Abstract
Background
While trouble sleeping is a common problem among women at mid-life, patterns in trouble sleeping relating to social and health-related risk factors are unclear. This analysis describes the dynamics of trouble sleeping among women at mid-life
Methods
The National Survey of Health and Development is a nationally representative study of births in 1946 in England, Scotland, and Wales followed up through mid-life. Multistate life table analysis utilised 893 women interviewed annually between ages 48 to 54 years.
Results
Women spent an average of 2.6 years with trouble sleeping, and the average length of a continuous episode of trouble sleeping was 2.4 years. Among women who reported at least one episode, the average number of episodes was 1.5. Health-related risk factors at age 43 of number of physical conditions, anxiety and depression symptoms, use of prescription medication, and current or past trouble sleeping were related to increased total and per episode duration of trouble sleeping over the seven year study interval and increased duration per episode. Differences associated with these risk factors ranged from 1.2 – 1.8 years for duration over the study interval and 0.5-0.8 years per episode. There was no association between average number of episodes per woman reporting at least one episode and these health-related risk factors at age 43.
Conclusions
This study provides support for association between increased duration of trouble sleeping, in total and per episode, and health risk factors at age 43, suggesting a long-term relationship between risk factors and sleep.
Keywords: sleep, multistate life tables, women’s health, duration of trouble sleeping
INTRODUCTION
Trouble sleeping is an indicator of health and well-being associated with poor daytime function, impaired psychomotor function, absenteeism from work, increased health care utilization,[1] industrial and motor vehicle accidents [2] and increased mortality.[3;4] Women disproportionately experience trouble sleeping compared to men from mid-life through later life, [5] with the sex ratio increasing from 1.4 to 1.7 after age 45.[6] An estimated 43% of women in the United Kingdom have experienced trouble sleeping.[7] Some studies have found that hormonal changes associated with menopause are related to trouble sleeping at mid-life [8-11] and may operate in part through vasomotor symptoms.[6;12-14] Alternatively, the sex difference in patterns of trouble sleeping may relate to greater exposure to social stress and more mental health problems at mid-life among women compared to men.[1]
There is little longitudinal evidence concerning predictors of the persistence and incidence of trouble sleeping [11;15-17] and the transition back to no trouble sleeping.[18] Because trouble sleeping is a health condition that may fluctuate, evidence over time is necessary to understand the dynamic nature of trouble sleeping. The objective of this paper is to describe patterns in trouble sleeping among women between the ages of 48 and 54 in relation to life course socioeconomic environment and health-related risk factors at age 43.
METHODS
Study population
The Medical Research Council National Survey of Health and Development (NSHD) is a longitudinal study of health of a cohort of 2547 women and 2815 men drawn in a social class-stratified, random sample of singleton births in England, Scotland, and Wales during one week in March 1946. During the ages of analysis, the cohort remained representative of the population born in Britain at the time.[19] Women study members responded to annual questionnaires between ages 47-54, and this analysis uses data between ages 48-54. By age 53, 6% of the original women cohort members had died, while 9% lived abroad, 3% were not traced, and 12% had refused to participate. Among the 1778 eligible women study members, 1572 returned at least one questionnaire. A total of 893 women provided complete information for the analysis. Ethical approval for this study was given by the North Thames Multicentre Research Committee.
Outcome: Trouble sleeping
The outcome of interest was whether the respondent reported bother from trouble sleeping in the past year for ages 48-54, for a total of seven waves. The category of “no trouble sleeping” is a combination of women who did not experience trouble sleeping and those who had experience but no bother. The category of “trouble sleeping” consists of women who reported a little or a lot of bother. [9] For those who responded to this question in 4 to 6 waves, missing values were imputed. If a year with missing data year fell between two years with the same response, that response was used for the missing year. Otherwise, the majority response of years with data was used for the missing year. In the case of an equal number of responses for sleep outcomes, “no trouble sleeping” was selected to replace the missing data. Trouble sleeping was imputed for 227 of the 893 women in the sample.
Risk Factors
The following categories of risk factors were selected for analysis because they were hypothesized to be associated with trouble sleeping or with other aspects of women’s health at mid-life: socioeconomic factors,[6;20] physical health,[1;6;18;21-24] mental health,[6;14;24] and health behaviours.[1;2;17;25]
Socioeconomic environment factors
Social class was categorised as non-manual or manual using the British Registrar General’s social class classification. Childhood social class was based on the father’s occupation when the cohort member was aged 4 years. Adult social class was derived on the basis of current or last occupation at 43 years. Educational level by age 26 was categorized as no qualifications, up to ordinary secondary qualifications (‘O’-levels usually attained at 16 years, and their training equivalents) advanced secondary qualifications (‘A’-levels, usually attained at 18 years, or degree level and their equivalents), and marital status at age 43 as single, married, widowed, separated, or divorced.
Health at Age 43
The Psychiatric Symptom Frequency Scale (PSF Scale) was used to assess symptoms relating to anxiety and depression.[26] The scale measured the frequency of problems in the past year according to 19 questions. Respondents reported number of current physical health conditions from a list of 27 conditions, including bronchitis, heart disease, diabetes, and severe headaches or migraine, for which they had seen medical professionals in the past year. The number of prescriptions used for the same list of conditions was also enumerated. Trouble sleeping status was derived from two questions. It indicated whether the respondent reported ever experiencing trouble sleeping at age 43 (past trouble sleeping) or experiencing the PSF Scale symptoms of trouble getting off to sleep or trouble with waking up and not being able to get back to sleep for a spell of a minimum of up to four months, once or twice a week, or three to ten times a month (current trouble sleeping). Past trouble sleeping was grouped with current trouble sleeping because few respondents reported only past trouble but not current trouble.
Health Behaviours at Age 43
Respondents reported number of prescription medications utilised. Exercise status was categorised as inactive, participation in vigorous activities once a week or less, or participation in vigorous activities more than once a week. Smoking status measured whether the respondent smoked for at least a year, regardless of number of cigarettes smoked, by age 43. Body mass index (weight/height2) was categorized as < 18.5, 18.5 – 24.9, 25-29.9, and 30 or greater. Alcohol consumption referred to 0 drinks/day (abstainers), 0.1-1.0 drinks/day (very light drinkers), 1.1-2.0 drinks/day (light drinkers), 2.1-4.0 drinks/day (moderate drinkers), and >4.1 drinks/day (heavy drinkers), and the last two groups were combined due to small numbers of respondents in each category[27].
Statistical and Demographic Analysis
Factors related to the distribution of number of waves in which trouble sleeping was reported between ages 48 and 54 were identified through a Kruskal-Wallis test for ordinal variables and a Wilcoxon-Mann-Whitney test for binary variables. Among those variables with significant relationships with distribution of number of waves of trouble sleeping, duration of trouble sleeping over the seven year study interval was investigated using multistate life table analysis with no absorbing state[28] (as cohort mortality was negligible during the study interval). To describe the nature of differences in total duration, duration per episode of trouble sleeping and average number of episodes of trouble sleeping per woman reporting at least one episode[29] were calculated. Duration of trouble sleeping is the sum of all years with trouble sleeping that women experienced on average, based on life table calculations. Duration per episode is a count of the consecutive years with trouble sleeping among all women, commencing either at the start of the study interval or when the respondent reported no trouble sleeping in the prior wave, and ending when there is a transition back to no trouble sleeping, divided by the number of episodes among all respondents. Average number of episodes of trouble sleeping per woman reporting at least one episode was calculated as the number of transitions from no trouble sleeping to trouble sleeping among all respondents plus the number of women who reported trouble sleeping in all waves, divided by the number of women who made at least one such transition plus the number of women who reported trouble sleeping in all waves. All life table measures demonstrate unadjusted relationships.
The model assumed that women’s sleep status changes were uniformly distributed events from age x to x + 1 and set the average age at transition between states to x + 0.5 for each value of x. Another key assumption was that reporting of trouble sleeping in the past year reflected status over the entire previous year, or in the case of a transition that it represented status for half of a year on average. Because the data were collected prospectively, values directly calculated from the data are reported, as opposed to implied values as in the case of life tables that use period data. Based on this method, the time interval under study is the six years that fall between the seven waves of data. STATA SE version 10 was utilized to determine distributions of number of waves with trouble sleeping by risk factor the data, and Microsoft Excel was used for the life table calculations.
RESULTS
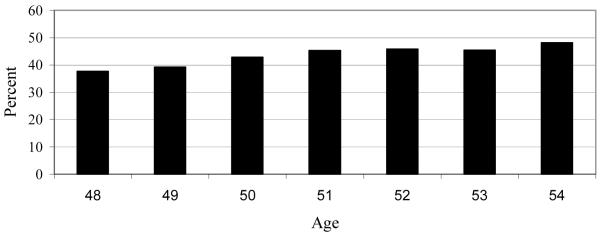
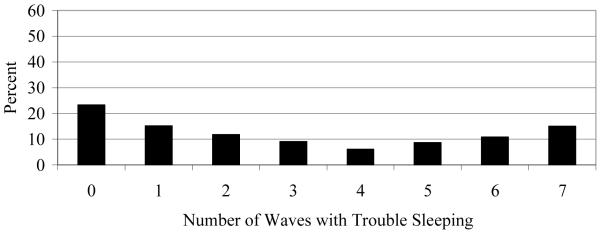
Figure 1 demonstrates that the prevalence of trouble sleeping rose slightly from 38% to 42% between ages 48 and 51 and then levelled around 45% between ages 52 and 54. As shown in Figure 2, across ages 48-54, 77% of women reported trouble sleeping during at least one wave, with 15% of women reporting trouble in every wave. Table 1 summarizes the relationships between the distributions of the number of waves of trouble sleeping and risk factor status. The table shows the distributions of trouble sleeping for those who reported trouble in 0 or 1 wave (combined), and those who reported trouble in 6 or 7 waves (combined). These values were selected because when the distribution shifted for significant risk factors, the changes came in the ends of the distributions, and not in the middle values. The relationships between responses of 3, 4, or 5 waves with sleep trouble and risk factors are not shown in Table 1.
Figure 1.
Percentage of Women Reporting Trouble Sleeping by age (N = 893)
Figure 2.
Percent Distribution of Waves with Trouble Sleeping, Age 48-54 (N= 893)
Table 1.
Unadjusted Relationships between Number of Waves of Trouble Sleeping (Age 48-54) and Life Course Risk Factors (N= 893)
| Variable (N) | Percentage reported trouble sleeping in 0 or 1 wave (Age 48- 54)* |
Percentage reported trouble sleeping in 6 or 7 Waves (Age 48- 54)* |
P value |
|---|---|---|---|
| Study Sample | 38.4 | 25.9 | - |
| Socioeconomic Factors | |||
| Childhood social class at age 4 | 0.14 | ||
| Manual (487) | 40.3 | 24.0 | |
| Non-manual (406) | 36.2 | 28.1 | |
| Adult social class at age 43 | 0.22 | ||
| Manual (224) | 36.6 | 29.5 | |
| Non-manual (669) | 39 | 24.7 | |
| Education at age 26 | 0.27 | ||
| None (293) | 39.9 | 25.6 | |
| Lower secondary level qualifications (324) | 40.4 | 21.9 | |
| Advanced secondary (219) | 34.7 | 29.7 | |
| Degree level or equivalent (57) | 33.3 | 35.1 | |
| Marital status at age 43 | 0.60 | ||
| Single (41) | 22.1 | 19.5 | |
| Married (737) | 38.1 | 26.1 | |
| Widowed (15) | 33.3 | 33.3 | |
| Separated (22) | 40.9 | 27.3 | |
| Divorced (78) | 34.6 | 25.6 | |
| Health at Age 43 | |||
| PSF Scale score (0 - 87) | <0.0001 | ||
| 0 to 4 (204) | 61.8 | 9.8 | |
| 5 to 9 (231) | 36.8 | 19.5 | |
| 10 to 16 (220) | 30.5 | 32.7 | |
| 17 to 87 (238) | 27.3 | 39.5 | |
| Number of physical conditions (0 to 9) | <0.0001 | ||
| 0 (401) | 45.1 | 21.0 | |
| 1 (282) | 34.8 | 27.3 | |
| 2 to 9 (210) | 30.5 | 33.3 | |
| Trouble sleeping status | <0.0001 | ||
| No (732) | 43.7 | 20.8 | |
| Yes (161) | 14.3 | 49.1 | |
| Health Behaviours at Age 43 | |||
| Use of prescription medication(0 - 7) | <0.0001 | ||
| 0 (497) | 43.3 | 21.9 | |
| 1 (256) | 32.4 | 29.3 | |
| 2 to 7 (140) | 32.1 | 33.6 | |
| Exercise at age 43 | 0.67 | ||
| Inactive (486) | 38.3 | 24.9 | |
| Vigorous < 1x/week (201) | 39.8 | 25.4 | |
| Vigorous > 1x/week (206) | 37.4 | 28.6 | |
| Smoking status by age 43 | 0.08 | ||
| Ever smoker (161) | 40.7 | 22.9 | |
| Never (732) | 36.1 | 28.9 | |
| Alcohol consumption in drinks/day | 0.56 | ||
| 0 (299) | 36.5 | 23.8 | |
| 0.1 - 1.0 (369) | 38.8 | 28.2 | |
| 1.1 - 2.0 (143) | 43.4 | 23.1 | |
| 2.1 or more (82) | 35.4 | 28.1 | |
| Body mass index | 0.61 | ||
| <18.5 (61) | 42.6 | 19.7 | |
| 18.5-24.9 (503) | 38.6 | 25.3 | |
| 25-29.9 (224) | 35.7 | 28.1 | |
| 30 or greater (105) | 41 | 27.6 |
Wilcoxon-Mann-Whitney test of differences in distributions used for binary exposure variables (social class at age 4 and age 43, history of trouble sleeping at age 43, and smoking status at age 43) and Kruskal-Wallis test of differences in distributions used for ordinal exposure variables (education at age 26, marital status at age 43, number of physical conditions at age 43, PSF Scale score at age 43, number of prescription medications at age 43, alcohol consumption at age 43, and body mass index at age 43). Distribution of respondents reporting 3,4, or 5 waves of trouble sleeping not shown because of consistency across risk factors. N = 893. P values correspond to the association between each exposure variable and trouble sleeping across all waves.
Row percentage
Women with higher PSF Scale scores at age 43, greater numbers of physical conditions at age 43, past or current trouble sleeping at age 43, and utilization of more prescription medications at age 43 were more likely to report higher numbers of waves with trouble sleeping between ages 48-54 than their counterparts. Social class during childhood and at age 43, educational attainment at age 26, marital status at age 43, exercise status at age 43, smoking status at age 43, alcohol consumption at age 43, and body mass index at age 43 were not related to number of waves of trouble sleeping between ages 48-54.
Table 2 shows duration of trouble sleeping, duration per episode, and average number of episodes per woman experiencing at least one episode for the risk factors that were significantly related to the frequency of reporting of trouble sleeping over age 48-54. Among the full sample, on average, each respondent was spent 2.6 years with trouble sleeping, with 2.4 years per episode, and each woman ever experiencing an episode had an average of 1.5 episodes. Women who reported higher scores on the PSF Scale at age 43, more physical conditions at age 43, who were experiencing trouble sleeping at age 43 or who had trouble sleeping at age 43, or who were taking more prescription medications at age 43 reported more years with trouble sleeping between ages 48-54. The highest duration was 4.0 years for women who reported past or current trouble sleeping at age 43, and the lowest duration was 1.5 years for a PSF Scale Score of 0 to 4. The highest and lowest levels of duration per episode were also associated with past or current trouble sleeping at age 43 (2.9 years) and a PSF Scale Score of 0 to 4 (1.9 years), respectively. While duration per episode also rose with increased levels of these risk factors, the average number of transitions remained relatively constant around 1.4. Differences in duration of trouble sleeping over the six year interval reflect the experience by women with certain risk factors of more consecutive years of trouble sleeping, as opposed to years accumulated over more frequent, shorter episodes.
Table 2.
Total Duration of Trouble Sleeping, Duration per Episode of Trouble Sleeping, and Average Number of Episodes per Woman Experiencing at Least One Episode, by Risk Factor Status (N=893)*
| Total duration of trouble sleeping (Years) |
Duration per episode of trouble sleeping (Years) |
Average number of episodes per woman experiencing at least one episode |
|
|---|---|---|---|
| Study Sample | 2.6 | 2.4 | 1.5 |
| Health at Age 43 | |||
| PSF Scale score (0 – 87) | |||
| 0 to 4 (204) | 1.5 | 1.9 | 1.4 |
| 5 to 9 (231) | 2.5 | 2.1 | 1.5 |
| 10 to 16 (220) | 3.0 | 2.5 | 1.5 |
| 17 to 87 (238) | 3.3 | 2.7 | 1.4 |
| Number of physical conditions (0 to 9) |
|||
| 0 (401) | 2.3 | 2.2 | 1.3 |
| 1 (282) | 2.7 | 2.3 | 1.5 |
| 2 to 9 (210) | 3.1 | 2.7 | 1.4 |
| Trouble sleeping status | |||
| No (732) | 2.3 | 2.2 | 1.4 |
| Yes (161) | 4.0 | 2.9 | 1.5 |
|
Health Behaviours at Age 43 |
|||
| Use of prescription medication(0 - 7) |
|||
| 0 (497) | 2.4 | 2.2 | 1.4 |
| 1 (256) | 2.9 | 2.4 | 1.5 |
| 2 to 7 (140) | 3.0 | 2.8 | 1.4 |
unadjusted
DISCUSSION
Principal findings
Prospective data from a British birth cohort showed that trouble sleeping is a common problem at mid-life among women that is related to prior health status. Reporting of higher numbers of waves with trouble sleeping, increased duration of trouble sleeping between ages 48-54, and higher average number of episodes were related to increased number of physical conditions, increased symptoms of depression and anxiety, use of prescription medications, and reporting of past or current trouble sleeping at age 43. Number of transitions per woman who reported at least one transition was not related to these four health risk factors. Health behaviours at age 43 and socioeconomic characteristics were not related to any measure of trouble sleeping.
Strengths and limitations
To our knowledge, this analysis is the first to study the relationships between the outcome of trouble sleeping and prospective measures of risk factors using multistate life table measures of duration, duration per episode, and average number of episodes.
There are several limitations concerning the life table model. First, the model makes a Markovian assumption in that the current state does not depend on the previous ones.[30] As the results here and in other studies[15;22;23] have shown, history of trouble sleeping predicts current trouble sleeping. Second, there are no standard methods for testing statistical significance among differences in life table values. Third, the models do not efficiently account for time-dependent variables and are best used in this context to study the relationships between a longitudinal outcome and the status of risk factors at one point in time. Therefore, results from the multistate life table analysis are purely descriptive.
There are also limitations to the data. The survey questions may not accurately capture trouble sleeping, as this concept is difficult to measure.[6] While the analysis assumes that the response to trouble sleeping in the past year represents the entire year, it is possible that respondents experienced the designated status for only a small portion of the year. Sensitivity analyses of imputing using responses concerning sleep trouble in the past month, when available, produced similar results as with imputation using the existing responses concerning trouble sleeping in the past year in other waves. However, as we rely on a binary indicator of sleep trouble, we may miss any more nuanced differences. The self-report of trouble sleeping may be another limitation of the study. Nevertheless, self-assessed report remains important since such perceptions may prompt the decision to seek medical help.[31]
Implications and future research
Results suggest that total duration and duration per episode of trouble sleeping at mid-life are predicted by prior health status. Risk factors are related to lengthening of the amount of consecutive time spent with trouble sleeping, as opposed to altering the number of times one experiences trouble sleeping. Prevention of risk factors may decrease future trouble sleeping. As the number of episodes does not appear to change according to risk factor status, an alternative strategy might focus on techniques to relieve trouble sleeping once an episode has begun. Results for the relationships between increased duration and duration per episode of trouble sleeping and problems with physical health,[2] use of prescription medications,[1;18] depression and anxiety,[16;18;21] and past trouble sleeping,[15;22;23] are consistent with previous studies that used other measures of trouble sleeping. Although previous findings have suggested relationships between trouble sleeping and smoking[6] and being divorced, widowed, or separated,[2] this analysis did not find similar relationships. While no relationship between these measures of sleep trouble and socioeconomic status in childhood or adulthood was detected, previous evidence has been mixed concerning these associations.[6] The lack of relationships between trouble sleeping and alcohol use[1] and weight[17] in our study may be due to reverse causation, which other studies have suggested. Discrepancies in results may also relate to different definitions of and time frames of trouble sleeping and use of multivariate results.
Future work will utilize alternative methods to investigate the multivariate relationships between dynamics of trouble sleeping at mid-life and risk factors measured repeatedly between ages 48-54. The multistate life table analysis has demonstrated a relationship between increased duration of trouble sleeping, in total and per episode, and health risk factors at age 43, suggesting a long-term relationship between risk factors and sleep.
What is already known on this topic
Trouble sleeping is common among women at mid-life. Women who experience difficulty with physical and mental health have a higher prevalence and incidence of trouble sleeping at mid-life.
What this study adds
This study uses multistate life table analysis, a technique from demography to study transitions, to estimate the duration over 7 years and duration per episode of trouble sleeping and average number of episodes per woman who experienced at least one episode, in relationship to life course socioeconomic characteristics and health-related risk factors at age 43. Risk factors related to physical and mental health at age 43 were also related to these longitudinal measures of trouble sleeping at mid-life. Results suggest that trouble sleeping should be viewed as a dynamic process.
Acknowledgments
Funding: The Medical Research Council provided funding for the National Survey of Health and Development and financial support for GM and DK. Supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Conflict of interest
None
Contributors: ST conducted the analyses and wrote the draft. DK, JG and GM contributed to the design of the analysis, interpretation of results, and revised the paper.
Competing interests: None declared
Ethical approval: North Thames Multicentre Research Ethics Committee.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JECH and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://jmg.bmj.com/ifora/licence.pdf).
References
- 1.Soares CN. Insomnia in women: an overlooked epidemic? Arch.Womens Ment.Health. 2005;8:205–13. doi: 10.1007/s00737-005-0100-1. [DOI] [PubMed] [Google Scholar]
- 2.Roth T. Prevalence, associated risks, and treatment patterns of insomnia. J.Clin.Psychiatry. 2005;66(Suppl 9):10–3. [PubMed] [Google Scholar]
- 3.Qureshi AI, Giles WH, Croft JB, Bliwise DL. Habitual sleep patterns and risk for stroke and coronary heart disease: a 10-year follow-up from NHANES I. Neurology. 1997;48:904–11. doi: 10.1212/wnl.48.4.904. [DOI] [PubMed] [Google Scholar]
- 4.Ferrie JE, Shipley MJ, Cappuccio FP, Brunner E, Miller MA, Kumari M, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–66. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamberg L. Menopause not always to blame for sleep problems in midlife women. JAMA. 2007;297:1865–6. doi: 10.1001/jama.297.17.1865. [DOI] [PubMed] [Google Scholar]
- 6.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med.Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 7.Stewart R, Besset A, Bebbington P, Brugha T, Lindesay J, Jenkins R, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29:1391–7. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 8.Ford K, Sowers M, Crutchfield M, Wilson A, Jannausch M. A longitudinal study of the predictors of prevalence and severity of symptoms commonly associated with menopause. Menopause. 2005;12:308–17. doi: 10.1097/01.gme.0000163869.89878.d9. [DOI] [PubMed] [Google Scholar]
- 9.Kuh DL, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br.J.Obstet.Gynaecol. 1997;104:923–33. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- 10.Chung KF, Tang MK. Subjective sleep disturbance and its correlates in middle-aged Hong Kong Chinese women. Maturitas. 2006;53:396–404. doi: 10.1016/j.maturitas.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Dennerstein L, Dudley EC, Hopper JL, Guthrie JR, Burger HG. A prospective population-based study of menopausal symptoms. Obstet.Gynecol. 2000;96:351–8. doi: 10.1016/s0029-7844(00)00930-3. [DOI] [PubMed] [Google Scholar]
- 12.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women’s Health Study. J.Womens Health (Larchmt.) 2007;16:667–77. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 13.Freedman RR, Roehrs TA. Sleep disturbance in menopause. Menopause. 2007;14:826–9. doi: 10.1097/GME.0b013e3180321a22. [DOI] [PubMed] [Google Scholar]
- 14.Kravitz HM, Ganz PA, Bromberger J, Powell LH, Sutton-Tyrrell K, Meyer PM. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10:19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Ganguli M, Reynolds CF, Gilby JE. Prevalence and persistence of sleep complaints in a rural older community sample: the MoVIES project. J.Am.Geriatr.Soc. 1996;44:778–84. doi: 10.1111/j.1532-5415.1996.tb03733.x. [DOI] [PubMed] [Google Scholar]
- 16.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30:274–80. [PubMed] [Google Scholar]
- 17.Stranges S, Cappuccio FP, Kandala NB, Miller MA, Taggart FM, Kumari M, et al. Cross-sectional versus prospective associations of sleep duration with changes in relative weight and body fat distribution: the Whitehall II Study. Am.J.Epidemiol. 2008;167:321–9. doi: 10.1093/aje/kwm302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byles JE, Mishra GD, Harris MA. The experience of insomnia among older women. Sleep. 2005;28:972–9. doi: 10.1093/sleep/28.8.972. [DOI] [PubMed] [Google Scholar]
- 19.Wadsworth ME, Butterworth SL, Hardy RJ, Kuh DJ, Richards M, Langenberg C, et al. The life course prospective design: an example of benefits and problems associated with study longevity. Soc.Sci.Med. 2003;57:2193–205. doi: 10.1016/s0277-9536(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 20.Hardy R, Kuh D. Social and environmental conditions across the life course and age at menopause in a British birth cohort study. BJOG. 2005;112:346–54. doi: 10.1111/j.1471-0528.2004.00348.x. [DOI] [PubMed] [Google Scholar]
- 21.Bromberger JT, Meyer PM, Kravitz HM, Sommer B, Cordal A, Powell L, et al. Psychologic distress and natural menopause: a multiethnic community study. Am.J.Public Health. 2001;91:1435–42. doi: 10.2105/ajph.91.9.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennerstein L, Lehert P, Guthrie JR, Burger HG. Modeling women’s health during the menopausal transition: a longitudinal analysis. Menopause. 2007;14:53–62. doi: 10.1097/01.gme.0000229574.67376.ba. [DOI] [PubMed] [Google Scholar]
- 23.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch.Gen.Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 24.Owens JF, Matthews KA. Sleep disturbance in healthy middle-aged women. Maturitas. 1998;30:41–50. doi: 10.1016/s0378-5122(98)00039-5. [DOI] [PubMed] [Google Scholar]
- 25.Kuh DJ, Cooper C. Physical activity at 36 years: patterns and childhood predictors in a longitudinal study. J.Epidemiol.Community Health. 1992;46:114–9. doi: 10.1136/jech.46.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindelow M, Hardy R, Rodgers B. Development of a scale to measure symptoms of anxiety and depression in the general UK population: the psychiatric symptom frequency scale. J.Epidemiol.Community Health. 1997;51:549–57. doi: 10.1136/jech.51.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richards M, Hardy R, Wadsworth ME. Alcohol consumption and midlife cognitive change in the British 1946 birth cohort study. Alcohol. 2005;40:112–7. doi: 10.1093/alcalc/agh126. [DOI] [PubMed] [Google Scholar]
- 28.Paloni A. Increment-Decrement Life Tables. In: Preston S, Heuveline P, Guillot M, editors. Demography: Measuring and Modeling Population Processes. Blackwell; Malden: 2001. pp. 256–71. [Google Scholar]
- 29.Espenshade TJ, Braun RE. Life Course Analysis and Multistate Demography: An Application to Marriage, Divorce, and Remarriage. Journal of Marriage and the Family. 1982;44:1025–1036. [Google Scholar]
- 30.Schoen R. Practical uses of multistate population models. Annu.Rev.Sociol. 1988;14:341–61. doi: 10.1146/annurev.so.14.080188.002013. [DOI] [PubMed] [Google Scholar]
- 31.Mishra G, Kuh D. Perceived change in quality of life during the menopause. Soc.Sci.Med. 2006;62:92–103. doi: 10.1016/j.socscimed.2005.05.015. [DOI] [PubMed] [Google Scholar]