Abstract
Objectives
To assess whether the core symptoms of Alzheimer’s disease (AD) consistently predict patient self-rated quality of life (QOL) as assessed by a variety of QOL measures in a large national sample of AD patients.
Design
Cross-sectional.
Setting
Fifteen dementia and geriatric clinics across Canada.
Participants
Community-living patients with AD (n = 370) with Mini-Mental State Examination (MMSE) scores > 10.
Measurements
Patients rated their QOL using two utility indexes, the EQ-5D, the Quality of Well-Being Scale, a global QOL visual analogue scale, and the disease-specific QOL-AD instrument. Cognition was assessed with the AD Assessment Scale-Cognitive subscale and MMSE, function with the Disability Assessment for Dementia, and behavioral and psychological symptoms with the Neuropsychiatric Inventory (NPI) and the Geriatric Depression Scale (GDS). One-way analysis of variance and fully adjusted multiple linear regression were used to assess the relationship between core dementia symptoms and QOL ratings.
Results
The QOL measures had only small to moderate correlations with each other. For all QOL measures, patient ratings were significantly lower among patients with more depressive symptoms. In multivariable analyses, the GDS score was the only significant independent predictor of patient self-ratings for all four QOL measures.
Conclusions
Self-rated symptoms of depression were a consistent independent predictor of patient-rated QOL across diverse QOL measures, while performance-based measures of cognition and informant-based functional status were not. These findings confirm the importance of identifying and treating depression in patients with AD and endorse the use of measures of self-rated depressive symptoms and QOL as outcomes in AD clinical trials.
Keywords: Alzheimer’s disease, dementia, quality of life, utility, depression
Alzheimer’s disease (AD) is characterized by three core features: cognitive impairment, functional limitations and behavioral and psychological symptoms.[1] A measure of cognition, the AD Assessment Scale-Cognitive Subscale (ADAS-Cog), has been the primary outcome in most published AD clinical drug trials.[2] Secondary outcomes in these studies have included measures of function and measures of behavioral and psychological symptoms, but quality of life (QOL) measures have been included infrequently.[2] There is a growing consensus that health-related QOL, which can capture elements of health not detected by standard symptom measures, should be included as an outcome in AD clinical trials.[3]
There are two basic types of QOL measures: generic instruments, including health profile and utility measures, and disease-specific instruments.[4] Disease-specific measures are considered more sensitive in detecting change over time since they focus on aspects of QOL that are particularly relevant to the disease of interest, while generic measures allow for comparisons across different disease states and can be used for economic analyses. Because of their differing strengths, investigators often elect to include both types of measures.[4]
Utility measures summarize the QOL of a disease state as a single number along a continuum that usually extends from death (0.0) to full health (1.0). Utilities can be measured by direct elicitation of an individual’s preferences or by utility indexes, which include a health state classification system and an algorithm to convert ratings into utility scores based on community-derived health preferences.[5] Utility indexes differ in terms of the content and structure of their health classification systems and how they are scored.[5]
Several previous studies have assessed predictors of patient-rated QOL in community-living patients with mild to moderate AD using multiple regression.[6–11] These showed that patient-rated depression was a significant independent predictor of QOL,[6–11] while cognition as assessed by the Mini-Mental State Examination (MMSE) was not.[6, 8, 10, 11] The influence of functional status was inconsistent.[6, 8, 11]. The majority of these studies included only a single measure of QOL,[6–10] most commonly the disease-specific QOL-AD.[6–8] Few of these studies included generic QOL measures such as global QOL rating scales or utility indexes.[10, 11]
Given that different types of QOL measures assess different dimensions of QOL and that utility scores for the same disease can vary substantially depending on the specific utility index used,[5] the primary objective of this study was to evaluate whether there are consistent predictors of patient-rated QOL across a spectrum of QOL measures (a disease-specific measure, two utility indexes and a global QOL rating scale) or whether predictors of patient-rated QOL vary with different QOL measures in a large national sample of community-living patients with mild to moderate AD. In addition, we wanted to address a few deficiencies in the literature on predictors of patient self-ratings of QOL in AD: little information on the predictive ability of the Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-Cog), which is a more sensitive measure of cognitive impairment in AD than the MMSE[12]; lack of data on the predictors of patient-rated QOL as measured by the Quality of Well Being (QWB) index[13]; and limited available research using multiple regression models that include a full spectrum of core dementia symptoms (cognition, function and behavioral and psychiatric symptoms) while adjusting for patient demographic factors, education and comorbidity.
Methods
Design
This report is based on cross-sectional analyses of baseline data derived from the longitudinal Canadian Alzheimer’s Disease Quality of Life (CADQOL) study.
Participants
Patients with AD and their family caregivers were recruited from 11 memory clinics and 4 geriatric assessment clinics across Canada. These are specialty clinics that provide diagnostic services and ongoing care for patients with dementia. Eligible participants were: persons with probable AD of any severity, as determined by the National Institute of Neurological and Communicative Diseases and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria[14]; not institutionalized at the time of enrolment; and had a family caregiver with a minimum of two contacts per week who provided emotional support and/or supervision/assistance with activities of daily living. Those with AD and their family caregivers had to be fluent in English. Patient self-rating of QOL was restricted to those with non-severe dementia, defined as a Mini-Mental State Examination (MMSE) score greater than 10.
Ethics
This study was approved by the Research Ethics Boards of the Toronto Rehabilitation Institute and the recruitment sites.
Demographic and Medical Data
We collected information about age, sex, first language spoken, marital status, living situation, employment status and medical conditions of the patient.
Measures of the Core Symptoms of Dementia
Cognition was measured using the 30-point MMSE[15] and the 70-point ADAS-Cog.[12] Lower MMSE scores and higher ADAS-Cog scores indicate greater cognitive impairment.
Function was measured using the Disability Assessment for Dementia (DAD)[16], a caregiver rated measure of basic and instrumental activities of daily living. Scores ranging from 0–100, with 0 representing complete dependence and 100 representing complete independence.
Behavioral and psychological symptoms were measured using the Neuropsychiatric Inventory (NPI)[17], which is a caregiver rated measure of 12 behavioral and psychological symptoms. The score ranges from 0–144, with higher scores representing more severe behavioral symptoms. In addition, patients and caregivers rated patients’ depressive symptoms using the 30-item Geriatric Depression Scale (GDS).[18] Higher scores indicate greater depressive symptoms, with scores greater than 10 suggestive of clinical depression.[19]
Quality of Life Measures
Disease-Specific
The QOL in AD (QOL-AD) is the most frequently used QOL measure used in AD research. It is a 13-item measure that covers the following domains: physical health, energy level, mood, living situation, memory, relationship with family members, caregiver and friends, ability to do usual/meaningful activities and financial situation. It has been shown to provide reliable and valid ratings by persons with mild and moderate AD.[6, 20] Scores range from 13–52, with higher scores indicating better QOL.
Generic Health Utility Indexes
The EQ-5D[21] and the Quality of Well-Being index (QWB)[22], differ in terms of content, structure, scoring function and completion time.[5, 13] Both have been shown to provide reliable and valid ratings by persons with mild and moderate AD.[11, 13] The EQ-5D is a brief instrument with only 5 items (mobility, self-care, usual activities, mood and pain), each rated on 3 levels. The QWB includes several functional scales (self-care, usual/social activities, mobility and physical activities) with 2 to 8 items per scale and incorporates 21 symptom/problem complexes pertaining to physical and emotional health, cognitive and sensory function, speech, general weakness and limb function.
Global QOL Rating Scale
We used a directly elicited visual analogue scale (VAS) in the form of a vertical “feeling thermometer” labelled from 0 (death) to 100 (full health).
Data Gathering
The assessment involved two sessions. In the first session, a research assistant administered the MMSE, ADAS-Cog and GDS to the patient, and then the GDS, DAD and NPI to the family caregiver. In the second session, the QOL measures were administered to the patient in a random order in a facilitated interview with the research assistant.
Statistical Analyses
Excluded and Missing Data
During the period of recruitment, a quality control site visit was unable to establish the validity of the data at one of the centers that allegedly recruited 42 participants. This center was closed, the data were excluded and recruitment was reallocated to the remaining sites.
All data were entered in duplicate and compared to minimize entry errors. Patients with complete scores on a QOL measure were retained for the analyses pertaining to that measure. Thirteen patients missing scores on all four QOL measures and one patient missing all demographic information were excluded from all analyses. For the remaining sample, when 20% or less of the total items was missing from a measure, scores were derived in accordance with scoring practices for the specific measure (e.g. prorated scores). Multiple imputation with the R package mi was used for any demographic or core dementia symptom predictor variables missing more than 20% of their total items.[23] This affected only 15 patient ratings; 4.1% of our analytic sample.
Data Analyses
We computed Pearson correlations between patient QOL ratings using the different QOL measures. We calculated mean patient QOL ratings for each severity level for the core dementia symptom measures. The ADAS-Cog and DAD scores were divided into four categories based on quartiles derived from our full dataset that included patients with severe dementia. Many patients had an NPI score of zero, so we treated zero as a separate category and broke the remaining scores into tertiles. For the GDS, we started with categories based on the literature (0–10, 11–13, 14–20 and > 20),[19, 24] but to accommodate the noted skewing of patient ratings, we used the following four groupings: 0–4, 5–10, 11–13 and 14–30. We conducted unadjusted analyses using an F-test from a one-way analysis of variance to compare the mean QOL across the levels of the symptom severity scores.
We used multiple linear regression to examine the relationships between each of the QOL outcomes and the core dementia symptom predictor variables. These adjusted models included age, sex (male vs. female), education (grade 9–13 or > grade 13 vs. < grade 9), marital status (other vs. married), first language spoken (English vs. other), comorbidity as measured by the Charlson score (2 or ≥ 3 vs. 1; all patients receive 1 point for having dementia),[25] and study center (5 centres each with less than 25 participants were grouped as a single center). Regression coefficients for the core dementia symptom predictor variables are expressed as the estimated mean difference in the QOL outcome per standard deviation of the predictor variable. Regression coefficients for age are expressed per 5 year increments. Parameter estimates were averaged across 10 imputed datasets and p-values were computed using a t-test for a single parameter and an F-test for group variables, using an estimated degrees of freedom that accounts for the imputation process.[26, 27] The relative importance of each variable to the QOL outcomes was summarized with the Lindeman, Merenda and Gold (LMG) R2, which is an average of the R2 increments from adding the variable of interest to models without the variable.[28] Because this relative importance measure is an average across all of the possible models, it is independent of the order of entry of the variable.
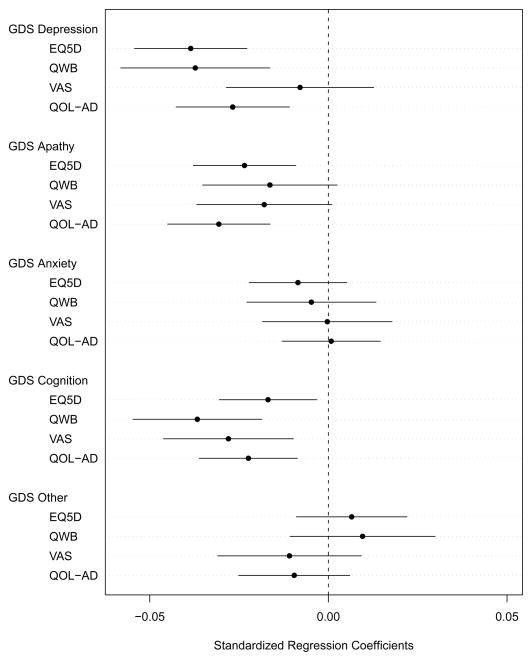
In an exploratory analysis to assess the relative impact on QOL ratings of GDS items that overlap with cognitive symptoms and those that relate to depression or other mood constructs, we used multiple linear regression as described above, but replaced the GDS total score variable with 5 GDS sub-scale scores (depressed mood, apathy, cognitive impairment, anxiety and other) based on a review of the literature on factor and component analyses of the GDS (the groupings of the GDS items and supplementary references are available at the journal website).[29, 30] For these exploratory analyses, we included only patients who had no missing values for GDS items so that we could reliably generate the sub-scale scores, but we used multiple imputation for other missing variables. Most estimated regression coefficients were similar to those from our main analysis, so we present (in a figure) only the coefficient estimates for the GDS sub-scale scores with 95% confidence intervals.
Figure. Estimated regression coefficients and their 95% confidence intervals for the GDS sub-scale variables related to the depressed mood, apathy, anxiety, cognitive impairment, and other items of the GDS.
GDS = Geriatric Depression Scale; EQ-5D = European Quality of Life-5 Dimensions; QWB = Quality of Well-Being Index; VAS = Global Quality of Life Visual Analog Scale; QOL-AD = Quality of Life-Alzheimer’s Disease.
For each quality of life outcome variable, the regression coefficients were calculated from four fully adjusted regression models. Each regression analysis was adjusted for patient age, sex, marital status, first language, Charlson score, Alzheimer’s Disease Assessment Scale-Cognitive Subscale, Disability Assessment for Dementia, Neuropsychiatric Inventory and study center, as well as for the other GDS sub-scale variables. The QOL-AD scores were normalized to a 0–1 scale. The degrees of freedom for calculating the 95% confidence intervals ranged from 288 to 291.
Data were analyzed using R, version 2.7.0 (April 22, 2008).[31] Since we carried out many hypothesis tests, statistical significance was set at p < 0.01. Estimates of minimal clinically important differences for the QOL measures are .06–.07 for the EQ-5D, .03 for the QWB,.07–.10 for the VAS, and half a standard deviation for the QOL-AD.[32–34]
Results
We carried out assessments on 384 patients. Fourteen were excluded because of extensive missing data. Table 1 shows the characteristics of the 370 patients in the analytic cohort. The excluded patients were more likely to be female, unmarried and more cognitively and functionally impaired. The analytic cohort had a mean age of 80.7 (standard deviation [SD]: 7.8), a mean MMSE of 22.3 (SD: 4.3) (9.2% with scores of 11–15, 19.7% with scores of 16–20 and 71.1% with scores >20), and 48.4% were female. Sixty-nine patients (18.6%) had DAD scores < 50, 50 (13.5%) had self-rated GDS scores suggestive of clinical depression, and 59 (15.9%) had an NPI score of 0 and only 93 (25.1%) had NPI scores > 14.
Table 1.
Patient Characteristics
| Characteristic | Analytic Sample (n = 370)
|
||
|---|---|---|---|
| N | Mean (SD)/% | Median (IQR) | |
| Age | 370 | 80.7 (7.8) | 8.1 (75.7 – 86.5) |
| Female | 179/370 | 48.4% | |
| First Language English | 303/370 | 81.9% | |
| Education | 367 | ||
| < 9 Grades | 34 | 9.3% | |
| 9–13 Grades | 134 | 36.5% | |
| College or University | 199 | 54.2% | |
| Married | 295/370 | 79.7% | |
| Live Alone | 39/369 | 10.6% | |
| * Private Home | 338/370 | 91.4% | |
| Comorbidity | 370 | ||
| Charlson = 1 | 210 | 56.8% | |
| Charlson = 2 | 81 | 21.9% | |
| Charlson ≥ 3 | 79 | 21.3% | |
| Years since AD diagnosis | 358 | 2.2 (1.4) | 2.0 (1.0 – 3.0) |
| MMSE (Cognition) | 370 | 22.3 (4.3) | 23.0 (20.0 – 25.0) |
| ADAS (Cognition) | 370 | 26.2 (8.4) | 24.7 (20.4 – 31.2) |
| DAD (Function) | 369 | 70.0 (22.0) | 71.4 (52.8 – 89.5) |
| NPI (Behavior) | 368 | 10.6 (12.0) | 7.0 (2.0 – 15.0) |
| GDS (Depression) | 361 | 5.6 (4.5) | 5.0 (2.0 – 8.0) |
SD = Standard Deviation; IQR = Interquartile Range; AD = Alzheimer’s disease; MMSE = Mini-Mental State Examination; ADAS = Alzheimer’s Disease Assessment Scale-Cognitive Subscale; DAD = Disability Assessment for Dementia; NPI = Neuropsychiatric Inventory; GDS = Geriatric Depression Scale (patient rated).
Private home represents a house, condominium or apartment that the patient owns or rents. Most patients not living in a private home lived in a retirement home or a relative’s home.
Table 2 displays the correlations between QOL ratings using the different measures. The correlations were only weak to moderate (0.24–0.49).
Table 2.
Correlations* (95% Confidence Intervals) Between Quality of Life Measures
| EQ-5D
|
QWB
|
VAS
|
|
|---|---|---|---|
| EQ-5D | |||
| QWB | 0.49(0.41–0.56) | ||
| VAS | 0.31(0.21–0.40) | 0.24 (0.14–0.33) | |
| QOL-AD | 0.48(0.40–0.56) | 0.36 (0.26–0.44) | 0.47 (0.38–0.54) |
The correlation values are Pearson’s correlation coefficients.
EQ-5D = European Quality of Life-5 Dimensions; QWB = Quality of Well-Being Index; VAS = global quality of life Visual Analog Scale; QOL-AD = Quality of Life-Alzheimer’s Disease.
Table 3 summarizes analyses exploring the relationship between dementia symptom severity scores and the patient QOL ratings. With increasing GDS scores, there were significantly lower mean QOL ratings for all four QOL measures. With more behavioral abnormalities, as measured by the NPI, there were lower mean QOL ratings for all four QOL measures, but this was statistically significant only for the QOL-AD. There were no significant relationships between mean QOL ratings and severity scores for cognition (ADAS-Cog or MMSE [results not shown]) or functional disability (DAD).
Table 3.
Mean QOL Scores for Different Severity Levels of the Core Symptoms Variables
| N† (%) | EQ-5D (n=368) Mean SD |
QWB (n=369) Mean SD |
VAS (n=362) Mean SD |
QOL-AD (n=367) Mean SD |
|
|---|---|---|---|---|---|
|
|
|
|
|||
| ADAS (Cognition) | |||||
| 8–21 | 111 (30.0) | 0.89 (0.12) | 0.63 (0.15) | 0.78 (0.14) | 40.6 (5.6) |
| 22–25 | 89 (24.1) | 0.87 (0.13) | 0.61 (0.15) | 0.77 (0.15) | 39.1 (5.3) |
| 26–34 | 108 (29.2) | 0.92 (0.11) | 0.65 (0.16) | 0.77 (0.14) | 40.3 (5.1) |
| 35–70 | 62 (16.8) | 0.87 (0.16) | 0.65 (0.18) | 0.71 (0.17) | 38.6 (5.3) |
| DAD (Function) | |||||
| 0–49 | 69 (18.6) | 0.90 (0.14) | 0.67 (0.18) | 0.75 (0.16) | 39.5 (5.1) |
| 50–68 | 92 (24.9) | 0.90 (0.14) | 0.64 (0.17) | 0.75 (0.17) | 38.7 (5.3) |
| 69–88 | 109 (29.5) | 0.88 (0.12) | 0.62 (0.15) | 0.77 (0.13) | 39.9 (4.8) |
| 89–100 | 100 (27.0) | 0.89(0.13) | 0.62(0.13) | 0.77(0.14) | 41.0(6.1) |
| NPI (Behavior) | |||||
| 0 | 59 (15.9) | 0.92 (0.11) | 0.69 (0.16) | 0.81 (0.12) | 42.1 (4.9) |
| 1–6 | 120 (32.4) | 0.91 (0.12) | 0.64 (0.15) | 0.77 (0.14) | 40.3 (5.5) |
| 7–14 | 98 (26.5) | 0.87 (0.13) | 0.63 (0.16) | 0.73 (0.16) | 39.2 (5.2) |
| 15–73 | 93 (25.1) | 0.87 (0.14) | 0.61 (0.15) | 0.76 (0.15) | 38.5 (5.3)* |
| GDS (Depression) | |||||
| 0–4 | 175 (47.3) | 0.94 (0.09) | 0.69 (0.15) | 0.81 (0.13) | 42.1 (4.6) |
| 5–10 | 145 (39.2) | 0.88 (0.12) | 0.60 (0.14) | 0.74 (0.14) | 38.9 (4.6) |
| 11–13 | 28 (7.6) | 0.80 (0.14) | 0.55 (0.14) | 0.69 (0.15) | 35.5 (5.4) |
| 14–30 | 22 (5.9) | 0.70 (0.21)** | 0.48 (0.12)** | 0.68 (0.19)** | 33.2 (5.9)** |
EQ-5D = European Quality of Life-5 Dimensions; QWB = Quality of Well-Being Index; VAS = global quality of life Visual Analog Scale; QOL-AD = Quality of Life-Alzheimer’s Disease; ADAS = Alzheimer’s Disease Assessment Scale-Cognitive sub-scale; DAD = Disability Assessment for Dementia; NPI = Neuropsychiatric Inventory; GDS = Geriatric Depression Scale.
The EQ-5D, QWB and VAS are rated on 0–1 scales; The QOL-AD is rated on a 13–52 scale.
p<0.001;
p<0.0001;
the p-values comparing the mean quality of life scores across the levels of the symptom severity scores were derived from the overall F-test in a one-way analysis of variance. All tests had numerator degrees of freedom (df) of 3 and denominator df of 365, 366, 359 and 364 for the EQ-5D, QWB, VAS and QOL-AD, respectively.
N = the number of participants from the full analytic cohort (n=370) in each severity level for each of the core dementia symptom measures. Because not all cohort participants completed each quality of life measure, the number of participants in each core symptom severity level varied slightly among the quality of life measures compared with the total cohort. These differences were 2 or less participants per severity level except for the following core symptom severity levels for the VAS: ADAS 35–70 (n=57, 15.7%), DAD 0–49 (n=64, 17.7%), NPI 0 (n=56, 15.5%), NPI 15–73 (n=90, 24.9%), GDS 0–4 (n=171, 47.2%), and GDS 5–10 (n=142, 39.2%).
Table 4 displays the results from fully-adjusted linear regression analyses exploring the predictors of patient ratings of their own QOL. Of the core dementia symptoms, the GDS score was the only consistent significant independent predictor of patients’ QOL ratings for the four QOL measures and the GDS explained by far the largest variance in the QOL scores of any of the predictor variables. The ADAS-Cog, MMSE (results not shown) and DAD scores were not significant independent predictors of QOL ratings for any of the measures. The NPI score was only a significant independent predictor of the QOL-AD, but it accounted for a very small amount of the variance of the QOL-AD ratings.
Table 4.
Predictors of QOL from Fully Adjusted Regression Analyses
| Variables | EQ-5D (n=368) (Model R2 = 0.33) |
QWB (n=369) (Model R2 = 0.28) |
VAS (n=362) (Model R2 = 0.17) |
QOL-AD (n=367) (Model R2 = 0.42) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| β | P | R2* | β | P | R2* | β | P | R2* | β | P | R2* | |
| Age | −0.011 | 0.007 | 0.01 | −0.005 | 0.299 | 0.00 | −0.001 | 0.923 | 0.00 | −0.220 | 0.173 | 0.00 |
| Sex (Male) | 0.050 | < 0.0001 | 0.02 | −0.018 | 0.275 | 0.01 | 0.001 | 0.944 | 0.00 | 1.033 | 0.037 | 0.00 |
| Education | 0.468 | 0.00 | 0.355 | 0.00 | 0.243 | 0.01 | 0.161 | 0.01 | ||||
| Marital Status | 0.040 | 0.013 | 0.00 | 0.018 | 0.363 | 0.00 | 0.022 | 0.278 | 0.00 | 0.762 | 0.221 | 0.00 |
| First Language | 0.001 | 0.942 | 0.00 | −0.023 | 0.243 | 0.00 | 0.009 | 0.673 | 0.00 | 0.790 | 0.203 | 0.01 |
| Charlson Score | 0.694 | 0.00 | 0.584 | 0.01 | 0.224 | 0.01 | 0.214 | 0.01 | ||||
| ADAS (Cognition) | −0.003 | 0.658 | 0.00 | 0.004 | 0.645 | 0.00 | −0.021 | 0.021 | 0.01 | −0.452 | 0.100 | 0.01 |
| DAD (Function) | −0.011 | 0.158 | 0.00 | −0.016 | 0.090 | 0.01 | 0.003 | 0.764 | 0.00 | 0.168 | 0.574 | 0.01 |
| NPI (Behavior) | −0.012 | 0.071 | 0.01 | −0.013 | 0.118 | 0.01 | 0.004 | 0.625 | 0.00 | −0.803 | 0.002 | 0.04 |
| GDS (Depression) | −0.068 | < 0.0001 | 0.25 | −0.066 | < 0.0001 | 0.17 | −0.048 | < 0.0001 | 0.10 | −2.825 | < 0.0001 | 0.28 |
| Study Center | 0.382 | 0.02 | 0.006 | 0.06 | 0.107 | 0.04 | 0.002 | 0.06 | ||||
EQ-5D = European Quality of Life-5 Dimensions; QWB = Quality of Well-Being Index; VAS = global quality of life Visual Analog Scale; QOL-AD = Quality of Life-Alzheimer’s Disease; β = β-coefficient; P = P-value; R2* = LMG R2, the average of R2 increments from adding the variable to models without the variable, across all of the possible models; Charlson score = measure of comorbidity; ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive sub-scale; DAD = Disability Assessment for Dementia; NPI = Neuropsychiatric Inventory; GDS = Geriatric Depression Scale.
All single parameter tests are t-tests with estimated degrees of freedom (df) ranging between 253 and 344. All multiple parameter tests are F-tests and these included: tests for education (numerator df = 2, denominator df > 300), the Charlson comorbidity score (numerator df = 2, denominator df > 300), and study center (numerator df = 9, denominator df > 300).
The Figure shows the regression coefficients for the five GDS sub-scale scores in predicting patient-rated QOL for the four QOL measures, adjusting for all patient demographic and comorbidity variables. Patient scores on the GDS cognition, depression and apathy items had an important impact on patient-rated QOL.
Discussion
We conducted a comprehensive study of the predictors of patient-rated QOL in AD using a wide range of predictor variables and a diversity of QOL measures that had only weak to moderate correlations with each other. Although our results are largely confirmatory, they add substantially to the literature. We show that in a large outpatient sample representative of AD clinical trial populations, the patient factors that most strongly predict patient-rated QOL are consistent across four distinct measures of QOL (i.e., a disease-specific measure, two utility indexes with differing classification and scoring systems and a directly elicited global QOL measure). Patient-rated depressive symptoms were an important independent predictor of patient-rated QOL for all four measures studied, while cognitive and functional status were not.
Our findings confirm previous reports that depressive symptoms are an important predictor of patient-rated QOL as measured by the QOL-AD,[6–8, 35] a single-item global QOL measure,[10] and the Health Utilities Index, version 2 (HUI2).[11] Our finding that depressive symptoms were an independent predictor of global QOL ratings measured on a visual analogue scale with no domains indicates that the relationship between depressive symptoms and patient-rated QOL cannot be explained entirely by inclusion bias (i.e. the inclusion of a mood domain in the other QOL measures). Despite the presence of a functional status domain in the EQ-5D, QWB and QOL-AD and a cognitive/memory domain in the QWB and QOL-AD, patient function and cognition were not independent predictors of QOL ratings for any of these measures. However, both the QOL and depression scores reflected patients’ perceptions, while the evaluation of the other core symptoms were either performance-based (cognition) or caregiver-derived (function and behavioral symptoms), which may have influenced our results. Of note, depressive symptoms remained a significant independent predictor of patient-rated QOL for all measures when caregiver ratings of patient depressive symptoms were substituted for patient self-ratings in the regression models (results not shown), substantiating their importance irrespective of the source of this information.
Our results also confirm previous findings that cognitive function measured by the MMSE is not an independent predictor of patient-rated QOL as measured by the QOL-AD, [6, 8, 35] a single-item global QOL measure,[10] the EQ-5D and the HUI2. [11] Hurt et al. recently reported that in a population with mild to moderate dementia, the ADAS-Cog, a more sensitive measure of cognitive impairment in AD than the MMSE and the primary outcome measure in most AD clinical trials, was not associated with patient QOL ratings using the disease-specific DEMQOL measure.[9] Our study adds to this finding by showing that cognitive function measured by the ADAS-Cog was not an independent predictor of patient-rated QOL for any of the 4 measures we used.
Although performance-based cognitive measures such as the ADAS-Cog and MMSE are useful outcomes in dementia clinical trials, the finding that scores on these measures do not predict patient-rated QOL raises concerns that these measures may not be assessing what is most valued by patients with AD. Therefore, the importance of performance-based cognitive measures in clinical trials should not be over-emphasized. Our finding that depressive symptoms strongly predict patient-rated QOL across a range of QOL measures supports the inclusion of a measure of depressive symptoms in AD clinical trials.
We did not find patient function measured by the DAD (caregiver elicited) to be an important independent predictor of patient-rated QOL. The literature is inconsistent regarding the relationship between measures of patient function and patient-rated QOL. [6, 8, 11, 35, 36] These inconsistencies may be at least partially related to differences in the source of information about the patient’s functional status and the measure used.
We found that patient behavioral symptoms, as measured by the total NPI score, were only a significant independent predictor of the disease-specific QOL-AD, but even for this measure the NPI accounted for a very small amount of the variance of the QOL ratings. A previous study did not find the NPI total score to be an independent predictor of patient-rated QOL-AD scores.[7] In addition, a recent review of the predictors of QOL in dementia noted that behavioral disturbance, as measured by various instruments, has not been found to be associated with patient self-ratings of their QOL.[37] However, the total NPI scores in both the current and previous studies have generally been low, reducing the chance of identifying a relationship.
In our multivariable analyses of the predictors of patient-rated QOL (Table 3), the model R2 values ranged from 0.17 to 0.42. Therefore, a large portion of the variance remains unexplained by the symptoms of dementia, as measured by the instruments used in our study, and by demographic and comorbidity factors. This supports the need to include direct measures of QOL in dementia clinical trials since QOL will capture dimensions beyond the typical dementia symptom outcomes.
In our exploratory analysis of the GDS sub-scales (Figure), we found that the GDS cognition items had a significant impact on patient-rated QOL for all four measures, which contrasted with our finding from the main analysis (Table 4) that performance-based cognitive measures did not predict patient-rated QOL. This may be because the patient-rated GDS cognition items reflected the patients’ perception of their cognitive ability, while the performance-based measures did not. Karlawish et al. similarly noted that patients’ awareness of cognitive problems on GDS memory items was associated with QOL ratings on the EQ-5D and HUI2, but a measure of insight into cognitive deficits was not.[11] Vogel et al. did not find a significant difference in QOL-AD or EQ-5D ratings between patients with full versus impaired cognitive insight.[38] The impact of insight on patient-rated QOL requires further study.
Major strengths of this study are that it is the largest of its kind, it included a comprehensive spectrum of QOL measures and predictor variables, and it used fully-adjusted regression analyses. This study also has some limitations. We excluded 14 patients from the analyses because of missing data. Although the excluded participants differed from those in the analytic sample, they made up only 3.4% of the total sample, so their exclusion is unlikely to have significantly affected the results. The results may not be generalizable to patients with severe dementia, but should be generalizable to typical AD clinical trial populations with mild-to-moderate dementia. Over half (53.6%) of the study sample had at least some college or university education. Therefore, the results may not be valid for patients with limited formal education.
In conclusion, in a large national sample of patients with mild to moderate AD, we showed that predictors of patient-rated QOL are consistent across diverse QOL measures that differ in methodological approach and content. We confirmed that patient-rated depressive symptoms are the most important predictor of patient-rated QOL and showed that the severity of performance-based cognitive impairment, even as measured by the ADAS-Cog, is not an independent predictor of patient-rated QOL. Our findings confirm the importance of identifying and treating depression in patients with AD in order to enhance their QOL and support the need to include outcomes in AD clinical trials that are of importance to patients, such as depressive symptoms and QOL.
Supplementary Material
Appendix.
| Depressed Mood |
| Satisfied with life |
| Hopeful about the future |
| In good spirits most of time |
| Happy most of the time |
| Often feel helpless |
| Wonderful to be alive |
| Often downhearted and blue |
| Feel pretty worthless |
| Situation is hopeless |
| Most people are better off |
| Frequently feel like crying |
| Enjoy getting up in the morning |
| Apathy |
| Dropped many activities, interests |
| Prefer to stay home |
| Hard to get started on a new project |
| Feel full of energy |
| Prefer to avoid social gatherings |
| Cognitive Impairment |
| Problems with memory |
| Have trouble concentrating |
| Mind as clear as it used to be |
| Anxiety |
| Bothered by thoughts |
| Afraid something bad is going to happen |
| Worry about the future |
| Worry about the past |
| Other |
| Life is empty |
| Often get bored |
| Find life exciting |
| Upset over little things |
| Easy to make decisions |
| Restless and fidgety |
Acknowledgments
Dr. Naglie receives funding support from the Mary Trimmer Chair in Geriatric Medicine, University of Toronto; Dr. Hogan receives funding support from the Brenda Strafford Foundation Chair in Geriatric Medicine, University of Calgary; Dr. Krahn receives funding support from the F. Norman Hughes Chair in Pharmacoeconomics; Dr. Black receives salary support from the Sunnybrook Research Institute, Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto, including the Brill Chair in Neurology, Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto; Dr. Freedman is supported by the Saul A. Silverman Family Foundation, Toronto, Ontario, Canada, as part of a Canada International Scientific Exchange Program (CISEPO) Project.
Funding for this study was provided by the Canadian Institutes of Health Research (MOP-57686 and MOP-77770) and the Alzheimer Society of Canada (#05-85).
We would like to acknowledge Dr. K. Jennifer Ingram of the Kawartha Regional Hospital Memory Clinic and Dr. Victoria Lee of the Toronto East General Hospital, for their assistance with patient recruitment. We would like to thank the research coordinators and staff at all of our study sites.
Footnotes
Data presented in part at the American Geriatrics Society Annual Scientific Meeting, Washington, DC, May 1–3, 2008 and the Canadian Geriatrics Society Annual Meeting, Montreal, Quebec, Canada, April 10–12, 2008.
There are no conflicts to report relating to this study.
Contributor Information
Gary Naglie, Division of General Internal Medicine, University Health Network; Geriatrics Program, Toronto Rehabilitation Institute; Departments of Medicine and Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada.
David B. Hogan, Brenda Strafford Foundation Chair in Geriatric Medicine, University of Calgary, Calgary, Alberta, Canada.
Murray Krahn, Toronto Health Economics and Technology Assessment Collaborative, Faculty of Pharmacy, University of Toronto; Division of General Internal Medicine, University Health Network, Toronto, Ontario, Canada.
B. Lynn Beattie, Division of Geriatric Medicine, University of British Columbia, Vancouver, British Columbia, Canada.
Sandra E. Black, Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, Ontario, Canada.
Christopher MacKnight, Queen Elizabeth II Health Sciences Centre; Division of Geriatric Medicine, Dalhousie University, Halifax, Nova Scotia, Canada.
Morris Freedman, Division of Neurology and Rotman Research Institute, Baycrest; Division of Neurology, Department of Medicine, University of Toronto; Division of Neurology, Department of Medicine, Mt. Sinai Hospital and University Health Network, Toronto, Ontario, Canada.
Christopher Patterson, Geriatric Services, Hamilton Health Sciences; Division of Geriatric Medicine, McMaster University, Hamilton, Ontario, Canada.
Michael Borrie, Division of Geriatric Medicine, Parkwood Hospital; Division of Geriatric Medicine, Schulich School of Medicine and Dentistry Medicine, University of Western Ontario, London, Ontario, Canada.
Howard Bergman, Division of Geriatric Medicine, McGill University and Jewish General Hospital, Montreal, Quebec, Canada.
Anna Byszewski, Division of Geriatrics, The Ottawa Hospital, Ottawa Health Research Institute and the University of Ottawa; Regional Geriatric Program of Eastern Ontario, Ottawa, Ontario, Canada.
David Streiner, Department of Psychiatry, University of Toronto; Kunin-Lunenfeld Applied Research Unit, Baycrest, Toronto, Ontario, Canada.
Jane Irvine, Department of Psychology, York University, Toronto, Ontario, Canada.
Paul Ritvo, Department of Psychology, York University; Division of Preventive Oncology, Cancer Care Ontario, Toronto, Ontario, Canada.
Janna Comrie, Department of Psychology, York University, Toronto, Ontario, Canada.
Matthew Kowgier, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada.
George Tomlinson, University Health Network; Dalla Lana School of Public Health, and Departments of Medicine, Medical Imaging, and Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada.
References
- 1.Burns A, Iliffe S. Alzheimer’s disease. BMJ. 2009;338:b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 2.Raina P, Santaguida P, Ismaila A, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: evidence review for a clnical practice guideline. Ann Intern Med. 2008;148:379–397. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 3.Mack JL, Whitehouse PJ. Quality of Life in Dementia: State of the Art - Report of the International Working Group for Harmonization of Dementia Drug Guidelines and the Alzheimer’s Society Satellite Meeting. Alzheimer Dis Assoc Disord. 2001;15:69–71. doi: 10.1097/00002093-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–9. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 5.Kopec JA, Willison KD. A comparative review of four preference-weighted measures of health-related quality of life. Journal of Clinical Epidemiology. 2003;56:317–325. doi: 10.1016/s0895-4356(02)00609-1. [DOI] [PubMed] [Google Scholar]
- 6.Logsdon R, Gibbons L, McCurry S, et al. Quality of life in Alzheimer’s Disease: patient and caregiver reports. Journal of Mental Health and Aging. 1999;5:21–32. [Google Scholar]
- 7.Fuh J-L, Wang S-J. Assessing quality of life in Taiwanese patients with Alzheimer’s disease. Int J Geriat Psychiatry. 2006;21:103–107. doi: 10.1002/gps.1425. [DOI] [PubMed] [Google Scholar]
- 8.Snow AL, Souchek RDJ, Sullivan G, et al. Comorbid psychosocial symptoms and quality of life in patients with dementia. Am J Geriatr Psychiatry. 2005;13:393–401. doi: 10.1176/appi.ajgp.13.5.393. [DOI] [PubMed] [Google Scholar]
- 9.Hurt CS, Banerjee S, Tunnard C, et al. Insight, cognition and quality of life in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2010;81:331–336. doi: 10.1136/jnnp.2009.184598. [DOI] [PubMed] [Google Scholar]
- 10.James BD, Kie SX, Karlawish JHT. How do patients with Alzheimer disease rate their overall quality of life. Am J Geriatr Psychiatry. 2005;13:484–490. doi: 10.1176/appi.ajgp.13.6.484. [DOI] [PubMed] [Google Scholar]
- 11.Karlawish JH, Zbrozek A, Kinosian B, et al. Preference-based quality of life in patients with Alzheimer’s disease. Alzheimer’s & Dementia. 2008;4:193–202. doi: 10.1016/j.jalz.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 13.Naglie G, Tomlinson G, Tansey C, et al. Utility-based quality of life measures in Alzheimer’s disease. Qual Life Res. 2006;15:631–643. doi: 10.1007/s11136-005-4364-8. [DOI] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Molloy DW, Alemayehu E, Roberts R. A Standardized Mini-Mental State Examination (SMMSE): its reliability compared to the traditional Mini-Mental State Examination (MMSE) Am J Psychiatry. 1991;148:102–105. doi: 10.1176/ajp.148.1.102. [DOI] [PubMed] [Google Scholar]
- 16.Gelinas I, Gauthier L, McIntyre M, et al. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471–81. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 17.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 18.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of Psychiatric Research. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 19.Brink TL, Yesavage JA, Lum O, et al. Screening tests for geriatric depression. Clinical Gerontologist. 1982;1:37–43. [Google Scholar]
- 20.Logsdon R, Gibbons L, McCurry S, et al. Assessing quality of life in older adults with cognitive impairment. Psychosom Med. 2002;64:510–519. doi: 10.1097/00006842-200205000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Dolan P. Modelling valuations for EuroQol health states. Med Care. 1997;35:1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan RM, Anderson JP. A general health policy model: update and applications. Health Serv Res. 1988;23:203–35. [PMC free article] [PubMed] [Google Scholar]
- 23.Gelman A, Hill J, Su YS, et al. mi: missing data imputation and model checking. R package version 0.09–02. 2010 http://CRAN.R-project.org/package=mi.
- 24.Wall JR, Lichtenberg PA, MacNeill SE, et al. Depression detection in geriatric rehabilitation: Geriatric Depression Scale short form vs. long form. Clinical Gerontologist. 1999;20:13–21. [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Barnard J, Rubin DB. Small-sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–955. [Google Scholar]
- 27.Li KH, Raghunathan TE, Rubin DB. Large-sample significance levels from multiply imputed data using moment-based statistics and an F reference distribution. J Amer Statistical Assoc. 1991;86:1065–1073. [Google Scholar]
- 28.Lindeman RH, Merenda PF, Gold RZ, editors. Introduction to Bivariate and Multivariate Analysis. Scott, Foresman & Co; Glenview, IL: 1980. p. 119. [Google Scholar]
- 29.Adams KB. Depressive symptoms, depletion, or developmental change? Withdrawal, apathy, and lack of vigor in the Geriatric Depression Scale. Gerontologist. 2001;41:768–777. doi: 10.1093/geront/41.6.768. [DOI] [PubMed] [Google Scholar]
- 30.Adams KB, Matto HC, Sanders S. Confirmatory factor analysis of the Geriatric Depression Scale. Gerontologist. 2004;44:818–826. doi: 10.1093/geront/44.6.818. [DOI] [PubMed] [Google Scholar]
- 31.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 32.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and Quality of Life Outcomes. 2007;5:70–77. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life. The remarkable universality of half a standard deviation. Med Care. 2003;41:582–592. doi: 10.1097/01.MLR.0000062554.74615.4C. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan RM. The minimally clinically important difference in generic utility-based measures. COPD: Journal of Chronic Obstructive Disease. 2005;2:91–97. doi: 10.1081/copd-200052090. [DOI] [PubMed] [Google Scholar]
- 35.Hoe J, Katona C, Orrell M, et al. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry. 2007;22:1031–1036. doi: 10.1002/gps.1786. [DOI] [PubMed] [Google Scholar]
- 36.Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, et al. Factors related to perceived quality of life in patients with Alzheimer’s disease: the patient’s perception compared with that of caregivers. Int J Geriat Psychiatry. 2009;24:585–594. doi: 10.1002/gps.2161. [DOI] [PubMed] [Google Scholar]
- 37.Banerjee S, Sanmsi K, Petrie CD, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and the explanatory value of disease specific measures of health related quality of life in people with dementia. Int J Geriat Psychiatry. 2009;24:15–24. doi: 10.1002/gps.2090. [DOI] [PubMed] [Google Scholar]
- 38.Vogel A, Mortensen EL, Hasselbalch SG, et al. Patient versus informant reported quality of life in the earliest phases of Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2006;21:1132–1138. doi: 10.1002/gps.1619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.