The reported prevalence and appearance of oral adverse events with tyrosine kinase inhibitors and mammalian target of rapamycin inhibitors and the current oral assessment tools commonly used in clinical trials are examined. Correlations between oral adverse events and hand–foot skin reaction and rash are discussed.
Keywords: Tyrosine kinase inhibitor, Mammalian target of rapamycin inhibitor, Oral adverse events, Mucositis, Aphthous stomatitis, Renal cell carcinoma
Learning Objectives
After completing this course, the reader will be able to:
Describe the oral manifestations that can appear with TKI/mTORI.
Describe the limitations of the current oral assessment tools in assessing these novel presentations and list items needed to assess the presentations properly.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Background.
Oral adverse events (OAEs) associated with multitargeted tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin inhibitors (mTORIs) are underestimated but frequent and novel presentations of mucosal manifestations. Because optimal antitumor activity requires maintaining the optimal dose, it is essential to avoid unintended treatment delays or interruptions.
Methods.
We review the reported prevalence and appearance of OAEs with TKIs and mTORIs and the current oral assessment tools commonly used in clinical trials. We discuss the correlations between OAEs and hand–foot skin reaction (HFSR) and rash.
Results.
The reported prevalence of oral mucositis/stomatitis of any grade is 4% for pazopanib, 28% for sorafenib, 38% for sunitinib, 41% for temsirolimus, and 44% for everolimus. Oral lesions associated with these agents have been reported to more closely resemble aphthous stomatitis than OM caused by conventional agents. In addition, these agents may result in symptoms such as oral mucosal pain, dysgeusia, and dysphagia, in the absence of clinical lesions. Because of these factors, OAEs secondary to targeted agents may be underreported. In addition, a correlation between OAEs and HFSR was identified.
Conclusions.
OAEs caused by TKIs and mTORIs may represent dose-limiting toxicities, especially considering the fact that even low grades of OAEs may be troubling to the patient. We discuss how these novel AEs can be assessed because current mucositis assessment tools have limitations. Prospective studies investigating the pathogenesis, risk factors, and management of OAEs are needed in order to minimize the impact on patient's health-related quality of life.
Introduction
As a result of the introduction of targeted anticancer therapy for advanced renal cell carcinoma (RCC) and metastatic RCC (mRCC), the overall survival time of patients with this disease has increased dramatically. Currently, six U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved targeted agents are available for treating RCC: sunitinib malate (Sutent®; Pfizer, New York), sorafenib tosylate (Nexavar/Nexxava®; Bayer HealthCare, Leverkusen, Germany), pazopanib (Votrient®; GlaxoSmithKline, Greenford, U.K.), temsirolimus (Torisel®; Wyeth Pharmaceuticals, Philadelphia), everolimus (Afinitor®; Novartis Pharmaceuticals, East Hanover, NJ), and bevacizumab (Avastin®; Genentech, Inc., South San Francisco, CA) plus interferon-α2a. These agents are indicated as first- and second-line therapies. Bevacizumab differs from the other agents reported here in that it blocks vascular endothelial growth factor, whereas the other agents block multiple receptors and intracellular pathways (Table 1).
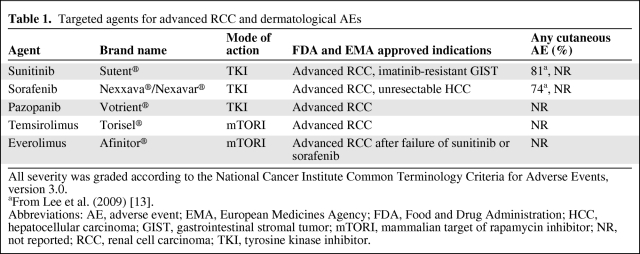
Table 1.
Targeted agents for advanced RCC and dermatological AEs
All severity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
aFrom Lee et al. (2009) [13].
Abbreviations: AE, adverse event; EMA, European Medicines Agency; FDA, Food and Drug Administration; HCC, hepatocellular carcinoma; GIST, gastrointestinal stromal tumor; mTORI, mammalian target of rapamycin inhibitor; NR, not reported; RCC, renal cell carcinoma; TKI, tyrosine kinase inhibitor.
With longer survival times, it has become even more important to optimize health-related quality of life (HRQoL) during treatment. These agents have a spectrum of mucocutaneous adverse events (AEs) with oral adverse events (OAEs), hand–foot skin reaction (HFSR) (for sunitinib, sorafenib, pazopanib, and everolimus), and rash as disabling and dose-limiting AEs. There are no evidence-based management options to prevent and treat these AEs.
Treatment of mRCC with Targeted Anticancer Agents
Targeted anticancer therapy is a general term that refers to drugs that target pathways in the growth and development of a tumor cell. Targeted therapies such as (multitargeted) tyrosine kinase inhibitors (TKIs) and mammalian target of rapamycin inhibitors (mTORIs) for RCC demonstrate a high level of efficacy with acceptable tolerability [1]. Targeted therapies may be continuously administered for their long-term ability to inhibit tumor growth, progression, cell proliferation, and angiogenesis.
In a short span of 4 years, the oral (multitargeted) TKIs sunitinib, sorafenib, and pazopanib, the i.v. mTORI temsirolimus, and the oral mTORI everolimus were approved by the FDA and EMA. Sorafenib received FDA and EMA approval in 2005 [2], sunitinib received approval in 2006 [3], temsirolimus received approval in 2007 [4], everolimus received approval in early 2009 [5], and pazopanib received approval in late 2009 [6]. Sunitinib also received FDA and EMA approval in 2006 for the treatment of gastrointestinal stromal tumor (GIST) [3], and sorafenib received approval in 2007 for the treatment of unresectable hepatocellular carcinoma (HCC) [2].
Treatment Delay, Dose Modifications, and Early Cessation
Optimal antitumor activity requires maintaining the optimal dose in individual patients. In order to improve HRQoL and adherence, AEs should be prevented if possible and treated if necessary. Current oral formulations of targeted agents consist of various schedules (e.g., continuous administration or 4 weeks on and 2 weeks off for sunitinib only) to optimize the risk–benefit profile.
Impaired HRQoL may have a negative impact on patient treatment adherence. Treatment over- or underadherence can have a significant impact on efficacy and the severity of treatment-related AEs [7]. Poor adherence may affect the therapeutic alliance, create skepticism in both the therapist and patient, induce resistance, worsen the disease or the prognosis attributed to missed doses, and increase health care costs [8]. Adherence to anticancer treatment is particularly important when prescribing self-administered oral therapies [9]. Because sorafenib, sunitinib, pazopanib, and everolimus are taken in the outpatient setting, patient education on the correct treatment dosing, usage, and the nature, recognition, and severity of AEs is essential to avoid unintended treatment delays or interruptions.
Conventional Cytotoxic Chemotherapy- and Radiotherapy-Induced OAEs
There are a number of cancer treatment–related, clinically important AEs that disrupt the function and integrity of the mouth. These AEs include OAEs characterized by redness, swelling, and ulceration; xerostomia (subjective dry mouth); and dysgeusia/ageusia (altered taste/taste loss). OAEs can result in significant clinical consequences, including oral sensitivity and pain, and can affect function, such as with difficulty in chewing and swallowing food, potentially leading to nutrient and caloric deficits, difficulty taking oral medications, and a higher risk for local and systemic infections [9, 10].
Stomatitis is a general term that includes inflammation and ulceration of the mucosal lining of the mouth resulting from any cause. Oral mucositis (OM) is the more specific term that is used to describe oral mucosal inflammation and ulceration induced by cancer therapies [11]. Conventional cytotoxic chemotherapy- or radiotherapy-induced OM is inflammatory-mediated damage of the mucosal membranes, most commonly involving nonkeratinized mucosa, that line the oral cavity; the ulcerative phase of development presents clinically with irregular and often confluent ulceration that is typically preceded by regional erythema. Whereas the first phases of mucositis involve the submucosal connective tissue, the epithelial cells of these mucosal tissues have a high turnover rate, which may make them susceptible to the effects of cancer chemotherapy and radiotherapy on the connective tissue and epithelium [12]. It is now recognized that it is not just the epithelium that is affected by cytotoxic treatment, but also the underlying connective tissue. OM develops almost exclusively on nonkeratinized mucosal surfaces (e.g., the buccal and labial mucosa, lateral tongue, floor of mouth, and soft palate).
The management of OAEs includes assessment, diagnosis, teaching oral care, administering interventions aimed at prevention and palliation of symptoms, and supporting patients in coping with symptom distress [9].
TKI- and mTORI-Induced Mucocutaneous AEs
Targeted therapy–related AEs, such as rash, xerosis, pruritus, mucosal, and hair abnormalities, occur in up to 81% of patients during treatment with TKIs or mTORIs [13]. Recognizing the fact that head-to-head comparisons are lacking and interpretation and scoring of AEs may not be univocal, Lee et al. [13] found that cutaneous reactions were more diverse in patients treated with sunitinib than in those treated with sorafenib. HFSR and OAEs were the most common mucocutaneous AEs (Tables 2 and 3).
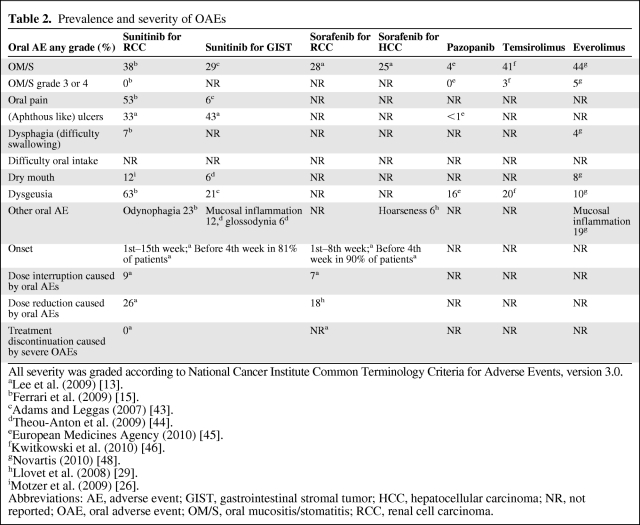
Table 2.
Prevalence and severity of OAEs
All severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
aLee et al. (2009) [13].
bFerrari et al. (2009) [15].
cAdams and Leggas (2007) [43].
dTheou-Anton et al. (2009) [44].
eEuropean Medicines Agency (2010) [45].
fKwitkowski et al. (2010) [46].
gNovartis (2010) [48].
hLlovet et al. (2008) [29].
iMotzer et al. (2009) [26].
Abbreviations: AE, adverse event; GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; NR, not reported; OAE, oral adverse event; OM/S, oral mucositis/stomatitis; RCC, renal cell carcinoma.
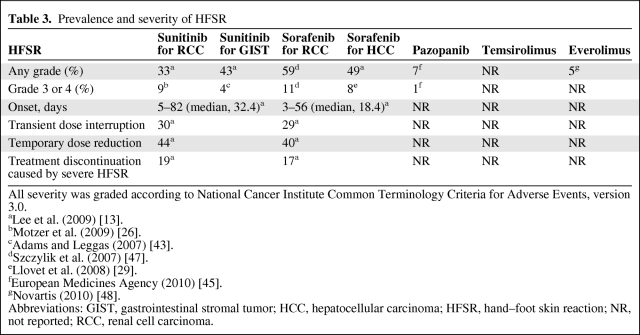
Table 3.
Prevalence and severity of HFSR
All severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
aLee et al. (2009) [13].
bMotzer et al. (2009) [26].
cAdams and Leggas (2007) [43].
dSzczylik et al. (2007) [47].
eLlovet et al. (2008) [29].
fEuropean Medicines Agency (2010) [45].
gNovartis (2010) [48].
Abbreviations: GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; HFSR, hand–foot skin reaction; NR, not reported; RCC, renal cell carcinoma.
TKI- and mTORI-Induced OAEs
To date, information on the pathobiology of the OAEs induced by targeted therapies is limited. In addition, there is no consensus on terminology, and in the literature on OAEs associated with targeted therapies, the terms mucositis and stomatitis are used interchangeably. This makes comparison of OAE data from different authors difficult.
An analysis of the appearance, course, and toxicity associations of mTORI-associated OAEs demonstrated that the condition is distinct from conventional mucositis and more closely resembles the presentation of aphthous stomatitis [14]. OAEs appeared within 5 days of deforolimus administration and were discrete, circular or ovoid, superficial, well demarcated, and surrounded by an erythematous halo primarily involving nonkeratinized mucosa. Their clinical appearance and distribution were similar to that of aphthous stomatitis but inconsistent with conventional mucositis. The lack of other gastrointestinal involvement but the presence of a higher prevalence of concomitant cutaneous AEs provided additional evidence to suggest a distinction between mTORI-associated OAEs and conventional cytotoxic therapy–induced OM [14].
In the study of Sonis et al. [14] of 78 solid tumor patients treated with deforolimus, OAEs, reported as mucositis, were dose-limiting toxicities for this new class of agents. OAEs were reported in 66% of the 78 study participants.
In a study of 30 mRCC patients treated with sunitinib, no correlation was found between the intensity of oral symptoms and clinical evidence of mucosal damage [15]. Patients were examined according to three standard assessments—the World Health Organization (WHO) Oral Toxicity Scale [16], National Cancer Institute Common Toxicity Criteria (NCI-CTC) [17], and Oral Mucositis Assessment Scale (OMAS) [16]—and according to an experimental assessment (EA) [15]. The EA consisted of an assessment of a number of symptoms using a visual analog scale (VAS) (range, 0–10) of dysgeusia, (subjective) dysphagia, odynophagia, and oral mucosal pain, which are subjective parameters, and objective mucosal erythema and ulceration. Whereas at the end of treatment the WHO Oral Toxicity Scale, NCI-CTC, and OMAS assessment were grade 0 in 62% of patients and grade 1 in 38% of patients, in the EA they observed no mucosal ulceration but 63% of patients experienced intense dysgeusia (VAS score, 7–10). Ten percent had intense (VAS score, 7–10) and 13% had moderate (VAS score, 4–6) odynophagia. Thirteen percent of the patients had acute pain (VAS score, 7–10) and 40% had intermediate pain (VAS score, 4–6). Three percent had moderate and 3% had severe dysphagia. Moderate erythema was observed in 40% of patients.
TKI- and mTORI-Induced HFSR
HFSR usually manifests as bilateral palmoplantar lesions, especially in areas of trauma or friction, such as over the interphalangeal joints, distal phalanges, or heels [18], and significantly affects patients' QoL [13]. Although most commonly associated with sorafenib and sunitinib, it is also reported with pazopanib and everolimus [19, 20].
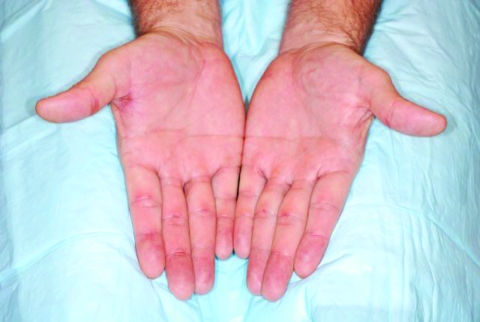
HFSR is associated with symptoms that are seen with OAEs too. Patients can develop localized, tender lesions that appear as blisters or hyperkeratosis, which in some cases can be surrounded by an erythematous halo (Fig. 1). Pain, dysesthesia, erythema, and edema [21, 22] are common symptoms on mechanically strained regions and can even appear without obvious skin alterations [23].
Figure 1.
Mammalian target of rapamycin inhibitor (mTORI)-induced hand–foot skin reaction (HFSR). HFSR caused by temsirolimus, an mTORI. HFSR is associated with symptoms that are seen with oral adverse events too. As shown in this picture, patients can develop localized, tender lesions that appear as blisters or hyperkeratosis, which in some cases can be surrounded by an erythematous halo.
In a meta-analysis by Chu et al. [24] on the incidence of and potential relationship between tumor type and sorafenib-associated HFSR, in total, 4,883 patients with metastatic tumors from 11 trials were included for analysis. They found that, among 3,252 patients with RCC, the prevalence of all-grade HFSR was 42.0% (95% confidence interval [CI], 24.9%–61.3%) and that of high-grade HFSR was 8.9% (95% CI, 6.3%–12.3%), whereas for 545 patients with malignancies other than RCC, the prevalence of all-grade HFSR was 27.6% (95% CI, 20.2%–36.4%) and the incidence of high-grade HFSR was 9.1% (95% CI, 7.2%–11.3%). There was a significant difference detected between patients with RCC and those with cancers other than RCC in terms of the prevalence of sorafenib-associated all-grade HFSR (relative risk [RR], 1.52; 95% CI, 1.32–1.75; p < .001). However, there was no significant difference between patients with RCC and those with cancers other than RCC in terms of the prevalence of high-grade HFSR (RR, 0.98; 95% CI, 0.76–1.26; p < .86) [24].
In a meta-analysis of HFSR with pazopanib [25], the overall incidences of all-grade and high-grade HFSR were 4.5% (95% CI, 2.5%–7.9%) and 1.5% (95% CI, 0.7%–3.1%), respectively. The RRs for all-grade and high-grade HFSR with pazopanib monotherapy in comparison with controls were greater, reaching statistical significance for all-grade (RR, 6.05; 95% CI, 1.11–33.12; p = .038) but not for high-grade (RR, 2.51; 95% CI, 0.12–51.9; p = .55) HFSR. We did not identify reports of HFSR caused by temsirolimus.
Because of the high prevalence of HFSR associated with TKI use (Table 3), early detection and timely treatment are vital in managing patients during their drug courses to allow continued treatment [13].
Objective
The aim of this study is to provide an overview of the prevalence and appearance of OAEs with TKI and mTORI treatment and the current oral assessment tools commonly used in clinical trials. We also wanted to find out if there is a correlation among OAEs, HFSR, and rash.
Methods
Search Strategy
We designed a search strategy to identify relevant literature that described OAEs resulting from targeted anticancer therapy among RCC patients in each database as outlined below. We performed our search in the electronic databases PubMed, Embase, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for literature published from 1980 through January 7, 2011, linking the subject search headings with text word, MESH terms, and substance name. We linked the key words “mucositis,” “stomatitis,” “ulcer,” “aphthous,” “oral pain,” “deglutition disorders,” “swallowing,” “dry mouth,” and “altered taste” to the generic agents and classes of drug. We didn't make language restrictions. OAEs in patients with cancer types other than RCC, GIST, or HCC were not appropriate. Because of the paucity of OAE studies on TKIs and mTORIs at the time of the search, all publication types were considered.
Selection Criteria
We were primarily interested in the clinical presentation of OAEs caused by TKIs and mTORIs. To be included, a paper had to be focused on OAEs, including assessment as (one of) the primary or secondary outcomes, and focusing on TKIs or mTORIs. Papers that only described the appearance of OAEs as a safety issue were excluded.
Results
Initial searching found a total of 630 citations; 239 hits in PubMed, 376 in Embase, and 15 in CINAHL. After removing duplicates, 501 citations remained; 472 were discarded based on title or abstract because they did not meet the inclusion criteria and 29 citations were included for review.
TKI- and mTORI-Induced AE Profiles
Although some targeted agents share a common mode of action, it should not be assumed that their AE profiles are comparable. Indeed, evidence indicates clinically relevant differences among the toxicity profiles of targeted therapies, including between agents with the same mode of action. For example, sorafenib and sunitinib are both multitargeted TKIs, but in patients with RCC, HFSR appears to occur more frequently with sorafenib (30%) than with sunitinib (19%) [13], whereas leukopenia, neutropenia, and anemia are common with sunitinib (78%, 77%, and 79%) but not with sorafenib. Febrile neutropenia or grade 4 thrombocytopenia did not occur with sorafenib. Grade 3 or 4 anemia occurred in 3% of patients and grade 3 or 4 lymphopenia occurred in 13% of patients [26–28]. It should also be noted that the AE profile for a targeted agent may differ among tumor types. For example, HFSR may occur less frequently with sorafenib in patients with HCC than in patients with RCC (Table 3) [27, 29]. In a meta-analysis performed by Chu et al. [30], it was found that patients with RCC had a significantly greater risk for all-grade HFSR than patients with a malignancy other than RCC, 42% (95% CI, 24.9%–63.3%) and 27.6% (95% CI, 20.2%–36.4%), respectively.
TKI- and mTORI-Induced OAEs
OAEs are associated with many targeted agents. The oral burden can be very difficult for patients, even when the treatment is effective in combating the cancer. These circumstances can lead to lower HRQoL, delay in treatment, dose modification, or early cessation of critical antineoplastic therapy [13].
Clinical Presentation of TKI and mTORI OAEs
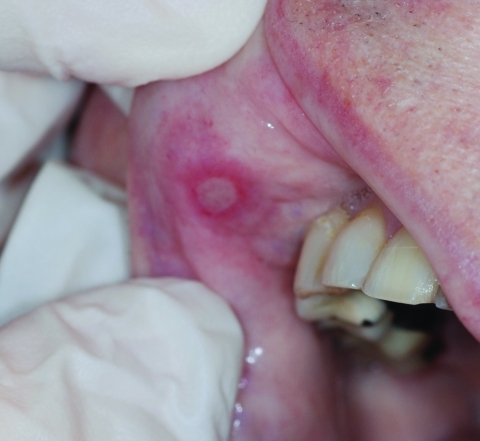
A variety of oral signs and symptoms have been described in association with the use of TKIs and mTORIs. For example, sunitinib treatment has been associated with oral mucosal hypersensitivity, oral ulcers, cheilitis, and taste alterations [23, 31]. Oral lesions associated with mTORIs have been described as discrete, oval, superficial ulcers with an erythematous halo (Fig. 2), an appearance similar to that of aphthous stomatitis and unlike that of OM secondary to conventional chemotherapeutic agents [14]. Interestingly, and also unlike oral mucosal toxicity associated with conventional chemotherapy, patients on such targeted agents may sometimes present with oral complaints such as mouth pain, dysgeusia, and dysphagia in the absence of any clinically apparent lesion [14, 21, 32]. Such symptoms have been reported to rapidly improve during treatment-free intervals [23] and may occur again with additional dosing of the targeted agent.
Figure 2.
Mammalian target of rapamycin inhibitor (mTORI)-induced oral adverse events. Aphthous stomatitis caused by temsirolimus, an mTORI. As shown in this picture, patients can develop localized, tender lesions that appear as aphthous stomatitis and that can be surrounded by an erythematous halo.
Prevalence of TKI- and mTORI-Induced OAEs
Current data on the frequency of the OAEs associated with each of the different targeted agents are highlighted in Table 2. OAEs are early symptoms, generally observed in sunitinib and sorafenib patients 1–15 weeks after initiation of treatment. As outlined in Table 2, many OAEs are not separately reported. The highest score of any-grade OM or stomatitis is reported with everolimus (44%) and the lowest score is reported with pazopanib (4%). OAEs generally appear 1–15 weeks after initiation of treatment; symptoms began before the fourth week of treatment in 81% and 90%, respectively, of sunitinib- and sorafenib-treated patients. The presence of OAEs required dose reduction in 26% of the sunitinib-treated patients and in 18% of the sorafenib-treated patients. No patient permanently discontinued treatment as a result of severe OAEs.
With mTORIs, oral lesions have a rapid onset (usually within 5 days) and are usually of mild to moderate severity (NCI-CTCAE grade 1–2). Lesions are usually found on the mucosa of the lips, lateral tongue, buccal mucosa, and soft palate. Unlike viral-induced ulcers, they are not commonly seen on the hard palate or outer aspects of the lip. They often present as individual ulcers, similar to aphthous ulcers (canker sores): distinct round-oval lesions with grayish-white necrotic centers surrounded by a ring of erythema. Unlike radiation- and chemotherapy-associated mucositis, there is no pseudomembrane formation (Fig. 2). Occasionally they are severe (grade 3), but generally they are reversible by withholding treatment. In many cases mucositis improves or resolves spontaneously despite treatment continuation [33].
Assessment of TKI- and mTORI-Induced OAEs
Numerous OM grading scales have been developed over the years to grade conventional mucositis [34]. The complexity and detail of these scales vary significantly and selection of a mucositis scale is influenced by the reason for assessing mucositis for either clinical care or OM research [35].
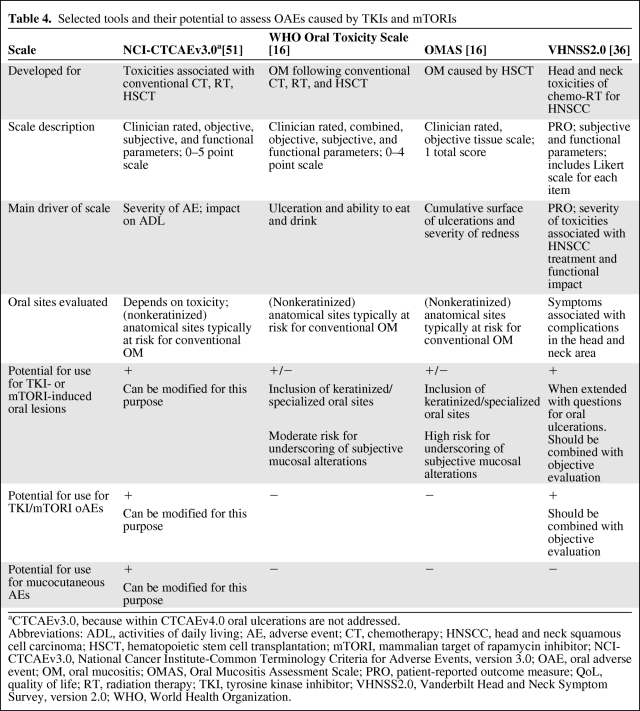
Targeted therapy may induce subjective symptoms of oral burden without significant clinical evidence [15]. No validated targeted therapy–specific grading scales are currently available. The frequently used OM scales like the WHO Oral Toxicity Scale, NCI-CTCAE, and OMAS are not designed to evaluate OAEs caused by TKIs and mTORIs and may result in underreporting and poor grading of OAEs in patients treated with these agents (Table 4). For example, the OMAS focuses on objective ulceration and redness, whereas the WHO Oral Toxicity Scale is mainly driven by the patient's ability to eat and drink. The EA suggested by Ferrari et al. [15] may be more adequate for scoring TKI- and mTORI-induced OAEs. The Vanderbilt Head and Neck Symptom Survey (VHNSS), version 2.0, is a tool developed for head and neck cancer patients treated with chemoradiation. It assesses patient-reported symptom burden in the head and neck area and function loss within symptom subscales, including nutrition, taste, pain, voice, swallow, and mucous/dry mouth [36]. The Multinational Association of Supportive Care in Cancer (MASCC) Skin Toxicity Study Group proposed a grading system for the most common epidermal growth factor receptor inhibitor (EGFRI)-induced mucocutaneous AEs [37]. That scale is consistent with the grading principles and language of the CTCAE, version 4.0, and may be formally integrated into future CTCAE versions.
Table 4.
Selected tools and their potential to assess OAEs caused by TKIs and mTORIs
aCTCAEv3.0, because within CTCAEv4.0 oral ulcerations are not addressed.
Abbreviations: ADL, activities of daily living; AE, adverse event; CT, chemotherapy; HNSCC, head and neck squamous cell carcinoma; HSCT, hematopoietic stem cell transplantation; mTORI, mammalian target of rapamycin inhibitor; NCI-CTCAEv3.0, National Cancer Institute-Common Terminology Criteria for Adverse Events, version 3.0; OAE, oral adverse event; OM, oral mucositis; OMAS, Oral Mucositis Assessment Scale; PRO, patient-reported outcome measure; QoL, quality of life; RT, radiation therapy; TKI, tyrosine kinase inhibitor; VHNSS2.0, Vanderbilt Head and Neck Symptom Survey, version 2.0; WHO, World Health Organization.
Management of OAEs
For the prevention of conventional OM, most recommendations begin with the use of oral care plans coupled with patient education [38]. A range of products is currently in development for the prevention and management of OAEs that fall into four main categories—cell resistance modifiers, mechanism-specific inhibitors, damage control agents, and healing accelerators. However, to date, proven approaches for the prevention and treatment of OAEs are limited [38, 39]. No trials have assessed the management of TKI- and mTORI-induced OAEs. Sonis et al. [14] suggested that mTORI-induced OAEs were distinct entities from conventional OM. The exact etiology of aphthous stomatitis has not been fully determined, but it is considered to involve immune mechanisms such as antibody-dependent cell-mediated cytotoxicity and immune complex formation; this is different from what is considered to occur with conventional OM [40]. Interventions for persistent TKI- or mTORI-related OAEs, therefore, may include the use of various agents such as topical corticosteroids and anti-inflammatory agents as well as supportive treatments such as local anesthetics and antimicrobials [40]. It is important, however, to avoid unfavorable drug interactions with TKI and mTORI drugs.
Correlation of OAEs with Dermatological AEs
Correlation Between OAEs and HFSR
Lee at al. [13] found a strong correlation between OAEs and HFSR in the patients they studied, who were treated with sunitinib and sorafenib. A significant correlation was found between the occurrence of stomatitis and severity of HFSR (p < .01, χ2 test for trend). OAEs were observed in 72% of patients with grade 3 HFSR and in 47% of patients with grade 2 HFSR. OAEs were more likely to occur in patients with severe HFSR than in those with mild HFSR. There was a significant relationship between the occurrence of stomatitis and severity of HFSR (p = .004, χ2 test for trend), although no significant correlation was found between HFSR severity and response to treatment [13].
Correlation Between OAEs and Rash
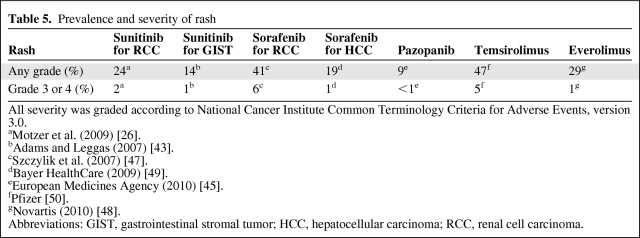
Rash caused by TKIs or mTORIs can affect 9%–47% of patients (Table 5). Because there was a significant relationship found between the occurrence of OAEs and severity of HFSR in sunitinib- and sorafenib-treated patients, it is interesting to assess the potential for OAEs occurring with rash. As far as we know, there is no literature addressing this possible correlation.
Table 5.
Prevalence and severity of rash
All severity was graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
aMotzer et al. (2009) [26].
bAdams and Leggas (2007) [43].
cSzczylik et al. (2007) [47].
dBayer HealthCare (2009) [49].
eEuropean Medicines Agency (2010) [45].
fPfizer [50].
gNovartis (2010) [48].
Abbreviations: GIST, gastrointestinal stromal tumor; HCC, hepatocellular carcinoma; RCC, renal cell carcinoma.
Discussion
TKI- and mTORI-related OAEs are underrecognized although they may represent a dose-limiting toxicity for this new class of agents, especially considering the fact that even low grades of OAEs with chronic daily dosing may result in morbidity that may lead to dose reductions [14]. With the longer survival times for RCC patients, it has become even more important to optimize HRQoL during treatment.
The prevalence of OAEs of any grade in renal cancer patients is 38% for sunitinib, 28% for sorafenib, 4% for pazopanib, 41% for temsirolimus, and 44% for everolimus. Interestingly, targeted therapy may induce subjective symptoms of oral burden without objective clinical evidence (e.g., mucosal sensitivity and pain, odynophagia, xerostomia, and taste alterations). Because of these symptoms and aphthous-like ulcerations being distinct from conventional ulcerative OM, current tools are of limited value for OAE assessment. The EA from Ferrari et al. [15] and a modified version of the VHNSS, version 2.0, are potentially useful to grade OAEs. There is a gap in the current literature related to assessing OAEs, HFSR, and rash resulting from therapy with TKIs and mTORIs. Therefore, development of a comprehensive grading system for TKI- and mTORI-associated mucocutaneous AEs similar to the MASCC EGFRI mucocutaneous AE–specific scale seems appropriate.
It is feasible that TKIs and mTORIs are associated with other less frequent or not yet investigated oral complications. For example, a case of jaw osteonecrosis associated with sunitinib has been reported [41], salivary gland function may be affected, resulting in hyposalivation and qualitative salivary alterations, and patients taking mTORIs may be at risk for periodontitis because these drugs induce immunosuppression and affect collagen synthesis.
A strong correlation was found between severe OAEs and HFSR. The results of the current review suggest that OAEs induced by TKIs and mTORIs are distinct from conventional chemotherapy- and radiotherapy-induced OM. More studies are necessary into the pathobiology of OAEs induced by TKIs and mTORIs. In addition, studies of individual patient characteristics predisposing for toxicities are promising, because these may lead to optimal treatment strategies. For example, a recent study indicated that polymorphisms in genes encoding metabolizing enzymes, efflux transporters, and drug targets are associated with sunitinib-related toxicities [42].
Targeted agents have mucocutaneous AEs in common, with OAEs, HFSR, and rash as the most disabling AEs. Evidence-based management guidelines to prevent and treat these complications are required; presently they are lacking.
Additional studies of management strategies may therefore be important for dose adherence to TKI and mTORI therapy and for the overall acceptance of this therapy for patients.
Educating patients on the importance of reporting all AEs and on compliance with the prescribed dose may increase early recognition and ensure adherence to treatment, allowing the most effective treatment strategy for the patient. There is currently only limited evidence for the prevention and management of OAEs caused by targeted agents, which indicates the need for more evidence derived from well-designed prospective clinical studies in order to improve management.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Christine B. Boers-Doets, Joel B. Epstein
Provision of study material or patients: Christine B. Boers-Doets
Collection and/or assembly of data: Christine B. Boers-Doets, Richard M. Logan
Data analysis and interpretation: Christine B. Boers-Doets, Joel B. Epstein, Judith E. Raber-Durlacher, Jan Ouwerkerk, Richard M. Logan, Jan A. Brakenhoff, Mario E. Lacouture, Hans Gelderblom
Manuscript writing: Christine B. Boers-Doets, Joel B. Epstein, Judith E. Raber-Durlacher, Jan Ouwerkerk, Richard M. Logan, Jan A. Brakenhoff, Mario E. Lacouture, Hans Gelderblom
Final approval of manuscript: Christine B. Boers-Doets, Joel B. Epstein, Judith E. Raber-Durlacher, Jan Ouwerkerk, Jan A. Brakenhoff, Mario E. Lacouture, Hans Gelderblom
References
- 1.Bukowski RM. Systemic therapy for metastatic renal cell carcinoma in treatment naïve patients: A risk-based approach. Expert Opin Pharmacother. 2010;11:2351–2362. doi: 10.1517/14656566.2010.499126. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. FDA Approval for Sorafenib Tosylate. 2007. Nov 19, [accessed October 20, 2010]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-sorafenib-tosylate#Anchor-Live-50484.
- 3.National Cancer Institute. FDA Approval for Sunitinib Malate. 2007. Feb 5, [accessed October 20, 2010]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-sunitinib-malate#kidney.
- 4.National Cancer Institute. FDA Approval for Temsirolimus. 2007. Jun 1, [accessed October 20, 2010]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-temsirolimus.
- 5.U.S. Food and Drug Administration. Everolimus. 2010. Jan 11, [accessed October 20, 2010]. Available at http://www.fda.gov/AboutFDA/CentersOffices/CDER/ucm127799.htm.
- 6.National Cancer Institute. FDA Approval for Pazopanib Hydrochloride. 2009. Oct 29, [accessed October 20, 2010]. Available at http://www.cancer.gov/cancertopics/druginfo/fda-pazopanibhydrochloride.
- 7.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 8.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 9.Harris DJ, Eilers J, Harriman A, et al. Putting evidence into practice: Evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs. 2008;12:141–152. doi: 10.1188/08.CJON.141-152. [DOI] [PubMed] [Google Scholar]
- 10.Wood LS. Managing the side effects of sorafenib and sunitinib. Commun Oncol. 2006;3:558–562. [Google Scholar]
- 11.Lalla RV, Sonis ST, Peterson DE. Management of oral mucositis in patients who have cancer. Dent Clin North Am. 2008;52:61–77. viii. doi: 10.1016/j.cden.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenstein EB, Peterson DE, Schubert M, et al. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100(9 suppl):2026–2046. doi: 10.1002/cncr.20163. [DOI] [PubMed] [Google Scholar]
- 13.Lee WJ, Lee JL, Chang SE, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161:1045–1051. doi: 10.1111/j.1365-2133.2009.09290.x. [DOI] [PubMed] [Google Scholar]
- 14.Sonis S, Treister N, Chawla S, et al. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210–215. doi: 10.1002/cncr.24696. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari V, Sgotti D, Amoroso V, et al. Oral mucositis a side effect in tyrosine-kinase inhibitor therapy (sunitinib): The role of assessment of symptoms in evaluation of toxicity [abstract] Eur J Cancer. 2009;2(suppl 7):195. [Google Scholar]
- 16.Sonis ST, Eilers JP, Epstein JB, et al. Validation of a new scoring system for the assessment of clinical trial research of oral mucositis induced by radiation or chemotherapy. Mucositis Study Group. Cancer. 1999;85:2103–2113. doi: 10.1002/(sici)1097-0142(19990515)85:10<2103::aid-cncr2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Common Toxicity Criteria (CTC), Version 2.0. 30–4-1999. [accessed November 9, 2009]. Available at www.eortc.be/services/doc/ctc/ctcv20_4-30-992.pdf.
- 18.Lacouture ME, Reilly LM, Gerami P, et al. Hand foot skin reaction in cancer patients treated with the multikinase inhibitors sorafenib and sunitinib. Ann Oncol. 2008;19:1951–1966. doi: 10.1093/annonc/mdn389. [DOI] [PubMed] [Google Scholar]
- 19.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: Results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 20.Novartis Pharmaceuticals Corporation. Afinitor® (everolimus) tablets [prescribing information] [accessed November 12, 2010]. Available at http://www.afinitor.com/index.jsp.
- 21.Porta C, Paglino C, Imarisio I, et al. Uncovering Pandora's vase: The growing problem of new toxicities from novel anticancer agents. The case of sorafenib and sunitinib. Clin Exp Med. 2007;7:127–134. doi: 10.1007/s10238-007-0145-8. [DOI] [PubMed] [Google Scholar]
- 22.Autier J, Escudier B, Wechsler J, et al. Prospective study of the cutaneous adverse effects of sorafenib, a novel multikinase inhibitor. Arch Dermatol. 2008;144:886–892. doi: 10.1001/archderm.144.7.886. [DOI] [PubMed] [Google Scholar]
- 23.Kollmannsberger C, Soulieres D, Wong R, et al. Sunitinib therapy for metastatic renal cell carcinoma: Recommendations for management of side effects. Can Urol Assoc J. 2007;1(2 suppl):S41–S54. doi: 10.5489/cuaj.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chu D, Lacouture ME, Fillos T, et al. Risk of hand-foot skin reaction with sorafenib: A systematic review and meta-analysis. Acta Oncol. 2008;47:176–186. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 25.Balagula Y, Wu S, Su X, et al. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: A systematic review of literature and meta-analysis. Invest New Drugs. 2011 Mar 11; doi: 10.1007/s10637-011-9652-2. [E-pub ahead of print]. DOI: 10.1007/s10637–011-9652–2. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–3318. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 28.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 30.Chu D, Lacouture ME, Weiner E, et al. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: A meta-analysis. Clin Genitourin Cancer. 2009;7:11–19. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 31.Grünwald V, Soltau J, Ivanyi P, et al. Molecular targeted therapies for solid tumors: Management of side effects. Onkologie. 2009;32:129–138. doi: 10.1159/000194949. [DOI] [PubMed] [Google Scholar]
- 32.Ivanyi P, Winkler T, Ganser A, et al. Novel therapies in advanced renal cell carcinoma: Management of adverse events of sorafenib and sunitinib. Dtsch Arztebl Int. 2008;105:232–237. doi: 10.3238/arztebl.2008.0232. In German. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankhala K, Mita A, Kelly K, et al. The emerging safety profile of mTOR inhibitors, a novel class of anticancer agents. Target Oncol. 2009;4:135–142. doi: 10.1007/s11523-009-0107-z. [DOI] [PubMed] [Google Scholar]
- 34.Sonis ST, Elting LS, Keefe D, et al. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100(9 suppl):1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 35.Brennan MT, von Bültzingslöwen I, Schubert MM, et al. Alimentary mucositis: Putting the guidelines into practice. Support Care Cancer. 2006;14:573–579. doi: 10.1007/s00520-006-0054-5. [DOI] [PubMed] [Google Scholar]
- 36.Cooperstein E, Epstein JB, Murphy BA, et al. Vanderbilt Head and Neck Symptom Survey Version 2.0 (VHNSS 2.0): A tool for the study of long-term oral health consequences of therapy for head and neck cancer (HNC) [abstract] J Clin Oncol. 2010;28(15 suppl):9055. [Google Scholar]
- 37.Lacouture ME, Maitland ML, Segaert S. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–522. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 38.Keefe DM, Schubert MM, Elting LS, et al. Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer. 2007;109:820–831. doi: 10.1002/cncr.22484. [DOI] [PubMed] [Google Scholar]
- 39.Al-Dasooqi N, Gibson RJ, Bowen JM, et al. Matrix metalloproteinases: Key regulators in the pathogenesis of chemotherapy-induced mucositis? Cancer Chemother Pharmacol. 2009;64:1–9. doi: 10.1007/s00280-009-0984-y. [DOI] [PubMed] [Google Scholar]
- 40.Messadi DV, Younai F. Aphthous ulcers. Dermatol Ther. 2010;23:281–290. doi: 10.1111/j.1529-8019.2010.01324.x. [DOI] [PubMed] [Google Scholar]
- 41.Koch FP, Walter C, Hansen T, et al. Osteonecrosis of the jaw related to sunitinib. Oral Maxillofac Surg. 2011;15:63–66. doi: 10.1007/s10006-010-0224-y. [DOI] [PubMed] [Google Scholar]
- 42.van Erp NP, Eechoute K, van der Veldt AA, et al. Pharmacogenetic pathway analysis for determination of sunitinib-induced toxicity. J Clin Oncol. 2009;27:4406–4412. doi: 10.1200/JCO.2008.21.7679. [DOI] [PubMed] [Google Scholar]
- 43.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther. 2007;29:1338–1353. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 44.Theou-Anton N, Faivre S, Dreyer C, et al. Benefit-risk assessment of sunitinib in gastrointestinal stromal tumours and renal cancer. Drug Saf. 2009;32:717–734. doi: 10.2165/00002018-200932090-00003. [DOI] [PubMed] [Google Scholar]
- 45.European Medicines Agency. Summary of Product Characteristics: Pazopanib (Votrient®) 2010. [accessed November 17, 2010]. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001141/WC500094272.pdf.
- 46.Kwitkowski VE, Prowell TM, Ibrahim A, et al. FDA approval summary: Temsirolimus as treatment for advanced renal cell carcinoma. The Oncologist. 2010;15:428–435. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Szczylik C, Demkow T, Staehler M, et al. Randomized phase II trial of first-line treatment with sorafenib versus interferon in patients with advanced renal cell carcinoma: Final results [abstract] J Clin Oncol. 2007;25(18 suppl):5025. doi: 10.1200/JCO.2008.19.3342. [DOI] [PubMed] [Google Scholar]
- 48.Novartis. Afinitor® (everolimus) [prescribing information] 2010. [accessed November 15, 2010]. Available at http://www.pharma.us.novartis.com/product/pi/pdf/afinitor.pdf.
- 49.Bayer HealthCare Pharmaceuticals Inc. Nexavar® (sorafenib [prescribing information] 2009. [accessed November 12, 2010]. Available at http://www.nexavar.com/html/download/Nexavar_PI.pdf.
- 50.Pfizer. Torisel® (temsirolimus) [prescribing information] 2010. [accessed November 12, 2010]. Available at http://www.pfizerpro.com/content/showlabeling.asp?id=490.
- 51.National Cancer Institute. Common Terminology Criteria for Adverse Events v3.0 (CTCAE) 2006. [accessed November 9, 2009]. Available at www.eortc.be/services/doc/ctc/ctcaev3.pdf.