Abstract
Rationale
Smoking rates are up to five times higher in people with schizophrenia than in the general population, placing these individuals at high risk for smoking-related health problems. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, is a promising aid for smoking cessation in this population. To optimize benefits to risks of treatment, it is critical to identify reliable predictors of positive response to varenicline in smokers with schizophrenia.
Objectives
Negative symptoms of schizophrenia are related to dysfunctions in the brain reward system, are associated with nicotine dependence, and may be improved by nicotine or nicotinic receptor agonists, suggesting that smoking cessation may be especially difficult for patients with substantial negative symptoms. The purpose of the study was to evaluate negative symptoms as predictors of response to varenicline.
Methods
Patients with schizophrenia (N=53) completed a 12-week smoking cessation trial combining varenicline with cognitive behavioral therapy. Negative symptoms were assessed via the Scale for the Assessment of Negative Symptoms (SANS; Andreasen 1983). Outcomes included smoking abstinence as assessed by self-report and expired carbon monoxide (CO). Change in performance on a probabilistic reward task was used as an index of change in reward sensitivity during treatment.
Results
At week 12, 32 participants met criteria for 14-day point-prevalence abstinence. Patients with lower baseline symptoms of affective flattening (more typical affect) were more likely to achieve smoking abstinence, and demonstrated larger increases in reward sensitivity during treatment.
Conclusions
These data suggest that affective flattening symptoms in smokers with schizophrenia may predict response to varenicline.
Keywords: Nicotine, Schizophrenia, Varenicline, Reward, Anhedonia, Affective flattening
INTRODUCTION
Cigarette smoking is a leading cause of preventable death worldwide (Fagerstrom 2002), predisposing smokers to a wide variety of diseases including cancer, coronary heart disease, and strokes. In schizophrenia, rates of smoking are two to five times higher than those of the general population (Bobes et al. 2010). Correspondingly, rates of mortality from vascular disease and cancer are elevated in people with schizophrenia (Bruce et al. 1994), who would benefit disproportionately from smoking cessation (Bobes et al. 2010).
Converging lines of evidence suggest that tobacco-derived nicotine reduces negative symptoms of schizophrenia (e.g., apathy, anhedonia, and affective flattening) (Dalack et al. 1998; Smith et al. 2002), and also cognitive symptoms characteristic of the illness acutely (e.g., difficulties with attention, concentration and memory), even in non-smokers (Barr et al. 2008a; Jubelt et al. 2008), and that people with schizophrenia may smoke in part for this reason (Winterer 2010). Smokers with schizophrenia report more negative symptoms than nonsmokers (Hall et al. 1995), and the severity of negative, but not positive, symptoms has been found to correlate positively with the severity of nicotine dependence (Fukui et al. 1995; Patkar et al. 2002). One potential explanation for this relationship is that nicotine may help to ameliorate a tonic hypodopaminergic state hypothesized to be associated with negative symptoms (Dalack et al. 1998) by increasing phasic dopamine release in the brain (Glassman 1993). Indeed, cigarette smoking stimulates transient dopamine release in the ventral striatum (Brody et al. 2004), a region critical to producing the affective and behavioral responses to rewards that are characteristically diminished in the negative symptoms of schizophrenia (Juckel et al. 2006). Along similar lines, nicotine has also been found to ameliorate some cognitive deficits in patients with schizophrenia (Barr et al. 2008a; Smith et al. 2002), which have been linked, in part, to reduced dopaminergic functioning in the prefrontal cortex (Davis et al. 1991). Smoking may be of additional therapeutic benefit to schizophrenia patients treated with typical neuroleptics, reducing side effects such as tardive dyskinesia (Silvestri et al. 2004) and Parkinsonism (Goff et al. 1992). These symptoms arise as a result of these medications’ tight binding to dopamine D2 receptors in the substantia nigra, which play a crucial role in motor functioning (Tuppurainen et al. 2009). In contrast, atypical antipsychotic medications demonstrate selective mesolimbic effects, bind loosely and are quickly released from D2 receptors, greatly reducing the risk of such side effects (Kinon and Lieberman 1996; Seeman 2006).
Evidence from experimental animals suggests that midbrain dopamine neurons, central to the brain’s reward system, fire according to two distinct but interacting systems (Bunney et al. 1991; Grace 1991). These include a slow, irregular pattern of tonic or sustained dopamine release activated by glutamate release from prefrontal cortical afferents, and complementary phasic, or transient dopamine burst responses to motivationally-relevant stimuli in the environment. In individuals with schizophrenia, reduced prefrontal cortical activity has been hypothesized to result in blunted tonic dopamine levels, a model which is consistent with observations of decreased glutamate levels in the cerebrospinal fluid of these patients (Kim et al. 1980). The tonic dopamine system regulates the intensity of phasic dopamine responses to motivationally-relevant stimuli via homeostatic mechanisms, and thus it is likely that the marked reduction in tonic dopamine release observed in schizophrenia elicits increased phasic dopamine responses to stimuli as a result of cigarette smoking (Grace 1991). In this way, tonic hypodopaminergic states can be phasically normalized through increased dopamine release in response to tobacco-derived nicotine. Importantly, phasic dopamine bursts are critically implicated in reinforcement learning (Montague et al. 1996; Schultz 2002) and motivational processes (Berridge et al. 2009), and are instrumental to the initial reinforcing properties of nicotine (Nissell et al. 1995) as well as reward-related learning (Barr et al. 2008b). Repeated voluntary smoking in response to these reinforcing effects may lead to habitual and eventually compulsive smoking behavior culminating in addiction (Everitt and Robbins 2005).
While such phasic dopamine bursts in response to nicotine may help to reduce negative symptoms in the short term, chronic stimulant exposure reduces dopamine receptor density in experimental animals (Ginovart et al. 1999; Moore et al. 1998), suggesting that chronic smoking may exacerbate dopaminergic dysfunction in people with negative symptoms of schizophrenia. Consistent with this hypothesis, chronic smoking has been associated with reduced dopamine D3 receptor expression in blood lymphocytes (Czermak et al. 2004) and reduced ventral striatal dopamine D1 receptor binding (Dagher et al. 2001). Such findings suggest that chronic smoking may result in blunted dopaminergic transmission and lend additional importance to early intervention for smokers with schizophrenia.
Varenicline is an FDA-approved smoking cessation aid which acts as a selective partial agonist at α4β2 nicotinic acetylcholine receptors (nAChR). It has been reported to be more effective than other treatments including bupropion and nicotine replacement therapy (Cahill et al. 2008). Varenicline binds to nAChR in the ventral tegmental area (VTA), stimulating moderate dopamine release and blocking nAChR (Foulds 2006). Because dopaminergic neurons originating in the VTA project throughout the mesocorticolimbic system, the effects of varenicline mimic those of nicotine on dopamine release in the nucleus accumbens, reducing nicotine self-administration (Fagerstrom and Balfour 2006). Thus, by blocking the binding of nicotine to nAChR, reducing the reward value of smoking, and by increasing dopamine release in the brain toward more normal levels during nicotine withdrawal, varenicline is an especially promising aid for smoking cessation in individuals with schizophrenia.
Given the centrality of the reward system to negative symptoms of schizophrenia, smoking behavior, and the mechanism of action of varenicline, changes in reward processing are likely to play a role in the efficacy of varenicline treatment for smoking cessation in patients with schizophrenia. The probabilistic reward task described by Pizzagalli et al (2005) is a robust, objective, laboratory-based measure of reward sensitivity. We have previously demonstrated that administration of nicotine in non-smokers enhances reward sensitivity as assessed with this task (Barr et al. 2008b). In this study, the task was administered as an objective index of change in brain reward system functioning, as a supplement to self-report of negative symptoms and reward responsiveness. In addition, because smoking behavior is subject to multiple factors outside the control of experimenters, the probabilistic reward task can provide an additional, more proximal measure of the effect of medication on reward responsiveness.
Prospective controlled trials of varenicline for smoking cessation in schizophrenia have not yet been published. Uncontrolled case reports and case series have reported inconsistent effects, with some reporting good efficacy and tolerability and even cognitive symptom improvement (Anghelescu 2009; Evins and Goff 2008; Fatemi 2008; Smith et al. 2009), while others have reported increased psychosis (Cinemre et al. 2010; Freedman 2007), paranoia and irritability (Gholson 2008), and mood symptoms (Alhatem and Black 2009; Popkin 2008). Thus, it is critical to identify reliable predictors of good response to varenicline in order to maximize efficacy while minimizing putative negative consequences of varenicline.
Based on the positive association between negative symptom severity and the severity of nicotine dependence (Fukui et al. 1995; Patkar et al. 2002), the effectiveness of nicotine as an agent of self-medication for negative symptoms (Smith et al. 2002), and demonstrated increases in negative symptoms in schizophrenia patients during nicotine withdrawal (Dalack et al. 1998), we hypothesized that individuals with less severe negative symptoms at baseline would respond most positively to varenicline, both in terms of increases in overall reward sensitivity and smoking cessation. To test this hypothesis, we analyzed the predictive validity of five validated subscales of the Schedule for the Assessment of Negative Symptoms (Andreason 1983), affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention, in predicting responses to varenicline in smokers with schizophrenia who took part in a 13-week prospective trial of varenicline.
METHODS
Study Population and Recruitment
The study protocol was approved by the Massachusetts General Hospital Institutional Review Board. Participants were recruited from five community mental health clinics in Massachusetts, New Hampshire, and Michigan. Prior to taking part in any study procedures, subjects underwent an informed consent process that included a full explanation of study procedures and its potential risks and benefits and completed a formal written determination of competency to consent to participate.
Eligible subjects were men and women, aged 18 – 70 with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder by SCID interview and chart review, who were clinically stable, on a stable dose of anti-psychotic medication for at least one month, and not acutely at risk for suicide. Eligible participants reported smoking 10 or more cigarettes per day for at least the prior 6 months, had an expired carbon monoxide (CO) level of > 9ppm and were willing to set a quit date within 2–3 weeks from enrollment. Women of childbearing potential were required to agree to an approved method of contraception for inclusion in the study.
Exclusion criteria included lifetime history of dementia, neurodegenerative disease, or other organic mental disorder, substance use disorder other than nicotine or caffeine in the prior 6 months, major depressive disorder in the prior 6 months, inpatient hospitalization for suicidal ideation in the prior 12 months, current suicidal or homicidal ideation, current unstable medical illness, renal insufficiency with estimated creatinine clearance <40 ml/min, plan to continue use of tobacco products other than cigarettes (e.g., cigar, pipe), or use of an investigational medication or device in the past 30 days.
Intervention
After initial screening, eligible participants were asked to return for a baseline visit in which clinical scales, neuropsychological tasks, and smoking measures were administered. Then, participants began varenicline and 12 weekly, one-hour group cognitive-behavioral therapy (CBT) sessions intended to promote smoking cessation. Smoking behavior, nicotine withdrawal, and adverse events were assessed weekly. Subjects received varenicline: 0.5 mg per day for 3 days, then 0.5 mg bid for 4 days, followed by 1 mg twice daily for 11 weeks. The target quit date was between weeks 4 and 5. Subjects who quit and then resumed smoking were encouraged to set a second quit date. At the end of open-phase, abstinence was assessed by self-report of smoking no cigarettes for the past 7 days and CO < 9 ppm for two consecutive weekly visits. At the end of 12 weeks, or at the time of early termination, clinical scales, neuropsychological tasks, and other measurements performed at baseline were repeated to assess change.
Measures
Probabilistic Reward Task
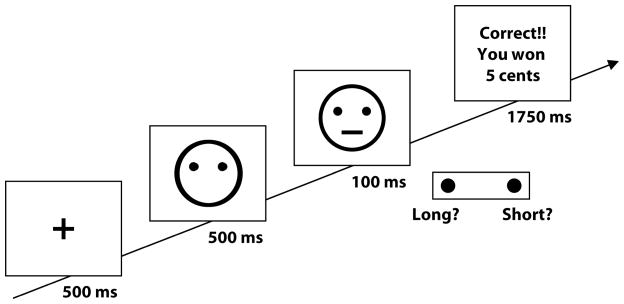
Participants completed a probabilistic reward task (see Figure I for task schematic) at baseline and week 12 or early termination. The task, adapted from Tripp and Alsop (1999), was designed to objectively assess reward responsiveness (i.e., participants’ propensity to modulate behavior as a function of reinforcement history). This task has been described in detail elsewhere (Pizzagalli et al. 2005), and validated in multiple, independent samples (e.g., Barr et al. 2008b; Pizzagalli et al. 2008, 2009). Participants completed three blocks of 100 trials, in which they determined whether a briefly presented mouth on a cartoon face was ‘long’ (13 mm) or ‘short’ (11.5 mm), and reported their decision by pressing one of two corresponding keys on a computer keyboard. An asymmetrical reinforcement ratio was implemented in each block so that one of the two stimuli (the ‘rich’ stimulus) was rewarded (“Correct!! You Won 5 Cents”) three times more frequently than the ‘lean’ stimulus (30/50 vs. 10/50 trials). In healthy participants, such asymmetric reinforcement schedule has been reliably found to induce a systematic preference (response bias) to choose the stimulus paired with more frequent reward (Pizzagalli et al. 2005, 2008, 2009). Patients with schizophrenia have displayed normal development of a response bias toward the more frequently rewarded stimulus in this task, suggesting intact reward sensitivity in this population (Heerey et al. 2008). In spite of these prior null findings, it is important to emphasize that this prior study did not provide information about possible differences in smoking status between healthy controls and individuals with schizophrenia (Heerey et al, 2008). In light of findings highlighting the effect of nicotine on response bias (Barr et al. 2008b), studies in schizophrenia controlling for smoking status are warranted.
Fig. I.
Schematic illustration of the Probabilistic Reward Task.
Scale for the Assessment of Negative Symptoms (SANS)
The SANS is a standard, clinician-rated assessment tool for negative symptoms of schizophrenia that contains five subscales pertaining to specific symptoms (Andreasen 1983). The affective flattening subscale assesses impoverished emotional expressions and reactivity. Affective flattening has been associated with dopamine receptor dysfunction and blunted motivation to seek rewards (Schmidt et al. 2001). The alogia subscale probes impoverished thinking and cognition, and has been linked to frontostriatal dysfunction (Joyce et al. 1996). The avolition/apathy subscale captures diminished energy, drive, and interest in relationships and the world, and high scores on have been related to reduced bilateral frontal lobe volumes (Roth et al. 2004). The anhedonia subscale assesses reduced experience of interest and pleasure. While one hypothesis proposes central dopaminergic dysfunctions as the neural basis of anhedonia (Heinz et al. 1998), a competing hypothesis suggests that dopaminergic stimulation encodes the desire to obtain a reward but is not involved in the consummatory experience of reward that is impaired in anhedonia (Robinson and Berridge 1993; Schmidt et al. 2001). The attention subscale measures attentional impairment, which has been associated with working memory deficits and linked to dysfunction in executive processing located in the dorsolateral prefrontal cortex (Silver and Feldman 2003).
To ensure high levels of inter-rater reliability throughout data collection, all assessors received extensive training by two doctorate-level psychologists experienced in SANS administration. The criterion for training to certification was 10 taped interviews with 85% agreement +/− 1 point of gold standard ratings, set by consensus ratings of the two doctorate-level psychologists and the PI of the study (AEE). Assessors administered SANS interviews independently only after achieving 85% or greater agreement on a minimum of ten SANS interviews and maintained inter-rater reliability through ratings of recorded interviews and discussion on biweekly conference calls. All ratings were archived, and inter-rater reliability correlations were calculated biweekly to assure fidelity. The weighted intraclass correlation alpha for SANS ratings over the course of data collection for all assessors is 0.87.
Smoking
Expired CO and self-reported tobacco use were assessed weekly. Expired CO was measured with a Bedfont Smokerlyzer (Kent, UK). Subjects exhaled into the device following a 15-second breath hold (Irving 1988). The Fagerstrom Test of Nicotine Dependence (FTND; Heatherton et al. 1991) was administered as an index of physiologic and psychological dependence on nicotine.
Wechsler Test of Adult Reading (WTAR)
The WTAR (Wechsler 2001), a reading test composed of 50 words, was administered as an estimate of cognitive functioning. In this task, participants are asked to read each word from a page aloud to an examiner. The number of correctly read words is the dependent variable, and the test is untimed.
Data Analyses
Probabilistic Reward Task
After removing trials with outlier reaction times (see Pizzagalli et al. 2005 for detail), response bias (log b) was computed according to the following formula:
As is evident from the formula, response bias reflects the participants’ preference for the stimulus paired with more frequent rewards. After calculating response bias separately for each block, difference scores between blocks 1 and 3 [ΔRB = RB(Block 3) – RB(Block 1)] were computed in order to capture the overall development of response bias over the course of the task as an index of reward sensitivity. In order to measure the change in reward sensitivity over the course of the intervention, a second difference score was calculated between ΔRB at baseline and endpoint (ΔΔRB = ΔRB(endpoint) – ΔRB(baseline)]. To test the hypothesis that individuals with less severe negative symptoms would demonstrate the largest increases in reward sensitivity, Pearson’s correlations were run between baseline scores on each SANS subscale and ΔΔRB. Hierarchical regression analyses predicting ΔΔRB were computed to test the specificity of any relationship emerging from correlational analyses.
Discriminability (log d), which indexes participants’ ability to perceptually discriminate between the rich and lean stimuli, was computed according to the following formula:
To test whether subjects’ changes in task performance over the course of treatment could be attributed to individual differences in their ability to differentiate between stimuli, baseline scores on discriminability across all three blocks of the task were entered into a separate linear regression predicting ΔΔRB.
Smoking Cessation
To test the hypothesis that patients with less severe negative symptoms at baseline would be more likely to achieve smoking abstinence, subjects were first divided into two outcome groups. Abstinence was defined as seven-day point prevalence abstinence at each of the last two group meetings, defined as self-report of smoking no cigarettes for the prior 7 days and expired CO < 9 ppm. Baseline SANS subscale scores were then entered into a multiple logistic regression predicting smoking outcome.
Medication Effects
Two additional regressions were run to determine whether antipsychotic medication type predicted treatment outcome or changes in reward sensitivity over the course of treatment. These included a binary logistic regression predicting treatment outcome and a linear regression predicting ΔΔRB from antipsychotic medication type (first generation, second generation, both first and second generation).
RESULTS
Participant Disposition
At the time of analysis, 102 subjects had either completed 13 weeks of open treatment with varenicline or terminated treatment early. Eighteen subjects did not perform the probabilistic reward task at one or both visits for the following reasons: ten subjects did not attend one of the data collection sessions; two subjects were unable to read and therefore could not perform the task; two subjects were unable to complete the task due to time limitations; two subjects declined to perform the task, and software malfunctions occurred in two instances. Nineteen subjects who had completed the reward task were lost due to poor data quality, including missing trials, at one or both visits. Twelve subjects had missing data due to administrative errors. Accordingly, 53 subjects performed the task at both study visits with data suitable for analysis. Subjects who met criteria for inclusion in the final analysis did not differ from those who did not in terms of age, gender, years of education, ethnicity, use of 1st or 2nd generation antipsychotics, antidepressants, anxiolytics, or mood stabilizing medications, WTAR score or SANS scores (ps > 0.05). Table I displays participant characteristics at baseline.
Table I.
Participant characteristics at baseline
| Baseline Participant Characteristics | ||||
|---|---|---|---|---|
| Included in Final Analysis (N = 53) | All Participants (N = 102) | |||
| Mean | S.D. | Mean | S.D. | |
| Age | 46.33 | 10.88 | 46.99 | 10.25 |
| Education (years) | 12.48 | 2.17 | 12.29 | 2.64 |
| Percent | Percent | |||
| Caucasian | 71.70% | N/A | 76.00% | N/A |
| Female | 45.28% | N/A | 38.24% | N/A |
| Mean | Mean | |||
| Years of Smoking | 26.31 | 12.41 | 28.00 | 11.37 |
| Cigarettes per Day | 15.14 | 12.59 | 16.66 | 11.58 |
| FTND Score | 6.08 | 1.81 | 6.16 | 1.94 |
| Expired CO | 23.68 | 16.50 | 23.89 | 18.93 |
| SANS Total Scores | 40.06 | 17.04 | 45.14 | 16.13 |
| Affective Flattening | 13.62 | 8.64 | 13.22 | 7.73 |
| Anhedonia | 11.38 | 5.45 | 10.78 | 5.00 |
| Attention | 5.38 | 3.92 | 5.61 | 3.95 |
| Apathy | 9.69 | 4.68 | 9.81 | 4.55 |
| Alogia | 5.37 | 3.96 | 5.72 | 4.12 |
| Percent | Percent | |||
| 1st Generation Antipsychotic(s) | 26.42% | N/A | 21.57% | N/A |
| 2nd Generation Antipsychotic(s) | 83.02% | N/A | 88.24% | N/A |
| 1st + 2nd Generation Antipsychotic(s) | 9.43% | N/A | 9.80% | N/A |
| Mood Stabilizer augmentation | 35.85% | N/A | 42.16% | N/A |
| Antidepressant augmentation | 58.49% | N/A | 59.80% | N/A |
| Anxiolytic augmentation* | 15.09% | N/A | 17.65% | N/A |
| Mean | Mean | |||
| Number of Medications | 2.55 | 1.07 | 2.81 | 1.25 |
Treatment Outcomes
Of the 53 participants included in the analysis, 32 (60.4%) met criteria for 14-day point prevalence abstinence at week 12. As displayed in Table II, subjects did not differ by abstinence status on years of regular cigarette smoking, baseline FTND scores, number of cigarettes smoked per day at baseline, expired CO concentration at baseline, or use of first or second generation antipsychotics, antidepressants, anxiolytics or mood stabilizers (ps > 0.14). Abstinent and smoking patients also did not differ on reward sensitivity indexed by the probabilistic reward task (ΔRB) at baseline (abstinent: 0.05 ± 0.21; smoking: 0.09 ± 0.35) or endpoint (abstinent: 0.10 ± 0.29; smoking: 0.10 ± 0.45) (ps > 0.57). A Group (abstinent, smoking at endpoint) × Time (pretreatment, endpoint) ANOVA on reward sensitivity (RB3-1) revealed no significant effects (ps >0.67). Paired samples t-tests revealed that participants achieved significant decreases in expired CO [t(52) = 7.08, p < 0.001; baseline: 23.68 ± 16.50; endpoint: 6.42 ± 11.98], and increases in overall response bias on the probabilistic reward task [t(52) = −2.10, p < 0.05; baseline: 0.07 ± 0.19; endpoint: 0.15 ± 0.17) over the course of treatment. No changes emerged from baseline to endpoint in SANS total scores or subscales (ps > 0.05).
Table II.
Baseline characteristics of abstinent vs. smoking participants at endpoint
| Baseline Participant Characteristics | ||||
|---|---|---|---|---|
| Abstinent (N = 32) | Smoking (N = 21) | |||
| Mean | S.D. | Mean | S.D. | |
| Age | 47.69 | 10.45 | 44.29 | 11.46 |
| Education (years) | 12.68 | 1.99 | 12.19 | 2.44 |
| Percent | Percent | |||
| Caucasian | 75.00% | N/A | 66.67% | N/A |
| Female | 43.75% | N/A | 47.62% | N/A |
| Mean | Mean | |||
| Years of Smoking | 26.92 | 12.19 | 25.38 | 13.00 |
| Cigarettes per Day | 16.62 | 12.79 | 13.10 | 12.32 |
| FTND Score | 5.93 | 1.82 | 6.29 | 1.82 |
| Expired CO | 25.06 | 16.84 | 21.57 | 16.1 |
| SANS Total Scores | 44.06 | 17.22 | 47.47 | 18.85 |
| Affective Flattening | 12.32 | 8.31 | 15.52 | 8.97 |
| Anhedonia | 11.68 | 4.92 | 10.95 | 6.26 |
| Attention | 4.87 | 4.12 | 6.14 | 3.57 |
| Apathy | 9.58 | 4.56 | 9.86 | 4.96 |
| Alogia | 5.61 | 4.62 | 5.00 | 2.76 |
| Percent | Percent | |||
| 1st Generation Antipsychotic(s) | 21.88% | N/A | 33.33% | N/A |
| 2nd Generation Antipsychotic(s) | 87.50% | N/A | 76.19% | N/A |
| 1st + 2nd Generation Antipsychotic(s) | 9.38% | N/A | 9.52% | N/A |
| Mood Stabilizer augmentation | 31.25% | N/A | 42.86% | N/A |
| Antidepressant augmentation | 65.63% | N/A | 47.62% | N/A |
| Anxiolytic augmentation* | 15.63% | N/A | 14.29% | N/A |
| Mean | Mean | |||
| Number of Medications | 2.56 | 0.95 | 2.52 | 1.25 |
Negative Symptom Predictors of Change in Reward Sensitivity
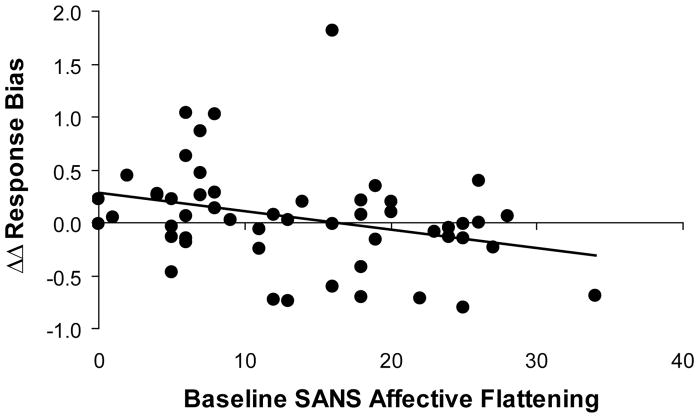
Pearson correlation analyses revealed a significant relationship between ΔΔRB and affective flattening (r = −0.31, p < 0.026, n = 52; Figure II), indicating that patients with lower baseline scores on the affective flattening subscale of the SANS (i.e., less severe affective flattening) demonstrated larger increases in reward sensitivity over the course of treatment. All other correlations were not significant (all rs < 0.16, all ps > 0.25). The correlation between ΔΔRB and affective flattening scores was confirmed when running a non-parametric Spearman Rank correlation (r = −0.33, p < 0.033). Highlighting the specificity of this finding, a hierarchical regression analysis indicated that affective flattening (entered in the second step) predicted ΔΔRB even when controlling for the other four SANS subscores (entered in the first step), (ΔR2 = 0.12, ΔF(1,46) = 6.81, p < 0.012). This effect remained when controlling for change in expired CO in addition to the other four SANS subscores (ΔR2 = 0.13, ΔF(1,45) = 7.27, p < 0.010), isolating the effect of varenicline on changes in reward sensitivity. Finally, all effects were confirmed after removing from the analyses the three subjects with changes in reward sensitivity more than two standard deviations outside the mean (Pearson r = −0.36, p < 0.010, n = 49; regression controlling for the four SANS subscores: ΔR2 = 0.15, ΔF(1,43) = 7.94, p < 0.007; regression controlling for the four SANS subscores and change in expired CO: ΔR2 = 0.15, ΔF(1,42) = 7.71, p < 0.008).
Fig. II.
Correlation between baseline affective flattening (as assessed by the SANS) and ΔΔRB [ΔΔRB = [ΔRB(endpoint) – ΔRB(baseline)], where ΔRB = [RB(Block 3) – RB(Block 1)]. N = 52.
Discriminability
The regression predicting ΔΔRB from baseline discriminability scores was not significant (p > 0.77), suggesting that changes in performance on the task could not be attributed to individual differences among subjects in the ability to perceptually discriminate between rich and lean stimuli.
Negative Symptom Predictors of Smoking Cessation
The multiple logistic regression predicting treatment outcome also revealed a significant effect for the affective flattening subscale of the SANS (β = 0.094, p = 0.044; Table III), such that those with lower baseline affective flattening scores were more likely to attain abstinence with varenicline and CBT. No other SANS subscales were predictive of treatment outcome (ps > 0.11). In addition, scores on the affective flattening subscale of the SANS were not significantly correlated with FTND scores or expired CO at baseline (ps > 0.39), suggesting that the effect could not be attributed to smoking or nicotine dependence severity at baseline.
Table III.
Regression predicting treatment outcome from baseline SANS scores
| SANS Subscale | B | SE B | p |
|---|---|---|---|
| Affective Flattening | 0.094 | 0.047 | 0.044 |
| Anhedonia | −0.084 | 0.069 | 0.228 |
| Attention | 0.089 | 0.078 | 0.257 |
| Apathy | 0.027 | 0.082 | 0.107 |
| Alogia | −0.151 | 0.095 | 0.111 |
Change in Reward Sensitivity as a Potential Mediator of Treatment Efficacy
In order to determine whether changes in reward sensitivity as measured by the probabilistic reward task might mediate the relationship between baseline symptoms of affective flattening and treatment efficacy with varenicline, a mediation analysis was performed according to the guidelines provided by Preacher and Hayes (2004). Sobel test results indicated that changes in probabilistic reward task performance did not mediate the relationship between baseline symptoms of affective flattening and smoking status at study endpoint (z = 0.0075, p = 0.99).
Effects of Antipsychotic Medication
The regressions predicting treatment outcome and ΔΔRB from antipsychotic medication type were not significant (ps > 0.43).
DISCUSSION
The goal of the study was to assess baseline negative symptoms as possible predictors of response to varenicline in a smoking cessation trial of patients with schizophrenia. In this sample, smokers with schizophrenia with less severe affective flattening were more successful in a smoking cessation attempt with varenicline and CBT. Less severe affective flattening at baseline was also predictive of improvement in reward responsiveness during the treatment trial, above and beyond the effects of the four additional subscales of the SANS and expired CO values. These results suggest that varenicline augmentation of behavioral treatment may be less effective as a smoking cessation aid in smokers with schizophrenia who have more severe pre-treatment symptoms of affective flattening. This finding may be best explained by examining the effects of varenicline on the dopaminergic dysfunctions implicated in affective flattening.
Several studies have found that symptoms of affective flattening are directly related to dopamine D2 receptor (DRD2) dysfunction (Heinz et al. 1998; Martinot et al. 1994; Schmidt et al. 2001). A study by Martinot et al (1994) using positron emission tomography (PET) demonstrated a negative correlation between striatal DRD2 density and a psychomotor dimension of schizophrenia involving flattened affect. In the same study, no relationship was found between DRD2 density and avolition, apathy, or anhedonia. A similar study using Single Photon Emission Computed Tomography (SPECT) found a negative correlation between dopamine D2/D3 receptor availability and affective flattening, but not anhedonia, attentional impairment, or positive symptoms in people with schizophrenia treated with antipsychotic medication (Heinz et al. 1998). Along similar lines, a study by Schmidt et al (2001) examining growth-hormone responses to the DRD2 agonist, apomorphine, found an association between central dopamine receptor dysfunction and affective flattening, but not anhedonia.
Berridge and colleagues argue that the role of dopamine in reward is to encode incentive-motivational stimuli, suggesting that DRD2 dysfunction associated with affective flattening could lead to reduced motivation to seek rewards in the environment (Berridge et al. 2009; Berridge and Kringelbach 2008). Varenicline has recently been shown to increase striatal DRD2 binding in rats (Crunelle et al. 2009), suggesting a possible mechanism by which varenicline may help to ameliorate reward-related deficits. In our study, patients with more severe pre-treatment affective flattening demonstrated relatively small improvements in reward responsiveness with varenicline treatment compared with those with less severe affective flattening. It is possible that those with high baseline symptomatology and poor improvement in reward responsiveness with antipsychotic treatment have more severe DRD2 dysfunction that is resistant to improvement with a NAChR partial agonist, varenicline.
Baseline severity of affective flattening was also predictive of smoking cessation outcomes, such that patients with more severe affective flattening at baseline were less likely to quit smoking. DRD2 dysfunctions associated with affective flattening may also be related to nicotine dependence. Nicotine dependence severity as assessed with FTND Scores (Heatherton et al. 1991) have been shown to correlate negatively with DRD2 neurotransmission (Scott et al. 2007). Similarly, DRD2 availability is reduced in nicotine-dependent smokers, and the severity of this reduction has been positively correlated with nicotine craving (Fehr et al. 2008), and reinforcing responses to psychostimulants more broadly (Volkow et al. 1999). DRD2 stimulation reduces smoking behavior acutely (Caskey et al. 1999), and thus increased striatal DRD2 binding may be one mechanism by which varenicline aids in smoking cessation. Thus, it is likely that the administered dose of varenicline was not sufficient to ameliorate DRD2 dysfunctions in the most severely affected patients, resulting in reduced treatment efficacy in these individuals.
In summary, the current study provides initial evidence that symptoms of affective flattening may predict smoking cessation response to varenicline treatment in individuals with schizophrenia. Albeit speculative, previous evidence for a link between affective flattening and DRD2 receptor dysfunctions suggest a possible neurochemical basis for this pattern. Limitations of the study include a relatively small sample size, and the concurrent administration of varenicline and CBT, which may limit the specificity of the results. Because our study did not include a control group of medication-only participants, the differential contributions of CBT vs. varenicline in predicting treatment outcome cannot be determined. Future studies should aim to evaluate the differential efficacy of varenicline and CBT monotherapy interventions as smoking cessation aids in schizophrenia patients. In addition, all participants were taking antipsychotic medication, so it is unclear whether some findings may be partially attributable to medication effects (e.g., Heinz et al. 1998); the observation that abstinent and relapsing patients did not differ in their medication regimen speaks, however, against confounding effects of concurrent medications. Finally, given the small sample size and modest effect of affective flattening severity as a predictor of response to treatment with varenicline, replication of these results will be needed before this finding can be used to inform treatment planning for smoking cessation in patients with schizophrenia. Future studies should examine whether elevated doses of varenicline, or adjunct therapy aimed at ameliorating affective flattening symptoms prior to treatment might optimize smoking cessation outcomes in individuals with schizophrenia.
Acknowledgments
The study was funded by NIDA R01 DA021245 (Evins). This grant supported Dr. Evins’ effort on the project. During the study, Dr. Stoeckel was partially supported by the Harvard Medical School Zinberg Fellowship in Clinical Addiction Research and a NIDA Loan Repayment Program Grant, and Dr. Pizzagalli was partially supported by NIMH grants R01MH68376 and R21MH078979.
Footnotes
Disclosures/Conflicts of Interest. The authors declare no competing financial interests. Dr. Evins has received research support from GSK, Janssen and Pfizer and honoraria or consulting fees from Boehringer-Ingelheim and Pfizer. Dr. Pizzagalli has received research support and consulting fees from ANT Inc. and honoraria and consulting fee from AstraZeneca.
References
- Alhatem F, Black JE. Varenicline-induced mania in a bipolar patient. Clin Neuropharm. 2009;32:117–118. doi: 10.1097/WNF.0b013e31816f75bc. [DOI] [PubMed] [Google Scholar]
- Andreasen N. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Anghelescu I. Successful smoking cessation and improvement of negative symptoms with varenicline in a stable schizophrenia patient. J Neuropsychiatry Clin Neurosci. 2009;21:102–110. doi: 10.1176/jnp.2009.21.1.102. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt JE, et al. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008a;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in non-smokers: Implications for development of dependence. Biol Psychiatry. 2008b;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, Aldridge JW. Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol. 2009;9:65–73. doi: 10.1016/j.coph.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobes J, Arango C, Garcia-Garcia M, Rejas J. Healthy lifestyle habits and 10-year cardiovascular risk in schizophrenia spectrum disorders: an analysis of the impact of smoking tobacco in the CLAMORS schizophrenia cohort. Schizophr Res. 2010;119:101–109. doi: 10.1016/j.schres.2010.02.1030. [DOI] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, et al. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Bruce ML, Leaf PJ, Florio L, Hoff RA. Psychiatric status and 9-year mortality data in the New Haven epidemiological catchment area study. Am J Psychiatry. 1994;151:716–721. doi: 10.1176/ajp.151.5.716. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Chiodo LA, Grace AA. Midbrain dopamine system electrophysiological functioning: a review and new hypothesis. Synapse. 1991;9:79–94. doi: 10.1002/syn.890090202. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2008;3:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Caskey NH, Jarvik ME, Wirshing WC. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp Clin Psychopharm. 1999;7:72–78. doi: 10.1037//1064-1297.7.1.72. [DOI] [PubMed] [Google Scholar]
- Cinemre B, Akdag ST, Metin O, Doganavsargil O. Varenicline-induced psychosis. CNS Spectr. 2010;15:469–472. doi: 10.1017/s1092852900000407. [DOI] [PubMed] [Google Scholar]
- Crunelle CL, Miller ML, De Bruin K, Van Den Brink W, Booij J. Varenicline increases striatal dopamine D2/3 receptor binding in rats. Addict Biol. 2009;14:500–502. doi: 10.1111/j.1369-1600.2009.00168.x. [DOI] [PubMed] [Google Scholar]
- Czermak C, Lehofer M, Wagner EM, et al. Dopamine D3 receptor expression in blood lymphocytes of smokers is negatively correlated with daily number of smoked cigarettes: A peripheral correlate of dopaminergic alterations in smokers. Nicotine Tob Res. 2004;6:49–54. doi: 10.1080/14622200310001656858. [DOI] [PubMed] [Google Scholar]
- Dagher A, Bleicher C, Aston JAD, Gunn RN, Clarke PBS, Cumming P. Reduced dopamine D1 receptor binding in the ventral striatum of cigarette smokers. Synapse. 2001;42:48–53. doi: 10.1002/syn.1098. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. Am J Psychiatry. 1998;155:1490–501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Evins AE, Goff DC. Varenicline treatment for smokers with schizophrenia: a case series. J Clin Psychiatry. 2008;69:1016. doi: 10.4088/jcp.v69n0620a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom K. The epidemiology of smoking: Health consequences and benefits of cessation. Drugs. 2002;62:1–9. doi: 10.2165/00003495-200262002-00001. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs. 2006;15:107–116. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- Fatemi SH. Varenicline efficacy and tolerability in a subject with schizophrenia. Schizophr Res. 2008;103:328–329. doi: 10.1016/j.schres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, et al. Association of low striatal dopamine D2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;16:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- Foulds J. The neurobiological basis for partial agonist treatment of nicotine dependence: Varenicline. Int J Clin Pract. 2006;60:571–576. doi: 10.1111/j.1368-5031.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- Freedman R. Exacerbation of schizophrenia by varenicline. Am J Psychiatry. 2007;164:1269. doi: 10.1176/appi.ajp.2007.07020326. [DOI] [PubMed] [Google Scholar]
- Fukui K, Kobayashi T, Hayakawa S, et al. Smoking habits in chronic schizophrenics. Arukoru Kenkyuto Yakabutsu Izon. 1995;30:447–454. [PubMed] [Google Scholar]
- Gholson LJ. Possible varenicline-induced paranoia and irritability in a patient with major depressive disorder, borderline personality disorder, and methamphetamine abuse in remission. J Clin Psychopharmacol. 2008;28:720–721. doi: 10.1097/JCP.0b013e31818db354. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Farde L, Halldin C, Swahn CG. Changes in striatal D2-receptor density following chronic treatment with amphetamine as assessed with PET in nonhuman primates. Synapse. 1999;31:154–162. doi: 10.1002/(SICI)1098-2396(199902)31:2<154::AID-SYN9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. Am J Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationships to psychopathology and medication side effects. Am J Psychiatry. 149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Hall RG, Duhamel M, McClanahan R, et al. Level of functioning, severity of illness, and smoking status among chronic psychiatric patients. J Nerv Ment Dis. 1995;183:468–471. doi: 10.1097/00005053-199507000-00008. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Bell-Warren KR, Gold JM. Decision-making impairments in the context of intact reward sensitivity in schizophrenia. Biol Psychiatry. 2008;64:62–69. doi: 10.1016/j.biopsych.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Knable MB, Coppola R, et al. Psychomotor slowing, negative symptoms and dopamine receptor availability – an IBZM SPECT study in neuroleptic-treated and drug-free schizophrenic patients. Schizophr Res. 1998;31:19–26. doi: 10.1016/s0920-9964(98)00003-6. [DOI] [PubMed] [Google Scholar]
- Irving JM, Clark EC, Crombie IK, Smith WC. Evaluation of a portable measure of expired-air carbon monoxide. Prev Med. 1988;17:109–115. doi: 10.1016/0091-7435(88)90076-x. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Collinson SL, Crichton P. Verbal fluency in schizophrenia: relationship with executive function, semantic memory and clinical alogia. Psychol Med. 1996;26:39–49. doi: 10.1017/s0033291700033705. [DOI] [PubMed] [Google Scholar]
- Jubelt L, Barr R, Goff D, Logvinenko T, Weiss A, Evins A. Effects of transdermal nicotine on episodic memory in non-smokers with and without schizophrenia. Psychopharmacology. 2008;199:89–98. doi: 10.1007/s00213-008-1133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. NeuroImage. 2006;29:409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Kinon BJ, Lieberman JA. Mechanisms of action of atypical antipsychotic drugs: A critical analysis. Psychopharmacology (Berl) 1996;124:2–34. doi: 10.1007/BF02245602. [DOI] [PubMed] [Google Scholar]
- Kim JS, Komhuber HH, Schmid-Burgk W, Holzmiller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Martinot JL, Palliere-Martinot ML, Loc’h C, et al. Central D2 receptors and negative symptoms of schizophrenia. Br J Psychiatry. 1994;164:27–34. doi: 10.1192/bjp.164.1.27. [DOI] [PubMed] [Google Scholar]
- Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse. 1998;28:1–9. doi: 10.1002/(SICI)1098-2396(199801)28:1<1::AID-SYN1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Basic Clin Pharmacol Toxicol. 1995;76:157–162. doi: 10.1111/j.1600-0773.1995.tb00123.x. [DOI] [PubMed] [Google Scholar]
- Patkar AA, Gopalakrishnan R, Lundy A, Leone FT, Weinstein SP. Relationship between tobacco smoking and positive and negative symptoms in schizophrenia. J Nerv Ment Dis. 2002;190:604–610. doi: 10.1097/00005053-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal detection approach. Biol Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Evins AE, Schetter EC, et al. Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 2008;196:221–232. doi: 10.1007/s00213-007-0957-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2009;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin MK. Exacerbation of recurrent depression as a result of treatment with varenicline. Am J Psychiatry. 2008;165:774. doi: 10.1176/appi.ajp.2008.07111735. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Meth Ins C. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Roth RM, Flashman LA, Saykin AJ, McAllister TW, Vidaver R. Apathy in schizophrenia: reduced frontal lobe volume and neuropsychological deficits. Am J Psychiatry. 2004;161:157–159. doi: 10.1176/appi.ajp.161.1.157. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Nolte-Zenker B, Patzer J, Bauer M, Schmidt LG, Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34:66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Domino EF, Heitzeg MM, et al. Smoking modulation of μ-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacol. 2007;32:450–457. doi: 10.1038/sj.npp.1301238. [DOI] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–531. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Silvestri S, Negrete JC, Seeman MG, Shammi CM, Seeman P. Does nicotine affect D2 receptor upregulation? A case control study. Acta Psychiatr Scand. 2004;109:313–317. doi: 10.1111/j.1600-0447.2004.00293.x. [DOI] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology. 2002;27:479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, et al. Cognitive and antismoking effects of varenicline in patients with schizophrenia or schizoaffective disorder. Schizophr Res. 2009;110:149–155. doi: 10.1016/j.schres.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28:366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Tupperainen H, Kuikka JT, Viinamaki H, Husso M, Tiihonen J. Dopamine D2/3 receptor binding potential and occupancy in midbrain and temporal cortex by haloperidol, olanzepine and clozapine. Psychiatry Clin Neurosci. 2010;63:529–537. doi: 10.1111/j.1440-1819.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, et al. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading (WTAR) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Curr Opin Psychiatr. 2010;23:112–119. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]