Abstract
Background
Human chromosomal region 8q24 contains several genes which could be functionally related to cancer, including the proto-oncogene c-MYC. However, the abundance of associations around 128 Mb on chromosome 8 could mask the appearance of a weaker, but important, association elsewhere on 8q24.
Methods
In this study, we completed a meta-analysis of results from nine genome-wide association studies for seven types of solid-tumor cancers (breast, prostate, pancreatic, lung, ovarian, colon, and glioma) to identify additional associations that were not apparent in any individual study.
Results
Fifteen SNPs in the 8q24 region had meta-analysis p-values < 1E-04. In particular, the region consisting of 120,576,000-120,627,000 bp contained 7 SNPs with p-values < 1.0E-4, including rs6993464 (p = 1.25E-07). This association lies in the region between two genes, NOV and ENPP2, which have been shown to play a role in tumor development and motility. An additional region consisting of 5 markers from 128,478,000 bp - 128,524,000 (around gene POU5F1B) had p-values < 1E-04, including rs6983267, which had the smallest p-value (p = 6.34E-08). This result replicates previous reports of association between rs6983267 and prostate and colon cancer.
Conclusions
Further research in this area is warranted as these results demonstrate that the chromosomal region 8q24 may contain a locus that influences general cancer susceptibility between 120,576 and 120,630 kb.
Background
Human chromosomal region 8q24 has been associated with many types of solid-tumor cancer, including cancers of the breast [1-5], prostate [6-10], bladder [11], colon [12-17], lung [18,19], ovaries [20], pancreas [21], and brain [22,23] (Additional file 1). The majority of these associations lie at approximately 128 Mb on chromosome 8, with one prominently associated SNP, rs6983267, shown to interact with the proto-oncogene c-MYC (128.82 Mb) [1,24]. Several studies suggest the possibility that some loci in 8q24 influence more than one type of cancer per locus: breast and pancreatic cancer [2]; prostate and colorectal cancer [25]; prostate, colorectal, and ovarian cancer [20]. These studies suggest that this region may contain loci that affect general cancer susceptibility, which interact with other loci (in or outside of 8q24) and/or environmental factors to determine cancer type.
Therefore, to fully understand the role of 8q24 in cancer development, it is important to determine any additional associations that lie within this region. For example, the region 8q24 contains several other genes which could be functionally related to cancer development, including NOV, which encodes a regulatory protein from the CCN family that has been associated with cancer development [26]. Therefore, we used data from nine genome-wide association studies (GWAS) for seven cancers to conduct a meta-analysis for general cancer risk loci in the entire region of 8q24.
Methods
Nine Genome-Wide Association Studies for Cancer
The meta-analysis consisted of solid-tumor cancer GWAS with data available via dbGaP or available to us through collaborations, including studies of lung [27], prostate [28], breast [29,30], and pancreatic [31] cancers from the Cancer Genetic Markers of Susceptibility (CGEMS) project, accessed from dbGaP; an additional lung cancer study [32]; the Cancer Family Registry (CFR) colon cancer study [33], a study of glioma [34], and two ovarian cancer studies from the United Kingdom [35] and the United States [36]. Table 1 presents a summary of the nine studies. The reader is referred to the primary papers for more extensive study details regarding recruitment, matching of controls and analysis methods.
Table 1.
Summary of the nine GWAS included in the meta-analysis for 8q24.
| Cancer | Ref | Study | Study size | Cases | Controls | Cancer subtypes | Study Notes |
|---|---|---|---|---|---|---|---|
| Ovarian | [35,38] | UK | 4170 | 1817 | 2353 | Majority of controls from UK 1958 birth cohort | |
| Ovarian | [36] | US | 3715 | 1815 | 1900 | epithelial ovarian cancer | Four incident case-control studies: Mayo Clinic Ovarian Cancer Study, Duke University's North Carolina Ovarian Cancer Study, University of Toronto Familial Ovarian Tumor Study, H. Lee Moffitt Cancer Center and Research Institute's Tampa Bay Ovarian Cancer Study |
| Glioma | [34] | Mayo Clinic | 350 | 176 | 174 | high-grade glioblastoma, anaplastic astrocytoma | |
| Lung | [32] | Mayo Clinic | 754 | 377 | 377 | adenocarcinoma, other | Cases and controls were never smokers (i.e., had smoked fewer than 100 cigarettes in their lifetime) |
| Lung | [27] | CGEMS/PLCO | 1629 | ~800 | ~800 | adenocarcinoma, squamous cell carcinoma, small-cell lung cancer, other | Cases and controls consisted of both smokers & non-smokers |
| Prostate | [28] | CGEMS/PLCO | 2252 | 1151 | 1101 | aggressive, non-aggressive | |
| Breast | [29,30] | CGEMS/Nurses' Health Study | 2287 | 1145 | 1142 | Postmenopausal women with matching based on use of hormone replacement therapy | |
| Pancreatic | [31] | CGEMS/PanScan | 7174 | 3532 | 3642 | primary adenocarcinoma of the exocrine pancreas | Consisted of 12 cohort and 8 case-control studies |
| Colon | [33] | Colon Family Registry (CFR) | 2190 | ~1000 | ~1000 | invasive, microsatellite stable or low microsatellite instability colorectal cancer | Cases had no germline mismatch repair mutations |
The CGEMS lung cancer GWAS [27] consisted of 1629 subjects from the Prostate, Lung, Colorectal, and Ovarian (PLCO) cancer study who provided consent for general cancer research. The study included adenocarcinoma, squamous cell carcinoma, small-cell lung cancer, and other histological types. The second lung cancer GWAS included 377 lung cancer cases and 377 matched controls, all of whom were never smokers (i.e., had smoked fewer than 100 cigarettes in their lifetime) [32]. Eighty-one percent of the cases had non-small-cell lung cancer (NSCLC); 68% had adenocarcinoma, a type of NSCLC. The CGEMS prostate cancer GWAS [28] included 1151 cases and 1101 male controls from the screening arm of the PLCO study. The cases included individuals with aggressive and nonaggressive cancer. The CGEMS breast cancer GWAS [29,30] consisted of 1145 cases and 1142 matched controls, all of whom were postmenopausal women of European ancestry from the Nurses' Health Study. The fourth CGEMS GWAS included was the pancreatic cancer GWAS [31], which included 7174 subjects drawn from cohort and case-control studies. All cases had primary adenocarcinoma of the exocrine pancreas.
The CFR colon cancer GWAS [33] consisted of 2190 individuals. The cases had invasive colorectal cancer and no identified germline mutations in mismatch repair proteins. The cases were self-identified as non-Hispanic white and had microsatellite stable or low microsatellite instability colorectal cancer (CRC) and/or MMR protein immunohistochemistry positive determined using standard methods [37]. The glioma GWAS [34] included 176 cases and 174 controls from the Mayo Clinic. The cases were adults with high-grade glioblastoma or anaplastic astrocytoma. Finally, the UK ovarian cancer GWAS [35,38] involved 1817 cases and 2353 controls, while the US ovarian GWAS [36] included 1,815 cases and 1,900 controls from four epithelial ovarian cancer (EOC) case-control studies.
Meta-analysis
The meta-analysis included 6686 SNPs across the 8q24 region, from rs6469653 (117.7 Mb) to rs7822726 (146.2 Mb), which were genotyped and/or imputed in at least five studies. P-values from an additive genetic model for the nine cancer GWAS were combined using Fisher's method [39] and Stouffer's (Lipták) method [40], which each use different transformations for combining m independent p-values (pi, i = 1, ..., m) into a test statistic. In particular, the test statistic for Fisher's method is , while the test statistic for Stouffer's method is , with Zi = Φ-1(pi), where Φ-1(·) is the inverse standard normal cumulative distribution function. Loughin [41] recommended that Fisher's method be used to emphasize small p-values, while Stouffer's method is preferable for putting equal weight on p-values at both extremes. Therefore, for the meta-analysis both Fisher's and Stouffer's methods were used to assess robustness of the findings.
Many meta-analyses weight results by the sample size of the studies included [42]. This practice is useful for meta-analyses based on averaging effect sizes, as larger studies are expected to have more accurate estimates of effect sizes [43]. However, meta-analyses based on p-values, such as this one, already incorporate sample size, as smaller studies contain less evidence and cannot have extremely small p-values. Simulations demonstrate that further weighting the analysis by sample size can bias the results toward the largest study and decrease power to detect effects shared only among the smaller studies (Additional file 2). For this reason, equal weight was given to each cancer type in the meta-analysis. Each of the two lung cancer studies and each of the two ovarian studies received a weight of one-half. The Fisher's meta-analysis was weighted according to the method of Hou [44].
In addition to the meta-analysis based on combination of p-values, analysis was also completed based on the number of individual studies in which the SNP had an individual association p-value less than 0.10, with the p-value based on a binomial distribution conditioned on the number of studies in which each SNP was genotyped. Linkage disequilibrium (LD) plots and statistics for Europeans (CEU) were derived from HapMap release 27 (http://hapmap.ncbi.nlm.nih.gov/downloads/ld_data/latest/; [45]). LocusZoom (http://csg.sph.umich.edu/locuszoom/; [46]) was used to plot association results.
Results
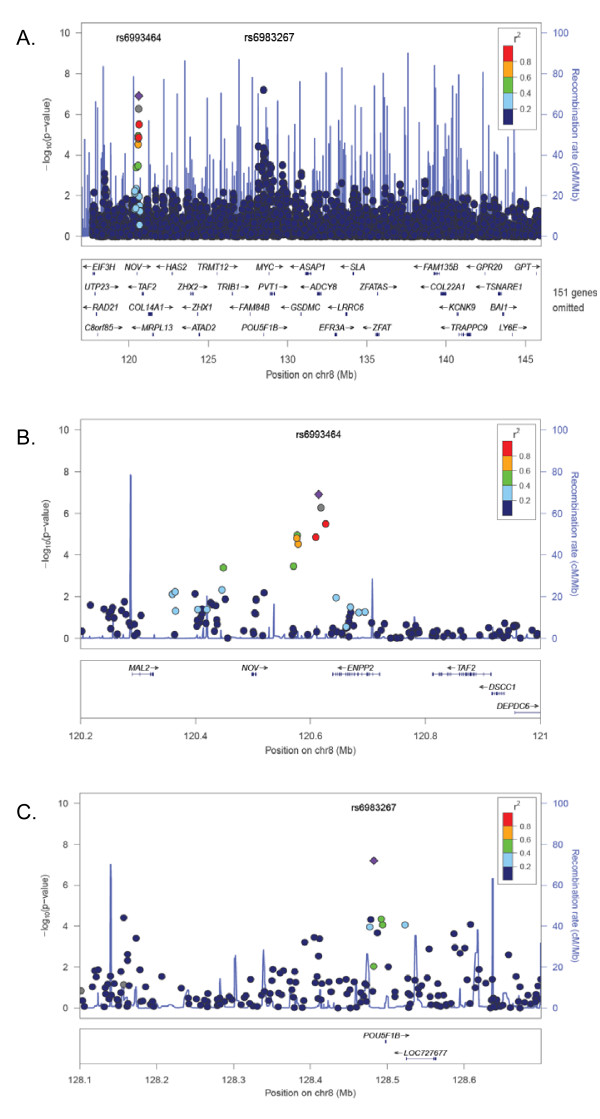
Fifteen SNPs had meta-analysis p-values below 1E-04, as shown in Table 2. The region chr8: 120,576,000-120,627,000 bp contains 7 SNPs with meta-analysis p-values < 1.0E-5 (Figures 1A and 1B), including rs6993464 (Fisher's p = 1.25E-07), which had the second-most significant p-value of the 6686 SNPs examined within 8q24. This p-value is significant after Bonferroni correction (p = 0.0025). In addition, two of the 7 SNPs had Stouffer's p-values < 1E-04, including rs6993464 (Stouffer's p = 6.92E-07). The agreement between the Fisher and Stouffer results demonstrates robustness of the result to the choice of transformation used in combining the p-values.
Table 2.
Top meta-analysis results.
| Individual Study P-value | Meta-analysis P-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | bp | Breast1 | Prostate1 | Pancreatic1 | Lung1 | Lung2 | Ovarian3 | Ovarian4 | Glioma5 | Colon6 | Fisher | Stouffer | Binomial |
| rs1695718 | 118458790 | 0.14 | 0.40 | 0.82 | 0.19 | - | 0.95 | 0.03 | 5.45E-05 | 0.23 | 7.94E-04 | 8.92E-03 | 0.19 |
| rs7846200 | 120576453 | 1.08E-03 | 0.18 | 7.52E-04 | 6.20E-03 | - | 0.87 | 0.61 | 0.40 | 0.39 | 1.51E-05 | 1.01E-04 | 0.04 |
| rs7000665 | 120576919 | 1.73E-03 | 0.22 | 1.12E-04 | 8.44E-03 | - | 0.90 | 0.54 | 0.62 | 0.45 | 1.11E-05 | 2.39E-04 | 0.04 |
| rs10505364 | 120578285 | 1.53E-03 | 0.27 | 2.97E-04 | 3.16E-03 | 0.75 | 0.82 | 0.46 | 0.37 | 0.39 | 2.94E-05 | 3.72E-04 | 0.05 |
| rs9643136 | 120609324 | 5.45E-04 | 0.34 | 1.15E-04 | 5.30E-03 | 0.50 | 0.99 | 0.58 | 0.70 | 0.36 | 1.36E-05 | 1.78E-03 | 0.05 |
| rs6993464 | 120614129 | 2.17E-03 | 0.17 | 6.39E-05 | 6.45E-04 | - | 0.81 | 0.22 | 0.21 | 0.24 | 1.25E-07 | 6.92E-07 | 0.04 |
| rs11782176 | 120617858 | 2.16E-03 | 0.20 | 5.55E-05 | 5.61E-04 | - | 0.76 | - | 0.24 | 0.25 | 5.24E-07 | 6.86E-06 | 0.03 |
| rs6991756 | 120626148 | 4.33E-03 | 0.24 | 5.01E-05 | 1.15E-03 | 1.00 | 0.69 | 0.24 | 0.24 | 0.23 | 3.17E-06 | 1.34E-04 | 0.05 |
| rs3870371 | 122766313 | 4.23E-05 | 0.57 | 0.62 | 0.38 | 0.82 | 0.23 | 0.16 | 0.69 | 0.72 | 9.12E-03 | 0.12 | 0.61 |
| rs920455 | 122769378 | 3.30E-05 | 0.65 | 0.64 | 0.41 | 0.68 | 0.16 | 0.18 | 0.85 | 0.76 | 9.26E-03 | 0.17 | 0.61 |
| rs6470494 | 128157086 | 9.43E-04 | 0.36 | 0.16 | 0.96 | 0.57 | 2.88E-03 | 0.93 | 0.57 | 5.85E-04 | 3.73E-05 | 1.60E-03 | 0.05 |
| rs17464492 | 128412048 | 0.21 | 0.04 | 0.08 | 0.92 | 0.09 | 6.14E-03 | 1.21E-02 | 0.72 | 1.18E-02 | 3.92E-04 | 4.80E-04 | 6.42E-05 |
| rs10808555 | 128478693 | 6.35E-03 | 1.29E-02 | 0.81 | 0.05 | 0.05 | 3.87E-03 | 0.78 | 0.53 | 8.82E-03 | 4.64E-05 | 1.91E-04 | 6.42E-05 |
| rs6983267 | 128482487 | 8.37E-03 | 1.05E-05 | 0.25 | 0.39 | 0.16 | 0.02 | 0.81 | 0.49 | 1.40E-03 | 6.34E-08 | 1.28E-06 | 8.33E-03 |
| rs7837328 | 128492309 | 3.88E-03 | 8.68E-04 | 0.63 | 0.23 | - | 9.83E-03 | 0.84 | 0.62 | 1.41E-02 | 4.40E-05 | 3.02E-04 | 5.02E-03 |
| rs7014346 | 128493974 | 6.19E-03 | 2.83E-03 | 0.69 | 0.18 | 0.23 | 0.02 | 0.98 | 0.49 | 1.02E-02 | 8.48E-05 | 6.32E-04 | 8.33E-03 |
| rs12334695 | 128523110 | 0.14 | 4.34E-04 | 0.02 | 0.53 | 0.96 | 0.03 | 0.74 | 3.09E-02 | 0.47 | 8.46E-05 | 4.43E-04 | 8.33E-03 |
| rs1447295 | 128554220 | 0.64 | 2.44E-05 | 0.52 | 0.46 | 0.24 | 0.17 | 0.27 | 0.12 | 0.73 | 1.26E-03 | 1.45E-02 | 0.61 |
| rs4242382 | 128586755 | 0.83 | 2.82E-06 | 0.43 | 0.60 | 0.13 | 0.19 | 0.16 | 0.13 | 0.99 | 2.26E-04 | 0.06 | 0.61 |
| rs4242384 | 128587736 | 0.87 | 2.66E-06 | 0.49 | 0.58 | - | 0.21 | 0.30 | 0.17 | 0.95 | 1.08E-03 | 0.11 | 0.57 |
| rs7017300 | 128594450 | 0.73 | 1.80E-05 | 0.85 | 0.95 | 0.15 | 0.39 | 0.60 | 8.45E-02 | 0.58 | 2.03E-03 | 0.09 | 0.23 |
| rs11988857 | 128601055 | 0.65 | 3.78E-06 | 0.97 | 0.87 | - | 0.16 | 0.37 | 5.68E-02 | 0.97 | 1.12E-03 | 0.29 | 0.19 |
| rs7837688 | 128608542 | 0.88 | 1.02E-06 | 0.65 | 0.92 | 0.11 | 0.22 | 0.23 | 5.37E-02 | 0.74 | 7.80E-05 | 0.02 | 0.23 |
| rs4404878 | 128867576 | 0.89 | 0.21 | 3.02E-05 | 0.06 | 0.50 | 0.05 | 0.17 | 0.10 | 0.97 | 3.20E-04 | 0.02 | 0.05 |
| rs7841347 | 128887490 | 0.82 | 0.09 | 3.25E-05 | 0.06 | 0.38 | 0.54 | 0.27 | 0.25 | 0.86 | 7.96E-04 | 1.68E-02 | 0.05 |
| rs16903097 | 129625538 | 0.59 | 0.92 | 0.39 | 0.05 | - | 1.98E-02 | 9.16E-05 | 0.14 | 0.33 | 1.08E-02 | 0.03 | 0.04 |
| rs2163951 | 130672539 | 0.43 | 0.81 | 0.56 | 0.46 | 0.51 | 0.87 | 0.98 | 0.65 | 9.72E-05 | 0.03 | 0.35 | 0.61 |
| rs6470863 | 132015998 | 0.55 | 0.32 | 0.87 | 5.38E-05 | 0.88 | 0.80 | 0.63 | 0.20 | 0.41 | 0.14 | 0.33 | 0.61 |
SNP markers with p ≤ .0001 (bold) for either the individual GWAS or the meta-analysis with SNP present in at least five studies, sorted based on base-pair position (bp) on chromosome 8. A dash indicates no data.
Results from 1CGEMS, 2Li et al [32], 3Song et al [35]; 4Permuth-Wey et al [36]; 5Wrensch et al [34]; 6CFR [33]
Figure 1.
Individual GWAS top results and meta-analysis results for 8q24 region. (A) -log10(p-values) from meta-analysis using Fisher's method of the 8q24 region; (B) -log10(p-values) from meta-analysis for the region around NOV and ENPP2. Purple diamond = rs6993464; gray = no LD data available in HapMap Phase II. Other colors indicate level of LD with rs6993464. (C) -log10(p-values) from meta-analysis using Fisher's method for the region around rs6983267. Purple diamond = rs6983267; colors indicate level of LD with rs6983267.
As shown in Table 2, rs6993464 is also the site of a weaker association peak in the CGEMS pancreatic cancer GWAS (p = 6.39E-05). However, the SNP rs6993464 retains a p-value of 7.94E-05 when the pancreatic cancer study is omitted from the analysis, suggesting that the association at rs6993464 is not solely driven by the pancreatic cancer study. Along with pancreatic cancer, the CGEMS lung and breast cancer datasets contribute the most to the significance of the meta-analysis p-values in this region, with study-specific p-values at these seven SNPs ranging from 0.008 to 5.45E-04. Unlike the CGEMS lung cancer GWAS, the never-smokers lung cancer GWAS did not contribute to the association signal at rs6993464 or nearby SNPs. This could be due to sample size, as the never-smokers dataset included fewer individuals (754 vs. 1629), or to gene-environment interaction, as the CGEMS lung cancer study included smokers, while the non-CGEMS lung cancer study did not.
An additional region consisting of 5 markers from 128,478,000 bp - 128,524,000 (around gene POU5F1B) had Fisher's p-values < 1E-04, including rs6983267, which had the smallest p-value in the meta-analysis (p = 6.34E-08) (Figure 1C). SNP rs10808555, and another SNP just outside the region (rs17464492; 128,412,048 bp), had p-values < 0.10 in 6 out of the 9 individual GWAS (binomial p = 6.4E-05). This result replicates previous reports of association between rs6983267 and prostate [20] and colon cancer [14-16,20], but provides conflicting evidence with respect to reports of association with ovarian cancer [20], as the UK ovarian study provided nominal support for association with rs6983267 (p = 0.022) while the US ovarian GWAS did not (p = 0.81).
Finally, two additional SNPs were found to be associated with cancer in the meta-analysis. An association at rs7837688 (128,608,542 bp; p = 7.80E-05) was primarily due to association with prostate cancer (individual study p = 1.02E-06). An association at rs6470494 (128,157,086 bp; p = 3.73E-05) showed some evidence of being a general cancer risk locus, as its significance was contributed by multiple data sets (colon, breast, and UK ovarian). However, this association was not as well supported by associations at neighboring SNPs.
This meta-analysis used equal weights for each cancer type; however, the studies included ranged in size from 350 subjects to 7174 subjects. As a sensitivity analysis, we also conducted a meta-analysis weighting each study by its sample size (Additional file 3). This analysis did not change the conclusions for rs6983267 (sample-weighted Fisher's p = 9.01E-05) or rs6993464 (p = 3.75E-07). However, the sample size-weighted analysis did result in a third region, 128,853 - 128,888 kb, showing p-values < 1E-04. The significance of this region was primarily due to the largest study, the pancreatic cancer data set. This region overlaps the gene PVT1, which has previously been implicated in breast and ovarian cancer [47]; however, in our analysis this region had nominal evidence of association with ovarian cancer (p = 0.017 at rs10956390 in the US ovarian data set) and no evidence of association with breast cancer.
Discussion
Previous studies of the 8q24 region have identified associations between multiple types of cancer and markers around 128 Mb; in particular, rs6983267 has been associated with colon [20], prostate [14-16,20], and ovarian cancer [20]. This meta-analysis reproduces the association between rs6983267 and cancer, with four additional SNPs in the region 128,158,000 - 128,524,000 bp having Fisher's meta-analysis p-values < 1E-04. SNP marker rs6983267 has been shown to exhibit long-range physical interaction with the proto-oncogene c-MYC (about 335 kb downstream) in colorectal, prostate, and breast cancer [1,24], providing a potential mechanism for the source of this association.
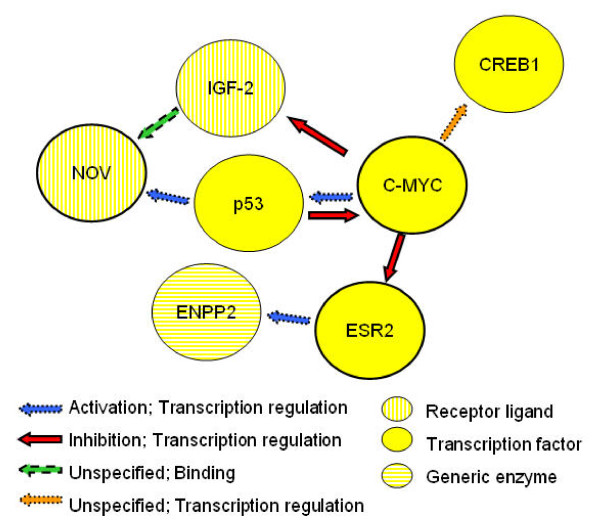
In addition to the association at rs6983267, we identified a second SNP, rs6993464, associated with cancer risk. This SNP is not in LD with rs6983267 or other loci that have previously been reported to be associated with various cancers (Additional file 1). It is possible that the association at rs6993464 is due to several distinct variants, each of which influences a different type of cancer, rather than a single locus that influences cancer development for multiple cancers. This result would also be of interest, as it would imply the existence of previously unreported cancer-specific variants in 8q24. As shown in Figure 1B, rs6993464 and six other SNPs with meta-analysis p-values < 1E-04 lie between the genes NOV (nephroblastoma overexpressed gene) and ENPP2 (ectonucleotide pyrophosphatase/phosphodiesterase 2). NOV encodes the regulatory protein CCN3, which plays an important role in cancer development [26]. ENPP2 encodes a phospholipase which stimulates tumor cell motility and catalyzes the production of lysophosphatidic acid, which stimulates cell proliferation [48]. ENPP2 has also been reported to have angiogenic properties and its expression is up-regulated in several kinds of carcinomas [49]. Furthermore, MetaCore (GeneGo Inc., St. Joseph, MI) regulatory network analysis [50] indicated that both NOV and ENPP2 are indirectly regulated by the 8q24 proto-oncogene c-MYC (Figure 2), via p53 (for NOV) [51,52] and ESR2 (for ENPP2) [53,54], indicating their potential involvement in a pathway for cancer susceptibility. Therefore, it is functionally plausible that this region contains a locus for general cancer susceptibility via cis regulation of NOV and/or ENPP2.
Figure 2.
Regulatory relationship between NOV, ENPP2, and c-MYC, reconstructed using MetaCore.
It is also possible that this region harbors an enhancer locus for a more distant oncogene. Several of the significant SNPs in this region are found in the SCAN database [55] as expression quantitative trait loci (eQTLs) for genes throughout the genome which are associated with various types of cancer. In particular, rs6993464 has been shown to be an eQTL for POLR2F, a gene on chromosome 22 which is up-regulated in colorectal cancer [56], while rs7000665 regulates expression of BRAF, a gene which plays an important role in tumorigenesis in thyroid cancer [57] and melanoma [58], as well as in survival in colon cancer [59]. Finally, rs7846200 has been reported to be an eQTL for multiple genes associated with cancer, including PTBP1, which affects the invasive capacity of cancer cell lines in a cell type-dependent manner [60], and HNRNPK, which interacts with the oncogene p53 and contributes to pancreatic cancer [61] and has been associated with prostate cancer [62].
An additional locus, rs11987056, which is in perfect LD with rs6993464 (r2 = 1), is highly conserved and only 170 bp downstream from the putative transcription factor binding sites V$S8_01 and V$NKX25_02 using the Transfac Matrix Database (v.7.0) [63]. This SNP is associated with expression of the proto-oncogene SRC, which is involved in the regulation of cell growth [64]. The SNP rs11987056 was not included in this analysis as it was genotyped in only two of the individual studies examined. Nonetheless, it is a strong functional candidate for the source of the association between rs6993464 and cancer development. Hence, there are several plausible mechanisms by which a locus in chromosome 8: 120,576,000 - 120,626,000 could affect general cancer susceptibility, explaining our meta-analysis findings.
Because Fisher's and Stouffer's tests are union-intersection tests [65], the meta-analysis peak at rs6993464 is not sufficient to conclude that the SNP is associated with multiple cancer types, only that it is associated with at least one type of cancer. However, the fact that rs6993464 retains a p-value of 7.94E-05 when pancreatic cancer, the study with the strongest association, is omitted from the analysis suggests that this locus is associated with more than one type of cancer in our study. Loci that affect general cancer susceptibility may act in a context-dependent manner; based on genetic background at other loci, tissue type, and other environmental factors, a particular variant could predispose for some types of cancers, while protecting against others. P-value based meta-analyses, in combination with two-sided tests of association such as those used the studies included here, are powered to detect opposite-directional associations. Among the three data sets which contributed the most significance at rs6993464, the T allele was associated with cancer risk in breast and pancreatic cancer, but with a protective effect in the CGEMS lung cancer data set. In the future, functional studies to establish the causal variant(s) that result in the association peak at rs6993464 may determine whether the opposite-directional association is due to context-dependent effects or to LD with distinct, tumor-specific modifiers.
A limitation of the current study is that the CGEMS lung and prostate cancer studies both drew samples from the PLCO Trial, and therefore the sets of controls overlapped. The pancreatic controls also included some individuals from PLCO (n ≤ 423), which may have overlapped with the lung and prostate cancer studies, and some controls from the Nurses' Health Study (n ≤ 166), which may overlapped with the breast cancer controls. Overlapping sets of controls could lead to inflation of -log10 p-values when the results are combined. However, when the prostate and pancreatic studies were omitted from the meta-analysis, thus removing any overlap in controls, rs6993464 retained a p-value of 6.89E-05. Similarly, marker rs6983267 had a p-value of 6.57E-08 when overlapping controls were removed by omitting the lung and pancreatic cancer data sets. This suggests that the overlapping sets of controls do not affect the overall conclusion of possible cancer risk loci around rs6983267 and rs6993464.
In the future, it would be beneficial to extend this analysis to include bladder cancer, another cancer type which has been associated with 8q24 [11]. It would also be advantageous to examine rs6993464 in the context of cancer-specific GWAS, conditioning on other loci which have been reported to be associated with these cancer types. This could clarify the role of rs6993464 among other loci that influence cancer susceptibility. This study demonstrates the power of meta-analysis for secondary phenotypes in identifying loci that may affect general cancer susceptibility. In the future, it would be valuable to conduct meta-analyses of subsets of solid-tumor cancer types with additional common features, such as breast, pancreatic, and colorectal cancer, which share the feature of frequent somatic amplification of 8q24 [20,25].
Conclusions
In summary, this meta-analysis of nine existing GWAS for solid-cancers indicates a possible cancer risk locus on 8q24 (120,576,000-120,626,000 bp) between NOV and ENPP2, both of which are involved in carcinogenesis and have a regulatory relationship with the proto-oncogene c-MYC. We were also able to replicate previous findings for rs6983267, which has been implicated in risk for multiple cancers and known to have long-range physical interaction with the proto-oncogene c-MYC. Future research in this area is warranted to determine the mechanism by which this region may influence general cancer risk, as well as its genetic and environmental interactions with other known risk factors.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AB performed the meta-analyses and drafted the manuscript. YWA performed the MetaCore and Transfac Matrix Database analyses. BLF conceived of the experiment and assisted in performing the meta-analyses and drafting the manuscript. HS, Y-YT, JAA, PY, RJ, PP, FS, DC, DJD, MJ, JH, SG, PN, GC, and TAS provided data and assisted in drafting the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Location of SNPs previously reported to be associated with cancer. The novel association, rs6993464, is indicated by the purple star. P-value plotted for previously reported associations is minimum from previous reports. Dark blue color indicates r2 < 0.2. Shape of points indicates type of cancer with which the locus has been associated; there is no symbol for ovarian cancer because rs6983267, rs10808556, and rs10505477 have been associated with multiple cancer types (downward-pointing triangles). Lung cancer has been associated with rs6983267 (multiple cancer associations, downward-pointing triangle) and deletion D8S272 (137.8 Mb, not shown).
Power of weighted and unweighted meta-analyses. Three studies of size 350, 350, and 5000 were simulated according to a logistic model with log(OR) = Beta*x, where x is the genotype (-1, 0, 1). Power at alpha = 0.05 (over 1000 simulations) is shown for Fisher's meta-analyses with weights proportional to study size and with equal weights.
Effect of sample size weighting on meta-analysis results. SNP markers with p ≤ .0001 (bold) for the Fisher or Stouffer meta-analysis, weighted by cancer type or sample size. SNPs are sorted based on base-pair position (bp) on chromosome.
Contributor Information
Abra G Brisbin, Email: brisbin.abra@mayo.edu.
Yan W Asmann, Email: asmann.yan@mayo.edu.
Honglin Song, Email: honglin@srl.cam.ac.uk.
Ya-Yu Tsai, Email: ya-yu.tsai@moffitt.org.
Jeremiah A Aakre, Email: aakre.jeremiah@mayo.edu.
Ping Yang, Email: yang.ping@mayo.edu.
Robert B Jenkins, Email: rjenkins@mayo.edu.
Paul Pharoah, Email: paul1@srl.cam.ac.uk.
Fredrick Schumacher, Email: fschumac@usc.edu.
David V Conti, Email: dconti@usc.edu.
David J Duggan, Email: dduggan@tgen.org.
Mark Jenkins, Email: m.jenkins@unimelb.edu.au.
John Hopper, Email: johnlh@unimelb.edu.au.
Steven Gallinger, Email: sgallinger@rogers.com.
Polly Newcomb, Email: pnewcomb@fhcrc.org.
Graham Casey, Email: gcasey@usc.edu.
Thomas A Sellers, Email: thomas.sellers@moffitt.org.
Brooke L Fridley, Email: fridley.brooke@mayo.edu.
Acknowledgements and Grants
We thank Margaret Wrensch and Paul Decker for access to the association results from the glioma case-control study; Ellen L. Goode, Joellen M. Schildkraut, Steven Narod, and Rebecca Sutphen for contribution of the US ovarian case-control association results; Jonathan Tyrer, Simon Gayther, and Susan Ramus for contribution of the UK ovarian case-control association results; and the Mayo Genotyping Shared Resource for genotyping the never-smokers lung cancer study. We also thank the Cancer Genetic Markers of Susceptibility (CGEMS) project for providing results for the breast, prostate, pancreatic and lung cancer for the 8q24 region via dbGaP.
The research was supported by R01 CA114343 (Sellers), P50 CA108961 (Mayo Clinic Brain Tumor SPORE), RC1 NS068222 (Jenkins), R01 CA80127 (Yang), R01 CA84354 (Yang), R21 CA140879 (Fridley), R21 GM86689 (Fridley), the Minnesota Partnership for Biotechnology and Medical Genomics (Fridley), and the Mayo Foundation. Colon cancer data were provided by the Australian Colorectal Cancer Family Registry (U01 CA097735), the Ontario Registry for Studies of Familial Colorectal Cancer (U01 CA074783), and the Seattle Colorectal Cancer Family Registry (U01 CA074794). This work was supported by the National Cancer Institute, National Institutes of Health under RFA # CA-95-011 and through cooperative agreements with members of the Colon Cancer Family Registry and PIs. The content of this manuscript does not necessarily reflect the views or policies of the National Cancer Institute or any of the collaborating centers in the CFRs, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the CFR. The funding bodies did not influence the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
References
- Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, He HH, Brown M, Liu XS, Davis M, Caswell JL, Beckwith CA, Hills A, Macconaill L, Coetzee GA, Regan MM, Freedman ML. 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci USA. 2010;107:9742–9746. doi: 10.1073/pnas.0910668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch FJ, Wang X, McWilliams RR, Bamlet WR, de Andrade M, Petersen GM. Association of breast cancer susceptibility variants with risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:3044–3048. doi: 10.1158/1055-9965.EPI-09-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher O, Johnson N, Gibson L, Coupland B, Fraser A, Leonard A, dos Santos Silva I, Ashworth A, Houlston R, Peto J. Association of genetic variants at 8q24 with breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:702–705. doi: 10.1158/1055-9965.EPI-07-2564. [DOI] [PubMed] [Google Scholar]
- Turnbull C, Ahmed S, Morrison J, Pernet D, Renwick A, Maranian M, Seal S, Ghoussaini M, Hines S, Healey CS, Hughes D, Warren-Perry M, Tapper W, Eccles D, Evans DG, Hooning M, Schutte M, van den Ouweland A, Houlston R, Ross G, Langford C, Pharoah PD, Stratton MR, Dunning AM, Rahman N, Easton DF. Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet. pp. 504–507. [DOI] [PMC free article] [PubMed]
- McInerney N, Colleran G, Rowan A, Walther A, Barclay E, Spain S, Jones AM, Tuohy S, Curran C, Miller N, Kerin M, Tomlinson I, Sawyer E. Low penetrance breast cancer predisposition SNPs are site specific. Breast Cancer Res Treat. 2009;117:151–159. doi: 10.1007/s10549-008-0235-7. [DOI] [PubMed] [Google Scholar]
- Gudmundsson J, Sulem P, Manolescu A, Amundadottir LT, Gudbjartsson D, Helgason A, Rafnar T, Bergthorsson JT, Agnarsson BA, Baker A, Sigurdsson A, Benediktsdottir KR, Jakobsdottir M, Xu J, Blondal T, Kostic J, Sun J, Ghosh S, Stacey SN, Mouy M, Saemundsdottir J, Backman VM, Kristjansson K, Tres A, Partin AW, Albers-Akkers MT, Godino-Ivan Marcos J, Walsh PC, Swinkels DW, Navarrete S, Isaacs SD, Aben KK, Graif T, Cashy J, Ruiz-Echarri M, Wiley KE, Suarez BK, Witjes JA, Frigge M, Ober C, Jonsson E, Einarsson GV, Mayordomo JI, Kiemeney LA, Isaacs WB, Catalona WJ, Barkardottir RB, Gulcher JR, Thorsteinsdottir U, Kong A, Stefansson K. Genome-wide association study identifies a second prostate cancer susceptibility variant at 8q24. Nat Genet. 2007;39:631–637. doi: 10.1038/ng1999. [DOI] [PubMed] [Google Scholar]
- Yeager M, Chatterjee N, Ciampa J, Jacobs KB, Gonzalez-Bosquet J, Hayes RB, Kraft P, Wacholder S, Orr N, Berndt S, Yu K, Hutchinson A, Wang Z, Amundadottir L, Feigelson HS, Thun MJ, Diver WR, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Crawford ED, Haiman CA, Henderson B, Kolonel L, Le Marchand L, Siddiq A, Riboli E, Key TJ, Kaaks R, Isaacs W, Isaacs S, Wiley KE, Gronberg H, Wiklund F, Stattin P, Xu J, Zheng SL, Sun J, Vatten LJ, Hveem K, Kumle M, Tucker M, Gerhard DS, Hoover RN, Fraumeni JF Jr, Hunter DJ, Thomas G, Chanock SJ. Identification of a new prostate cancer susceptibility locus on chromosome 8q24. Nat Genet. 2009;41:1055–1057. doi: 10.1038/ng.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Olama AA, Kote-Jarai Z, Giles GG, Guy M, Morrison J, Severi G, Leongamornlert DA, Tymrakiewicz M, Jhavar S, Saunders E, Hopper JL, Southey MC, Muir KR, English DR, Dearnaley DP, Ardern-Jones AT, Hall AL, O'Brien LT, Wilkinson RA, Sawyer E, Lophatananon A, Horwich A, Huddart RA, Khoo VS, Parker CC, Woodhouse CJ, Thompson A, Christmas T, Ogden C, Cooper C, Donovan JL, Hamdy FC, Neal DE, Eeles RA, Easton DF. Multiple loci on 8q24 associated with prostate cancer susceptibility. Nat Genet. 2009;41:1058–1060. doi: 10.1038/ng.452. [DOI] [PubMed] [Google Scholar]
- Pomerantz MM, Beckwith CA, Regan MM, Wyman SK, Petrovics G, Chen Y, Hawksworth DJ, Schumacher FR, Mucci L, Penney KL, Stampfer MJ, Chan JA, Ardlie KG, Fritz BR, Parkin RK, Lin DW, Dyke M, Herman P, Lee S, Oh WK, Kantoff PW, Tewari M, McLeod DG, Srivastava S, Freedman ML. Evaluation of the 8q24 prostate cancer risk locus and MYC expression. Cancer Res. 2009;69:5568–5574. doi: 10.1158/0008-5472.CAN-09-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Watahiki A, Bayani J, Zhang F, Liu L, Ling V, Sadar MD, English J, Fazli L, So A, Gout PW, Gleave M, Squire JA, Wang YZ. ASAP1, a gene at 8q24, is associated with prostate cancer metastasis. Cancer Res. 2008;68:4352–4359. doi: 10.1158/0008-5472.CAN-07-5237. [DOI] [PubMed] [Google Scholar]
- Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KK, Stacey SN, Gudmundsson J, Jakobsdottir M, Bergthorsson JT, Sigurdsson A, Blondal T, Witjes JA, Vermeulen SH, Hulsbergen-van de Kaa CA, Swinkels DW, Ploeg M, Cornel EB, Vergunst H, Thorgeirsson TE, Gudbjartsson D, Gudjonsson SA, Thorleifsson G, Kristinsson KT, Mouy M, Snorradottir S, Placidi D, Campagna M, Arici C, Koppova K, Gurzau E, Rudnai P, Kellen E, Polidoro S, Guarrera S, Sacerdote C, Sanchez M, Saez B, Valdivia G, Ryk C, de Verdier P, Lindblom A, Golka K, Bishop DT, Knowles MA, Nikulasson S, Petursdottir V, Jonsson E, Geirsson G, Kristjansson B, Mayordomo JI, Steineck G, Porru S, Buntinx F, Zeegers MP, Fletcher T, Kumar R, Matullo G, Vineis P, Kiltie AE, Gulcher JR, Thorsteinsdottir U, Kong A, Rafnar T, Stefansson K. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet. 2008;40:1307–1312. doi: 10.1038/ng.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanke BW, Greenwood CM, Rangrej J, Kustra R, Tenesa A, Farrington SM, Prendergast J, Olschwang S, Chiang T, Crowdy E, Ferretti V, Laflamme P, Sundararajan S, Roumy S, Olivier J-F, Robidoux F, Sladek R, Montpetit A, Campbell P, Bezieau S, O'Shea AM, Zogopoulos G, Cotterchio M, Newcomb P, McLaughlin J, Younghusband B, Green R, Green J, Porteous ME, Campbell H, Blanche H, Sahbatou M, Tubacher E, Bonaiti-Pellie C, Buecher B, Riboli E, Kury S, Chanock SJ, Potter J, Thomas G, Gallinger S, Hudson TJ, Dunlop MG. Genome-wide assocation scan identifies a colorectal cancer susceptibility locus on chromosome 8q24. Nat Genet. 2007;39:989–994. doi: 10.1038/ng2089. [DOI] [PubMed] [Google Scholar]
- Tenesa A, Farrington SM, Prendergast JG, Porteous ME, Walker M, Haq N, Barnetson RA, Theodoratou E, Cetnarskyj R, Cartwright N, Semple C, Clark AJ, Reid FJ, Smith LA, Kavoussanakis K, Koessler T, Pharoah PD, Buch S, Schafmayer C, Tepel J, Schreiber S, Volzke H, Schmidt CO, Hampe J, Chang-Claude J, Hoffmeister M, Brenner H, Wilkening S, Canzian F, Capella G, Moreno V, Deary IJ, Starr JM, Tomlinson IP, Kemp Z, Howarth K, Carvajal-Carmona L, Webb E, Broderick P, Vijayakrishnan J, Houlston RS, Rennert G, Ballinger D, Rozek L, Gruber SB, Matsuda K, Kidokoro T, Nakamura Y, Zanke BW, Greenwood CM, Rangrej J, Kustra R, Montpetit A, Hudson TJ, Gallinger S, Campbell H, Dunlop MG. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet. 2008;40:631–637. doi: 10.1038/ng.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Plummer SJ, Thompson CL, Merkulova A, Acheson LS, Tucker TC, Casey G. A common 8q24 variant and the risk of colon cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:339–342. doi: 10.1158/1055-9965.EPI-07-0713. [DOI] [PubMed] [Google Scholar]
- Poynter JN, Figueiredo JC, Conti DV, Kennedy K, Gallinger S, Siegmund KD, Casey G, Thibodeau SN, Jenkins MA, Hopper JL, Byrnes GB, Baron JA, Goode EL, Tiirikainen M, Lindor N, Grove J, Newcomb P, Jass J, Young J, Potter JD, Haile RW, Duggan DJ, Le Marchand L. Variants on 9p24 and 8q24 are associated with risk of colorectal cancer: results from the Colon Cancer Family Registry. Cancer Res. 2007;67:11128–11132. doi: 10.1158/0008-5472.CAN-07-3239. [DOI] [PubMed] [Google Scholar]
- Tomlinson I, Webb E, Carvajal-Carmona L, Broderick P, Kemp Z, Spain S, Penegar S, Chandler I, Gorman M, Wood W, Barclay E, Lubbe S, Martin L, Sellick G, Jaeger E, Hubner R, Wild R, Rowan A, Fielding S, Howarth K, Silver A, Atkin W, Muir K, Logan R, Kerr D, Johnstone E, Sieber O, Gray R, Thomas H, Peto J, Cazier JB, Houlston R. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39:984–988. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- Gruber SB, Moreno V, Rozek LS, Rennerts HS, Lejbkowicz F, Bonner JD, Greenson JK, Giordano TJ, Fearson ER, Rennert G. Genetic variation in 8q24 associated with risk of colorectal cancer. Cancer Biol Ther. 2007;6:1143–1147. doi: 10.4161/cbt.6.7.4704. [DOI] [PubMed] [Google Scholar]
- Girard L, Zochbauer-Muller S, Virmani AK, Gazdar AF, Minna JD. Genome-wide allelotyping of lung cancer identifies new regions of allelic loss, differences between small cell lung cancer and non-small cell lung cancer, and loci clustering. Cancer Res. 2000;60:4894–4906. [PubMed] [Google Scholar]
- Park SL, Chang SC, Cai L, Cordon-Cardo C, Ding BG, Greenland S, Hussain SK, Jiang Q, Liu S, Lu ML, Mao JT, Morgenstern H, Mu LN, Ng LJ, Pantuck A, Rao J, Reuter VE, Tashkin DP, You NC, Yu CQ, Yu SZ, Zhao JK, Belldegrun A, Zhang ZF. Associations between variants of the 8q24 chromosome and nine smoking-related cancer sites. Cancer Epidemiol Biomarkers Prev. 2008;17:3193–3202. doi: 10.1158/1055-9965.EPI-08-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoussaini M, Song H, Koessler T, Al Olama AA, Kote-Jarai Z, Driver KE, Pooley KA, Ramus SJ, Kjaer SK, Hogdall E, DiCioccio RA, Whittemore AS, Gayther SA, Giles GG, Guy M, Edwards SM, Morrison J, Donovan JL, Hamdy FC, Dearnaley DP, Ardern-Jones AT, Hall AL, O'Brien LT, Gehr-Swain BN, Wilkinson RA, Brown PM, Hopper JL, Neal DE, Pharoah PD, Ponder BA, Eeles RA, Easton DF, Dunning AM. Multiple loci with different cancer specificities within the 8q24 gene desert. J Natl Cancer Inst. 2008;100:962–966. doi: 10.1093/jnci/djn190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagk D, Schaefer KL, Eisenacher M, Braun Y, Wai DH, Schleicher C, Diallo-Danebrock R, Bojar H, Roeder G, Gabbert HE, Domschke W, Poremba C. Expression analysis of pancreatic cancer cell lines reveals association of enhanced gene transcription and genomic amplifications at the 8q22.1 and 8q24.22 loci. Oncol Rep. 2007;17:399–407. [PubMed] [Google Scholar]
- Shete S, Hosking FJ, Robertson LB, Dobbins SE, Sanson M, Malmer B, Simon M, Marie Y, Boisselier B, Delattre JY, Hoang-Xuan K, El Hallani S, Idbaih A, Zelenika D, Andersson U, Henriksson R, Bergenheim AT, Feychting M, Lonn S, Ahlbom A, Schramm J, Linnebank M, Hemminki K, Kumar R, Hepworth SJ, Price A, Armstrong G, Liu Y, Gu X, Yu R, Lau C, Schoemaker M, Muir K, Swerdlow A, Lathrop M, Bondy M, Houlston RS. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemaker MJ, Robertson L, Wigertz A, Jones ME, Hosking FJ, Feychting M, Lonn S, McKinney PA, Hepworth SJ, Muir KR, Auvinen A, Salminen T, Kiuru A, Johansen C, Houlston RS, Swerdlow AJ. Interaction between 5 genetic variants and allergy in glioma risk. Am J Epidemiol. pp. 1165–1173. [DOI] [PubMed]
- Pomerantz MM, Ahmadiyeh N, Jia L, Herman P, Verzi MP, Doddapaneni H, Beckwith CA, Chan JA, Hills A, Davis M, Yao K, Kehoe SM, Lenz HJ, Haiman CA, Yan C, Henderson BE, Frenkel B, Barretina J, Bass A, Tabernero J, Baselga J, Regan MM, Manak JR, Shivdasani R, Coetzee GA, Freedman ML. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet. 2009;41:882–884. doi: 10.1038/ng.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiman CA, Le Marchand L, Yamamato J, Stram DO, Sheng X, Kolonel LN, Wu AH, Reich D, Henderson BE. A common genetic risk factor for colorectal and prostate cancer. Nat Genet. 2007;39:954–956. doi: 10.1038/ng2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo GW, Kohls CD, He BC, Chen L, Zhang W, Shi Q, Zhang BQ, Kang Q, Luo J, Luo X, Wagner ER, Kim SH, Restegar F, Haydon RC, Deng ZL, Luu HH, He TC, Luo Q. The CCN proteins: important signaling mediators in stem cell differentiation and tumorigenesis. Histol Histopathol. 2010;25:795–806. doi: 10.14670/hh-25.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, Mirabello L, Jacobs K, Wheeler W, Yeager M, Bergen AW, Li Q, Consonni D, Pesatori AC, Wacholder S, Thun M, Diver R, Oken M, Virtamo J, Albanes D, Wang Z, Burdette L, Doheny KF, Pugh EW, Laurie C, Brennan P, Hung R, Gaborieau V, McKay JD, Lathrop M, McLaughlin J, Wang Y, Tsao MS, Spitz MR, Wang Y, Krokan H, Vatten L, Skorpen F, Arnesen E, Benhamou S, Bouchard C, Metsapalu A, Vooder T, Nelis M, Valk K, Field JK, Chen C, Goodman G, Sulem P, Thorleifsson G, Rafnar T, Eisen T, Sauter W, Rosenberger A, Bickeboller H, Risch A, Chang-Claude J, Wichmann HE, Stefansson K, Houlston R, Amos CI, Fraumeni JF Jr, Savage SA, Bertazzi PA, Tucker MA, Chanock S, Caporaso NE. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Kraft P, Jacobs KB, Cox DG, Yeager M, Hankinson SE, Wacholder S, Wang Z, Welch R, Hutchinson A, Wang J, Yu K, Chatterjee N, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover RN, Thomas G, Chanock SJ. A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet. 2007;39:870–874. doi: 10.1038/ng2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Jacobs KB, Kraft P, Yeager M, Wacholder S, Cox DG, Hankinson SE, Hutchinson A, Wang Z, Yu K, Chatterjee N, Garcia-Closas M, Gonzalez-Bosquet J, Prokunina-Olsson L, Orr N, Willett WC, Colditz GA, Ziegler RG, Berg CD, Buys SS, McCarty CA, Feigelson HS, Calle EE, Thun MJ, Diver R, Prentice R, Jackson R, Kooperberg C, Chlebowski R, Lissowska J, Peplonska B, Brinton LA, Sigurdson A, Doody M, Bhatti P, Alexander BH, Buring J, Lee IM, Vatten LJ, Hveem K, Kumle M, Hayes RB, Tucker M, Gerhard DS, Fraumeni JF Jr, Hoover RN, Chanock SJ, Hunter DJ. A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1) Nat Genet. 2009;41:579–584. doi: 10.1038/ng.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, Fuchs CS, Petersen GM, Arslan AA, Bueno-de-Mesquita HB, Gross M, Helzlsouer K, Jacobs EJ, LaCroix A, Zheng W, Albanes D, Bamlet W, Berg CD, Berrino F, Bingham S, Buring JE, Bracci PM, Canzian F, Clavel-Chapelon F, Clipp S, Cotterchio M, de Andrade M, Duell EJ, Fox JW Jr, Gallinger S, Gaziano JM, Giovannucci EL, Goggins M, Gonzalez CA, Hallmans G, Hankinson SE, Hassan M, Holly EA, Hunter DJ, Hutchinson A, Jackson R, Jacobs KB, Jenab M, Kaaks R, Klein AP, Kooperberg C, Kurtz RC, Li D, Lynch SM, Mandelson M, McWilliams RR, Mendelsohn JB, Michaud DS, Olson SH, Overvad K, Patel AV, Peeters PH, Rajkovic A, Riboli E, Risch HA, Shu XO, Thomas G, Tobias GS, Trichopoulos D, Van Den Eeden SK, Virtamo J, Wactawski-Wende J, Wolpin BM, Yu H, Yu K, Zeleniuch-Jacquotte A, Chanock SJ, Hartge P, Hoover RN. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet. 2009;41:986–990. doi: 10.1038/ng.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sheu CC, Ye Y, de Andrade M, Wang L, Chang SC, Aubry MC, Aakre JA, Allen MS, Chen F, Cunningham JM, Deschamps C, Jiang R, Lin J, Marks RS, Pankratz VS, Su L, Li Y, Sun Z, Tang H, Vasmatzis G, Harris CC, Spitz MR, Jen J, Wang R, Zhang ZF, Christiani DC, Wu X, Yang P. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010;11:321–330. doi: 10.1016/S1470-2045(10)70042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, Hall D, Hopper JL, Jass J, Le Marchand L, Limburg P, Lindor N, Potter JD, Templeton AS, Thibodeau S, Seminara D. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–2343. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- Wrensch M, Jenkins RB, Chang JS, Yeh RF, Xiao Y, Decker PA, Ballman KV, Berger M, Buckner JC, Chang S, Giannini C, Halder C, Kollmeyer TM, Kosel ML, LaChance DH, McCoy L, O'Neill BP, Patoka J, Pico AR, Prados M, Quesenberry C, Rice T, Rynearson AL, Smirnov I, Tihan T, Wiemels J, Yang P, Wiencke JK. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Ramus SJ, Tyrer J, Bolton KL, Gentry-Maharaj A, Wozniak E, Anton-Culver H, Chang-Claude J, Cramer DW, DiCioccio R, Dork T, Goode EL, Goodman MT, Schildkraut JM, Sellers T, Baglietto L, Beckmann MW, Beesley J, Blaakaer J, Carney ME, Chanock S, Chen Z, Cunningham JM, Dicks E, Doherty JA, Durst M, Ekici AB, Fenstermacher D, Fridley BL, Giles G, Gore ME, De Vivo I, Hillemanns P, Hogdall C, Hogdall E, Iversen ES, Jacobs IJ, Jakubowska A, Li D, Lissowska J, Lubinski J, Lurie G, McGuire V, McLaughlin J, Medrek K, Moorman PG, Moysich K, Narod S, Phelan C, Pye C, Risch H, Runnebaum IB, Severi G, Southey M, Stram DO, Thiel FC, Terry KL, Tsai YY, Tworoger SS, Van Den Berg DJ, Vierkant RA, Wang-Gohrke S, Webb PM, Wilkens LR, Wu AH, Yang H, Brewster W, Ziogas A, Houlston R, Tomlinson I, Whittemore AS, Rossing MA, Ponder BA, Pearce CL, Ness RB, Menon U, Kjaer SK, Gronwald J, Garcia-Closas M, Fasching PA, Easton DF, Chenevix-Trench G, Berchuck A, Pharoah PD, Gayther SA. A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nat Genet. 2009;41:996–1000. doi: 10.1038/ng.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permuth-Wey J, Kim D, Tsai YY, Lin HY, Chen YA, Barnholtz-Sloan J, Birrer MJ, Bloom G, Chanock SJ, Chen Z, Cramer DW, Cunningham JM, Dagne G, Ebbert-Syfrett J, Fenstermacher D, Fridley BL, Garcia-Closas M, Gayther SA, Ge W, Gentry-Maharaj A, Gonzalez-Bosquet J, Goode EL, Iversen E, Jim H, Kong W, McLaughlin J, Menon U, Monteiro AN, Narod SA, Pharoah PD, Phelan CM, Qu X, Ramus SJ, Risch H, Schildkraut JM, Song H, Stockwell H, Sutphen R, Terry KL, Tyrer J, Vierkant RA, Wentzensen N, Lancaster JM, Cheng JQ, Sellers TA. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Res. 2011;71:3896–3903. doi: 10.1158/0008-5472.CAN-10-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor NM, Rabe K, Petersen GM, Haile R, Casey G, Baron J, Gallinger S, Bapat B, Aronson M, Hopper J, Jass J, LeMarchand L, Grove J, Potter J, Newcomb P, Terdiman JP, Conrad P, Moslein G, Goldberg R, Ziogas A, Anton-Culver H, de Andrade M, Siegmund K, Thibodeau SN, Boardman LA, Seminara D. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA. 2005;293:1979–1985. doi: 10.1001/jama.293.16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, Sher T, Gentry-Maharaj A, Wozniak E, Tsai YY, Weidhaas J, Paik D, Van Den Berg DJ, Stram DO, Pearce CL, Wu AH, Brewster W, Anton-Culver H, Ziogas A, Narod SA, Levine DA, Kaye SB, Brown R, Paul J, Flanagan J, Sieh W, McGuire V, Whittemore AS, Campbell I, Gore ME, Lissowska J, Yang HP, Medrek K, Gronwald J, Lubinski J, Jakubowska A, Le ND, Cook LS, Kelemen LE, Brook-Wilson A, Massuger LF, Kiemeney LA, Aben KK, van Altena AM, Houlston R, Tomlinson I, Palmieri RT, Moorman PG, Schildkraut J, Iversen ES, Phelan C, Vierkant RA, Cunningham JM, Goode EL, Fridley BL, Kruger-Kjaer S, Blaeker J, Hogdall E, Hogdall C, Gross J, Karlan BY, Ness RB, Edwards RP, Odunsi K, Moyisch KB, Baker JA, Modugno F, Heikkinenen T, Butzow R, Nevanlinna H, Leminen A, Bogdanova N, Antonenkova N, Doerk T, Hillemanns P, Durst M, Runnebaum I, Thompson PJ, Carney ME, Goodman MT, Lurie G, Wang-Gohrke S, Hein R, Chang-Claude J, Rossing MA, Cushing-Haugen KL, Doherty J, Chen C, Rafnar T, Besenbacher S, Sulem P, Stefansson K, Birrer MJ, Terry KL, Hernandez D, Cramer DW, Vergote I, Amant F, Lambrechts D, Despierre E, Fasching PA, Beckmann MW, Thiel FC, Ekici AB, Chen X, Johnatty SE, Webb PM, Beesley J, Chanock S, Garcia-Closas M, Sellers T, Easton DF, Berchuck A, Chenevix-Trench G, Pharoah PD, Gayther SA. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42:880–884. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methods for Research Workers. London: Oliver and Boyd; 1932. [Google Scholar]
- Stouffer SA, Suchman EA, DeVinney LC, Star SA, Williams RMJ. The American Soldier, Vol 1:Adjustment during Army Life. Princeton: Princeton University Press; 1949. [Google Scholar]
- Loughin TM. A systematic comparison of methods for combining p-values from independent tests. Comp Stat Data Analysis. 2004;47:467–485. doi: 10.1016/j.csda.2003.11.020. [DOI] [Google Scholar]
- Guerra R, Goldstein DR. Meta-analysis and combining information in genetics and genomics. Chapman & Hall/CRC; 2009. [Google Scholar]
- Cooper HM, Hedges LV, Valentine JC. The handbook of research synthesis and meta-analysis. Russell Sage Foundation Publications; 2009. [Google Scholar]
- Hou CD. A simple approximation for the distribution of the weighted combination of non-independent or independent probabilities. Stat Prob Letters. 2005;73:179–187. doi: 10.1016/j.spl.2004.11.028. [DOI] [Google Scholar]
- Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, Peltonen L, Dermitzakis E, Bonnen PE, Altshuler DM, Gibbs RA, de Bakker PI, Deloukas P, Gabriel SB, Gwilliam R, Hunt S, Inouye M, Jia X, Palotie A, Parkin M, Whittaker P, Yu F, Chang K, Hawes A, Lewis LR, Ren Y, Wheeler D, Gibbs RA, Muzny DM, Barnes C, Darvishi K, Hurles M, Korn JM, Kristiansson K, Lee C, McCarrol SA, Nemesh J, Dermitzakis E, Keinan A, Montgomery SB, Pollack S, Price AL, Soranzo N, Bonnen PE, Gibbs RA, Gonzaga-Jauregui C, Keinan A, Price AL, Yu F, Anttila V, Brodeur W, Daly MJ, Leslie S, McVean G, Moutsianas L, Nguyen H, Schaffner SF, Zhang Q, Ghori MJ, McGinnis R, McLaren W, Pollack S, Price AL, Schaffner SF, Takeuchi F, Grossman SR, Shlyakhter I, Hostetter EB, Sabeti PC, Adebamowo CA, Foster MW, Gordon DR, Licinio J, Manca MC, Marshall PA, Matsuda I, Ngare D, Wang VO, Reddy D, Rotimi CN, Royal CD, Sharp RR, Zeng C, Brooks LD, McEwen JE. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Kuo WL, Stilwell JL, Takano H, Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, Kalloger SE, Carlson JW, Ginzinger DG, Celniker SE, Mills GB, Huntsman DG, Gray JW. Amplification of PVT1 contributes to the pathophysiology of ovarian and breast cancer. Clin Cancer Res. 2007;13:5745–5755. doi: 10.1158/1078-0432.CCR-06-2882. [DOI] [PubMed] [Google Scholar]
- Gaetano CG, Samadi N, Tomsig JL, Macdonald TL, Lynch KR, Brindley DN. Inhibition of autotaxin production or activity blocks lysophosphatidylcholine-induced migration of human breast cancer and melanoma cells. Mol Carcinog. 2009;48:801–809. doi: 10.1002/mc.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCBI Nucleotide Database: Homo sapiens ectonucleotide pyrophosphatase/phosphodiesterase 2 (ENPP2), transcript variant 2, mRNA. http://www.ncbi.nlm.nih.gov/nuccore/NM_001040092?
- Nikolsky Y, Ekins S, Nikolskaya T, Bugrim A. A novel method for generation of signature networks as biomarkers from complex high throughput data. Toxicol Lett. 2005;158:20–29. doi: 10.1016/j.toxlet.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Bohlig L, Metzger R, Rother K, Till H, Engeland K. The CCN3 gene coding for an extracellular adhesion-related protein is transcriptionally activated by the p53 tumor suppressor. Cell Cycle. 2008;7:1254–1261. doi: 10.4161/cc.7.9.5812. [DOI] [PubMed] [Google Scholar]
- Westermann F, Muth D, Benner A, Bauer T, Henrich KO, Oberthuer A, Brors B, Beissbarth T, Vandesompele J, Pattyn F, Hero B, Konig R, Fischer M, Schwab M. Distinct transcriptional MYCN/c-MYC activities are associated with spontaneous regression or malignant progression in neuroblastomas. Genome Biol. 2008;9:R150. doi: 10.1186/gb-2008-9-10-r150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller KI, Zhao X, Lee CW, Chiu KP, Yao F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, Fu Y, Weng Z, Kuznetsov VA, Sung WK, Ruan Y, Dang CV, Wei CL. Global mapping of c-Myc binding sites and target gene networks in human B cells. Proc Natl Acad Sci USA. 2006;103:17834–17839. doi: 10.1073/pnas.0604129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossi F, Barnett DH, Frasor J, Komm B, Lyttle CR, Katzenellenbogen BS. Transcriptional profiling of estrogen-regulated gene expression via estrogen receptor (ER) alpha or ERbeta in human osteosarcoma cells: distinct and common target genes for these receptors. Endocrinology. 2004;145:3473–3486. doi: 10.1210/en.2003-1682. [DOI] [PubMed] [Google Scholar]
- Gamazon ER, Zhang W, Konkashbaev A, Duan S, Kistner EO, Nicolae DL, Dolan ME, Cox NJ. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26:259–262. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonacopoulou AG, Grivas PD, Skarlas L, Kalofonos M, Scopa CD, Kalofonos HP. POLR2F, ATP6V0A1 and PRNP expression in colorectal cancer: new molecules with prognostic significance? Anticancer Res. 2008;28:1221–1227. [PubMed] [Google Scholar]
- Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, Refetoff S, Nikiforov YE, Fagin JA. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005;65:4238–4245. doi: 10.1158/0008-5472.CAN-05-0047. [DOI] [PubMed] [Google Scholar]
- Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Farina-Sarasqueta A, van Lijnschoten G, Moerland E, Creemers GJ, Lemmens VE, Rutten HJ, van den Brule AJ. The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients. Ann Oncol. 2010;21:2396–2402. doi: 10.1093/annonc/mdq258. [DOI] [PubMed] [Google Scholar]
- Wang C, Norton JT, Ghosh S, Kim J, Fushimi K, Wu JY, Stack MS, Huang S. Polypyrimidine tract-binding protein (PTB) differentially affects malignancy in a cell line-dependent manner. J Biol Chem. 2008;283:20277–20287. doi: 10.1074/jbc.M803682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Shanas R, Nelson MA, Bhattacharyya A, Shi J. Increased expression of the heterogeneous nuclear ribonucleoprotein K in pancreatic cancer and its association with the mutant p53. Int J Cancer. 2010;126:395–404. doi: 10.1002/ijc.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboro P, Repaci E, Rubagotti A, Salvi S, Boccardo S, Spina B, Truini M, Introini C, Puppo P, Ferrari N, Carmignani G, Boccardo F, Balbi C. Heterogeneous nuclear ribonucleoprotein K: altered pattern of expression associated with diagnosis and prognosis of prostate cancer. Br J Cancer. 2009;100:1608–1616. doi: 10.1038/sj.bjc.6605057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gene regulation TRANSFAC database. http://www.gene-regulation.com/pub/databases.html
- Roskoski R Jr. Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–1164. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- Erickson S, Kim K, Allison DB. In: Meta-analysis and Combining Information in Genetics and Genomics. Guerra R, Goldstein D, editor. New York: Chapman & Hall/CRC; 2010. Composite hypothesis testing: an approach built on intersection-union tests and Bayesian posterior probabilities; pp. 83–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Location of SNPs previously reported to be associated with cancer. The novel association, rs6993464, is indicated by the purple star. P-value plotted for previously reported associations is minimum from previous reports. Dark blue color indicates r2 < 0.2. Shape of points indicates type of cancer with which the locus has been associated; there is no symbol for ovarian cancer because rs6983267, rs10808556, and rs10505477 have been associated with multiple cancer types (downward-pointing triangles). Lung cancer has been associated with rs6983267 (multiple cancer associations, downward-pointing triangle) and deletion D8S272 (137.8 Mb, not shown).
Power of weighted and unweighted meta-analyses. Three studies of size 350, 350, and 5000 were simulated according to a logistic model with log(OR) = Beta*x, where x is the genotype (-1, 0, 1). Power at alpha = 0.05 (over 1000 simulations) is shown for Fisher's meta-analyses with weights proportional to study size and with equal weights.
Effect of sample size weighting on meta-analysis results. SNP markers with p ≤ .0001 (bold) for the Fisher or Stouffer meta-analysis, weighted by cancer type or sample size. SNPs are sorted based on base-pair position (bp) on chromosome.