search for any missing data regarding the hazard ratio (hr) for treatment effect. Trials published in abstract form were included, provided that relevant outcomes data were reported.
We included all randomized controlled trials that compared pemetrexed with another active treatment or with placebo, and that reported a survival outcome with a minimum follow up of 12 months. Trials were conducted exclusively in advanced nsclc (stages iii and iv) and were not evaluating a radiation treatment question. Information for the histologic subgroup analyses was extracted from the published data. The primary outcome of interest was overall survival, and not objective response rate, because the response rate does not always translate into a survival benefit 10.
2.1. Selection of Trials and Data Abstraction
The search, conducted by KA, yielded a total of 1062 citations. The initial title and abstract screen, conducted by KA and CQ, selected 100 citations for further evaluation and a possible full-text review. Five trials 11–15 fulfilled the selection criteria and were included in the final analysis. Trial quality assessment was conducted in accordance with the Cochrane handbook guidelines 16 and the Grading of Recommendations Assessment, Development and Evaluation Working Group guidelines 17. To avoid bias, the data were abstracted and assessed by two authors (KA, CQ), with any discrepancies being resolved by discussion.
2.2. Statistical Analysis
The analysis was conducted using the Review Manager software (RevMan, version 5.0: The Cochrane Collaboration, http://www.cochrane.org). A pooled analysis of the hrs, calculated from survival curve analyses, was conducted to assess any survival advantage for pemetrexed-based chemotherapy compared with other treatments 18. The crude log hr value and its standard error in each trial was calculated using a published tool 19. We assumed constant hazard for the two types of therapy within an individual trial in which the hr represents the instantaneous reduction in risk of death. A hr lower than unity indicates that the pemetrexed-based chemotherapy is better than the comparator treatment. This method is similar to a method previously published in the literature 20 in advanced nsclc.
Using this inverse hr method, data were compiled by RevMan using a random effects model to allow for between-study variations. A heterogeneity score was calculated using the Cochran Q and the I2 score to examine any possible heterogeneity between studies.
Given the differences in study design, a priori hypotheses were established to explore differences in the effectiveness of pemetrexed according to histology (squamous or non-squamous), line of therapy (first or second), and comparator arm (active treatment versus placebo). Adverse events were compared using the Mantel–Haenszel method, with a random effects model accounting for any variability between studies.
There was a plan to calculate publication bias using Begg funnel plots and the Egger test if 10 or more studies were identified, but given that just five studies were identified, publication bias was not calculated, the feeling being that this calculation is less informative with fewer papers 21.
3. RESULTS
Five studies met the inclusion criteria (Four studies compared pemetrexed with another treatment (three in first-line therapy and one in second-line therapy). One study compared pemetrexed as maintenance therapy with a placebo control arm. The study by Obasaju et al. 15 was a three-arm study. Only arms B and C (carboplatin, and pemetrexed with docetaxel respectively) were selected for analysis because enzastaurin was not considered a standard treatment for nsclc.
Table i sets out t he characteristics of t he five trials, which together contributed 3541 patients to the analysis. Outcomes data by histology were available for both squamous and non-squamous histology in three trials and for non-squamous histology in one study. In all the studies, more than 50% of the participants had been diagnosed with non-squamous histology, which is similar to the expected histology distribution.
TABLE I.
Studies included in the meta-analysis
| Reference | Pts (n) | Regimen | Remarks | Grade and quality |
|---|---|---|---|---|
| Hanna et al., 200411 | 288 | Docetaxel 75 mg/m2 every 21 days until disease progression (median number of cycles: 4) | Second line ps 0–2 |
Moderate No important study limitations Direct |
| 283 | Pemetrexed 500 mg/m2 every 21 days until disease progression (median number of cycles: 4) | No important imprecision Unlikely publication bias +++ |
||
| Scagliotti et al., 200812 | 863 | Cisplatin 75 mg/m2 on day 1 and gemcitabine 1250 mg/m2 on days 1 and 8 for 6 cycles | First line ps 0–1 |
Moderate-high Few important study limitations No important inconsistencies |
| 862 | Cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 on day 1 for 6 cycles | Direct No important imprecision Unlikely publication bias ++++ |
||
| Ciuleanu et al., 200914 | 441 | Pemetrexed 500 mg/m2 on day 1 every 21 days till disease progression (median number of cycles: 5) | Maintenance therapy ps 0–1 |
Moderate-high No important study limitations No important inconsistency |
| 222 | Placebo | Direct No important imprecision Possible publication bias (sponsor heavily involved) +++ |
||
| Grønberg et al., 200913 | 217 | Gemcitabine 1000 mg/m2 on days 1 and 8 plus carboplatin AUC 5 for 4 cycles | First line ps 0–2 |
Moderate-high Few important study limitations No important inconsistencies |
| 219 | Pemetrexed 500 mg/m2 plus carboplatin AUC 5 for 4 cycles | Direct No important imprecision Unlikely publication bias +++ |
||
| Obasaju et al., 200915 | 74 | Pemetrexed 500 mg/m2 and carboplatin AUC 6 every 3 weeks for 6 cycles | First line Abstract only 3-Arm trial |
Low Serious study limitations No important inconsistency |
| 72 | Docetaxel 75 mg/m2 and carboplatin AUC 6 every 3 weeks for 6 cycles | Direct Imprecision Unlikely publication bias + |
ps = Performance status.
Two studies warrant special mention. The first study, by Ciuleanu et al. 14, was labelled a maintenance pemetrexed study and would be best considered to be an early second-line versus placebo study in patients who did not progress after 4 cycles of cisplatin-based chemotherapy (that is, high-risk patients were eliminated). The placebo comparator might exaggerate the effect of the chemotherapy. The trial by Obasaju et al. had been published only in abstract form, with two reported endpoints at 12 and 24 months. We used arms B and C because those arms involved a comparison between pemetrexed and another standard treatment. The study did not include data on histologic subtype.
3.1. Overall Survival
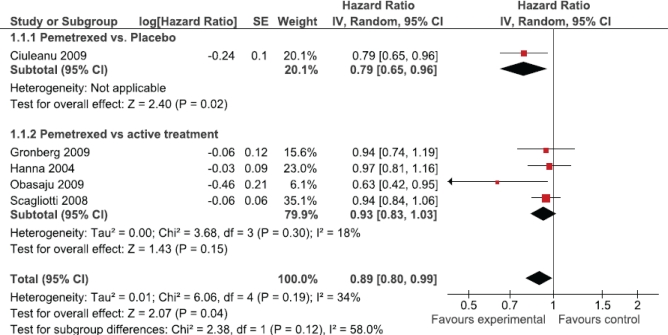
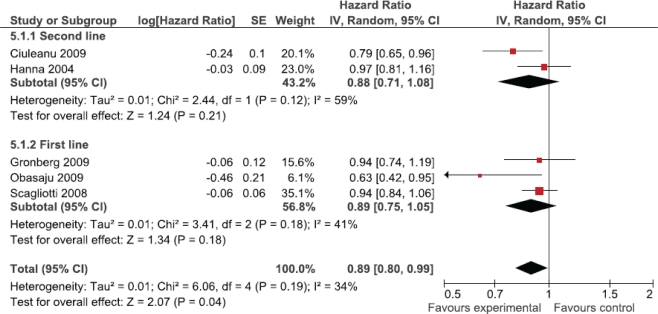
The primary analysis demonstrated an improvement in overall survival for patients treated with pemetrexed compared with either another treatment or placebo [hr: 0.89; 95% confidence interval (ci): 0.80 to 0.99; Figure 1]. Although we observed no statistically significant heterogeneity (p = 0.19), a priori sensitivity analyses were conducted to explore differences in effect between comparator arms (active treatment vs. placebo), line of treatment (first vs. second), and histology (non-squamous vs. squamous). If the trial of maintenance pemetrexed versus placebo was omitted, overall survival showed no significant improvement (pemetrexed vs. active treatment subgroup, hr: 0.93; 95% ci: 0.83 to 1.03; Figure 1). The hr for overall survival was similar whether pemetrexed was used as first- or second-line therapy (hr: 0.89 vs. 0.88; Figure 2).
FIGURE 1.
Overall effect of pemetrexed treatment.
FIGURE 2.
First-line compared with second-line pemetrexed.
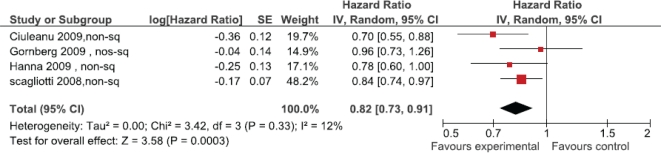
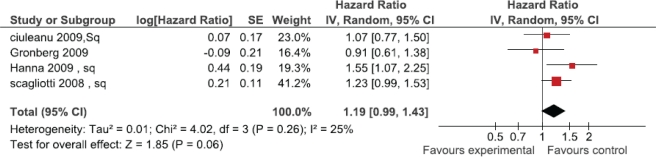
Survival outcomes based on histologic subtype were provided in four studies (Figure 3). A significant improvement in survival was observed for patients with non-squamous histology receiving pemetrexed (hr: 0.82; 95% ci: 0.73 to 0.91; I2 = 12%). Three studies reported survival for patients with squamous histology (Figure 4). There was a trend toward inferior survival for patients with squamous histology treated with pemetrexed (hr: 1.19; 95% ci: 0.99 to 1.43) that did not reach statistical significance.
FIGURE 3.
Pemetrexed in non-squamous histology.
FIGURE 4.
Pemetrexed in squamous histology.
3.2. Toxicity
We excluded the trial of Ciuleanu et al. from the toxicity analysis, because the comparator arm was placebo, which would have introduced significant heterogeneity into the analysis. The remaining studies all demonstrated fewer side effects for patients treated with pemetrexed. In particular, a lower rate of hematologic toxicity was observed in patients treated with pemetrexed. Significantly less neutropenia was observed [odds ratio (or): 0.41; 95% ci: 0.18 to 0.93], keeping in mind that all studies mandated vitamin B12 and folic acid supplementation for patients receiving pemetrexed. Additionally, more elevation of alanine aminotransferase was observed (or: 11.68; 95% ci: 0.64 to 212.19), although the confidence interval was wide and statistically nonsignificant. We observed no significant difference in the incidence of anemia for patients treated with pemetrexed (or: 1.36; 95% ci: 0.73 to 2.52).
4. DISCUSSION
This meta-analysis systemically examined the effect of pemetrexed on overall survival in patients with advanced nsclc. A significant improvement in overall survival was observed, but the effect was limited to patients with non-squamous histology. This finding is consistent with biologic data demonstrating differential expression of ts, the enzyme target of pemetrexed.
Our meta-analysis has some limitations, given that it used published and abstract data 22 and approximation methods for calculating hrs. This latter method may be less reliable than an analysis of individual patient data. Also, not all the included studies prospectively reported outcomes based on histology; the subgroup analysis is therefore susceptible to bias. However, such bias is less likely, given that the observed overall benefit in survival was driven mostly by the effect of histology. A similar finding was also made in a reanalysis of the combined results of two randomized trials 23 (also possibly subject to bias). Nevertheless, our literature review was exhaustive and provided the best reliable estimate of the effect.
Our findings suggest that, in patients with nonsquamous histology, pemetrexed in various combinations is superior to other chemotherapy regimens for the treatment of advanced nsclc. Patients with squamous cancer treated with pemetrexed appear to have inferior survival. Together, those results support the conclusion that histology is an important determinant in the selection of treatment options in advanced nsclc. Additionally, other treatment decisions—including the addition of bevacizumab to platinum-based chemotherapy 24—rely on appropriate histologic subtyping. Furthermore, molecular profiling is important in appropriately selecting treatment in patients with gene mutations, including those involving the epidermal growth factor receptor 25 and translocations of the EML4 and ALK genes 26.
These data highlight an important issue in the diagnosis of lung cancer. Establishment of the histologic subtype is a crucial factor in determining the most appropriate treatment option for patients with nsclc. Historically, however, treatment options for patients with lung cancer have largely rested on the distinction between small-cell lung cancer and nsclc. As a result, many lung cancer pathology diagnoses are limited to “nsclc not otherwise specified.” One important conclusion from these data is that it is critical to determine the histologic subtype when making a diagnosis of nsclc, with implications not only for the pathologists making the diagnosis, but also for the physicians obtaining the diagnostic material. Samples must be sufficient to establish a histologic diagnosis based on examination of hematoxylin and eosin staining, or alternatively, appropriate immunohistochemical testing that makes the distinction between the squamous and non-squamous histologies. It may no longer be appropriate to make a lung cancer diagnosis based on minimal cytology material obtained from bronchial washings or brushings.
5. ACKNOWLEDGMENT
The authors thank Diane Heels–Ansdell and Kristin Thorlund for statistical advice and Drs. Bjørn H. Grønberg and Coleman K. Obasaju for providing additional study information.
Footnotes
6. CONFLICT OF INTEREST DISCLOSURES
PE has received honoraria and research funding from Eli Lilly and Company. The remaining authors have no financial conflicts of interest to declare.
7. REFERENCES
- 1.Canadian Cancer Society’s Steering Committee . Canadian Cancer Statistics 2009. Toronto, ON: Canadian Cancer Society; 2009. pp. 13–14. [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Fort Washington, PA: NCCN; 2011. Ver. 2.2012. [Available online at: http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (free registration required); cited December 9, 2011] [Google Scholar]
- 3.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.De Marinis F, De Petris L. Pemetrexed in second-line treatment of non-small-cell lung cancer. Oncology (Williston Park) 2004;18(suppl 8):38–42. [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues Pereira J, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–32. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Jones RJ, Twelves CJ. Pemetrexed: a multitargeted antifolate (Alimta, LY-231514) Expert Rev Anticancer Ther. 2002;2:13–22. doi: 10.1586/14737140.2.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Calvert AH. Biochemical pharmacology of pemetrexed. Oncology (Williston Park) 2004;18(suppl 8):13–17. [PubMed] [Google Scholar]
- 9.McKibbon KA, Wilczynski NL, Haynes RB, on behalf of the Hedges Team Retrieving randomized controlled trials from medline: a comparison of 38 published search filters. Health Info Libr J. 2009;26:187–202. doi: 10.1111/j.1471-1842.2008.00827.x. [DOI] [PubMed] [Google Scholar]
- 10.George SL. Response rate as an endpoint in clinical trials. J Natl Cancer Inst. 2007;99:98–9. doi: 10.1093/jnci/djk024. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase iii trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–97. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 12.Scagliotti GV, Parikh P, von Pawel J, et al. Phase iii study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naïve patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–51. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 13.Grønberg BH, Bremnes RM, Fløtten O, et al. Phase iii study by the Norwegian Lung Cancer Study Group: pemetrexed plus carboplatin compared with gemcitabine plus carboplatin as first-line chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27:3217–24. doi: 10.1200/JCO.2008.20.9114. [DOI] [PubMed] [Google Scholar]
- 14.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–40. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 15.Obasaju CK, Raju RN, Stinchcombe T, et al. Final results of a randomized phase ii trial of pemetrexed (p) + carboplatin (cb) ± enzastaurin (e) versus docetaxel (d) + cb as first-line treatment of patients (pts) with stage iiib/iv non-small cell lung cancer (nsclc) [abstract 8037] J Clin Oncol. 2009. p. 27. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=65&abstractID=34353; cited December 7, 2011]
- 16.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, U.K: John Wiley and Sons; 2009. Ver. 5.0.2. [Google Scholar]
- 17.Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck–Ytter Y, Schünemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8. [Erratum in: Stat Med 2004;23:1817] [DOI] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-smallcell lung cancer. J Clin Oncol. 2004;22:3852–9. doi: 10.1200/JCO.2004.02.109. [DOI] [PubMed] [Google Scholar]
- 21.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. BMJ. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam VC, Hotte SJ. Consistency of phase iii clinical trial abstracts presented at an annual meeting of the American Society of Clinical Oncology compared with their subsequent full-text publications. J Clin Oncol. 2008;26:2205–11. doi: 10.1200/JCO.2007.14.6795. [DOI] [PubMed] [Google Scholar]
- 23.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to nsclc histology: a review of two phase iii studies. Oncologist. 2009;14:253–63. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 24.Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 25.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 26.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4–ALK. J Clin Oncol. 2009;27:4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]