Abstract
Objectives
To evaluate the association between inflammation and heart failure (HF) risk in older adults.
Background
Inflammation is associated with HF risk factors and also directly affects myocardial function.
Methods
The association of baseline serum concentrations of interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP) with incident HF was assessed with Cox models among 2610 older persons without prevalent HF enrolled in the Health ABC Study (age, 73.6±2.9 years; 48.3% men; 59.6% white).
Results
During follow-up (median, 9.4 years), 311 participants (11.9%) developed HF. In models controlling for clinical characteristics, ankle-arm index, and incident coronary heart disease, doubling of IL-6, TNF-α, and CRP concentrations was associated with 29% (95% CI, 13 to 47%; P<.001), 46% (95% CI, 17 to 84%; P=.001), and 9% (95% CI, -1 to 24%; P=.087) increase in HF risk, respectively. In models including all three markers, IL-6 and TNF- α, but not CRP, remained significant. These associations were similar across sex and race and persisted in models accounting for death as a competing event. Post-HF ejection fraction was available in 239 (76.8%) cases; inflammatory markers had stronger association with HF with preserved ejection fraction. Repeat IL-6 and CRP determinations at 1-year follow-up did not provide incremental information. Addition of IL-6 to the clinical Health ABC HF model improved model discrimination (C index from 0.717 to 0.734; P=.001) and fit (decreased Bayes information criterion by 17.8; P<.001).
Conclusions
Inflammatory markers are associated with HF risk among older adults and may improve HF risk stratification.
Key words (MeSH): Heart Failure, Elderly, Inflammation
Inflammatory markers, including interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP), are elevated in patients with heart failure (HF) and are associated with outcomes regardless of etiology (1–6). Inflammatory markers are also elevated in individuals with asymptomatic left ventricular systolic and diastolic dysfunction (7–9), and experimental studies have suggested that IL-6 and TNF-α are associated with left ventricular remodeling, fetal gene expression, myocyte hypertrophy, and myocyte apoptosis (10). These observations raise the possibility that inflammation may be related to a heightened risk for HF, and previous studies have indeed suggested such an association (11–13). However, the number of incident HF events in these studies was small precluding an opportunity to assess for possible differential association across major demographic groups and adequately control for important predictors of HF including incident coronary events. Finally, the incremental value of inflammatory markers for HF risk determination was not assessed in these studies.
We recently developed and validated a prediction model for incident HF in the elderly, the Health ABC HF model (14), based on nine routinely available variables including age, history of coronary heart disease (CHD), smoking, systolic blood pressure, heart rate, and serum concentrations of fasting glucose, creatinine, and albumin. Most of these risk factors have been associated with inflammation. For example, the association of inflammation with CHD and subclinical atherosclerosis is well documented (15,16), as is the association between inflammation and diabetes mellitus (17). Systemic low-grade inflammation has been reported in smokers (18). Both inflammation and oxidative stress have been correlated with chronic kidney disease (19). Similar associations have been reported for hypertension and hypoalbuminemia (20,21). Thus, beyond the direct effects on myocardial cells, inflammation may also drive HF risk through the creation of a biologic milieu that predisposes individuals to HF.
In this study, we assessed the association between baseline levels of inflammatory markers and risk for HF among the elderly participants of the Health, Aging, and Body Composition (Health ABC) Study in the total cohort and in predefined subgroups of interest, as well as the possible incremental value of these markers for incident HF prediction. In addition, we assessed the impact of multiple inflammatory marker elevation and the incremental value of serial biomarker measurements for incident HF prediction.
METHODS
Study Population
Study population for this investigation included participants in the Health ABC Study, a population-based cohort of 3075 community-dwelling men and women aged 70 to 79 years at inception. To be eligible, participants had to report no difficulty in walking mile or climbing 10 stairs without resting. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible black community residents in designated zip codes areas surrounding Pittsburgh and Memphis. Exclusion criteria included difficulties with activities of daily living, obvious cognitive impairment, inability to communicate with the interviewer, or intention of moving within 3 years. All participants gave written informed consent; the Institutional Review Boards at both study sites approved the protocol. Baseline data were collected in 1997–1998 and these results represent the outcomes during ten years of follow-up. The presence of cardiovascular disease at baseline was based on ICD 9-CM codes, as reported by Medicare and Medicaid Services for the years 1995–1998, self-reported history, and the use of selected drugs (22). Participants with HF or missing data on HF at baseline (n=140) were excluded from this analysis. Of the 2935 participants without HF at baseline, 2612 (89.0%) had available IL-6, TNF-α, and CRP serum concentrations. Two participants were excluded because of extreme outlier values of TNF-α; the remaining 2610 participants were included in this study.
Serum Biomarker Measurements
In the Health ABC Study, concentrations of biomarkers were obtained from frozen stored serum or plasma collected at baseline after an overnight fast. Samples were obtained in the morning and after processing, the specimens were frozen at −70°C, and shipped to the Core Laboratory at the University of Vermont. Cytokines were measured in duplicate by ELISA. The detectable limit for IL-6 was 0.10 pg/mL and for TNF-α 0.18 pg/mL. Serum concentrations of CRP were also measured in duplicate by ELISA on the basis of purified protein and polyclonal anti-CRP antibodies. The CRP assay was standardized according to World Health Organization First International Reference Standard with a sensitivity of 0.08 μg/mL. Blind duplicate analyses (n=150) for IL-6, CRP, and TNF-α showed inter-assay coefficients of variation of 10.3%, 8.0%, and 15.8%, respectively.
Repeat determinations of IL-6 and CRP serum levels at year 2 visit (i.e., at first 1-year follow-up) were available in 2372 (90.9%) and 2370 (90.8%) of the 2610 participants with inflammatory marker levels at baseline. Repeat determinations were not available for TNF-α. The year 2 samples of IL-6 and CRP were analyzed in a different laboratory (Wake Forest University) than the baseline samples (University of Vermont) and calibrated based on 150 samples that were analyzed both at Wake Forest University and University of Vermont. The intra-class correlation coefficient for the calibrated samples was 0.93 for IL-6 and 0.89 for CRP.
Study Definitions
Race was self-defined by the participant. Hypertension was defined as self-reported history of physician diagnosis accompanied by use of antihypertensive medications. Diabetes was defined as self-reported history of diabetes or use of antidiabetic medications. Smoking status was classified as current, past (≥100 lifetime cigarettes), or never. Left ventricular hypertrophy was diagnosed based on electrocardiogram using the following voltage criteria; R amplitude >26 mm in either V5 or V6, or R amplitude >20 mm in any of leads I, II, III, aVF, or R amplitude >12 mm in lead aVL or R amplitude in V5 or V6 plus S amplitude in V1 >35 mm. Definite CHD was defined as (1) history of coronary artery bypass graft or percutaneous coronary angioplasty; or (2) electrocardiographic evidence of myocardial infarction; or (3) history of myocardial infarction or angina accompanied by use of antianginal medications. Probable CHD was defined as self-reported history of myocardial infarction or angina without use of antianginal medications. Cerebrovascular disease was defined as history of stroke or transient ischemic attack or carotid endarterectomy. Peripheral arterial disease was defined as history of claudication or lower extremity bypass or angioplasty. Methods of assessment of ankle-arm index in Health ABC have been previously published (23).
Study Outcomes
All participants were asked to report any hospitalizations and every 6 months were asked direct questions to elicit information about interim events. All first admissions with an overnight stay confirmed to be related to HF by local adjudicators were classified as incident HF. Adjudication criteria for HF required, in addition to a physician diagnosis of HF, (1) medical record documentation of symptoms (e.g. shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea) and physical signs (e.g. edema, pulmonary rales, gallop rhythm,); (2) supporting clinical findings such as evidence of pulmonary edema on chest radiography or evidence supporting HF on echocardiography; and (3) medical therapy for HF, including at least a diuretic and a vasodilator and/or digitalis. Information on left ventricular ejection fraction (EF) post-HF development was abstracted from any imaging report in the medical record. Incident CHD was defined as hospitalization for myocardial infarction, angina pectoris, or elective coronary revascularization, either surgical or percutaneous.
Statistical Analysis
The proportion of variance of inflammatory markers explained by baseline characteristics was assessed using multiple regression. The association of inflammatory markers with HF risk was assessed with Cox proportional hazards models. Inflammatory marker concentrations were log transformed prior to regression analyses because of skewed distributions. We opted for log2 transform (i.e. logarithm with basis 2) to facilitate communication, because the hazard ratio per log2-transformed increase denotes the increase in hazard associated with doubling of the original value. The proportionality assumption in Cox models was assessed with the Schoenfeld residuals.
In multivariable models, we controlled for (1) baseline characteristics, including use of antihypertensive and anti-inflammatory medications (entered as a binary indicator variable for each class), and (2) ankle-arm index, as a measure of subclinical atherosclerosis, and time-varying incident CHD evaluated at 1-year intervals, in nested models. We repeated these analyses in subgroups defined by the presence or absence of any atherosclerotic disease (definite or probable CHD, cerebrovascular disease, or peripheral arterial disease). Finally, we repeated these analyses with death as a competing event, using the competing risks model (proportional subhazards model) proposed by Fine and Gray (24).
In secondary analyses, we assessed the association between inflammatory markers and risk for HF with reduced (≤45%) vs. preserved (>45%) left ventricular EF. Although there is no universally accepted cutoff point for EF, an EF>45% is considered to represent preserved systolic function by most investigators and has also been used in recent trials (25,26). For this analysis, only new HF cases with documented left ventricular EF were considered as events in separate (reduced vs. preserved) Cox models. In addition, we assessed the effect of combined elevations of multiple inflammatory markers on HF risk. For this analysis, we empirically divided biomarker values using the cohort median. Finally, we evaluated the prognostic value of changes in IL-6 and CRP at 1-year follow-up for future incident HF among the 2370 participants who had repeat IL-6 and CRP determinations and were still free of HF at 1-year follow-up.
The incremental value of inflammatory markers for HF risk prediction over the Health ABC HF model (14), was assessed with the Harrell’s C index for survival models and the change in Bayes information criterion, a likelihood-based measure of model fit that penalizes for the number of variables in the model (27,28). For calculation of the Bayes information criterion, we applied the Volinsky and Raftery modification for censored data (28), and corresponding P values for improvement were calculated according to Wagenmakers (29).
For multivariable models, missing values of covariates were imputed using the multiple imputation by chained equations procedure as introduced by van Buuren et al (30,31). Parameter estimates were obtained by combining 5 imputed datasets using the method described by Barnard and Rubin to account for possible error in missing value analysis (32). A two-sided p<0.05 was accepted as statistically significant for all analyses. Analyses were performed with STATA versions10 and 11 (StataCorp, College Station, TX).
RESULTS
Baseline Participant Characteristics and Inflammatory Marker Distribution
Table 1 shows the baseline participant characteristics and Table 2 shows the distribution of biomarkers overall and by HF development. The mean age of participants was 73.6±2.9 years; 48.3% were men and 59.6% were white. During a median follow-up of 9.4 years (interquartile range [IQR], 7.0–9.4), 311 participants (11.9%) developed HF (15.1 cases per 1000 person-years) and 790 participants (30.3%) died (annual mortality, 3.7%).
Table 1.
Baseline Participant Characteristics (N=2610)
| Characteristic | Value |
|---|---|
| Age, years | 73.6 ± 2.9 |
| Male sex, % | 48.3 |
| Black race, % | 40.4 |
| Body mass index, kg/m2 | 27.3 ± 4.7 |
| Waist/thigh circumference ratio | 1.95 ± 0.21 |
| Smoking, % | |
| Current | 10.5 |
| Past | 44.6 |
| Alcohol consumption, % | |
| Never | 49.5 |
| <1 drink/week | 21.5 |
| 1–7 drinks/week | 21.9 |
| >7 drinks/week | 7.0 |
| Diabetes mellitus, % | 14.8 |
| Hypertension, % | 53.1 |
| Coronary heart disease, % | |
| Definite | 16.2 |
| Probable | 3.3 |
| Cerebrovascular disease, % | 7.7 |
| Peripheral arterial disease, % | 4.8 |
| Left ventricular hypertrophy, % | 12.1 |
| Systolic blood pressure, mg/dl | 136 ± 21 |
| Diastolic blood pressure, mg/dl | 71 ± 12 |
| Heart rate, bpm | 65 ± 11 |
| Fasting glucose, mg/dl* | 94 (87, 105) |
| Albumin, gm/dl | 4.0 ± 0.3 |
| Creatinine, mg/dl* | 1.0 (0.9, 1.2) |
| Total cholesterol, mg/dl | 203 ± 38 |
| Low density lipoprotein, mg/dl | 122 ± 34 |
| High density lipoprotein, mg/dl | 54 ± 17 |
| Triglycerides, mg/dl* | 118 (88, 161) |
| Medications | |
| Beta blockers, % | 12.2 |
| Angiotensin converting enzyme inhibitors, % | 14.3 |
| Calcium channel blockers, % | 22.2 |
| Thiazide diuretics, % | 17.6 |
| Other antihypertensive, % | 8.5 |
| Statins, % | 12.5 |
| Steroids, % | 2.2 |
| Non-steroid anti-inflammatory agents, %† | 51.4 |
Value expressed as median (interquartile range) because of highly skewed distributions
Including salicylates
Table 2.
Inflammatory Markers According to Incident Heart Failure Development
| Marker | Total cohort (N=2610) | Participants without incident HF (N=2299) | Participants with incident HF (N=311) | P value |
|---|---|---|---|---|
| Interleukin-6, pg/mL* | 1.80 (1.23, 2.76) | 1.75 (1.20, 2.67) | 2.31 (1.56, 3.43) | <.001 |
| Tumor necrosis factor α, pg/mL* | 3.14 (2.41, 4.06) | 3.10 (2.40, 3.96) | 3.49 (2.58, 4.76) | <.001 |
| C-reactive protein, μg/mL* | 1.64 (0.99, 3.04) | 1.60 (0.97, 2.97) | 1.94 (1.18, 3.81) | <.001 |
Values are expressed as median (interquartile range) because of highly skewed distributions and compared with the Mann-Whitney test
Table 3 presents the correlation between the inflammatory markers and baseline participant characteristics. Multiple statistically significant correlations were detected; however, these were modest. In multiple regression analyses, baseline characteristics were able to explain 16.2%, 27.6%, and 16.2% of the variance of log-transformed IL-6, TNF-a, and CRP, respectively.
Table 3.
Correlation between Inflammatory Markers and Baseline Characteristics
| Variable | IL-6 | TNF-α | CRP | |||
|---|---|---|---|---|---|---|
| rho | P | rho | P | rho | P | |
|
|
|
|
||||
| Age | 0.036 | .064 | 0.082 | <.001 | −0.059 | .003 |
| Sex (men=0, women=1) | −0.061 | .002 | −0.068 | <.001 | 0.142 | <.001 |
| Race (white=0, black=1) | 0.098 | <.001 | −0.125 | <.001 | 0.161 | <.001 |
| Body mass index | 0.167 | <.001 | 0.077 | <.001 | 0.244 | <.001 |
| Waist/thigh circumference ratio | 0.107 | <.001 | 0.163 | <.001 | 0.019 | .33 |
| Smoking (no=0, past=1, current=2) | 0.128 | <.001 | 0.031 | .12 | 0.070 | <.001 |
| Alcohol consumption (drinks/wk) | −0.046 | .020 | 0.004 | .84 | −0.043 | .029 |
| Diabetes mellitus (no=0, yes=1) | 0.121 | <.001 | 0.075 | <.001 | 0.089 | <.001 |
| Hypertension (no=0, yes=1) | 0.099 | <.001 | 0.070 | <.001 | 0.104 | <.001 |
| Coronary heart disease (no=0, probable=1, definite=2) | 0.114 | <.001 | 0.138 | <.001 | 0.011 | .57 |
| Cerebrovascular disease (no=0, yes=1) | 0.042 | .032 | 0.032 | 0.10 | 0.028 | .15 |
| Peripheral vascular disease (no=0, yes=1) | 0.090 | <.001 | 0.068 | <.001 | 0.053 | .007 |
| Left ventricular hypertrophy (no=0, yes=1) | 0.064 | .001 | 0.023 | .25 | 0.028 | .15 |
| Systolic blood pressure | 0.044 | .026 | 0.010 | .62 | 0.039 | .049 |
| Diastolic blood pressure | −0.029 | .14 | −0.025 | .20 | −0.003 | .87 |
| Heart Rate | 0.152 | <.001 | 0.031 | .11 | 0.145 | <.001 |
| Fasting glucose | 0.139 | <.001 | 0.093 | <.001 | 0.088 | <.001 |
| Albumin | −0.140 | <.001 | −0.038 | .05 | −0.101 | <.001 |
| Creatinine | 0.124 | <.001 | 0.274 | <.001 | 0.017 | .37 |
| Total cholesterol | −0.119 | <.001 | −0.025 | .19 | 0.052 | .008 |
| Low density lipoprotein | −0.068 | <.001 | −0.012 | .54 | 0.155 | .43 |
| High density lipoprotein | −0.168 | <.001 | −0.304 | <.001 | 0.002 | .93 |
| Triglycerides | 0.053 | .007 | 0.309 | <.001 | 0.097 | <.001 |
All correlation (rho) values refer to Spearman nonparametric rank correlation coefficients.
Inflammatory Markers and Incident Heart Failure
All inflammatory markers were associated with HF risk in unadjusted analyses; HR per doubling of levels was 1.61 (95% CI, 1.43–1.80; P<.001) for IL-6, 1.83 (95% CI, 1.51–2.21; P<.001) for TNF-α, and 1.25 (95% CI, 1.14–1.36; P<.001) for CRP. The proportional hazards assumption was valid for all three markers (P value for the χ2 test of Schoenfeld residuals was .55, .73, and .45, for IL-6, TNF-α, and CRP, respectively) and association with HF risk was similar across sex and race (P values for interaction terms >.15). In models controlling for all baseline characteristics, the association of IL-6 and TNF-α with HF risk remained significant, whereas that of CRP was attenuated (Table 4). Adding baseline ankle-arm index (as a measure of baseline subclinical atherosclerosis) and incident CHD as a time-varying covariate in the model did not materially affect the estimates; doubling of concentrations of IL-6, TNF-α, and CRP was associated with 29% (95% CI, 13 to 47%), 46% (95% CI, 17 to 84%), and 9% (95% CI, −1 to 24%) increase in HF risk, respectively, in fully adjusted models (Table 4). When all markers were entered simultaneously in adjusted models, only IL-6 and TNF-α, but not CRP, remained independently associated with HF risk.
Table 4.
Inflammatory Biomarkers and Incident Heart Failure Risk
| Inflammatory marker | All (n=2610) | Present atherosclerotic disease at baseline (n=665) | Absent atherosclerotic disease at baseline (n=1945) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Individual markers – Model 1* | ||||||
| Interleukin-6, per log2 | 1.32 (1.16–1.50) | <.001 | 1.23 (1.02–1.48) | .032 | 1.40 (1.18–1.66) | <.001 |
| Tumor necrosis factor-α, per log2 | 1.49 (1.19–1.87) | .001 | 1.73 (1.26–2.38) | .001 | 1.40 (1.06–1.84) | .018 |
| C-reactive protein, per log2 | 1.11 (1.00–1.23) | .051 | 1.02 (0.88–1.17) | .82 | 1.19 (1.05–1.36) | .009 |
|
| ||||||
| Individual markers – Model 2† | ||||||
| Interleukin-6, per log2 | 1.29 (1.13–1.47) | <.001 | 1.18 (0.98–1.42) | .090 | 1.38 (1.17–1.64) | <.001 |
| Tumor necrosis factor-α, per log2 | 1.46 (1.17–1.84) | .001 | 1.71 (1.25–2.35) | .001 | 1.36 (1.04–1.82) | .025 |
| C-reactive protein, per log2 | 1.09 (0.99–1.21) | .087 | 0.98 (0.85–1.14) | .84 | 1.19 (1.04–1.35) | .010 |
|
| ||||||
| Pooled markers – Model 1* | ||||||
| Interleukin-6, per log2 | 1.26 (1.09–1.46) | .002 | 1.23 (0.99–1.52) | .062 | 1.30 (1.07–1.58) | .009 |
| Tumor necrosis factor-α, per log2 | 1.39 (1.10–1.74) | .005 | 1.65 (1.20–2.27) | .002 | 1.25 (0.94–1.66) | .13 |
| C-reactive protein, per log2 | 1.02 (0.91–1.14) | .73 | 0.95 (0.81–1.11) | .52 | 1.08 (0.94–1.25) | .28 |
| Joint significance | <.001 | .002 | <.001 | |||
|
| ||||||
| Pooled markers – Model 2† | ||||||
| Interleukin-6, per log2 | 1.24 (1.07–1.44) | .004 | 1.19 (0.96–1.48) | .10 | 1.28 (1.06–1.56) | .013 |
| Tumor necrosis factor-α, per log2 | 1.41 (1.12–1.77) | .003 | 1.65 (1.20–2.27) | .002 | 1.23 (0.92–1.63) | .16 |
| C-reactive protein, per log2 | 1.01 (0.90–1.13) | .91 | 0.92 (0.78–1.08) | .31 | 1.08 (0.93–1.25) | .30 |
| Joint significance | <.001 | .004 | <.001 | |||
CI=Confidence interval; HR=Hazard ratio
HR expressed per log2 (logarithm with basis 2) - equivalent to the HR per doubling of the original value of the parameter.
Atherosclerotic disease at baseline was defined as presence of definite or probable coronary heart disease, cerebrovascular disease, or peripheral arterial disease at baseline.
Model 1: Adjusted for baseline characteristics and medication use as described in Table 1.
Model 2: Model 1 variables plus ankle-arm index and time-varying incident coronary events.
In subgroups defined by the presence or absence of any atherosclerotic (CHD, cerebro-vascular or peripheral) disease at baseline, IL-6 and TNF-α were associated with incident HF risk in both subgroups. Notably, TNF-α appeared to be a stronger predictor of HF among participants with prevalent atherosclerotic disease, whereas IL-6 was a stronger predictor among participants without atherosclerotic disease at baseline. Considering that this is a post-hoc analysis based on a posteriori definition, these results need to be interpreted with caution.
When death was accounted for as a competing event using the Fine and Gray proportional subhazards models, IL-6 and TNF-α were still strongly associated with incident HF risk and both provided complementary information in models with all markers included (Table 5), whereas CRP was not associated with incident HF in competing risks models.
Table 5.
Inflammatory Biomarkers and Incident Heart Failure with Death as a Competing Risk
| Inflammatory marker | Model 1* | Model 2† | ||
|---|---|---|---|---|
| sHR (95% CI) | P value | sHR (95% CI) | P value | |
| Individual markers | ||||
| Interleukin-6, per log2 | 1.23 (1.08–1.41) | .002 | 1.21 (1.06–1.38) | .005 |
| Tumor necrosis factor-α, per log2 | 1.45 (1.15–1.82) | .001 | 1.43 (1.13–1.80) | .002 |
| C-reactive protein, per log2 | 1.07 (0.97–1.19) | .17 | 1.06 (0.96–1.17) | .26 |
|
| ||||
| Pooled markers | ||||
| Interleukin-6, per log2 | 1.19 (1.03–1.38) | .020 | 1.17 (1.01–1.35) | .037 |
| Tumor necrosis factor-α, per log2 | 1.37 (1.09–1.73) | .008 | 1.39 (1.07–1.72) | .011 |
| C-reactive protein, per log2 | 1.01 (0.91–1.12) | .88 | 1.00 (0.90–1.11) | .99 |
| Joint significance | <.001 | .003 | ||
CI=Confidence interval; sHR=Subhazard ratio
sHR expressed per log2 (logarithm with basis 2) - equivalent to the sHR per doubling of the original value of the parameter.
Model 1: Adjusted for baseline characteristics and medication use as described in Table 1.
Model 2: Model 1 variables plus ankle-arm index and time-varying incident coronary events.
Incident Heart Failure with Preserved and Reduced EF
Information on post-HF left ventricular EF was available for 239 of 311 (76.8%) incident HF cases. Median EF was 43% (IQR, 27 to 55%). When only cases with preserved EF were considered (105 of 239; 43.9%), IL-6 (HR per doubling, 1.49; 95% CI, 1.19–1.86; P<.001) and TNF-α (HR per doubling, 1.81; 95% CI, 1.23–2.68; P=.003) were strongly associated with HF risk in models controlling for baseline characteristics; association was less robust for CRP (HR per doubling, 1.15; 95% CI, 0.96–1.36; P=.12). When all three markers were included in the model, IL-6 (HR per doubling, 1.40; 95% CI, 1.09–1.80; P=.008) and TNF-α (HR per doubling, 1.60; 95% CI, 1.08–2.38; P=.020) were independently associated with HF risk, whereas CRP was not (HR per doubling, 1.01; 95% CI; 0.84–1.22; P=.90). The associations of IL-6 and TNF-α with incident HF did not materially change in models additionally controlling for ankle-arm index at baseline and incident CHD as time-varying covariate (data not shown). When only HF cases with reduced EF (134 of 239; 56.1%) were considered, inflammatory markers were marginally associated with HF risk; HR per doubling of levels was 1.21 (95% CI, 0.99–1.48; P=.067) for IL-6, 1.36 (95% CI, 0.96–1.93; P=.081) for TNF-α, and 1.09 (95% CI, 0.93–1.27; P=.28) for CRP in models controlling for baseline characteristics. This association was further attenuated in models that additionally controlled for ankle-arm index at baseline and incident CHD (data not shown).
Multiple Inflammatory Marker Elevations and Incident Heart Failure Risk
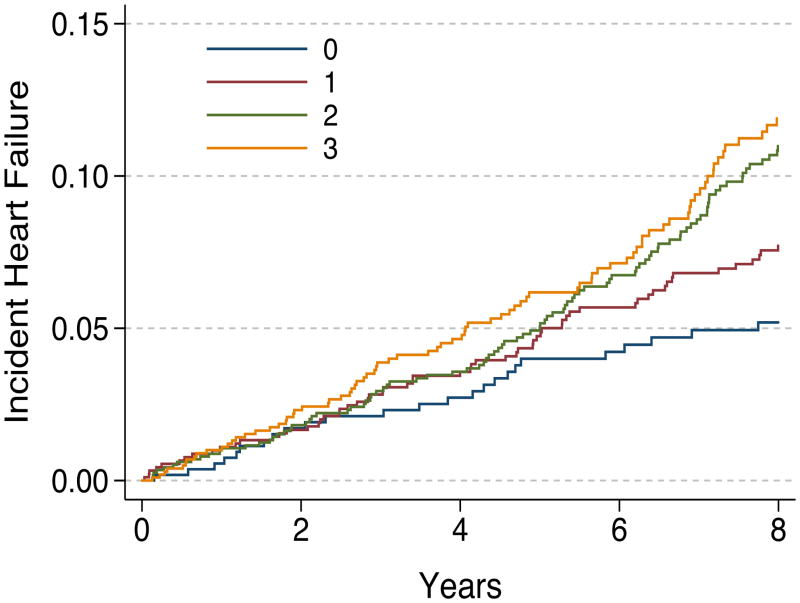
Compared with participants who had concentrations below the cohort median for all three biomarkers (n=515), those with one (n=795), two (n=776), or three (n=524) biomarkers elevated at baseline had increased risk for HF in unadjusted analyses (HR, 1.45; 95% CI, 0.97–2.17; P=.074; HR, 2.37; 95% CI, 1.61–3.48; P<.001; and HR, 3.20; 95% CI, 2.16–4.74; P<.001, respectively; P for trend <.001). The increased risk persisted for those with two or three biomarkers elevated in models adjusted for baseline characteristics (HR, 1.26; 95% CI, 0.83–1.90; P=.27; HR, 1.65; 95% CI, 1.10–2.47; P=.014; and HR, 1.76; 95% CI, 1.14–2.70; P=.010, for participants with one, two, or three biomarkers elevated, respectively; P for trend =.003) (Figure 1). These results did not change when ankle-arm index and incident CHD was included in the model (data not shown).
Figure 1. Heart Failure Rates According to the Number of Elevated Inflammatory Markers at Baseline.
Adjusted Kaplan-Meier plots illustrating cumulative heart failure incidence among Health ABC Study participants according to the number of elevated inflammatory markers (interleukin-6, tumor necrosis factor alpha, or C-reactive protein) at baseline. Adjustment model included all baseline characteristics as described in Table 1.
Serial Biomarkers and Incident Heart Failure Risk
Among the 2370 participants who had repeat determinations of IL-6 and CRP at year 2 and were still free from HF, median increase in IL-6 and CRP levels from year 1 was 0.30 pg/mL (IQR, −0.39 to 1.25 pg/mL) and 0.51 μg/mL (IQR, −1.07 to 2.08 μg/mL), respectively. Repeat IL-6 and CRP levels were still associated with future HF risk (HR per doubling, 1.21; 95% CI, 1.09–1.36; P=.001 and 1.12; 95% CI, 1.03–1.21; P=.006, respectively). However, the change in IL-6 or CRP from baseline to 1-year follow-up was not associated with HF risk (both P>.5).
Incremental Value for Incident Heart Failure Prediction
We have previously developed a HF risk prediction model, the Health ABC HF model (14). Addition of all 3 inflammatory markers to this clinical model increased the C index from 0.717 (95% CI, 0.686–0.747) to 0.737 (95% CI, 0.709–0.765), P=.001 for the comparison, and improved model fit as assessed with the Bayes information criterion (decreased by 10.9 units; P=.004). The improvement was mainly due to IL-6; adding only IL-6 to clinical predictors improved C index to 0.734 (95% CI, 0.706–0.763; P=.001 for the comparison), and improved Bayes information criterion by 17.8 units (P<.001) because of a more parsimonious model.
DISCUSSION
In this study, we validate earlier observations suggesting that inflammatory cytokines are associated with incident HF risk. In our study, IL-6 and TNF-α were independently associated with HF development whereas CRP demonstrated a weaker association. These findings were consistent in both sexes, in blacks and whites, and persisted in models controlling for clinical characteristics, ankle-arm index, and incident coronary events. In addition, both IL-6 and TNF-α were associated with incident HF in models with death as a competing risk. The association between inflammatory markers and HF risk was stronger for HF with preserved as opposed to reduced EF; this however needs cautious interpretation as post-HF EF was not uniformly assessed in the Health ABC Study. Finally, serial determinations of IL-6 and CRP added little information. Using the clinical Health ABC HF model as baseline, we found that addition of IL-6 in the model improved model performance. These observations underscore the relation between inflammation and HF risk, and may have screening and prevention implications.
The rate of incident HF in our study was similar to a recent cohort study reporting on HF incidence among older adults (33). Importantly, these rates are lower compared to administrative data (34,35). Varying rates of incident HF have also been reported by the Framingham Heart Study, the Cardiovascular Health Study, and from the Olmsted County, Minnesota (36–38). These differences are likely related to different age, racial mix, geographic variation etc but also to varying diagnostic criteria. Regardless of the precise estimate, a high incidence rate, worsening profile with aging, and either stagnant or increasing incidence rates over the last decades are common findings across these studies.
Inflammation may contribute to HF development in multiple ways. Enhanced inflammatory state may create a milieu that increases risk for HF since many HF risk factors are associated with inflammation. Indeed, in our analysis, all risk factors for HF in the Health ABC cohort (14), albeit modestly, were correlated with inflammatory marker levels. However, our data suggest that inflammation may also have a more direct role in HF development since the association between inflammation and HF persisted despite controlling for known HF risk factors. Previous data support our findings. The Framingham investigators have shown that there was a 60% (with TNF-α) to 68% (with IL-6) increase in HF risk per tertile increase in cytokine concentration (11). Two other studies have suggested a similar association (12,13); however, all three studies had small number of HF cases (n<100). In this respect, the large number of incident HF cases, and longer follow-up in our study (median, 9.4 years) allowed us to control for multiple risk factors and incident CHD, and perform important subgroup analyses.
Previous reports support a potential direct role for inflammation in HF development. Both IL-6 and TNF-α affect cardiomyocyte contractility (39–41). These cytokines also influence left ventricular remodeling and hypertrophy (42–45). Interestingly, unlike the previous studies (11–13), CRP levels had a weak association with HF risk in our study, and were not associated with HF in models including IL-6 and TNF-α. This is likely related to the rigorous controlling for confounders in our study. Although CRP may promote left ventricular remodeling by stimulating IL-6 production (46), there are no data to suggest a direct association between CRP levels and left ventricular remodeling. Moreover, elevated CRP levels may reflect increased hepatic synthesis under the influence of elevated IL-6 levels and thus represent a “distal” phenomenon secondary to a more “proximal” primary excess production of IL-6 (47,48).
Several additional observations from our study merit attention. First, our findings were consistent across sex and in white and black participants. Second, inflammatory marker levels had a strong association with HF with preserved EF. This association is plausible because inflammation has been linked with diastolic dysfunction in patients with hypertension (49) and CHD (8,9), and inhibition of inflammatory pathways prevents diastolic dysfunction in experimental diabetic cardiomyopathy (50,51). This finding has important implications as HF is primarily a disease of the elderly and many of these individuals develop HF with preserved EF. However, cautious interpretation is needed because post-HF left ventricular function was not systematically assessed in the Health ABC Study and the corresponding findings are therefore subject to possible selection bias.
What are the implications of these findings? First, it provides pathophysiologic insights into HF development and raises the possibility that inflammation might be a target for prevention interventions in selected individuals. For example, agents with anti-inflammatory properties e.g. statins might be able to modulate HF risk among individuals with elevated levels of inflammatory biomarkers who are at high risk for developing heart failure. If proven, this might be especially important for HF with preserved EF, for which no effective therapy currently exists. Indeed, in the statin trials for secondary prevention, incident HF reduction has been reported (52,53), and statins have been shown to prevent ventricular remodeling in experimental studies (54,55). However, whether targeted administration of statins would attenuate HF risk among high-risk individuals is speculative and needs further study. Also, if IL-6 assays were to be developed and commercially available, it is possible that adding IL-6 to clinical predictors might facilitate earlier identification of high-risk individuals and implementation of preventive strategies.
Our study has several limitations. Diagnosis of HF was based on HF hospitalization. Because some participants may have developed HF without requiring hospitalization, HF rates are likely underestimated. Echocardiography was not performed at baseline in the Health ABC Study and thus participants with asymptomatic structural heart abnormalities may have been included in the analysis. It may be argued, therefore, that the risk associated with elevated inflammatory marker levels is merely because of inclusion of participants with yet undetected HF at baseline. However, the association with HF risk was consistent over time as shown by the proportionality tests, i.e. hazards did not converge over time. Because HF is unlikely to remain undiagnosed for several years, we contend that the observed association cannot be ascribed merely to undetected HF at baseline. Also, left ventricular function during hospitalization for HF was not prospectively assessed in the Health ABC Study and information on left ventricular EF was based on chart review in a subset (76.8%) of participants with incident HF; thus, the corresponding results should be interpreted with caution.
In conclusion, in this study we demonstrate a significant association between inflammatory markers and risk for incident HF among older persons. These findings were consistent across sex and race, and persisted after controlling for HF risk factors and incident CHD. Multiple biomarker elevations at baseline were associated with pronounced risk for HF. Inclusion of IL-6 in a clinical model for HF risk prediction significantly improved the predictive properties of the model. The practical implications of our findings, including screening individuals for HF risk determination or targeting inflammation to reduce HF risk, need further study.
Acknowledgments
Research Support: This research was supported in part by the Intramural Research Program of the National Institute of Aging, National Institutes of Health, Bethesda, Maryland, and by grants N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. Support for this research was also partially funded through an Emory University Heart and Vascular Board grant titled ‘Novel Risk Markers and Prognosis Determination in Heart Failure’.
ABBREVIATIONS
- CHD
Coronary heart disease
- CI
Confidence interval
- CRP
C-reactive protein
- EF
Ejection fraction
- HF
Heart failure
- HR
Hazard ratio
- IL-6
Interleukin-6
- IQR
Interquartile range
- sHR
Subhazard ratio
- TNF-α
Tumor necrosis factor alpha
Footnotes
Conflict of interest: None
References
- 1.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: a report from the Studies of Left Ventricular Dysfunction (SOLVD) J Am Coll Cardiol. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 2.Tsutamoto T, Hisanaga T, Wada A, et al. Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–8. doi: 10.1016/s0735-1097(97)00494-4. [DOI] [PubMed] [Google Scholar]
- 3.Roig E, Orus J, Pare C, et al. Serum interleukin-6 in congestive heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 1998;82:688–90. A8. doi: 10.1016/s0002-9149(98)00388-9. [DOI] [PubMed] [Google Scholar]
- 4.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen KH, Lassus J, Harjola VP, et al. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396–403. doi: 10.1016/j.ejheart.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Anand IS, Latini R, Florea VG, et al. C-reactive protein in heart failure: prognostic value and the effect of valsartan. Circulation. 2005;112:1428–34. doi: 10.1161/CIRCULATIONAHA.104.508465. [DOI] [PubMed] [Google Scholar]
- 7.Raymond RJ, Dehmer GJ, Theoharides TC, Deliargyris EN. Elevated interleukin-6 levels in patients with asymptomatic left ventricular systolic dysfunction. Am Heart J. 2001;141:435–8. doi: 10.1067/mhj.2001.113078. [DOI] [PubMed] [Google Scholar]
- 8.Kosmala W, Derzhko R, Przewlocka-Kosmala M, Orda A, Mazurek W. Plasma levels of TNF-alpha, IL-6, and IL-10 and their relationship with left ventricular diastolic function in patients with stable angina pectoris and preserved left ventricular systolic performance. Coron Artery Dis. 2008;19:375–82. doi: 10.1097/MCA.0b013e3282fc617c. [DOI] [PubMed] [Google Scholar]
- 9.Williams ES, Shah SJ, Ali S, Na BY, Schiller NB, Whooley MA. C-reactive protein, diastolic dysfunction, and risk of heart failure in patients with coronary disease: Heart and Soul Study. Eur J Heart Fail. 2008;10:63–9. doi: 10.1016/j.ejheart.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Menyar AA. Cytokines and myocardial dysfunction: state of the art. J Card Fail. 2008;14:61–74. doi: 10.1016/j.cardfail.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 11.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–91. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 12.Cesari M, Penninx BW, Newman AB, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–22. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 13.Bahrami H, Bluemke DA, Kronmal R, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51:1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 14.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident Heart Failure Prediction in the Elderly: The Health ABC Heart Failure Score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 16.van der Meer IM, de Maat MP, Bots ML, et al. Inflammatory mediators and cell adhesion molecules as indicators of severity of atherosclerosis: the Rotterdam Study. Arterioscler Thromb Vasc Biol. 2002;22:838–42. doi: 10.1161/01.atv.0000016249.96529.b8. [DOI] [PubMed] [Google Scholar]
- 17.Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’? Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- 18.Yanbaeva DG, Dentener MA, Creutzberg EC, Wesseling G, Wouters EF. Systemic effects of smoking. Chest. 2007;131:1557–66. doi: 10.1378/chest.06-2179. [DOI] [PubMed] [Google Scholar]
- 19.Annuk M, Soveri I, Zilmer M, Lind L, Hulthe J, Fellstrom B. Endothelial function, CRP and oxidative stress in chronic kidney disease. J Nephrol. 2005;18:721–6. [PubMed] [Google Scholar]
- 20.Wong LY, Leung RY, Ong KL, Cheung BM. Plasma levels of fibrinogen and C-reactive protein are related to interleukin-6 gene -572C>G polymorphism in subjects with and without hypertension. J Hum Hypertens. 2007;21:875–82. doi: 10.1038/sj.jhh.1002233. [DOI] [PubMed] [Google Scholar]
- 21.Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 23.Li R, Nicklas B, Pahor M, et al. Polymorphisms of angiotensinogen and angiotensin-converting enzyme associated with lower extremity arterial disease in the Health, Aging and Body Composition study. J Hum Hypertens. 2007;21:673–82. doi: 10.1038/sj.jhh.1002198. [DOI] [PubMed] [Google Scholar]
- 24.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 25.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 26.Wang J, Nagueh SF. Current perspectives on cardiac function in patients with diastolic heart failure. Circulation. 2009;119:1146–57. doi: 10.1161/CIRCULATIONAHA.108.822676. [DOI] [PubMed] [Google Scholar]
- 27.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 28.Volinsky CT, Raftery AE. Bayesian information criterion for censored survival models. Biometrics. 2000;56:256–62. doi: 10.1111/j.0006-341x.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- 29.Wagenmakers EJ. A practical solution to the pervasive problems of p values. Psychon Bull Rev. 2007;14:779–804. doi: 10.3758/bf03194105. [DOI] [PubMed] [Google Scholar]
- 30.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–94. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 31.Ambler G, Omar RZ, Royston P. A comparison of imputation techniques for handling missing predictor values in a risk model with a binary outcome. Stat Methods Med Res. 2007;16:277–98. doi: 10.1177/0962280206074466. [DOI] [PubMed] [Google Scholar]
- 32.Barnard J, Rubin B. Small-sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–955. [Google Scholar]
- 33.Barker WH, Mullooly JP, Getchell W. Changing incidence and survival for heart failure in a well-defined older population, 1970–1974 and 1990–1994. Circulation. 2006;113:799–805. doi: 10.1161/CIRCULATIONAHA.104.492033. [DOI] [PubMed] [Google Scholar]
- 34.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–9. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 35.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–24. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 36.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–37. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 37.Levy D, Kenchaiah S, Larson MG, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 38.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 39.Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992;257:387–9. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- 40.Muller-Werdan U, Schumann H, Fuchs R, et al. Tumor necrosis factor alpha (TNF alpha) is cardiodepressant in pathophysiologically relevant concentrations without inducing inducible nitric oxide-(NO)-synthase (iNOS) or triggering serious cytotoxicity. J Mol Cell Cardiol. 1997;29:2915–23. doi: 10.1006/jmcc.1997.0526. [DOI] [PubMed] [Google Scholar]
- 41.Janssen SP, Gayan-Ramirez G, Van den Bergh A, et al. Interleukin-6 causes myocardial failure and skeletal muscle atrophy in rats. Circulation. 2005;111:996–1005. doi: 10.1161/01.CIR.0000156469.96135.0D. [DOI] [PubMed] [Google Scholar]
- 42.Hirota H, Yoshida K, Kishimoto T, Taga T. Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice. Proc Natl Acad Sci U S A. 1995;92:4862–6. doi: 10.1073/pnas.92.11.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bozkurt B, Kribbs SB, Clubb FJ, Jr, et al. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 44.Bryant D, Becker L, Richardson J, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998;97:1375–81. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 45.Bradham WS, Moe G, Wendt KA, et al. TNF-alpha and myocardial matrix metalloproteinases in heart failure: relationship to LV remodeling. Am J Physiol Heart Circ Physiol. 2002;282:H1288–95. doi: 10.1152/ajpheart.00526.2001. [DOI] [PubMed] [Google Scholar]
- 46.Verma S, Li SH, Badiwala MV, et al. Endothelin antagonism and interleukin-6 inhibition attenuate the proatherogenic effects of C-reactive protein. Circulation. 2002;105:1890–6. doi: 10.1161/01.cir.0000015126.83143.b4. [DOI] [PubMed] [Google Scholar]
- 47.Yudkin JS, Kumari M, Humphries SE, Mohamed-Ali V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis. 2000;148:209–14. doi: 10.1016/s0021-9150(99)00463-3. [DOI] [PubMed] [Google Scholar]
- 48.Zambon A, Gervois P, Pauletto P, Fruchart JC, Staels B. Modulation of hepatic inflammatory risk markers of cardiovascular diseases by PPAR-alpha activators: clinical and experimental evidence. Arterioscler Thromb Vasc Biol. 2006;26:977–86. doi: 10.1161/01.ATV.0000204327.96431.9a. [DOI] [PubMed] [Google Scholar]
- 49.Sciarretta S, Ferrucci A, Ciavarella GM, et al. Markers of inflammation and fibrosis are related to cardiovascular damage in hypertensive patients with metabolic syndrome. Am J Hypertens. 2007;20:784–91. doi: 10.1016/j.amjhyper.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 50.Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–7. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 51.Van Linthout S, Riad A, Dhayat N, et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007;50:1977–86. doi: 10.1007/s00125-007-0719-8. [DOI] [PubMed] [Google Scholar]
- 52.Kumar A, Cannon CP. The role of statins in the prevention of heart failure after acute coronary syndrome. Heart Fail Clin. 2008;4:129–39. doi: 10.1016/j.hfc.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Udell JA, Ray JG. Primary and secondary prevention of heart failure with statins. Expert Rev Cardiovasc Ther. 2006;4:917–26. doi: 10.1586/14779072.4.6.917. [DOI] [PubMed] [Google Scholar]
- 54.Liao Y, Zhao H, Ogai A, et al. Atorvastatin slows the progression of cardiac remodeling in mice with pressure overload and inhibits epidermal growth factor receptor activation. Hypertens Res. 2008;31:335–44. doi: 10.1291/hypres.31.335. [DOI] [PubMed] [Google Scholar]
- 55.Hayashidani S, Tsutsui H, Shiomi T, et al. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;105:868–73. doi: 10.1161/hc0702.104164. [DOI] [PubMed] [Google Scholar]