New developments in the treatment of patients with relapsed/refractory Hodgkin's lymphoma undergoing autologous stem cell transplantation are summarized including modern prognostic markers, the role of functional imaging, the role of newer drugs, different conditioning regimens, and maintenance therapy.
Keywords: Autologous stem cell transplant, Hodgkin's lymphoma, High-dose chemotherapy
Abstract
Despite the relatively high long-term disease-free survival (DFS) rate for patients with Hodgkin lymphoma (HL) with modern combination chemotherapy or combined modality regimens, ∼20% of patients die from progressive or relapsed disease. The standard treatment for relapsed and primary refractory HL is salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (ASCT), which has shown a 5-year progression-free survival rate of ∼50%–60%. Recent developments in a number of diagnostic and therapeutic modalities have begun to improve these results. Functional imaging, refinement of clinical prognostic factors, and development of novel biomarkers have improved the predictive algorithms, allowing better patient selection and timing for ASCT. In addition, these algorithms have begun to identify a group of patients who are candidates for more aggressive treatment beyond standard ASCT. Novel salvage regimens may potentially improve the rate of complete remission prior to ASCT, and the use of maintenance therapy after ASCT has become a subject of current investigation. We present a summary of developments in each of these areas.
Introduction
Over the last 20 years, sequential generations of clinical trials honing in on the appropriate use of chemotherapy and radiotherapy (RT) for both limited and advanced stage Hodgkin's lymphoma (HL) have allowed the development of treatment strategies that achieve excellent response rates and long progression-free survival (PFS) intervals while also decreasing the late effects of therapy [1, 2]. However, up to 15% of patients with limited stage disease and 35%–40% of patients with advanced stage disease either do not achieve complete remission (CR) initially or relapse within the first few years after initial treatment [3]. To maximize the efficacy of initial therapy, more intensive therapeutic regimens have been tested by the German Hodgkin Study Group (GHSG) [4, 5]. In a competing trend, positron emission tomography (PET) response–adapted therapy is currently being tested in an effort to decrease therapeutic intensity in patients with an early disease response [6].
Although no overall survival (OS) benefit has ever been demonstrated in a prospective, randomized clinical trial, salvage chemotherapy followed by high-dose chemotherapy and autologous stem cell transplantation (HDT–ASCT) is the treatment of choice for patients with chemosensitive refractory or relapsed HL [7–9]. For patients able to undergo HDT–ASCT, several clinical trials and single-center series have suggested a long-term disease-free survival (DFS) rate of ∼50%–60% [7, 8, 10–13]. Patients who relapse after HDT–ASCT have traditionally had a poor prognosis. In the context of improvements in initial therapy, significant research in the last few years has focused on maximizing outcomes with HDT–ASCT. These efforts, which are summarized here, include defining better prognostic factors at relapse and the incorporation of functional imaging (FI) and biomarkers to improve patient selection and timing of HDT–ASCT, the development of novel salvage chemotherapy and conditioning regimens prior to ASCT, and the first trials to test maintenance therapy after ASCT.
Prognostic Factors at Relapse
Clinical Prognostic Factors
The International Prognostic Score developed by Hasenclever and Diehl [14] has been a useful tool for risk stratification at initial presentation of advanced stage HL but has not been shown to be useful for predicting prognosis in refractory and relapsed HL patients [15]. Other retrospective and prospective studies have identified prognostic factors in patients with relapsed and primary refractory HL. The Société Française de Greffe de Moelle (SFGM) performed a retrospective analysis on 218 patients who underwent ASCT for relapsed HL and developed a prognostic model based on risk factors of duration of initial remission (≤12 months or >12 months) and nodal or extranodal relapse. In the presence of zero, one, or two risk factors, the 4-year OS rates were 93%, 59%, and 43%, respectively [16]. The GHSG developed a prognostic score based on 422 patients enrolled in sequential clinical trials who relapsed after standard treatment. With multivariate analysis, three significant prognostic factors for OS at relapse were identified: the presence of anemia, advanced clinical stage at relapse (III or IV), and duration of initial remission (≤12 months or >12 months). The freedom from second treatment failure (FF2F) rates were 45%, 32%, and 18% with zero or one, two, or three prognostic factors, respectively [17]. In a smaller retrospective analysis of 100 patients treated with HDT–ASCT for failure or progression after initial therapy, the need for more than one chemotherapy regimen before HDT–ASCT and the presence of extranodal disease at relapse were independent risk factors for outcome in patients at first relapse (after >3 months following the completion of first-line treatment) [13].
In another series of 357 patients who underwent ASCT for HL at first relapse, advanced stage at diagnosis, the use of RT during initial treatment, duration of CR, and the presence of disease at the time of transplant were indicators of a poor outcome after transplant. For patients with zero or one risk factor, the 5-year time to failure (TTF) rate was 71%, but in the presence of three or more risk factors, the 5-year TTF rate was 18% [18]. A Memorial Sloan-Kettering Cancer Center prospective study on salvage therapy for relapsed and refractory HL found that the presence of B symptoms at relapse, extranodal disease, and duration of initial response <12 months independently predicted worse outcome. The event-free survival (EFS) rates were 83%, 27%, and 10% for patients with zero or one, two, or three factors, respectively [11]. To improve outcomes in the high-risk relapsed patient cohort, the same group developed a risk-adapted strategy with intensified salvage and transplant procedures for less favorable patients and this seemed to eliminate the differences between the high- and low-risk groups [19]. In summary, there is no single standard prognostic system for relapsed HL though the most common factors appear to be a short duration of initial remission and advanced clinical stage at relapse. The development of such a consensus tool validated in large numbers of patients would be helpful for future clinical trials, either in developing risk-adapted approaches or for enrollment into trials testing novel strategies beyond ASCT.
Role of Functional Imaging in Predicting Outcome after ASCT
In addition to clinical factors, the expanded use of FI (most commonly PET scanning) after salvage therapy and before ASCT has been clearly identified as a critical predictor of outcome. A retrospective analysis of 211 patients with relapsed HL demonstrated significant differences in terms of both PFS and OS outcomes based on pretransplant FI status. The 3-year PFS rate were 69% and 23% for FI− and FI+ patients, respectively, and the 3-year OS rates were 87% and 58%, respectively [20]. Other series have demonstrated similar results, although some of these are confounded by the inclusion of both HL and non-Hodgkin lymphoma (NHL) patients [21–25]. More recently, an analysis performed on 153 patients with relapsed and refractory HL proceeding to ASCT after ifosfamide, carboplatin, and etoposide (ICE)-based salvage therapy revealed a 5-year EFS rate of 31% for FI+ patients before ASCT, compared with 75% for FI− patients [26]. From these studies, it is clear that FI status prior to HDT–ASCT is a very powerful predictor of outcome after HDT–ASCT, and some institutions have already altered their standard practice to continuing with salvage chemotherapy until FI is negative before proceeding to ASCT. However, it is unclear how FI status interacts with the clinical prognostic factors mentioned above, and proceeding with further potentially ineffective salvage chemotherapy may allow the disease to progress in the interim. Given the widespread availability of FI, it would be ideal to conduct a prospective trial to attempt to answer this question before changing standard practice. Currently, we routinely attempt to achieve an FI− status before ASCT, but do proceed with ASCT in certain FI+ cases as well.
Novel Biologic Markers
In addition to clinical risk factors and FI status, specific biomarkers expressed by malignant Reed-Sternberg (RS) cells or by the tumor microenvironment have been identified as predictors of outcome, either at the time of initial diagnosis or after primary treatment failure [27]. In the microenvironment, greater expression of CD68 on tumor-infiltrating macrophages was associated with a shorter survival duration and the failure of second-line treatment [28]. Several centers are now pursuing routine measurement of CD68 on resection specimens, but prospective trials are needed before this can be widely adopted.
The prognostic importance of novel biomarkers has not been assessed in a population undergoing ASCT. In addition, it is not clear if previously identified prognostic factors will maintain their relevance as initial and salvage treatment strategies evolve [29]. The real challenge, of course, in identifying all these different prognostic factors is how to incorporate them along with clinical factors and FI into a consensus index that can accurately predict the outcome of patients with relapsed or refractory HL. The ability to translate this type of index into therapeutic improvements in the care of relapsed patients also requires prospective clinical trials that demonstrate that stratification by such an index into different risk groups with different therapies can lead to better outcomes and less toxicity.
Treatment Options
Salvage Chemotherapy Regimens Prior to ASCT
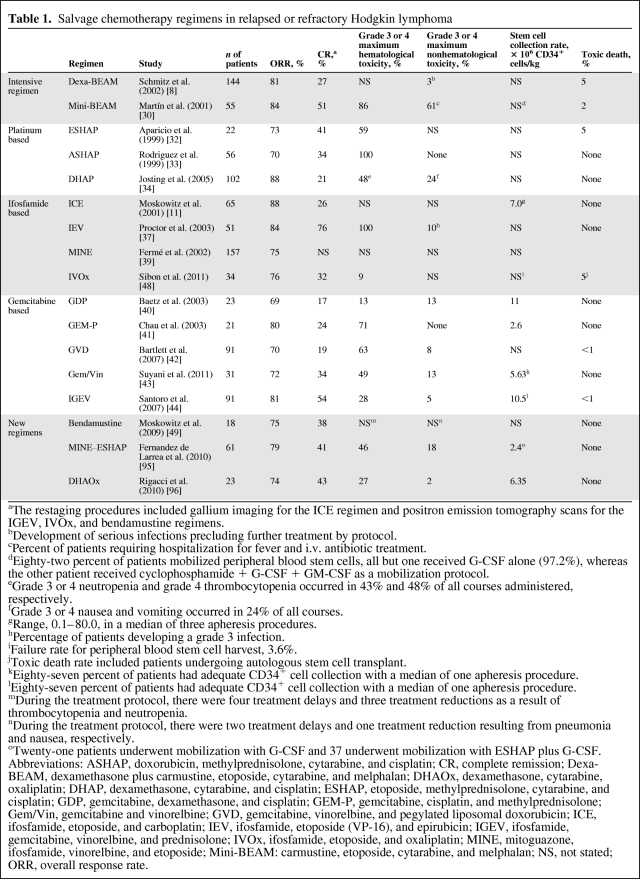
As we discussed, disease status before HDT–ASCT by both clinical and PET metrics has been proven to be one of the most important predictors of outcome [26]. In spite of a multitude of phase II studies reporting the results of various salvage regimens, no prospective randomized trials have compared one salvage regimen with another. The optimal salvage regimen should be highly effective with acceptable hematological and nonhematological toxicities, but should also ideally not inhibit future peripheral blood stem cell (PBSC) mobilization. Traditional regimens can be divided into intensive conventional therapies, such as low-dose etoposide, carmustine, cytarabine, and melphalan (mini-BEAM) or dexamethasone plus BEAM (dexa-BEAM) [8, 30, 31]; platinum-based regimens, such as etoposide, methylprednisolone, cytarabine, and cisplatin (the ESHAP regimen), dexamethasone, cytarabine, and cisplatin (the DHAP regimen), and doxorubicin, methylprednisolone, cisplatin, and cytarabine (the ASHAP regimen) [32–34]; ifosfamide and etoposide–based protocols (ICE; ifosfamide, epirubicin, and etoposide [the IEV regimen]; mesna, ifosfamide, novantrone, and etoposide [the MINE regimen]) [11, 35–39]; and, more recently, gemcitabine-based combinations (gemcitabine, dexamethasone, and cisplatin [GDP]; gemcitabine, cisplatin, and methylprednisolone [GEM-P]; gemcitabine, navelbine (vinorelbine), and pegylated liposomal doxorubicin [GND]; gemcitabine plus vinorelbine; ifosfamide, gemcitabine, vinorelbine, and prednisolone [IGEV]) [40–44]. Intensive conventional regimens are effective but are associated with severe hematological toxicity and significant treatment-related mortality. Indeed, a retrospective analysis comparing an intensive conventional regimen (mini-BEAM) with a gemcitabine-based regimen (GDP) demonstrated a superior PFS outcome with GDP, although there was no difference in the OS outcome. In addition, adequate stem cell collection was more likely after GDP [45]. Another retrospective study of platinum-based regimens in a relapsed and refractory population, which included some HL patients, suggested that ICE seemed to be more effective than DHAP, with a similar toxicity profile [46], yet a large German study showed an impressive response rate and relatively low toxicity with a time-intensified DHAP regimen [47]. An oxaliplatin-based regimen combining ifosfamide, etoposide, and oxaliplatin (IVOx) showed promising activity in a series of 34 HL patients in first relapse and refractory patients, with a relatively low toxicity profile and PBSC mobilization failure rate [48]. Remarkably, the overall response rate (ORR) and CR rate after two cycles of IVOx were 76% and 32%, respectively, and 26 patients were able to proceed to the HDT–ASCT. Most recently, an interim analysis of an ongoing phase II trial evaluating the activity of single-agent bendamustine in patients with relapsed or primary refractory HL showed very promising activity, with a 75% ORR in highly pretreated patients [49]. Longer follow-up and complete data on the ORR, toxicity, and rate of mobilization failure are awaited before bendamustine can be accepted as a standard option for salvage. Results with most of the commonly used salvage chemotherapy regimens are summarized in Table 1.
Table 1.
Salvage chemotherapy regimens in relapsed or refractory Hodgkin lymphoma
aThe restaging procedures included gallium imaging for the ICE regimen and positron emission tomography scans for the IGEV, IVOx, and bendamustine regimens.
bDevelopment of serious infections precluding further treatment by protocol.
cPercent of patients requiring hospitalization for fever and i.v. antibiotic treatment.
dEighty-two percent of patients mobilized peripheral blood stem cells, all but one received G-CSF alone (97.2%), whereas the other patient received cyclophosphamide + G-CSF + GM-CSF as a mobilization protocol.
eGrade 3 or 4 neutropenia and grade 4 thrombocytopenia occurred in 43% and 48% of all courses administered, respectively.
fGrade 3 or 4 nausea and vomiting occurred in 24% of all courses.
gRange, 0.1–80.0, in a median of three apheresis procedures.
hPercentage of patients developing a grade 3 infection.
iFailure rate for peripheral blood stem cell harvest, 3.6%.
jToxic death rate included patients undergoing autologous stem cell transplant.
kEighty-seven percent of patients had adequate CD34+ cell collection with a median of one apheresis procedure.
lEighty-seven percent of patients had adequate CD34+ cell collection with a median of one apheresis procedure.
mDuring the treatment protocol, there were four treatment delays and three treatment reductions as a result of thrombocytopenia and neutropenia.
nDuring the treatment protocol, there were two treatment delays and one treatment reduction resulting from pneumonia and nausea, respectively.
oTwenty-one patients underwent mobilization with G-CSF and 37 underwent mobilization with ESHAP plus G-CSF.
Abbreviations: ASHAP, doxorubicin, methylprednisolone, cytarabine, and cisplatin; CR, complete remission; Dexa-BEAM, dexamethasone plus carmustine, etoposide, cytarabine, and melphalan; DHAOx, dexamethasone, cytarabine, oxaliplatin; DHAP, dexamethasone, cytarabine, and cisplatin; ESHAP, etoposide, methylprednisolone, cytarabine, and cisplatin; GDP, gemcitabine, dexamethasone, and cisplatin; GEM-P, gemcitabine, cisplatin, and methylprednisolone; Gem/Vin, gemcitabine and vinorelbine; GVD, gemcitabine, vinorelbine, and pegylated liposomal doxorubicin; ICE, ifosfamide, etoposide, and carboplatin; IEV, ifosfamide, etoposide (VP-16), and epirubicin; IGEV, ifosfamide, gemcitabine, vinorelbine, and prednisolone; IVOx, ifosfamide, etoposide, and oxaliplatin; MINE, mitoguazone, ifosfamide, vinorelbine, and etoposide; Mini-BEAM: carmustine, etoposide, cytarabine, and melphalan; NS, not stated; ORR, overall response rate.
Some centers have explored intensive sequential chemotherapeutic combinations prior to HDT–ASCT. A prospective trial investigated the feasibility and efficacy of sequential high-dose ifosfamide, carboplatin, and etoposide in patients with relapsed or refractory NHL and HL. That regimen was more toxic but no more effective than the same drugs administered at standard doses (the ICE regimen) [50]. A multicenter Italian retrospective study using a high-dose sequential chemotherapy regimen prior to ASCT reported a benefit in terms of PFS for patients at first relapse, with no differences between patients who had an early (<12 months) and those who had a late (>12 months) relapse, suggesting that this poor prognostic factor can be overcome [51]. More recently, a prospective randomized trial from the GHSG randomized patients with relapsed HL who had been treated with two cycles of DHAP to a standard arm and an intensified arm. Patients in the intensified arm were treated with sequential HDT consisting of high-dose cyclophosphamide, methotrexate, and etoposide before BEAM–ASCT, whereas the standard arm proceeded directly to BEAM–ASCT. At 3 years, the OS rates were 87% and 80%, the PFS rates were 72% and 67%, and the freedom from treatment failure rates were 71% and 65%, respectively, thus failing to demonstrate any benefit from intensified therapy [52]. Overall, there is no clear prospective evidence showing the superiority of one salvage regimen over another, and choice should be based on institutional experience, preference, and individual patient characteristics. We routinely use either ICE or GND at first relapse, although we eagerly await further data on the use of bendamustine and future clinical trials with brentuximab vedotin in this setting (see below). There is no evidence to suggest that intensive sequential chemotherapy prior to ASCT adds any benefit.
HDT Regimens
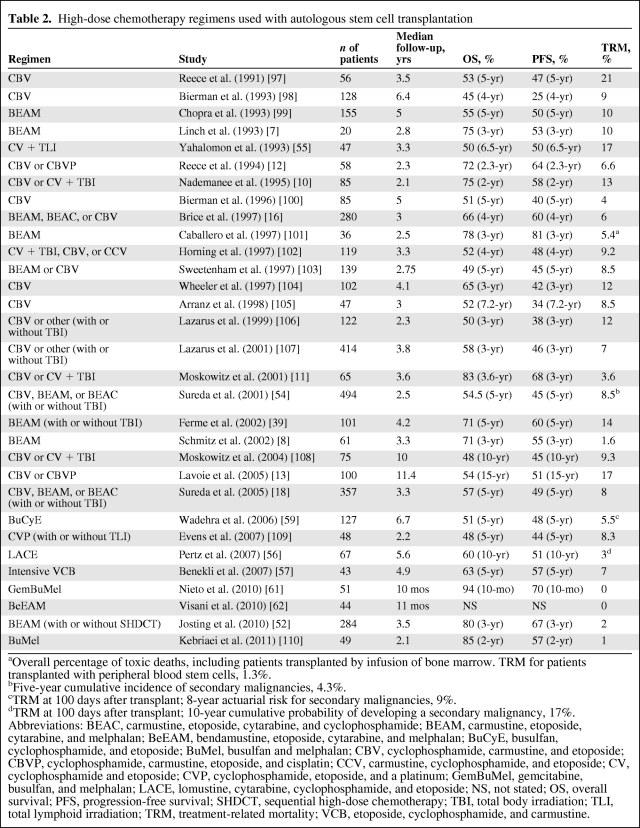
There have been no prospective trials comparing HDT regimens as part of ASCT and the choice is usually based on institutional preference and experience. Total body irradiation (TBI)-based regimens for patients with HL have been largely abandoned in favor of chemotherapy-based regimens because of a high incidence of secondary malignancies and transplant-related mortality [53–55]. The two early randomized trials that compared traditional salvage chemotherapy with HDT–ASCT both used BEAM as conditioning for ASCT [7, 8], and BEAM remains the most popular regimen used worldwide. Other conditioning regimens with comparable toxicities and outcomes have been reported in single-institution studies and include cyclophosphamide, carmustine, and etoposide (CBV) and CBV with reduced doses of carmustine with cisplatin (CBVP) [13]; lomustine, cytarabine, cyclophosphamide, and etoposide (the LACE regimen) [56]l and intensified VCB (a CBV modification) [57] (Table 2). Although busulfan-based regimens are commonly used for conditioning prior to allogeneic SCT and have been described in ASCT for NHL [58], there are limited data regarding its use in HDT regimens for patients with HL. The combination of busulfan, cyclophosphamide, and etoposide (BuCyE) was investigated in patients with relapsed and refractory HL, with results reported from the Cleveland Clinic in 2006. With a median follow-up of 6.7 years, the 5-year PFS rate was 48% and the 5-year OS rate was 51% [59]. A more recent report from Emory University showed comparable results [60].
Table 2.
High-dose chemotherapy regimens used with autologous stem cell transplantation
aOverall percentage of toxic deaths, including patients transplanted by infusion of bone marrow. TRM for patients transplanted with peripheral blood stem cells, 1.3%.
bFive-year cumulative incidence of secondary malignancies, 4.3%.
cTRM at 100 days after transplant; 8-year actuarial risk for secondary malignancies, 9%.
dTRM at 100 days after transplant; 10-year cumulative probability of developing a secondary malignancy, 17%.
Abbreviations: BEAC, carmustine, etoposide, cytarabine, and cyclophosphamide; BEAM, carmustine, etoposide, cytarabine, and melphalan; BeEAM, bendamustine, etoposide, cytarabine, and melphalan; BuCyE, busulfan, cyclophosphamide, and etoposide; BuMel, busulfan and melphalan; CBV, cyclophosphamide, carmustine, and etoposide; CBVP, cyclophosphamide, carmustine, etoposide, and cisplatin; CCV, carmustine, cyclophosphamide, and etoposide; CV, cyclophosphamide and etoposide; CVP, cyclophosphamide, etoposide, and a platinum; GemBuMel, gemcitabine, busulfan, and melphalan; LACE, lomustine, cytarabine, cyclophosphamide, and etoposide; NS, not stated; OS, overall survival; PFS, progression-free survival; SHDCT, sequential high-dose chemotherapy; TBI, total body irradiation; TLI, total lymphoid irradiation; TRM, treatment-related mortality; VCB, etoposide, cyclophosphamide, and carmustine.
Newer conditioning regimens have incorporated agents shown to have high activity against HL. Nieto et al. [61] reported on 51 patients who underwent ASCT after gemcitabine, busulfan, and melphalan (GemBuMel) and suggested an outcome superior to that of patients receiving BEAM or BuMel in a single-institution retrospective cohort comparison. Recently, a phase I/II study for relapsed lymphoma patients, including 15 with HL, evaluated the safety and efficacy of bendamustine as a component of conditioning for ASCT. This new conditioning regimen combined high doses of bendamustine with etoposide, cytarabine, and melphalan (the BeEAM regimen) and appeared to be safe and efficacious in heavily pretreated lymphoma patients [62].
Much like the experience with salvage chemotherapy, there are no prospective data to suggest the superiority of one conditioning regimen over another. Given that the therapeutic value of HDT–ASCT is the inherent cytotoxicity of HDT, a logical regimen would include agents that have been shown to be active in HL patients. Choice at this time should be based on institutional experience, preference, individual patient comorbidities, and exhibited responsiveness to specific agents for the individual patient. Like many other institutions, we no longer use TBI-based regimens because of concerns over long-term toxicity in this population. Our standard regimen remains CBV and we have experience with busulfan-based regimens [63], especially in the elderly population. We look forward to more data regarding newer regimens incorporating active agents such as bendamustine and gemcitabine.
Role of RT
Salvage RT alone is a treatment option in selected patients. In a retrospective analysis of 100 patients with localized relapsed disease without clinical risk factors treated with RT alone, 77% achieved a CR, the 5-year OS rate was 51%, and the FF2F rate was 28% [64]. However, given the current safety of ASCT, we rarely offer RT alone for relapsed or refractory disease, with the exceptions being if a patient is not fit for ASCT or if only one refractory site of disease remains after initial therapy. RT was investigated in combination with chemotherapy in order to obtain maximal cytoreduction before ASCT or consolidate response after ASCT. The traditional concern with adding RT has been toxicity, both acute and long term, given that ASCT itself has been shown to predispose HL patients to secondary malignancies [65].
With modern combined-modality therapy using significantly lower doses and smaller fields of involved-field radiotherapy (IFRT), both acute and chronic toxicities have diminished. Several single-institution retrospective series suggested that the use of IFRT in the peritransplant period (immediately before or after ASCT) results in lower rates of relapse and longer DFS times. However, other reports have not definitively confirmed these results [66–68]. A Memorial Sloan-Kettering Cancer Center dose-dense and dose-intense multimodality program for refractory and relapsed HL patients included IFRT to nodal sites of disease that initially measured ≥5 cm or to sites of residual disease after two cycles of ICE chemotherapy. Previously unirradiated patients received total lymphoid irradiation (TLI) on completion of IFRT, prior to high-dose cyclophosphamide plus etoposide, whereas high-dose carmustine was given to patients who had previous RT. In 65 patients, 88% responded to ICE–IFRT and almost all underwent HDT–ASCT. At a median follow-up of 43 months, the EFS and OS rates of those who responded to ICE–IFRT were 68% and 83%, respectively. Most failures occurred in unirradiated extranodal sites or in nodal sites that could not be irradiated, suggesting a benefit with IFRT [11]. As previously mentioned, the same group conducted a phase II study of risk-adapted salvage treatment including IFRT and TLI in previously unirradiated patients, which appeared to lead to a longer EFS interval in high-risk patients, without a higher treatment-related mortality rate [19]. In our practice, we routinely offer consolidative IFRT either before or after ASCT to patients with limited-stage relapse, with sites of residual fluorodeoxyglucose (FDG) avidity on PET scans, and with initial bulky sites of disease. Ideally, large, randomized clinical trials, designed in a risk-adapted fashion, can be conducted to determine whether integrating RT into the peri-ASCT period is beneficial.
Strategies to Improve Outcome After HDT–ASCT
Long-term outcomes after HDT–ASCT of patients who remain in remission for ≥2 years following transplantation are favorable, with a 10-year OS rate of 77% [69]. The majority of patients who relapse after HDT–ASCT do so within one year, with a median time to progression of 5.7 months and a median survival time from second-line treatment failure of only 25 months [70]. Relapse within 6 months after ASCT is especially worrisome and is associated with a median OS time in the range of 4–22 months [71]. Novel therapeutic strategies beyond HDT–ASCT for patients who are predicted to have early relapse are clearly needed.
Tandem Autologous Transplantation
Tandem autologous transplantation has been used successfully in multiple myeloma patients and in the therapy of patients with germ cell tumors [72, 73] and has been attempted in a few trials with patients with HL. The Groupe d'Étude des Lymphomes de l'Adulte (GELA)–SFGM study group conducted a prospective study of 43 patients with induction failure, early relapse, or disseminated relapse. Conditioning was with cyclophosphamide, carmustine, etoposide, and mitoxantrone (CBVMx) for the first ASCT, followed by TBI (or busulfan in previously irradiated patients) plus cytarabine and melphalan for the second ASCT, with a median interval of 58 days between the two transplants. Seventy-four percent of patients completed the tandem transplantation procedure and the 2-year survival rate was 74% for those who were able to tolerate both transplants, versus 40% for patients who received only one ASCT [74]. In another multicenter study involving 46 patients with recurrent HL, patients were treated first with high-dose melphalan and then with TBI (or carmustine for prior irradiated patients) in combination with etoposide and cyclophosphamide, with a median of 64 days between the two procedures. The majority of patients (83%) completed the entire treatment program, and at a median follow-up of 5.3 years, the 5-year PFS and OS rates were 49% and 54%, respectively [75].
Subsequently, the GELA–SFGM study group used a risk-adapted approach, reserving tandem ASCT for patients with two or more adverse risk factors, namely, short time to relapse or progression <12 months, stage III or stage IV at relapse, and relapse in a previously irradiated site. The outcomes for these patients were compared with those of low-risk patients who underwent a single BEAM–ASCT. In the intensified group, the first conditioning regimen consisted of CBVMx and the second conditioning regimen consisted of TBI (or busulfan for patients who had received prior dose-limiting radiation) and melphalan. Despite the intensified strategy, the outcomes for the poor-risk patients remained inferior to those of the intermediate-risk patients, with 5-year FF2F rates of 46% and 73% and OS rates of 57% and 85%, respectively [76]. Most recently, a large single-arm multicenter U.S. clinical trial of tandem ASCT was completed through the Bone Marrow Transplant Clinical Trials Network (BMT CTN) (BMT CTN protocol 0703/SWOG 0410). Conditioning was with melphalan for the first ASCT and TBI (or carmustine) plus etoposide and cyclophosphamide for the second ASCT. The results of that trial are not yet available. Ultimately, a prospective randomized trial will be required to compare tandem ASCT with a single ASCT; however, we do not foresee enthusiasm for tandem ASCT and do not recommend this approach outside a clinical trial given the additional toxicity involved. Rather, adding novel agents as maintenance therapy will likely occupy research avenues in the near future as post-ASCT intervention, especially in the population who are at increased risk for relapse.
Limited subsets of patients who relapse after ASCT are candidates for allogeneic SCT. The role for allogeneic SCT in patients with relapsed HL is beyond the scope of this review, but it is worth mentioning that recent series have substantiated a potent graft-versus-lymphoma effect [77]. In accordance, our institution has an ongoing clinical trial using a tandem ASCT–reduced intensity allogeneic SCT approach for patients with poor risk lymphoma, including patients with HL who have been refractory to at least one salvage regimen (Chen, personal communication).
Role of New Agents in Salvage Therapy and HDT
Several novel therapeutic agents are currently under clinical investigation as single agents or in combination with conventional therapies in patients with relapsed and refractory HL. All of these therapies are generally less toxic than conventional chemotherapy and, thus, could either be added to current salvage therapy or HDT regimens or considered as candidates for use as maintenance therapy after ASCT. The historical experience with delivering cytotoxic therapy after HDT–ASCT has not been compelling. One early study investigated the role of consolidative chemoradiotherapy after HDT–ASCT in 37 patients with relapsed or refractory HL. Treatment consisted of high-dose gemcitabine, carmustine, and melphalan with ASCT, followed by IFRT to any pre-existing mass >2 cm. This was then followed by up to four cycles of consolidative chemotherapy, alternating the DCEP-G regimen, consisting of dexamethasone, cyclophosphamide, etoposide, cisplatin, and gemcitabine), with dexamethasone, cisplatin, and paclitaxel (the DPP regimen), administered at 3 months, 6 months, 9 months, and 12 months post-transplant. The 2.5-year PFS and OS rates were 59.1% and 86.3%, respectively. Of note, the majority of patients did not receive the planned post-ASCT therapy because of refusal, early relapse, or other complications [78].
Brentuximab vedotin (SGN-35) is an antibody–drug conjugate (anti-CD30 conjugated to the antitubulin agent auristatin E) that has shown remarkable activity in relapsed and refractory HL patients and was recently approved by the U.S. Food and Drug Administration. A phase I, open-label trial in 42 HL patients who had failed a median of three previous chemotherapies, including 73% with prior ASCT, showed durable objective responses, with mild toxicity in the majority of patients [79]. Recently a pivotal phase II study confirmed the efficacy and safety of this agent in 102 patients with relapsed or refractory HL after ASCT [80]. An ongoing, randomized, placebo-controlled, multicenter phase III trial is evaluating the efficacy and safety of brentuximab vedotin in patients who have received ASCT in the previous 30–45 days and are at high risk for residual HL post-ASCT, defined as those with a history of refractory disease who relapse or progress within 1 year from receiving frontline chemotherapy and those who have extranodal disease at the time of relapse (ClinicalTrials.gov identifier, NCT01100502). Future studies incorporating brentuximab vedotin into earlier phases of therapy, ASCT conditioning regimens, and allogeneic SCT are planned.
A variety of histone deacetylase inhibitors (HDACIs) are currently being evaluated in patients with relapsed HL. Epigenetic changes are thought to be partly responsible for the loss of B-cell phenotype in RS cells and may allow RS cells to evade immunosurveillance [81]. Givinostat is a selective class I and class II HDACI with encouraging activity as a single agent. A phase II trial of givinostat combined with mechloretamine in patients with relapsed or refractory HL was conducted at the National Tumors Institute of Milan and showed a median survival duration of 28 months with a projected 2-year survival rate of 52%. Interestingly, decreased serum thymus and activation-regulated cytokine (TARC) levels after the first cycle of therapy were very predictive of responsiveness [82]. Sureda et al. [83] presented preliminary results of a phase II trial using single-agent panobinostat in 129 heavily pretreated patients who had all failed ASCT, reporting a disease control rate (CR rate plus partial response rate plus stable disease rate) of 82% and estimated duration of response of 6.9 months. Based on this promising activity as a single agent, a phase I/II trial is evaluating the combination of the pan-HDACI panobinostat and ICE chemotherapy in the salvage setting (ClinicalTrials gov identifier, NCT01169636). A phase III trial, designed to evaluate the efficacy of maintenance panobinostat in patients with HL who achieved a CR following ASCT (ClinicalTrials.gov identifier, NCT01034163), was closed as a result of slow accrual.
Mammalian target of rapamycin (mTOR) is another promising therapeutic target because the phosphoinositide 3-kinase–AKT–mTOR signaling pathway is constitutively active in HL cell lines [84]. Everolimus is an oral antineoplastic agent that targets mTOR, and phase II studies using everolimus demonstrated promising results in patients with relapsed or refractory HL, as a single agent [85] and in combination with panobinostat [86]. If this activity is confirmed, everolimus may also be a candidate for use in salvage therapy or as maintenance therapy after HDT–ASCT.
Lenalidomide is an immunomodulatory drug that is approved for the treatment of 5q-myelodysplastic syndrome and multiple myeloma. Lenalidomide has multiple proposed modes of action, including direct induction of apoptosis, antiangiogenic effects, and activation of T cells and natural killer cells. There have been small studies using lenalidomide in patients with relapsed or refractory HL. Böll et al. [87] described 12 patients, all of whom derived some clinical benefit in terms of disease stabilization, including one CR. Other preliminary results have been presented as well using single-agent lenalidomide in the relapsed or refractory setting [88, 89], which have shown evidence of some activity, and larger studies are being planned.
Preliminary data have shown that the anti-CD20 monoclonal antibody rituximab may have therapeutic potential in patients who have recurrent, classic HL regardless of the expression of CD20 by RS cells, as a single agent [90] and in combination with gemcitabine [91], or with the multidrug combination of gemcitabine, ifosfamide, and oxaliplatin may also have therapeutic potential [92]. Moreover, clonotypic small B cells can be found in the blood of HL patients and could constitute “cancer stem cells” [93]. A phase I/II study of HDT and immunotherapy for relapsed HL patients is currently under way using high-dose cyclophosphamide, rituximab, and a novel HL vaccine as an alternative regimen to HDT–ASCT. On preliminary analysis, that regimen produced encouraging outcomes in relapsed HL patients and could be an alternative approach for patients not eligible for conventional ASCT [94].
The immediate future of novel agents in the therapy of patients with relapsed HL is bright. We expect to see widespread use of brentuximab vedotin, initially as a single agent both as a bridge to transplantation in relapsed or chemotherapy-refractory patients and as salvage therapy after ASCT. This will be followed rapidly by trials exploring combination regimens with chemotherapy in conjunction with brentuximab both as salvage therapy and in the frontline setting for HL. Some caution will need to be paid to the potential for synergistic neuropathy combining this agent with vinca alkaloids. The maintenance question is currently being explored in a phase III clinical trial. The apparent dramatic responses to this agent will set the bar high for HDACIs, inhibitors of the mTOR pathway, lenalidomide, and other novel agents. These agents will likely enter clinical practice in the limited arena of brentuximab failure.
Conclusions
HDT–ASCT for patients with relapsed and refractory HL provides a 50%–60% chance for a long DFS time. Historically, the outcome is better for patients in chemosensitive relapse and those whose duration of first remission has been relatively long. Large studies have suggested that early relapse, chemotherapy-refractory disease, and the presence of extranodal disease are all clinical factors associated with a high risk for relapse after ASCT. Recently, the presence of residual FDG avidity after salvage chemotherapy and prior to ASCT was shown to have a remarkable ability to stratify risk, and novel biomarkers are also being analyzed for their ability to predict prognosis. A prognostic index incorporating a clinical risk score, FI, and biological measures of intrinsic chemotherapy resistance as well as microenvironmental response still needs to be developed. If we are able to better predict prognosis after HDT–ASCT then risk-adapted protocols can be developed and higher risk patients can be entered into clinical trials beyond conventional ASCT. More effective salvage regimens incorporating newer agents including gemcitabine, bendamustine, and brentuximab vedotin will allow patients to proceed to ASCT in minimal residual disease states, and this will likely translate to superior outcomes. Similarly, using these agents in HDT regimens with ASCT may also improve outcomes over those seen with traditional regimens. Lastly, given that less toxic and more specific agents such as brentuximab vedotin and panobinostat are being developed, the concept of maintenance therapy after HDT–ASCT is actively being tested and may one day become a reality.
Acknowledgments
Dr. Chen is supported by a career development award in clinical research from the Leukemia and Lymphoma Society.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Yi-Bin Chen, Ephraim Hochberg, Anna Colpo
Manuscript writing: Yi-Bin Chen, Ephraim Hochberg, Anna Colpo
Final approval of manuscript: Yi-Bin Chen, Ephraim Hochberg, Anna Colpo
References
- 1.Engert A, Plẗschow A, Eich HT, et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N Engl J Med. 2010;363:640–652. doi: 10.1056/NEJMoa1000067. [DOI] [PubMed] [Google Scholar]
- 2.Eich HT, Diehl V, Görgen H, et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin's lymphoma: Final Analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. [DOI] [PubMed] [Google Scholar]
- 3.Bonadonna G, Viviani S, Bonfante V, et al. Survival in Hodgkin's disease patients—report of 25 years of experience at the Milan Cancer Institute. Eur J Cancer. 2005;41:998–1006. doi: 10.1016/j.ejca.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin's Disease. N Engl J Med. 2003;348:2386–2395. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 5.Duggan DB, Petroni GR, Johnson JL, et al. Randomized comparison of ABVD and MOPP/ABV hybrid for the treatment of advanced Hodgkin's disease: Report of an Intergroup trial. J Clin Oncol. 2003;21:607–614. doi: 10.1200/JCO.2003.12.086. [DOI] [PubMed] [Google Scholar]
- 6.Gallamini A, Patti C, Viviani S, et al. Early chemotherapy intensification with BEACOPP in advanced-stage Hodgkin lymphoma patients with a interim-PET positive after two ABVD courses. Br J Haematol. 2011;152:551–560. doi: 10.1111/j.1365-2141.2010.08485.x. [DOI] [PubMed] [Google Scholar]
- 7.Linch DC, Winfield D, Goldstone AH, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant Hodgkin's disease: Results of a BNLI randomised trial. Lancet. 1993;341:1051–1054. doi: 10.1016/0140-6736(93)92411-l. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: A randomised trial. Lancet. 2002;359:2065–2071. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 9.Puig N, Pintilie M, Seshadri T, et al. Different response to salvage chemotherapy but similar post-transplant outcomes in patients with relapsed and refractory Hodgkin's lymphoma. Haematologica. 2010;95:1496–1502. doi: 10.3324/haematol.2009.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nademanee A, O'Donnell MR, Snyder D, et al. High-dose chemotherapy with or without total body irradiation followed by autologous bone marrow and/or peripheral blood stem cell transplantation for patients with relapsed and refractory Hodgkin's disease: Results in 85 patients with analysis of prognostic factors. Blood. 1995;85:1381–1390. [PubMed] [Google Scholar]
- 11.Moskowitz CH, Nimer SD, Zelenetz AD, et al. A 2-step comprehensive high-dose chemoradiotherapy second-line program for relapsed and refractory Hodgkin disease: Analysis by intent to treat and development of a prognostic model. Blood. 2001;97:616–623. doi: 10.1182/blood.v97.3.616. [DOI] [PubMed] [Google Scholar]
- 12.Reece DE, Connors JM, Spinelli JJ, et al. Intensive therapy with cyclophosphamide, carmustine, etoposide +/- cisplatin, and autologous bone marrow transplantation for Hodgkin's disease in first relapse after combination chemotherapy. Blood. 1994;83:1193–1199. [PubMed] [Google Scholar]
- 13.Lavoie JC, Connors JM, Phillips GL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: Long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005;106:1473–1478. doi: 10.1182/blood-2004-12-4689. [DOI] [PubMed] [Google Scholar]
- 14.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin's disease. N Engl J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 15.Bierman PJ, Lynch JC, Bociek RG, et al. The International Prognostic Factors Project score for advanced Hodgkin's disease is useful for predicting outcome of autologous hematopoietic stem cell transplantation. Ann Oncol. 2002;13:1370–1377. doi: 10.1093/annonc/mdf228. [DOI] [PubMed] [Google Scholar]
- 16.Brice P, Bouabdallah R, Moreau P, et al. Prognostic factors for survival after high-dose therapy and autologous stem cell transplantation for patients with relapsing Hodgkin's disease: Analysis of 280 patients from the French registry. Société Frana̧ise de Greffe de Moëlle. Bone Marrow Transplant. 1997;20:21–26. doi: 10.1038/sj.bmt.1700838. [DOI] [PubMed] [Google Scholar]
- 17.Josting A, Franklin J, May M, et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's Lymphoma Study Group. J Clin Oncol. 2002;20:221–230. doi: 10.1200/JCO.2002.20.1.221. [DOI] [PubMed] [Google Scholar]
- 18.Sureda A, Constans M, Iriondo A, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005;16:625–633. doi: 10.1093/annonc/mdi119. [DOI] [PubMed] [Google Scholar]
- 19.Moskowitz CH, Yahalom J, Zelenetz AD, et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging. Br J Haematol. 2010;148:890–897. doi: 10.1111/j.1365-2141.2009.08037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabbour E, Hosing C, Ayers G, et al. Pretransplant positive positron emission tomography/gallium scans predict poor outcome in patients with recurrent/refractory Hodgkin lymphoma. Cancer. 2007;109:2481–2489. doi: 10.1002/cncr.22714. [DOI] [PubMed] [Google Scholar]
- 21.Becherer A, Mitterbauer M, Jaeger U, et al. Positron emission tomography with [18F]2-fluoro-D-2-deoxyglucose (FDG-PET) predicts relapse of malignant lymphoma after high-dose therapy with stem cell transplantation. Leukemia. 2002;16:260–267. doi: 10.1038/sj.leu.2402342. [DOI] [PubMed] [Google Scholar]
- 22.Spaepen K, Stroobants S, Dupont P, et al. Prognostic value of pretransplantation positron emission tomography using fluorine 18-fluorodeoxyglucose in patients with aggressive lymphoma treated with high-dose chemotherapy and stem cell transplantation. Blood. 2003;102:53–59. doi: 10.1182/blood-2002-12-3842. [DOI] [PubMed] [Google Scholar]
- 23.Svoboda J, Andreadis C, Elstrom R, et al. Prognostic value of FDG-PET scan imaging in lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant. 2006;38:211–216. doi: 10.1038/sj.bmt.1705416. [DOI] [PubMed] [Google Scholar]
- 24.Filmont JE, Gisselbrecht C, Cuenca X, et al. The impact of pre- and post-transplantation positron emission tomography using 18-fluorodeoxyglucose on poor-prognosis lymphoma patients undergoing autologous stem cell transplantation. Cancer. 2007;110:1361–1369. doi: 10.1002/cncr.22911. [DOI] [PubMed] [Google Scholar]
- 25.Schot BW, Zijlstra JM, Sluiter WJ, et al. Early FDG-PET assessment in combination with clinical risk scores determines prognosis in recurring lymphoma. Blood. 2007;109:486–491. doi: 10.1182/blood-2005-11-006957. [DOI] [PubMed] [Google Scholar]
- 26.Moskowitz AJ, Yahalom J, Kewalramani T, et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood. 2010;116:4934–4937. doi: 10.1182/blood-2010-05-282756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banerjee D. Recent advances in the pathobiology of Hodgkin's lymphoma: Potential impact on diagnostic, predictive, and therapeutic strategies. Adv Hematol. 2011;2011:439–456. doi: 10.1155/2011/439456. Epub 2011 Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith SD, Moskowitz CH, Dean R, et al. Autologous stem cell transplant for early relapsed/refractory Hodgkin lymphoma: Results from two transplant centres. Br J Haematol. 2011;153:358–363. doi: 10.1111/j.1365-2141.2011.08616.x. [DOI] [PubMed] [Google Scholar]
- 30.Martín A, Fernàndez-Jiménez MC, Caballero MD, et al. Long-term follow-up in patients treated with Mini-BEAM as salvage therapy for relapsed or refractory Hodgkin's disease. Br J Haematol. 2001;113:161–171. doi: 10.1046/j.1365-2141.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- 31.Fernàndez-Jiménez MC, Canales MA, Ojeda E, et al. Salvage chemotherapy with mini-BEAM for relapsed or refractory Hodgkin's disease prior to autologous peripheral blood stem cell transplantation. Haematologica. 1999;84:1007–1011. [PubMed] [Google Scholar]
- 32.Aparicio J, Segura A, Garcerà S, et al. ESHAP is an active regimen for relapsing Hodgkin's disease. Ann Oncol. 1999;10:593–595. doi: 10.1023/a:1026454831340. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez J, Rodriguez MA, Fayad L, et al. ASHAP: A regimen for cytoreduction of refractory or recurrent Hodgkin's disease. Blood. 1999;93:3632–3636. [PubMed] [Google Scholar]
- 34.Josting A, Rudolph C, Mapara M, et al. Cologne high-dose sequential chemotherapy in relapsed and refractory Hodgkin lymphoma: Results of a large multicenter study of the German Hodgkin Lymphoma Study Group (GHSG) Ann Oncol. 2005;16:116–123. doi: 10.1093/annonc/mdi003. [DOI] [PubMed] [Google Scholar]
- 35.Hertzberg MS, Crombie C, Benson W, et al. Outpatient fractionated ifosfamide, carboplatin and etoposide as salvage therapy in relapsed and refractory non-Hodgkin's and Hodgkin's lymphoma. Ann Oncol. 2006;17(suppl 4):iv25–iv30. doi: 10.1093/annonc/mdj995. [DOI] [PubMed] [Google Scholar]
- 36.Zinzani PL, Tani M, Molinari AL, et al. Ifosfamide, epirubicin and etoposide regimen as salvage and mobilizing therapy for relapsed/refractory lymphoma patients. Haematologica. 2002;87:816–821. [PubMed] [Google Scholar]
- 37.Proctor SJ, Jackson GH, Lennard A, et al. Strategic approach to the management of Hodgkin's disease incorporating salvage therapy with high-dose ifosfamide, etoposide and epirubicin: A Northern Region Lymphoma Group study (UK) Ann Oncol. 2003;14(suppl 1):i47–i50. doi: 10.1093/annonc/mdg710. [DOI] [PubMed] [Google Scholar]
- 38.Fermé C, Bastion Y, Lepage E, et al. The MINE regimen as intensive salvage chemotherapy for relapsed and refractory Hodgkin's disease. Ann Oncol. 1995;6:543–549. doi: 10.1093/oxfordjournals.annonc.a059242. [DOI] [PubMed] [Google Scholar]
- 39.Fermé C, Mounier N, Diviné M, et al. Intensive salvage therapy with high-dose chemotherapy for patients with advanced Hodgkin's disease in relapse or failure after initial chemotherapy: Results of the Groupe d'tudes des Lymphomes de l'Adulte H89 trial. J Clin Oncol. 2002;20:467–475. doi: 10.1200/JCO.2002.20.2.467. [DOI] [PubMed] [Google Scholar]
- 40.Baetz T, Belch A, Couban S, et al. Gemcitabine, dexamethasone and cisplatin is an active and non-toxic chemotherapy regimen in relapsed or refractory Hodgkin's disease: A phase II study by the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 2003;14:1762–1767. doi: 10.1093/annonc/mdg496. [DOI] [PubMed] [Google Scholar]
- 41.Chau I, Harries M, Cunningham D, et al. Gemcitabine, cisplatin and methylprednisolone chemotherapy (GEM-P) is an effective regimen in patients with poor prognostic primary progressive or multiply relapsed Hodgkin's and non-Hodgkin's lymphoma. Br J Haematol. 2003;120:970–977. doi: 10.1046/j.1365-2141.2003.04226.x. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett NL, Niedzwiecki D, Johnson JL, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007;18:1071–1079. doi: 10.1093/annonc/mdm090. [DOI] [PubMed] [Google Scholar]
- 43.Suyani E, Sucak GT, Aki SZ, et al. Gemcitabine and vinorelbine combination is effective in both as a salvage and mobilization regimen in relapsed or refractory Hodgkin lymphoma prior to ASCT. Ann Hematol. 2011;90:685–691. doi: 10.1007/s00277-010-1113-z. [DOI] [PubMed] [Google Scholar]
- 44.Santoro A, Magagnoli M, Spina M, et al. Ifosfamide, gemcitabine, and vinorelbine: A new induction regimen for refractory and relapsed Hodgkin's lymphoma. Haematologica. 2007;92:35–41. doi: 10.3324/haematol.10661. [DOI] [PubMed] [Google Scholar]
- 45.Kuruvilla J, Nagy T, Pintilie M, et al. Similar response rates and superior early progression-free survival with gemcitabine, dexamethasone, and cisplatin salvage therapy compared with carmustine, etoposide, cytarabine, and melphalan salvage therapy prior to autologous stem cell transplantation for recurrent or refractory Hodgkin lymphoma. Cancer. 2006;106:353–360. doi: 10.1002/cncr.21587. [DOI] [PubMed] [Google Scholar]
- 46.Abali H, Urn̈ Y, Oksz̈oğlu B, et al. Comparison of ICE (ifosfamide-carboplatin-etoposide) versus DHAP (cytosine arabinoside-cisplatin-dexamethasone) as salvage chemotherapy in patients with relapsed or refractory lymphoma. Cancer Invest. 2008;26:401–406. doi: 10.1080/07357900701788098. [DOI] [PubMed] [Google Scholar]
- 47.Josting A, Rudolph C, Reiser M, et al. Time-intensified dexamethasone/cisplatin/cytarabine: An effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol. 2002;13:1628–1635. doi: 10.1093/annonc/mdf221. [DOI] [PubMed] [Google Scholar]
- 48.Sibon D, Ertault M, Al Nawakil C, et al. Combined ifosfamide, etoposide and oxaliplatin chemotherapy, a low-toxicity regimen for first-relapsed or refractory Hodgkin lymphoma after ABVD/EBVP: A prospective monocentre study on 34 patients. Br J Haemat. 2011 Mar 8; doi: 10.1111/j.1365-2141.2011.08594.x. [Epub ahead of print]. doi: 10.1111/j.1365–2141.2011.08594.x. [DOI] [PubMed] [Google Scholar]
- 49.Moskowitz AJ, Hamlin PA, Jr, Gerecitano J, et al. Bendamustine is highly active in heavily pre-treated relapsed and refractory Hodgkin lymphoma and serves as a bridge to allogeneic stem cell transplant. Blood. 2009;114:720. [Google Scholar]
- 50.Shea TC, Beaven AW, Moore DT, et al. Sequential high-dose ifosfamide, carboplatin and etoposide with rituximab for relapsed Hodgkin and large B-cell non-Hodgkin lymphoma: Increased toxicity without improvement in progression-free survival. Leuk Lymphoma. 2009;50:741–748. doi: 10.1080/10428190902853136. [DOI] [PubMed] [Google Scholar]
- 51.Tarella C, Cuttica A, Vitolo U, et al. High-dose sequential chemotherapy and peripheral blood progenitor cell autografting in patients with refractory and/or recurrent Hodgkin lymphoma: A multicenter study of the intergruppo Italiano Linfomi showing prolonged disease free survival in patients treated at first recurrence. Cancer. 2003;97:2748–2759. doi: 10.1002/cncr.11414. [DOI] [PubMed] [Google Scholar]
- 52.Josting A, Ml̈ler H, Borchmann P, et al. Dose intensity of chemotherapy in patients with relapsed Hodgkin's lymphoma. J Clin Oncol. 2010;28:5074–5080. doi: 10.1200/JCO.2010.30.5771. [DOI] [PubMed] [Google Scholar]
- 53.Darrington DL, Vose JM, Anderson JR, et al. Incidence and characterization of secondary myelodysplastic syndrome and acute myelogenous leukemia following high-dose chemoradiotherapy and autologous stem-cell transplantation for lymphoid malignancies. J Clin Oncol. 1994;12:2527–2534. doi: 10.1200/JCO.1994.12.12.2527. [DOI] [PubMed] [Google Scholar]
- 54.Sureda A, Arranz R, Iriondo A, et al. Autologous stem-cell transplantation for Hodgkin's disease: Results and prognostic factors in 494 patients from the Grupo Español de Linfomas/Transplante Autológo de Médula Osea Spanish Cooperative Group. J Clin Oncol. 2001;19:1395–1404. doi: 10.1200/JCO.2001.19.5.1395. [DOI] [PubMed] [Google Scholar]
- 55.Yahalom J, Gulati SC, Toia M, et al. Accelerated hyperfractionated total-lymphoid irradiation, high-dose chemotherapy, and autologous bone marrow transplantation for refractory and relapsing patients with Hodgkin's disease. J Clin Oncol. 1993;11:1062–1070. doi: 10.1200/JCO.1993.11.6.1062. [DOI] [PubMed] [Google Scholar]
- 56.Perz JB, Giles C, Szydlo R, et al. LACE-conditioned autologous stem cell transplantation for relapsed or refractory Hodgkin's lymphoma: Treatment outcome and risk factor analysis in 67 patients from a single centre. Bone Marrow Transplant. 2007;39:41–47. doi: 10.1038/sj.bmt.1705544. [DOI] [PubMed] [Google Scholar]
- 57.Benekli M, Smiley SL, Younis T, et al. Intensive conditioning regimen of etoposide (VP-16), cyclophosphamide and carmustine (VCB) followed by autologous hematopoietic stem cell transplantation for relapsed and refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2008;41:613–619. doi: 10.1038/sj.bmt.1705951. [DOI] [PubMed] [Google Scholar]
- 58.Ulrickson M, Aldridge J, Kim HT, et al. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: A single-institution experience. Biol Blood Marrow Transplant. 2009;15:1447–1454. doi: 10.1016/j.bbmt.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 59.Wadehra N, Farag S, Bolwell B, et al. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:1343–1349. doi: 10.1016/j.bbmt.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 60.Santos EC, Sessions J, Hutcherson D, et al. Long-term outcome of Hodgkin disease patients following high-dose busulfan, etoposide, cyclophosphamide, and autologous stem cell transplantation—a similar experience. Biol Blood Marrow Transplant. 2007;13:746–747. doi: 10.1016/j.bbmt.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 61.Nieto Y, Anderlini P, Popat U, et al. Gemcitabine, busulfan and melphalan (GemBuMel) is a new high-dose chemotherapy (HDC) regimen with high activity in refractory Hodgkin's lymphoma (HL) patients receiving an autologous stem-cell transplant (ASCT): A contemporaneous comparison with BEAM and busulfan/melphalan (BuMel) Blood. 2010;116:690. [Google Scholar]
- 62.Visani G, Malerba L, Stefani PM, et al. A novel high dose chemotherapy strategy with bendamustine in adjunct to etoposide, cytarabine and melphalan (BeEAM) followed by autologous stem cell rescue is safe and highly effective for the treatment of resistant/relapsed lymphoma patients: A phase I-II study on 44 patients. Blood. 2010;116(21):31. [Google Scholar]
- 63.Lane AA, McAfee SL, Kennedy J, et al. High-dose chemotherapy with busulfan and cyclophosphamide and autologous stem cell rescue in patients with Hodgkin lymphoma. Leuk Lymphoma. 2011;52:1363–1366. doi: 10.3109/10428194.2011.572324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Josting A, Nogovà L, Franklin J, et al. Salvage radiotherapy in patients with relapsed and refractory Hodgkin's lymphoma: A retrospective analysis from the German Hodgkin Lymphoma Study Group. J Clin Oncol. 2005;23:1522–1529. doi: 10.1200/JCO.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 65.Goodman KA, Riedel E, Serrano V, et al. Long-term effects of high-dose chemotherapy and radiation for relapsed and refractory Hodgkin's lymphoma. J Clin Oncol. 2008;26:5240–5247. doi: 10.1200/JCO.2007.15.5507. [DOI] [PubMed] [Google Scholar]
- 66.Lazarus HM, Crilley P, Ciobanu N, et al. High-dose carmustine, etoposide, and cisplatin and autologous bone marrow transplantation for relapsed and refractory lymphoma. J Clin Oncol. 1992;10:1682–1689. doi: 10.1200/JCO.1992.10.11.1682. [DOI] [PubMed] [Google Scholar]
- 67.Crump M, Smith AM, Brandwein J, et al. High-dose etoposide and melphalan, and autologous bone marrow transplantation for patients with advanced Hodgkin's disease: Importance of disease status at transplant. J Clin Oncol. 1993;11:704–711. doi: 10.1200/JCO.1993.11.4.704. [DOI] [PubMed] [Google Scholar]
- 68.Wendland MM, Asch JD, Pulsipher MA, et al. The impact of involved field radiation therapy for patients receiving high-dose chemotherapy followed by hematopoietic progenitor cell transplant for the treatment of relapsed or refractory Hodgkin disease. Am J Clin Oncol. 2006;29:189–195. doi: 10.1097/01.coc.0000209370.61355.8e. [DOI] [PubMed] [Google Scholar]
- 69.Majhail NS, Bajorunaite R, Lazarus HM, et al. Long-term survival and late relapse in 2-year survivors of autologous haematopoietic cell transplantation for Hodgkin and non-Hodgkin lymphoma. Br J Haematol. 2009;147:129–139. doi: 10.1111/j.1365-2141.2009.07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moskowitz AJ, Perales MA, Kewalramani T, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009;146:158–163. doi: 10.1111/j.1365-2141.2009.07727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horning S, Fanale M, deVos S, et al. Defining a population of Hodgkin lymphoma patients for novel therapeutics: An international effort [abstract 18] Ann Oncol. 2008;19(suppl 4):iv120–iv1. [Google Scholar]
- 72.Regelink JC, van Roessel CHM, van Galen KPM, et al. Long-term follow-up of tandem autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2010;28:e741–e743. doi: 10.1200/JCO.2010.31.5515. [DOI] [PubMed] [Google Scholar]
- 73.Lazarus HM, Stiff PJ, Carreras J, et al. Utility of single versus tandem autotransplants for advanced testes/germ cell cancer: A Center for International Blood and Marrow Transplant Research (CIBMTR) analysis. Biol Blood Marrow Transplant. 2007;13:778–789. doi: 10.1016/j.bbmt.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 74.Brice P, Divine M, Simon D, et al. Feasibility of tandem autologous stem-cell transplantation (ASCT) in induction failure or very unfavorable (UF) relapse from Hodgkin's disease (HD). SFGM/GELA Study Group. Ann Oncol. 1999;10:1485–1488. doi: 10.1023/a:1008343823292. [DOI] [PubMed] [Google Scholar]
- 75.Fung HC, Stiff P, Schriber J, et al. Tandem autologous stem cell transplantation for patients with primary refractory or poor risk recurrent Hodgkin lymphoma. Biol Blood Marrow Transplant. 2007;13:594–600. doi: 10.1016/j.bbmt.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 76.Morschhauser F, Brice P, Fermé C, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin's lymphoma: Results of the prospective multicenter H96 trial by the GELA/SFGM Study Group. J Clin Oncol. 2008;26:5980–5987. doi: 10.1200/JCO.2007.15.5887. [DOI] [PubMed] [Google Scholar]
- 77.Corradini P, Sarina B, Farina L. Allogeneic transplantation for Hodgkin's lymphoma. Br J Haematol. 2011;152:261–272. doi: 10.1111/j.1365-2141.2010.08492.x. [DOI] [PubMed] [Google Scholar]
- 78.Rapoport AP, Guo C, Badros A, et al. Autologous stem cell transplantation followed by consolidation chemotherapy for relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004;34:883–890. doi: 10.1038/sj.bmt.1704661. [DOI] [PubMed] [Google Scholar]
- 79.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 80.Chen R, Gopal AK, Smith SE, et al. Results of a pivotal phase 2 study of brentuximab vedotin (SGN-35) in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2010;116:283. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwering I, Bräuninger A, Klein U, et al. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101:1505–1512. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- 82.Carlo-Stella C, Guidetti A, Viviani S, et al. Safety and clinical activity of the histone deacetylase inhibitor givinostat in combination with meclorethamine in relapsed/refractory Hodgkin lymphoma (HL) J Clin Oncol. 2010;28(15 suppl):3068. [Google Scholar]
- 83.Sureda A, Younes A, Ben-Yehuda D, et al. Final analysis: Phase II study of oral panobinostat in relapsed/refractory Hodgkin lymphoma patients following autologous hematopoietic stem cell transplant. Blood. 2010;116:419. [Google Scholar]
- 84.Dutton A, Reynolds GM, Dawson CW, et al. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin's lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 85.Johnston PB, Inwards DJ, Colgan JP, et al. A phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010;85:320–324. doi: 10.1002/ajh.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Younes A, Copeland A, Fanale MA, et al. Phase I/II study of the novel combination of panobinostat (LBH589) and everolimus (RAD001) in relapsed/refractory Hodgkin and non-Hodgkin lymphoma. Blood. 2010;116:3964. [Google Scholar]
- 87.Böll B, Borchmann P, Topp MS, et al. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Br J Haematol. 2010;148:480–482. doi: 10.1111/j.1365-2141.2009.07963.x. [DOI] [PubMed] [Google Scholar]
- 88.Kuruvilla J, Taylor D, Wang L, et al. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2008;112:3052. [Google Scholar]
- 89.Fehniger TA, Larson S, Trinkaus K, et al. A Phase II multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2009;114:3693. doi: 10.1182/blood-2011-07-362475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Younes A, Romaguera J, Hagemeister F, et al. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98:310–314. doi: 10.1002/cncr.11511. [DOI] [PubMed] [Google Scholar]
- 91.Oki Y, Pro B, Fayad LE, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008;112:831–836. doi: 10.1002/cncr.23237. [DOI] [PubMed] [Google Scholar]
- 92.Corazzelli G, Capobianco G, Arcamone M, et al. Rituximab plus gemcitabine, ifosfamide, oxaliplatin (R-GIFOX), a new effective cytoreductive/mobilizing salvage regimen for relapsed and refractory Hodgkin lymphoma. Blood. 2008;112:2601. [Google Scholar]
- 93.Jones RJ, Gocke CD, Kasamon YL, et al. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kasamon YL, Jones RJ, Levitsky HI, et al. High-dose cyclophosphamide (Cy), rituximab, and a cancer vaccine for relapsed classical Hodgkin's lymphoma (cHL) Blood. 2010;116:3954. [Google Scholar]
- 95.Fernàndez de Larrea C, Martínez C, Gaya A, et al. Salvage chemotherapy with alternating MINE–ESHAP regimen in relapsed or refractory Hodgkin's lymphoma followed by autologous stem-cell transplantation. Ann Oncol. 2010;21:1211–1216. doi: 10.1093/annonc/mdp487. [DOI] [PubMed] [Google Scholar]
- 96.Rigacci L, Fabbri A, Puccini B, et al. Oxaliplatin-based chemotherapy (dexamethasone, high-dose cytarabine, and oxaliplatin) ± rituximab is an effective salvage regimen in patients with relapsed or refractory lymphoma. Cancer. 2010;116:4573–4579. doi: 10.1002/cncr.25216. [DOI] [PubMed] [Google Scholar]
- 97.Reece DE, Barnett MJ, Connors JM, et al. Intensive chemotherapy with cyclophosphamide, carmustine, and etoposide followed by autologous bone marrow transplantation for relapsed Hodgkin's disease. J Clin Oncol 1991;9:1871–1879. Erratum in J Clin Oncol. 1992;10:170. doi: 10.1200/JCO.1991.9.10.1871. [DOI] [PubMed] [Google Scholar]
- 98.Bierman PJ, Bagin RG, Jagannath S, et al. High dose chemotherapy followed by autologous hematopoietic rescue in Hodgkin's disease: Long-term follow-up in 128 patients. Ann Oncol. 1993;4:767–773. doi: 10.1093/oxfordjournals.annonc.a058662. [DOI] [PubMed] [Google Scholar]
- 99.Chopra R, McMillan AK, Linch DC, et al. The place of high-dose BEAM therapy and autologous bone marrow transplantation in poor-risk Hodgkin's disease. A single-center eight- year study of 155 patients. Blood. 1993;81:1137–1145. [PubMed] [Google Scholar]
- 100.Bierman PJ, Anderson JR, Freeman MB, et al. High-dose chemotherapy followed by autologous hematopoietic rescue for Hodgkin's disease patients following first relapse after chemotherapy. Ann Oncol. 1996;7:151–156. doi: 10.1093/oxfordjournals.annonc.a010542. [DOI] [PubMed] [Google Scholar]
- 101.Caballero MD, Rubio V, Rifon J, et al. BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: Analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant. 1997;20:451–458. doi: 10.1038/sj.bmt.1700913. [DOI] [PubMed] [Google Scholar]
- 102.Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin's disease: Analysis of the Stanford University results and prognostic indices. Blood. 1997;89:801–813. [PubMed] [Google Scholar]
- 103.Sweetenham JW, Taghipour G, Milligan D, et al. High-dose therapy and autologous stem cell rescue for patients with Hodgkin's disease in first relapse after chemotherapy: Results from the EBMT. Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1997;20:745–752. doi: 10.1038/sj.bmt.1700963. [DOI] [PubMed] [Google Scholar]
- 104.Wheeler C, Eickhoff C, Elias A, et al. High-dose cyclophosphamide, carmustine, and etoposide with autologous transplantation in Hodgkin's disease: A prognostic model for treatment outcomes. Biol Blood Marrow Transplant. 1997;3:98–106. [PubMed] [Google Scholar]
- 105.Arranz R, Tomàs JF, Gil-Fernàndez JJ, et al. Autologous stem cell transplantation (ASCT) for poor prognostic Hodgkin's disease (HD): Comparative results with two CBV regimens and importance of disease status at transplant. Bone Marrow Transplant. 1998;21:779–786. doi: 10.1038/sj.bmt.1701186. [DOI] [PubMed] [Google Scholar]
- 106.Lazarus HM, Rowlings PA, Zhang MJ, et al. Autotransplants for Hodgkin's disease in patients never achieving remission: A report from the Autologous Blood and Marrow Transplant Registry. J Clin Oncol. 1999;17:534–545. doi: 10.1200/JCO.1999.17.2.534. [DOI] [PubMed] [Google Scholar]
- 107.Lazarus HM, Loberiza FR, Jr, Zhang MJ, et al. Autotransplants for Hodgkin's disease in first relapse or second remission: A report from the Autologous Blood and Marrow Transplant Registry (ABMTR) Bone Marrow Transplant. 2001;27:387–396. doi: 10.1038/sj.bmt.1702796. [DOI] [PubMed] [Google Scholar]
- 108.Moskowitz CH, Kewalramani T, Nimer SD, et al. Effectiveness of high dose chemoradiotherapy and autologous stem cell transplantation for patients with biopsy-proven primary refractory Hodgkin's disease. Br J Haematol. 2004;124:645–652. doi: 10.1111/j.1365-2141.2003.04828.x. [DOI] [PubMed] [Google Scholar]
- 109.Evens AM, Altman JK, Mittal BB, et al. Phase I/II trial of total lymphoid irradiation and high-dose chemotherapy with autologous stem-cell transplantation for relapsed and refractory Hodgkin's lymphoma. Ann Oncol. 2007;18:679–688. doi: 10.1093/annonc/mdl496. [DOI] [PubMed] [Google Scholar]
- 110.Kebriaei P, Madden T, Kazerooni R, et al. Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biology Blood and Marrow Transplant. 2011;17:412–420. doi: 10.1016/j.bbmt.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]