Abstract
We have synthesized a family of double-stranded cDNAs (ds cDNAs) using as a template the family of highly purified protamine mRNAs from rainbow trout testis. Individual pure protamine cDNA components were isolated by cloning this family of protamine ds cDNAs in a plasmid vector (pMB9). Clones containing protamine sequences were characterized by restriction mapping and by a positive hybrid-selected translation assay, which allowed us to correlate particular cDNAs with particular protein components. To allow more detailed comparisons, complete nucleotide sequences were determined for selected protamine clones. We have detected at least 5 distinctly different coding sequences, which nevertheless show at least 82% homology, and which have probably arisen by repeated gene duplication. These very highly conserved coding sequences do however contain a distinctly variable region near the 5'-end of the mRNA (N-terminus of the protein), corresponding to the major sites of serine phosphorylation. Since the amino acid sequences predicted by our DNA sequences were slightly different from those previously published (1), we have independently determined the amino acid sequences of protamine components CI, CII, CIII from our own source of trout testis. These new peptide sequences are completely consistent with those predicted by our nucleotide sequences. The 3'-untranslated regions of the protamine mRNAs are, surprisingly almost as highly conserved as the coding regions. Both coding and 3'-noncoding portions appear to be under a similar degree of selective pressure and evolutionary constraint to remain constant.
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Watanabe S. A new method for fractionation of protamines and the amino acid sequences of salmine and three components of iridine. Int J Protein Res. 1969;1(3):221–224. doi: 10.1111/j.1399-3011.1969.tb01646.x. [DOI] [PubMed] [Google Scholar]
- Bailey G. S., Cocks G. T., Wilson A. C. Gene duplication in fishes: malate dehydrogenases of salmon and trout. Biochem Biophys Res Commun. 1969 Mar 10;34(5):605–612. doi: 10.1016/0006-291x(69)90781-5. [DOI] [PubMed] [Google Scholar]
- Black J. A., Dixon G. H. Evolution of protamine: a further example of partial gene duplication. Nature. 1967 Oct 14;216(5111):152–154. doi: 10.1038/216152a0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brenner M., Tisdale D., Loomis W. F., Jr Techniques for rapid biochemical screening of large numbers of cell clones. Exp Cell Res. 1975 Feb;90(2):249–252. doi: 10.1016/0014-4827(75)90313-4. [DOI] [PubMed] [Google Scholar]
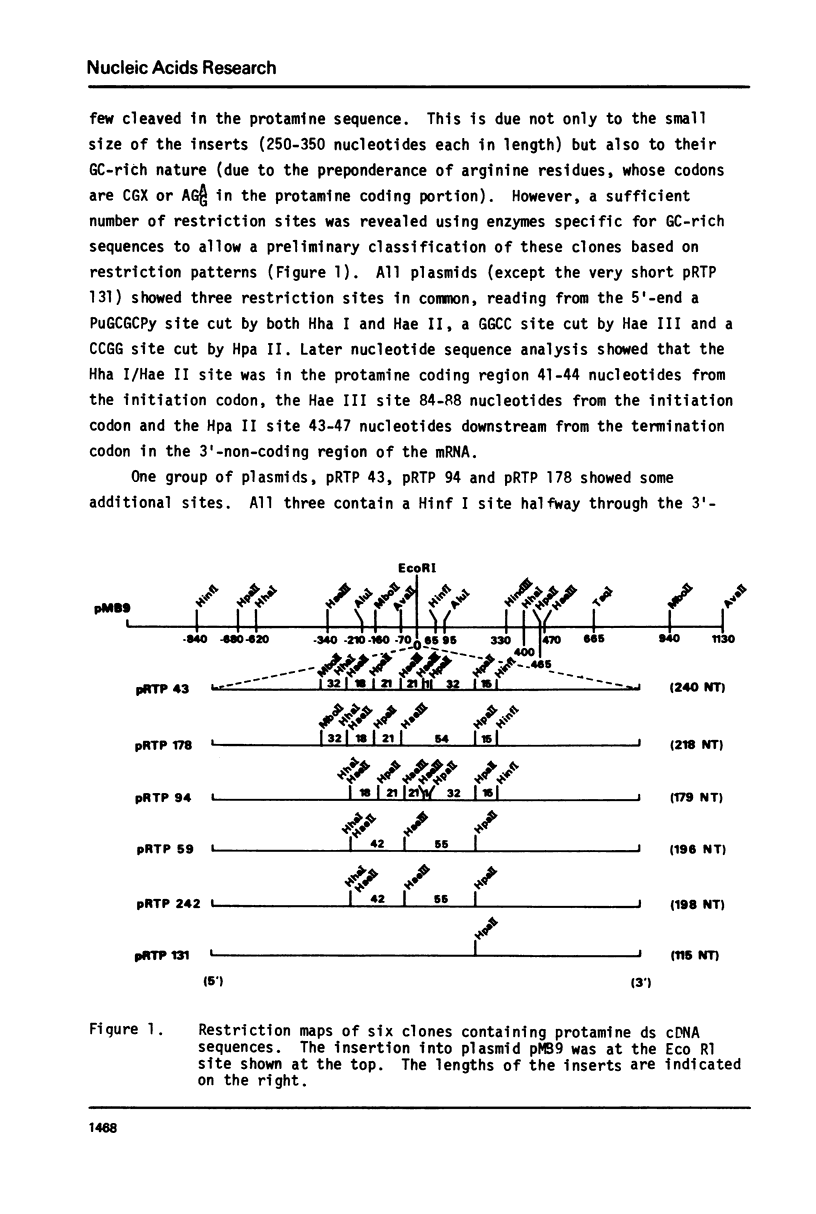
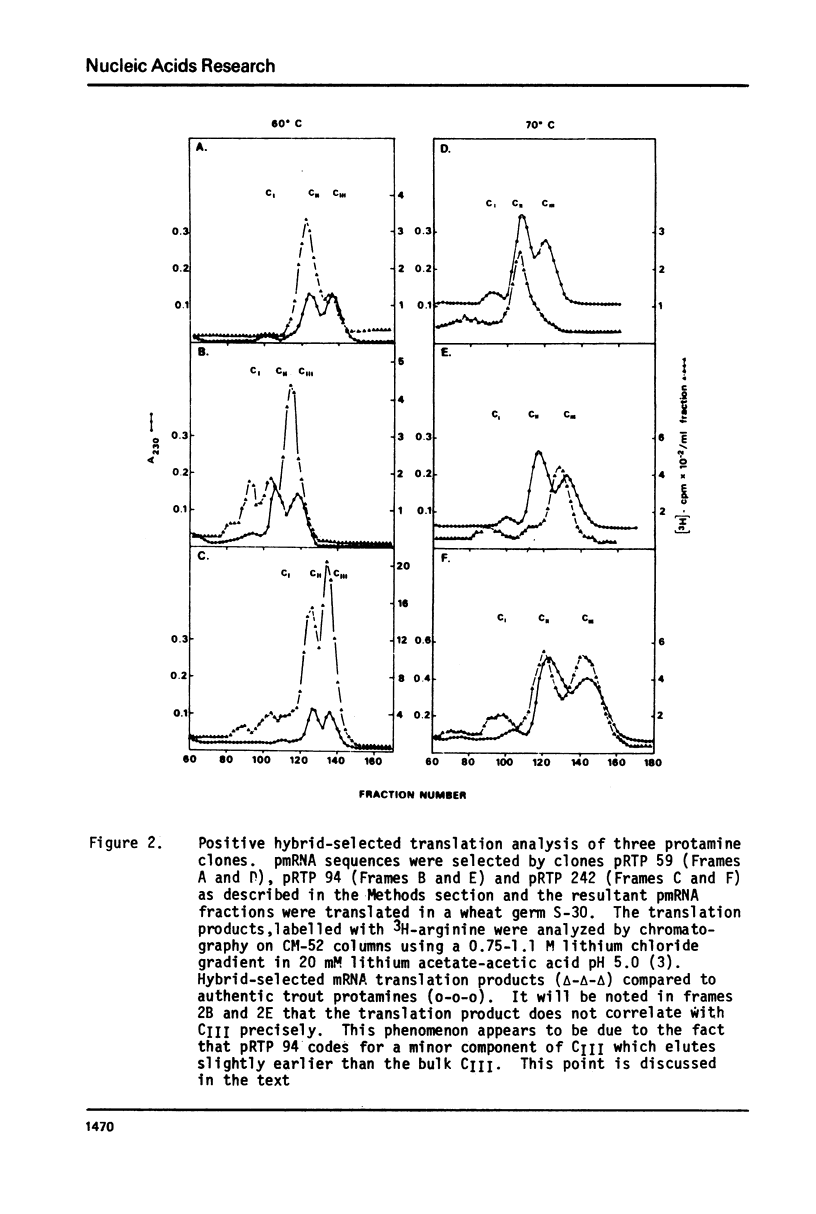
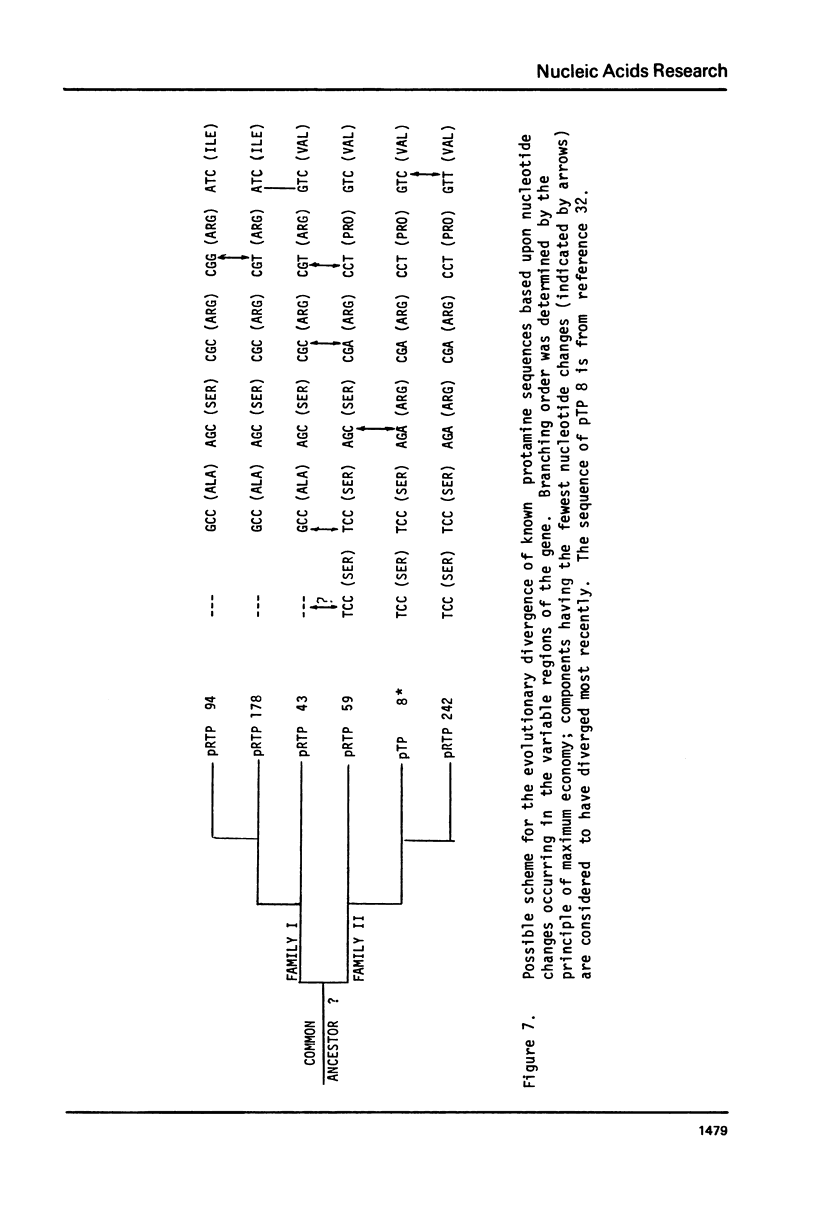
- Davies P. L., Dixon G. H., Ferrier L. N., Gedamu L., Iatrou K. The structure and function of protamine mRNA from developing trout testis. Prog Nucleic Acid Res Mol Biol. 1976;19:135–155. doi: 10.1016/s0079-6603(08)60915-0. [DOI] [PubMed] [Google Scholar]
- Davies P. L., Dixon G. H., Simoncsits A., Brownlee G. C. Sequences of large T1 ribonuclease-resistant oligoribonucleotides from protamine mRNA: the overall architecture of protamine mRNA. Nucleic Acids Res. 1979 Dec 20;7(8):2323–2345. doi: 10.1093/nar/7.8.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. L., Ferrier L. N., Dixon G. H. Sequence analysis of protamine mRNA from the rainbow trout. Depurination and nearest neighbor analysis of protamine cDNA. J Biol Chem. 1977 Feb 25;252(4):1386–1393. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Ferrier L. N., Davies P. L., Dixon G. H. Protamine messenger RNA from rainbow trout testis contains the nucleotide sequence A-A-U-A-A-A in an untranslated region. Biochim Biophys Acta. 1977 Dec 14;479(4):460–470. doi: 10.1016/0005-2787(77)90039-9. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Effect of enzymatic "decapping" on protamine mRNA translation in wheat germ S-30. Biochem Biophys Res Commun. 1978 Nov 14;85(1):114–124. doi: 10.1016/s0006-291x(78)80018-7. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Heterogeneity of biologically active deadenylated protamine mRNA components isolated from rainbow trout testes. Nucleic Acids Res. 1979 Aug 10;6(11):3661–3672. doi: 10.1093/nar/6.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedamu L., Dixon G. H. Purification and properties of biologically active rainbow trout testis protamine mRNA. J Biol Chem. 1976 Mar 10;251(5):1455–1463. [PubMed] [Google Scholar]
- Gedamu L., Iatrou K., Dixon G. H. A simple procedure for the isolation and purification of protamine messenger ribonucleic acid from trout testis. Biochem J. 1978 Jun 1;171(3):589–599. doi: 10.1042/bj1710589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedamu L., Iatrou K., Dixon G. H. Isolation and characterization of trout testis protamine mRNAs lacking poly (A). Cell. 1977 Mar;10(3):443–451. doi: 10.1016/0092-8674(77)90031-9. [DOI] [PubMed] [Google Scholar]
- Gedamu L., Iatrou K., Dixon G. H. Translation of partially purified poly(A)+ protamine messenger RNA components in wheat germ and rabbit reticulocyte cell-free systems. Evidence for translational control mechanisms. Biochim Biophys Acta. 1979 May 24;562(3):481–494. doi: 10.1016/0005-2787(79)90111-4. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatrou K., Dixon G. H. The distribution of poly(A)+ and poly(A)- protamine messenger RNA sequences in the developing trout testis. Cell. 1977 Mar;10(3):433–441. doi: 10.1016/0092-8674(77)90030-7. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Gedamu L., Dixon G. H. Protamine messenger RNA: partial purification and characterization of a heterogeneous family of polyadenylated messenger components. Can J Biochem. 1979 Jun;57(6):945–956. doi: 10.1139/o79-115. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Tsitilou S. G., Goldsmith M. R., Kafatos F. C. Molecular analysis of the GrB mutation in Bombyx mori through the use of chorion cDNA library. Cell. 1980 Jul;20(3):659–669. doi: 10.1016/0092-8674(80)90312-8. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R. Sequence divergence of rainbow trout protamine mRNAs; comparison of coding and non-coding nucleotide sequences in three protamine cDNA plasmids. Nature. 1979 Jun 28;279(5716):809–811. doi: 10.1038/279809a0. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy W. B., Dixon G. H. Reiteration frequency of the protamine genes in rainbow trout (Salmo gairdnerii). J Biol Chem. 1977 Nov 25;252(22):8062–8065. [PubMed] [Google Scholar]
- Ling V., Jergil B., Dixon G. H. The biosynthesis of protamine in trout testis. 3. Characterization of protamine components and their synthesis during testis development. J Biol Chem. 1971 Feb 25;246(4):1168–1176. [PubMed] [Google Scholar]
- Marushige K., Dixon G. H. Developmental changes in chromosomal composition and template activity during spermatogenesis in trout testis. Dev Biol. 1969 Apr;19(4):397–414. doi: 10.1016/0012-1606(69)90050-5. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai M., Fujii-Kuriyama Y., Muramatsu M. Number and frequency of protamine genes in rainbow trout testis. Biochemistry. 1978 Dec 12;17(25):5510–5515. doi: 10.1021/bi00618a028. [DOI] [PubMed] [Google Scholar]
- Sanders M. M., Dixon G. H. The biosynthesis of protamine in trout testis. IV. Sites of phosphorylation. J Biol Chem. 1972 Feb 10;247(3):851–855. [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Weiss B., Milcarek C. Mass screening for mutants with altered DNases by microassay techniques. Methods Enzymol. 1974;29:180–193. doi: 10.1016/0076-6879(74)29020-7. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]



