Abstract
Background & Aims
Integrity of the intestinal epithelium is required for nutrition absorption and defense against pathogens. Claudins are cell adhesion molecules that localize at tight junctions (TJs); many are expressed in the intestinal tract, but little is known about their functions. Claudin-7 is unique in that it has a stronger basolateral membrane distribution than other claudins, which localize primarily to apical TJs in the intestinal epithelium. We investigated the basolateral functions of claudin-7 and assessed the effects of disruption of Cldn7 in intestines of mice.
Methods
We generated Cldn7−/− mice and examined their intestines by histology, molecular and cellular biology, and biochemistry approaches. We carried out gene silencing experiments in epithelial cell lines using small interfering (si)RNAs.
Results
The Cldn7−/− mice had severe intestinal defects that included mucosal ulcerations, epithelial cell sloughing, and inflammation. Intestines of Cldn7−/− mice produced significantly higher levels of cytokines, the NF-κB p65 subunit, and COX-2; they also upregulated expression of matrix metalloproteinases (MMPs)-3 and -7. siRNA in epithelial cell lines demonstrated that the increased expression of MMP-3 resulted directly from claudin-7 depletion, whereas that of MMP-7 resulted from inflammation. Electron microscopy analysis showed that intestines of Cldn7−/− mice had intercellular gaps below TJs and cell-matrix loosening. Deletion of Cldn7 reduced expression and altered localization of the integrin α2 subunit; disrupted formation of complexes of claudin-7, integrin α2, and claudin-1 that normally form in epithelial basolateral compartments of intestines.
Conclusion
In mice, claudin-7 has non-TJ functions, including maintenance of epithelial cell–matrix interactions and intestinal homeostasis.
Keywords: Mucosal integrity, epithelial barrier, mouse model, permeability, IBD
Background
Disruption of intestinal epithelial tissue integrity leads to various intestinal disorders such as inflammatory bowel disease (IBD).1 Cell adhesion molecules play a fundamental role in maintaining intestinal epithelial integrity.2 Claudins are a family of cell adhesion molecules mainly localized at apical tight junctions (TJs).3 Among 24 claudin members in mammals, at least 10 of them are expressed in the mouse intestines.4 Although multiple claudins are expressed in the intestinal tract, their exact roles in the intestinal system remain largely unknown. Although several claudin gene-deficient mouse models, which include claudin-1, -5, -11, -14, -15, -16, and -19, are currently available, none of these mouse models show IBD-like symptoms. The only claudin member reported to affect intestinal structure so far is claudin-15. Claudin-15-deficient mice formed megaintestines in which the upper small intestine was two times larger than the normal intestine, indicating the importance of claudin-15 in regulating normal-sized morphogenesis of the small intestine.4 Despite the lack of animal models in specific claudin-deficient mice, defective epithelial barrier function or increased intestinal permeability has been implicated in IBD.5 Moreover, altered expression and distribution of claudin-2, -5, and -8 have been reported in biopsy samples from patients with Crohn’s disease.6 However, whether the epithelial permeability defect observed is a cause or a consequence of IBD remains to be determined.
Studies have established that claudins are the most important structural and functional components of TJs and are crucial for the paracellular flux of ions and small molecules.7–8 Nevertheless, recent antibody-based studies indicated that several claudins, including claudin-7, are not only localized at apical TJs but also have a strong basolateral membrane distribution in the epithelia of various tissues, including intestines.9–11 It is essentially unknown what the functions of these non-TJ claudins are. In this study, using our claudin-7-deficient mouse model, we discovered that claudin-7 interacts with integrin α2 at the epithelial basolateral compartment of intestines. Deletion of claudin-7 gene reduces integrin α2 expression and disrupts integrin α2 localization and claudin-7/integrin α2/claudin-1 complexes that formed in normal intestines, leading to MMPs upregulation, intestinal epithelial tissue damage, and invoking the inflammatory response, which resemble IBD-like symptoms.
Materials and Methods
Antibodies
Claudin-1, -2, and -4 antibodies were purchased from Invitrogen (Carlsbad, CA). Claudin-7 antibody was obtained from Immuno-Biological Laboratories (Japan). The antibodies against MMP-3, MMP-7, and MMP-8 were from Abcam (Cambridge, MA). Anti-macrophage antibody CD11b (Mac-1) was from Miltenyi Biotech (Auburn, CA). Monoclonal and polyclonal antibodies against integrin α2 were from Chemicon (Millipore, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. All other antibodies were from Cell Signaling Technology (Beverly, MA).
Cldn7−/− Mice
The generation of Cldn7−/− mice by gene targeting was described previously.12 The Cldn7−/− mouse colony was housed in the microisolation barrier. To determine mouse genotypes, tail snips were obtained while newborn mice were tattooed. All animal experiments were approved by the East Carolina University Animal Care and Use Committee.
RNA Isolation, PCRArrays, and Real-Time qRT-PCR
Total RNA was isolated from Cldn7+/+ and Cldn7−/− intestines, T84 and HCC827 cells using Qiagen RNeasy kit (Qiagen, Valencia, CA) following the manufacturer’s instructions. PCRArray and real-time qRT-PCR experiments were performed using RT2 Profiler PCR Array system from SABiosciences (Qiagen). The relative changes in gene expression from real-time qRT-PCR experiments were analyzed using 2−ΔΔCt method.13
Cell Culture and siRNA Transfection
T84 intestinal and HCC827 lung epithelial cell lines were obtained from American Type Culture Collection (Rockville, MD). The cells were grown in DMEM/F-12 (T84) or RPMI 1640 (HCC827) medium supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% heat-inactivated fetal bovine serum. After reaching 70–80% confluence, the cells were transfected with the specific siRNA against claudin-7 or with the scrambled siRNA (control) using Nucleofector solution and instrument (Lonza, Walkersville, MD). After 96 h transfection, the transfected cells and their culture media were collected separately for Western blotting analysis. The culture media were concentrated using the concentrator (Novagen, CA).
Zymography
Cldn7+/+ and Cldn7−/− intestinal tissues were homogenized in RIPA Buffer and prepared in the sample buffer without reducing agent for electrophoresis. The equal amount of each protein sample was loaded and separated by 12% zymogram gel containing casein (Invitrogen). After electrophoresis, the SDS was removed from the gel by incubation in unbuffered Triton-X-100, followed by incubation in an digestion buffer (Invitrogen) for overnight at 37°C. The gels were then stained with Coomassie Brilliant Blue and photographed using a Chemiluminescent Imaging with the Chemi DOC XRS (Bio-Rad, Hercules, CA).
BrdU Injection and Detection
Cldn7+/+ and Cldn7−/− pups were intraperitoneally injected with BrdU (50 mg/kg, 1 mg/mL) dissolved in PBS. The pups were killed 2 h after injection. Swiss-rolled intestines were fixed in Carnoy’s fixative14 overnight and embedded in paraffin. Sections were treated with 1 N HCl at 60°C for 8 min to denature DNA. The slides were incubated with monoclonal anti-BrdU antibody (Sigma) diluted to 1:1000. The signal was detected by biotinylated anti-mouse IgG and peroxidase-conjugated strepavidin (Vector Laboratories, Burlingame, CA).
Statistical analysis
The data for real-time qRT-PCR assays were expressed as means ± s.e.m. The differences between the two groups were analyzed using the unpaired Student’s t-test. A P-value of < 0.05 was considered significant.
Results
Deletion of claudin-7 gene disrupted intestinal epithelial tissue integrity
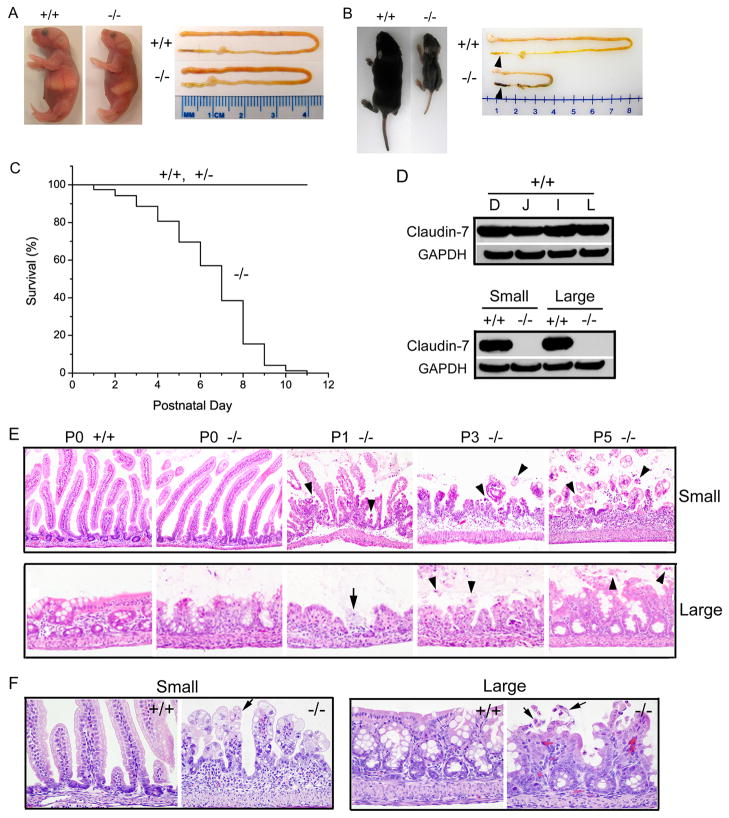
Cldn7−/− mice were born viable and showed no obvious difference in appearance at birth (postnatal day 0, P0) (Fig. 1A, left). The appearance of intestines between Cldn7+/+ and Cldn7−/− was quite similar (Fig. 1A, right). However, the weight of one-week old Cldn7−/− pup was only 40% of that of Cldn7+/+ as shown in Fig. 1B. Bloody loose stools were always observed in Cldn7−/− large intestine, but not in Cldn7+/+ (Fig. 1B, arrowheads). The survival curve indicated that most of Cldn7−/− pups died within 9 days after birth (Fig. 1C). Claudin-7 was highly expressed in both small and large intestines in Cldn7+/+ but was completely absent in Cldn7−/− as confirmed by Western blotting (Fig. 1D). Besides showing salt wasting phenotype as we reported previously, 12 Cldn7−/− mice displayed severe intestinal defects with mucosal ulcerations and villi disruption. Histopathological examinations revealed that on day P0, there were no differences in villous length and morphology between Cldn7+/+ and Cldn7−/− small intestines (Fig. 1E, top panel, P0). On day P1, villi in Cldn7−/− mice were minimally blunted and individual enterocytes sloughed into the intestinal lumen. On day P3, many poorly adhesive enterocytes prematurely sloughed into the lumen as individual cells or in small clusters. On day 5, intestines from Cldn7−/− mice demonstrated multifocal full thickness mucosal ulcers bordered by strikingly blunted villi. The underlying lamina propria was expanded by mixed inflammatory infiltrates and was covered by a layer of degenerate neutrophils and macrophages. Remaining villi were blunted and the intestinal lumen contained sloughed cells and cellular debris (Fig. 1E, top panel, P5). On day P0, the medial and distal segments of the colon in Cldn7−/− mice showed the occasional swelling and sloughing of the epithelium into the lumen. On day P1 and P3, epithelial cells lining the colon of Cldn7−/− mice continued to slough and small mucosal ulcers appeared (Fig. 1E, bottom panel, arrow in P1). An inflammatory infiltrate comprised primarily of macrophages and neutrophils bordered mucosal ulcerations. On day P5, rafts of sloughed epithelial cells were present in the colonic lumen of Cldn7−/− mice. Figure 1F revealed normal finger-like villi in day P4 Cldn7+/+ small intestines, but a distorted mucosal architecture with short and disorganized villi in day P4 Cldn7−/− small intestines. Similarly, the epithelial lining of Cldn7−/− large intestine was also disrupted and the surface erosion was obvious when compared with the normal morphology of Cldn7+/+ large intestine (Fig. 1F). Both small and large intestines of Cldn7−/− pups showed epithelial cell sloughing as indicated by arrows in Figure 1F.
Figure 1.
Deletion of claudin-7 disrupted the intestinal epithelial structure in mice. (A) New born (P0) Cldn7+/+ and Cldn7−/− pups and their isolated intestines. (B) Size comparison of one-week old Cldn7+/+ and Cldn7−/− littermates and their dissected intestinal tracts. The arrowheads indicated the normal shaped stool in Cldn7+/+ large intestine, but melena in Cldn7−/−. (C) Survival curve of Cldn7−/− mice. About 95% of 279 Cldn7−/− mice died within 10 days after birth. (D) Immunoblotting revealed the similar amount of claudin-7 expression in Cldn7+/+ duodenum (D), jejunum (J), ileum (I), and large intestine (L) and the absence of claudin-7 in Cldn7−/− intestines. (E) Light micrographs of H&E stained small and large intestines from P0, P1, P3, and P5 Cldn7+/+ and Cldn7−/− pups. The sections showed the normal Cldn7+/+ (+/+) and timely deteriorated Cldn7−/− (−/−) mucosa. The arrowheads indicated the sloughing epithelial cells. The arrow indicated the site of ulcer. (F) Enlarged H&E stained images to show that the epithelial cells lining the villi in P4 Cldn7−/− mice lost their polarity and culomnar orientation and became polygonal and markedly swollen. The arrows pointed to the sloughing epithelial cells in small and large Cldn7−/− intestines. Magnifications: (E) top, ×200; bottom, ×400; (F) ×600.
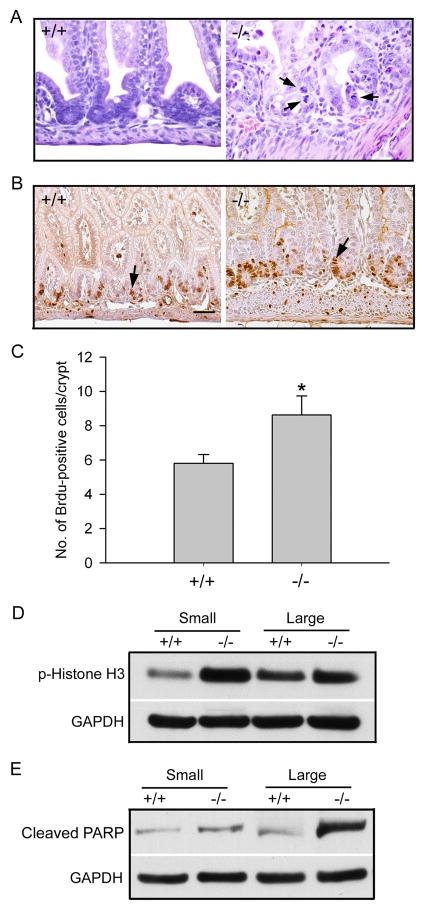
The intestinal epithelial loss was accompanied by increased cell proliferation. As shown in Figure 2, the crypts of day P4 Cldn7−/− intestines displayed an abnormally high cell proliferation rate. The mitotic figures in Cldn7−/− crypt regions can be easily observed (Fig. 2A, −/−, arrows). This high cell proliferation was also confirmed by the BrdU injection experiments. Figures 2B and 2C revealed that the number of dividing cells labeled by BrdU incorporation was greatly increased in Cldn7−/− intestines compared to that of Cldn7+/+ intestines. However, the number of dividing cells did not increase in P0 Cldn7−/− intestines (Fig. S1). Phosphorylation of histone H3 was another indication of cell proliferation. Consistent with the BrdU results, the signal of phosphorylated histone H3 was significantly higher in both small and large intestines of Cldn7−/− pups than those of Cldn7+/+ when analyzed by Western blotting (Fig. 2D). At the same time, cell apoptosis was also increased in Cldn7−/− intestines as indicated by the stronger anti-cleaved PARP signal (Fig. 2E). There was no significant difference in cell apoptosis between day P0 Cldn7+/+ and Cldn7−/− intestines (Fig. S2).
Figure 2.
Increased cell proliferation in Cldn7−/− intestines. (A) H&E staining of small intestines from day P4 Cldn7+/+ and Cldn7−/− pups. Arrows indicated the mitotic figures in Cldn7−/− crypt regions. Magnification: ×600. (B) Cldn7+/+ and Cldn7−/− were injected with BrdU (50mg/kg) and sacrificed 2 h after injection. Sections of the small intestine were stained with anti-BrdU antibody. Bar: 40 μm. (C) Statistical analysis revealed the increased numbers of mitotic cells in Cldn7−/− small intestines (−/−) compared to those of Cldn7+/+ (+/+). Data was obtained from three independent experiments. (D) and (E) Intestinal lysates from Cldn7+/+ and Cldn7−/− pups were immunoblotted with anti-phospho-Histone H3 and anti-cleaved PARP antibodies. GAPDH served as a protein loading control.
Inflammatory responses in Cldn7−/− mice
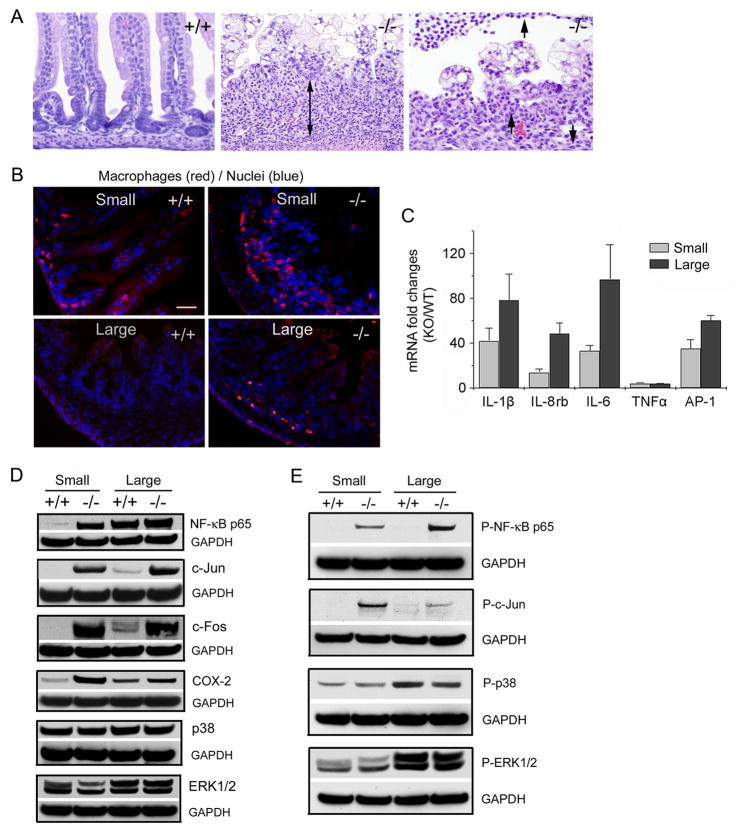
Histological examinations of small intestines from day P4 Cldn7−/− pups revealed that the lamina propria contained an increased number of leukocytes compared to that in Cldn7+/+, indicating an immune response in Cldn7−/− pups (Fig. 3A, the double arrow and arrows in −/−). This observation was confirmed by an antibody recognizing macrophages. The immunostaining signals in both small and large intestines were greatly increased in P4 Cldn7−/− compared to those in P4 Cldn7+/+ (Fig. 3B). The macrophages were not increased in P0 Cldn7−/− intestines (Fig. S3). To determine whether the inflammatory cytokines were upregulated in Cldn7−/− intestines, qRT-PCR based arrays were performed and then further verified by real-time RT-PCR (Fig. 3C). Indeed, mRNAs of IL-1β, IL-8 rb, IL-6, and AP-1, a transcription factor regulating gene transcription, were dramatically increased in day P4 Cldn7−/− small and large intestines when compared to those of Cldn7+/+. The TNFα mRNA was also elevated more than 5-fold in Cldn7−/− intestines. The protein expression levels of NF-κB, c-Jun, c-Fos, and COX-2 were all significantly increased in Cldn7−/− intestines, while p38 and ERK1/2 expressions were not (Fig. 3D). The phosphorylations of NF-κB and c-Jun in Cldn7−/− intestines were also greatly elevated, but p38 and ERK1/2 phosphorylation levels were unchanged (Fig. 3E). NF-κB has been identified as one of the key regulators in mucosal inflammation in IBD, and its activation was markedly induced in IBD patients.15–16 Increased expression of COX-2 has been reported in many active human IBD patient samples.17 C-Jun and c-Fos are the components of AP-1, which is the downstream element of NF-κB. The significant increase of these proteins indicated that mice lacking claudin-7 evoked the intestinal inflammation. Although histological data did not show inflammation in P0 Cldn7−/− intestines, and NF-κB p65 subunit and COX-2 were not increased in P0 Cldn7−/− intestines, several cytokine mRNA levels, especially the IL-1β mRNA level, were elevated, indicating that the inflammation was initiated at the message level (Fig. S4).
Figure 3.
The inflammatory response in Cldn7−/− intestines. (A) Light micrographs of H&E stained small intestines from day P4 Cldn7+/+ (+/+) and Cldn7−/− (−/−) pups. The Cldn7−/− section showed the large amount of mixed inflammatory cells in the lamina propria area (double arrow in −/−). At the high magnification, neutrophils can be observed (arrows in −/−). Magnifications: left and middle images, ×400; right image, ×600. (B) Frozen sections of intestinal tissues were stained with an antibody recognizing macrophages and DAPI. Bar: 20 μm. (C) The mRNA levels of cytokines and AP-1 were greatly increased in Cldn7−/− intestines measured by qRT-PCR. This increase was expressed as fold changes when comparing Cldn7−/− (KO) values to that of Cldn7+/+ (WT) after they were normalized to the GAPDH value. The data were from five independent experiments. (D) The expression levels of NF-κB p65, c-Jun, c-Fos, and COX-2 were greatly increased in Cldn7−/− intestines by immunoblotting analysis. The p38 and ERK1/2 expressions were not enhanced in Cldn7−/− intestines. GAPDH served as a loading control. (E) The immunoblotting results showed that NF-κB p65 and c-Jun proteins in Cldn7−/− intestines were highly phosphorylated using anti-phospho-NF-κB p65 and anti-phospho-c-Jun antibodies. The levels of phospho-p38 and phospho-ERK1/2 did not have significant changes between Cldn7+/+ and Cldn7−/− intestines.
MMP-3 increase in Cldn7−/− intestines was the direct consequence of claudin-7 deletion
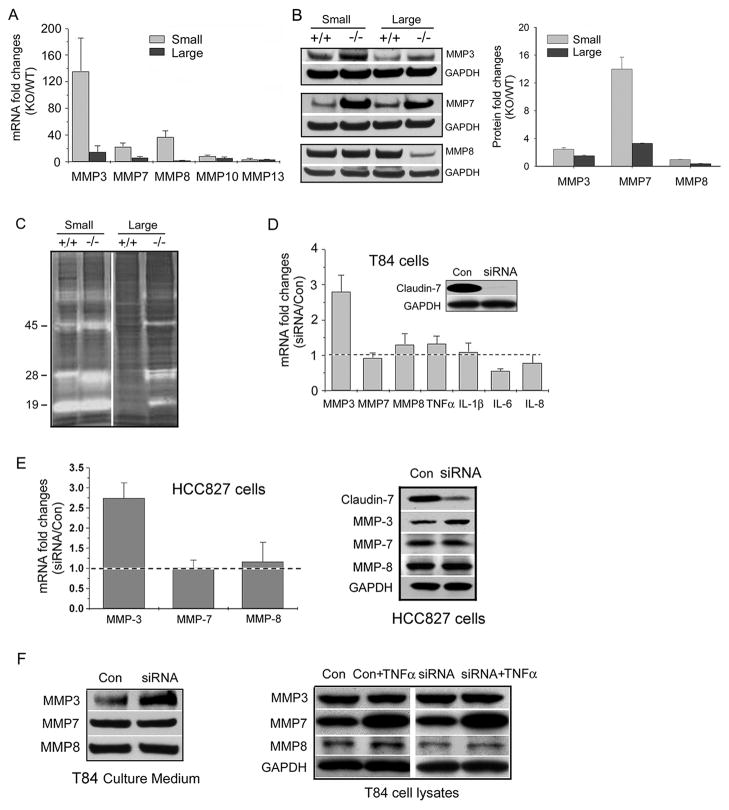
It has been demonstrated that uncontrolled activation of MMPs can result in tissue injury and inflammation.18 In an attempt to investigate the potential mechanism underlying the damage of intestines in Cldn7−/− mice, we examined a panel of MMPs using a real-time RT-PCR approach. We found that MMP-3 mRNA was increased more than 120-fold in P4 Cldn7−/− small intestine compared to that of Cldn7+/+ (Fig. 4A). MMP-7 and MMP-8 mRNAs were enhanced 20 to 40-fold in Cldn7−/− small intestine. In day P0 intestines, only MMP-3 mRNA level was dramatically increased (Fig. S5 A). At the protein level, MMP-3 and MMP-7 were significantly increased in both small and large intestines of day P4 Cldn7−/− mice (Fig. 4B). Interestingly, MMP-8 protein expression was not elevated in Cldn7−/− intestines. MMP-7 is secreted as a 28 kDa proenzyme that can be activated in vivo by MMP-3 to form a 19 kDa active MMP-7.19 The active form of MMP-3 is at 45 kDa. The enzymatic activity of MMP-3 and MMP-7 can be detected by using zymography based on molecular weight shift from latent to activated forms. Figure 4C showed the casein substrate zymography detecting latent and activated MMPs from tissue lysates of Cldn7+/+ and Cldn7−/− intestines. The increased band intensity can be observed at 28 and 45 kDa in Cldn7−/− small intestine and 19, 28, and 45 kDa in Cldn7−/− large intestine.
Figure 4.
Upregulation of MMPs in day P4 Cldn7−/− intestines. (A) The mRNA levels of MMPs in Cldn7−/− intestines were greatly increased by qRT-PCR analysis. Each data point was obtained from five independent experiments. (B) MMP-3 and MMP-7 expressions, but not MMP-8, were significantly enhanced in Cldn7−/− intestines by immunoblotting examinations. The band intensity measurements (present as KO/WT) were shown on the right. (C) Increased enzymatic activities of MMPs in Cldn7−/− intestines were revealed by casein zymography gel. (D) Upregulation of MMP-3 in T84 epithelial cells with claudin-7 knockdown (KD). The insert showed the claudin-7 KD by 50 μM siRNA against claudin-7 for 72 h. The scramble siRNA was used in control cells (Con). Only MMP-3 mRNA was significantly increased with claudin-7 KD by qRT-PCR analysis. The data represented the mean values of five independent experiments and were normalized with endogenous GAPDH values. The dotted line represents no change when the ratio equals 1. (E) Real-time qRT-PCR data also showed a significant increase of MMP-3 mRNA (left) and protein (right) in human lung HCC827 cells with claudin-7 KD by specific siRNA. (F) Culture media were collected from T84 cells treated with scramble siRNA (Con) or specific siRNA against claudin-7 (siRNA). Immunoblotting displayed the increased level of MMP-3, but not MMP-7 and MMP-8 (left). MMP-7 expression was greatly increased in both control and claudin-7 KD cells after the addition of cytokine TNFα (20 ng/mL) to the culture medium for 24 h (right).
To investigate whether MMPs increase was caused by claudin-7 deletion or by the secondary effect of inflammation, we silenced claudin-7 in T84 intestinal epithelial cell line using a siRNA approach. Under the condition in which claudin-7 expression was largely suppressed by the siRNA against claudin-7 (Fig. 4D, insert), only MMP-3 mRNA was increased almost 3-fold without significant changes in the mRNA levels of MMP-7, MMP-8, and other cytokines (Fig. 4D). The effect of claudin-7 on MMP-3 expression was also examined in HCC827 human lung cancer cells, and similar results were obtained. When claudin-7 expression was suppressed in HCC827 cells by claudin-7 specific siRNA, the MMP-3 mRNA and protein levels were significantly increased without changes in the mRNA and protein levels of MMP-7 and MMP-8 (Fig. 4E). We also identified that the MMP-3 level in the culture medium was considerably higher in the cells with claudin-7 knockdown (KD), while the MMP-7 and MMP-8 levels were unchanged in T84 cells (Fig. 4F, left panel). The activity of MMP-3 can be inhibited by a MMP-3 inhibitor (Fig. S5 B). To test our hypothesis that the MMP-7 increase in Cldn7−/− intestines was caused by inflammatory response, TNFα was added to the culture medium. Our results demonstrated that MMP-7 expression was greatly upregulated by the addition of TNFα, while MMP-3 and MMP-8 were not in T84 cells (Fig. 4F, right panel). Therefore, we concluded that MMP-3 upregulation in Cldn7−/− intestines was due to claudin-7 deletion, while MMP-7 increase was caused by inflammatory response.
Deletion of claudin-7 altered integrin α2 expression and localization and disrupted the integrin α2/claudin-1 protein complex
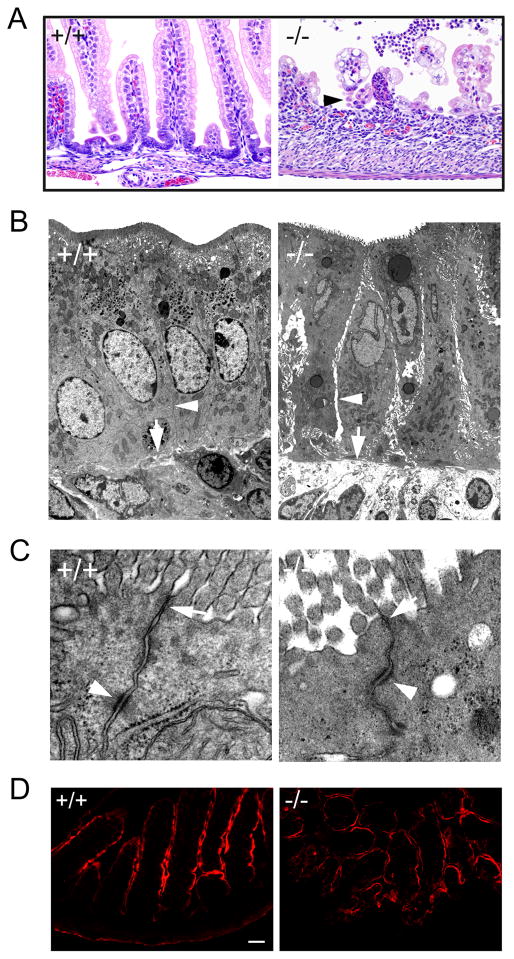
Cldn7−/− intestines showed severe epithelial cell sloughing (Fig. 5A, −/−, arrowhead). Electron microscopic examination revealed the significant intercellular gaps between adjacent epithelial cells and the loosening between cell and matrix in day P5 Cldn7−/− small intestine compared to that of Cldn7+/+ (Fig. 5B, arrowheads and arrows, respectively), while the apical TJs were largely intact in Cldn7−/− small intestine (Fig. 5C, arrow). In addition, the desmosome was visualized in both Cldn7+/+ and Cldn7−/− small intestines (Figure 5C, arrowhead). We found that deletion of claudin-7 did not affect the localization of ezrin, an apical marker protein (Fig. 5D). The localization of basolateral marker Na-K ATPase α1 was also similar in Cldn7−/− small intestines compared to that of Cldn7+/+ (Fig. S6). No TJ leakage was detected in P0 Cldn7−/− intestines when using biotin as a trace molecule although we sometimes observed the disruption of epithelial barrier function at a later stage of Cldn7−/−’s life, such as day P5 (Fig. S7). The intercellular gap was not observed in P0 Cldn7−/− intestines (Fig. S8).
Figure 5.
Disruption of cell-cell adhesion in Cldn7−/− intestines. (A) Light micrographs of H&E stained day P5 Cldn7+/+ (+/+) and Cldn7−/− (−/−) small intestines. The arrowhead in (−/−) pointed to the sloughing epithelial cells. Magnification: ×400. (B) Electron micrographs of day P5 Cldn7+/+ (+/+) and Cldn7−/− (−/−) small intestines. The arrowhead in (−/−) pointed to the intercellular gap along the Cldn7−/− lateral membrane compared to that of Cldn7+/+ (+/+, arrowhead). The arrow in (−/−) revealed the loosening of cell-matrix connection in Cldn7−/− versus the close contact between cell and matrix in Cldn7+/+ intestines (+/+, arrow). (C) The arrows in (+/+) and (−/−) pointed to the apical TJ. The arrowheads indicated the desmosome. Magnifications: (B), ×5,000; (C), ×50,000. (D) Frozen sections of Cldn7+/+ (+/+) and Cldn7−/− (−/−) small intestines were immunostained with an apical protein marker, Ezrin. Bar: 40 μm.
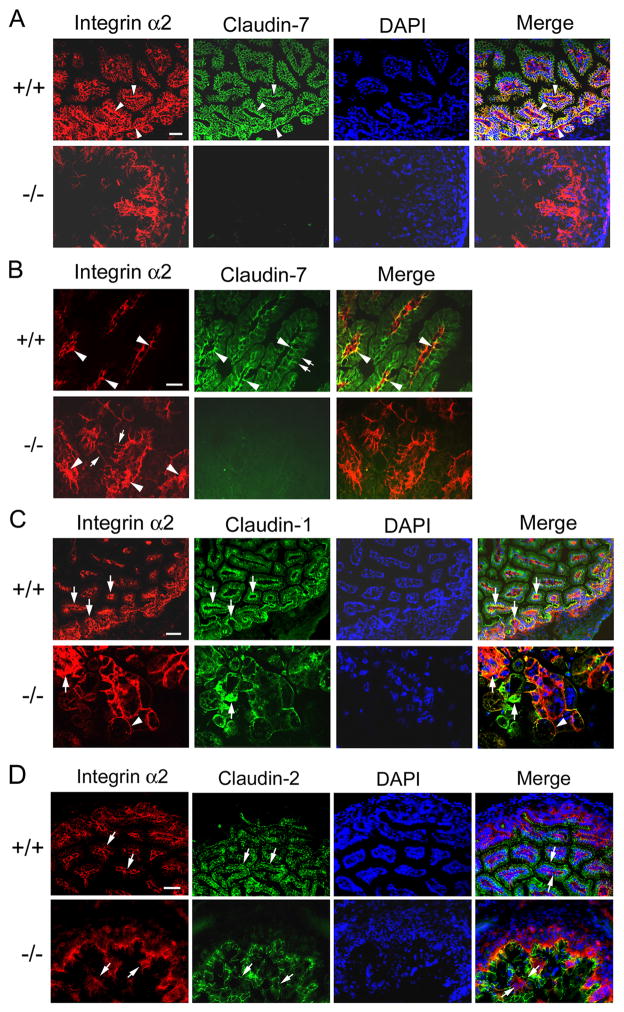
Immunofluorescent light microscopy results showed that claudin-7 was strongly expressed at the basolateral membrane and was partially co-localized with integrin α2 in Cldn7+/+ intestines (Fig. 6A, +/+, arrowheads). The distribution of integrin α2 was disorganized in Cldn7−/− intestines (Fig. 6A, −/−). At the high magnification, the co-localization of claudin-7 and integrin α2 in Cldn7+/+ intestines were clearly visualized at the basal membrane (Fig. 6B, +/+, arrowheads). In Cldn7−/− intestines, integrin α2 was either forming clusters (Fig. 6B, −/−, arrowheads) or moving towards the apical lateral surface (Fig. 6B, −/−, arrows). Claudin-1 was also partially co-localized with integrin α2 especially at the basal membrane of crypt region in Cldn7+/+ intestines (Fig. 6C, +/+, arrows). However, in Cldn7−/− intestines, the normal localization of integrin α2 and claudin-1 was disrupted and signal of cluster was observed (Fig. 6C, −/−, arrows). Integrin α2 signal was more toward apical lateral membrane than at the basal membrane (Fig. 6C, −/−, arrowhead). In contrast, claudin-2 was largely localized at the apical membrane, which did not co-localize with integrin α2 (Fig. 6D, +/+, arrows). Although claudin-7 deletion altered the normal distribution pattern of integrin α2, claudin-2 was still localized at the apical membrane (Fig. 6D, −/−, arrows).
Figure 6.
Disruption of integrin α2 localization in Cldn7−/− intestines. (A) Arrowheads in (+/+) showed examples of double immunostaining signals where claudin-7 and integrin α2 were co-localized at the basal membrane. Integrin α2 signals were disorganized in (−/−). Bar: 40 μm. (B) High magnification to show the co-localization of claudin-7 and integrin α2 in Cldn7+/+ (arrowheads in +/+) and disorganized integrin α2 signal in Cldn7−/− intestines (arrowheads in −/−). The double arrows in (+/+) indicated the apical surface of the villus and arrows in (−/−) pointed to the integrin α2 signal moving towards apical lateral surface. Bar: 20 μm. (C) Integrin α2 and claudin-1 were partially co-localized at the basolateral region of Cldn7+/+ villi (arrows in +/+). Arrows in (−/−) indicated the clustered signals of integrin α2 and claudin-1, while the arrowhead pointed to integrin α2 moving towards the apical lateral surface in the absence of claudin-7. Bar: 40 μm for the top panel and 20 μm for the bottom panel. (D) Claudin-2 and integrin α2 were not co-localized since claudin-2 signal was largely localized at the apical membrane even in the absence of claudin-7 (claudin-2, arrows). Integrin α2 signal (integrin α2, arrows) was mainly in the basolateral region in Cldn7+/+, but was disrupted in Cldn7−/−. Arrows in merge images pointed to claudin-2 (top) and integrin α2 (bottom) signals. Bar: 20 μm.
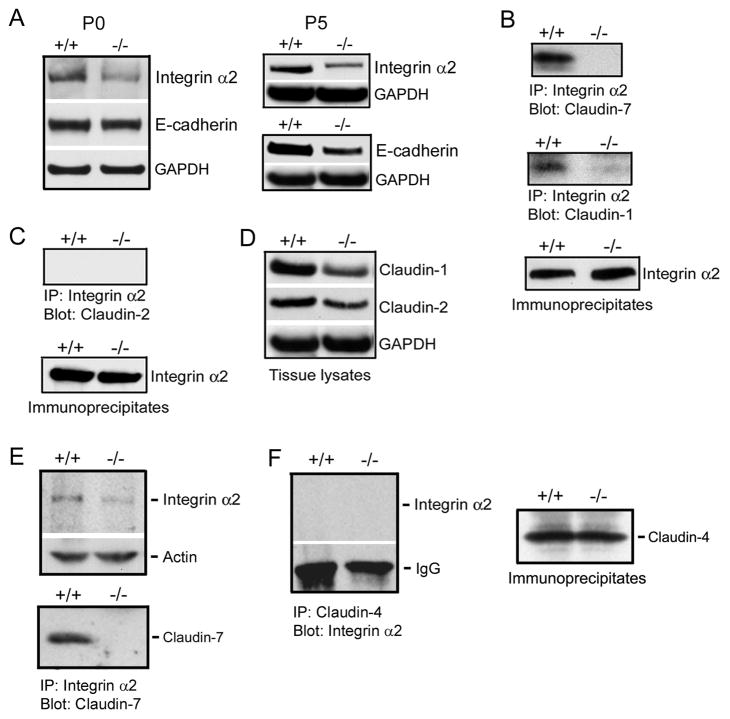
To test our hypothesis that claudin-7 and claudin-1 interacted with integrin α2 in Cldn7+/+ intestines, we performed the biochemical analysis. Western blotting results showed that integrin α2 expression was reduced in both P0 and P5 Cldn7−/− intestines, while E-cadherin expression was only decreased in P5 Cldn7−/− intestines (Fig. 7A). We also found that the expression of desmoglein was reduced in P5 Cldn7−/− intestines as well (Fig. S9). Co-immunoprecipitation results demonstrated that integrin α2 formed a protein complex with both claudin-7 and claudin-1 in Cldn7+/+ intestines (Fig. 7B, +/+, top and middle panels, respectively). However, the interaction between claudin-1 and integrin α2 was almost diminished in the absence of claudin-7 (Fig. 7B, −/−, middle panel). In the immunoprecipitates, integrin α2 was clearly present in both Cldn7+/+ and Cldn7−/− tissue lysates (Fig. 7B, bottom panel). In contrast, claudin-2 did not form an immunoprecipitable complex with integrin α2, which suggested that they were not interacting with each other even though integrin α2 was present in the immunoprecipitates (Fig. 7C). Figure 7D showed the expression levels of claudin-1 and claudin-2 in P5 Cldn7+/+ and Cldn7−/− intestinal lysates. The expression levels of other claudins were shown in Fig. S10. These results indicated that claudin-7/integrin α2/claudin-1 formed an interacting complex in Cldn7+/+ intestines. Reduction of integrin α2 expression (Fig. 7E, top) and interaction of claudin-7 with integrin α2 (Fig. 7E, bottom) were also confirmed in Cldn7−/− and Cldn7+/+ kidneys. As a negative control, claudin-4 did not interact with integrin α2 in kidneys (Fig. 7F) because claudin-4 was localized at apical TJ area (data not shown).
Figure 7.
Disruption of integrin α2/claudin-1 complex in Cldn7−/− intestines. (A) Immunoblotting results showed that integrin α2 expression, but not E-cadherin, was decreased in P0 Cldn7−/− intestines (left), while both integrin α2 and E-cadherin signals were reduced in P5 Cldn7−/− intestines (right). (B) Claudin-7 was co-immunoprecipitated with integrin α2 in Cldn7+/+ (top). Claudin-1 also formed a complex with integrin α2 in Cldn7+/+, but this complex was disrupted in Cldn7−/− (middle). The co-immunoprecipitation experiments were working since integrin α2 was present in the immunoprecipitates (bottom). (C) Claudin-2 did not interact with integrin α2 (top) even though integrin α2 was present in the immunoprecipitates (bottom). (D) Expression levels of claudin-1 and claudin-2 in Cldn7+/+ and Cldn7−/− intestines. (E) Interaction of claudin-7 with integrin α2 in Cldn7+/+ kidneys. Claudin-7 deletion also led to the reduced integrin α2 expression in kidneys (top). Claudin-7 formed an immunoprecipitable protein complex with integrin α2 in Cldn7+/+ kidneys (bottom). (F) Integrin α2 did not interact with claudin-4 (left) even though claudin-4 was present in the immunoprecipitates (right).
Discussion
This study demonstrated that deletion of claudin-7 led to the disruption of intestinal architecture with mucosal ulcerations, epithelial sloughing, and inflammation. We found that in normal intestines, integrin α2 formed a stable protein complex with claudin-7 and claudin-1. This complex was disrupted in Cldn7−/− intestines, and the expression and localization of integrin α2 were also affected, indicating the importance of claudin-7 in mediating cell-matrix interactions. Deletion of claudin-7 also markedly upregulated the expression levels of MMP-3 and MMP-7 that could cause intestinal tissue damage and inflammation.18, 20–21 However, we were unable to rescue Cldn7−/− mice by inhibiting MMP-3 and MMP-7 activities using MMP inhibitors (data not shown). This suggests that MMP-3 and MMP-7 upregulation contributed to the phenotypes of Cldn7−/− mice, but was not the sole reason causing mucosal damage. We propose that disruption of claudin-7 interaction with integrins/claudin-1/ECM components could be the main driving force leading to the severe intestinal defect that cannot be corrected by MMP inhibitors alone.
MMPs are a family of zinc-dependent endopeptidases and are responsible for the tissue remodeling and the degradation of extracellular matrix components.22 Excessive or abnormal expression of MMPs contributes to the pathological processes of tissue injury, organ dysfunction, and chronic disease.23 An inflammatory response or bacterial infection could also modulate MMPs expression or their activity. For example, MMP-7 expression was significantly increased in inflamed mucosa after Helicobacter pylori strain infection.24 Our results showed that claudin-7 depletion in both intestinal T84 and lung HCC827 cell lines significantly upregulated MMP-3 expression, while MMP-7 and MMP-8 expression remained unchanged. However, MMP-7 expression was dramatically increased when T84 cells were treated with TNFα (Fig. 4F). This suggests that the increase of MMP-7 expression in Cldn7−/− intestines could be due to the inflammatory response. Indeed, the inflammatory and immune responses were clearly observed in Cldn7−/− intestines (Fig. 3). It is possible that claudin-7 deletion leads to MMP-3 expression and activation, which results in the degradation of extracellular matrix components. This action could induce the production of cytokines and stimulate the immune response. Another alternative explanation could be that claudin-7 deletion also affects the epithelial barrier function. The pathogens and microorganisms could enter the body through the defective barrier. Although our biotin permeability assay did not observe the barrier leakage in P0 Cldn7−/− intestines, the barrier function was disrupted in P5 Cldn7−/− intestines (Fig. S7).
Studies suggest that barrier defects contribute to the intestine disease.25–26 Recently, it has been reported that p120-catenin is essential for maintenance of intestinal barrier function in mice.27 Increased mucosal permeability has also been reported in JAM-A deficient mice.28 However, the work of Turner’s group indicates that the intestinal epithelial barrier dysfunction alone may not be sufficient to cause intestinal diseases in mice.29 Although several specific claudin deletion or mutation mouse models are available now, none of them show the intestine defects similar to IBD in humans.30–32 Therefore, our Cldn7−/− mouse model provides a unique tool for studying the roles of claudins in intestines and the pathogenesis of IBD-like intestinal disorders. Clearly, claudin-7 functions in intestines are irreplaceable. Although multiple claudins, including claudin-1, -2, -3, -4, and -15, are expressed in the intestines, none of them are able to compensate for the loss of claudin-7 functions. Since the majority of claudin-7 is localized at the basolateral membrane of epithelial cells, which is different from most of the claudins with the apical localization, claudin-7 mediated cell-matrix interaction is indispensible in the intestines
We found that there was no change at the mRNA levels for integrin α2 and claudins. It is possible that deletion of claudin-7 affects integrin binding to the extracellular components and causes integrin internalization and increased degradation. It is also possible that the increased MMP expression can lead to the degradation of integrin α2 and claudin-1 since both of them have extracellular domains. Although intercellular gaps were not present in P0 Cldn7−/− intestines, molecular alterations (increased MMP-3 and reduced integrin α2) did occur at the birth of Cldn7−/− mice (Fig. S5 A and Fig. 7A). Certainly, the subsequent inflammatory response and increased TJ permeability contributed to Cldn7−/− intestinal phenotypes and escalated the mucosa damage.
Our current study provides the strong evidence that claudin-7 has a non-TJ function mediating cell-matrix interactions. First, claudin-7’s major location is at the basolateral membrane compartment; second, claudin-7 interacts with integrin α2 at the basolateral membrane of normal intestinal epithelia; third, TJ morphology is similar in Cldn7+/+ and Cldn7−/− intestines, while intercellular gaps below TJs are evident when examined by transmission electron microscopy; last, claudin-7 silencing by siRNA in lung HCC827 cells reduces integrin expression and cell adhesion to the matrix. This defect can be partially rescued by ectopically introducing affected integrin (data not shown). Therefore, Cldn7−/− mouse model has provided us with the new opportunity and challenge to investigate the non-TJ functions of claudins and presents a new animal model to study the pathogenesis of intestinal disorders and inflammation.
Supplementary Material
Acknowledgments
We thank Beverly G. Jeansonne and Joani T. Zary for their technical assistance. This work is supported by National Institutes of Health grant HL085752 and ES016888 to Y.H.C.
Abbreviations used in this paper
- TJ
tight junction
- IBD
inflammatory bowel disease
- qRT-PCR
quantitative reverse-transcription polymerase chain reaction
- MMP
matrix metalloproteinase
- BrdU
bromodeoxyuridine
- IL
interleukin
- NF-κB
nuclear factor-κB
- TNF
tumor necrosis factor
Footnotes
Conflicts of Interest Statement: All authors declare that no financial interest exists in relation to this submission.
Author contribution: Lei Ding and Zhe Lu were involved in the acquisition, statistical analysis, and interpretation of data as well as drafting of the manuscript. Oded Foreman characterized claudin-7-deficient mice and provided H&E intestinal data. Rodney Tatum performed experiments, analysis and interpretation of data. Qun Lu provided technical and material support and was involved in experimental design and interpretation. Randall Renegar performed experiments and critically revised the manuscript for important intellectual content. Jian Cao provided technical and material support and critically revised the manuscript for important intellectual content. Yan-Hua Chen provided funding, the study concept, experimental design, study supervision, and finalized manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Smalley-Freed WG, Efimov A, Burnett PE, et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 2010;120:1824–35. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends Cell Biol. 2006;16:181–8. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Tamura A, Kitano Y, Hata M, et al. Megaintestine in claudin-15-deficient mice. Gastroenterology. 2008;134:523–34. doi: 10.1053/j.gastro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Weber CR, Turner JR. Inflammatory bowel disease: is it really just another break in the wall? Gut. 2007;56:6–8. doi: 10.1136/gut.2006.104182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeissig S, Burgel N, Gunzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118:5109–18. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 8.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 9.Alexandre MD, Lu Q, Chen YH. Overexpression of claudin-7 decreases the paracellular Cl− conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J Cell Sci. 2005;118:2683–93. doi: 10.1242/jcs.02406. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Mariscal L, Namorado Mdel C, Martin D, et al. The tight junction proteins claudin-7 and -8 display a different subcellular localization at Henle’s loops and collecting ducts of rabbit kidney. Nephrol Dial Transplant. 2006;21:2391–8. doi: 10.1093/ndt/gfl255. [DOI] [PubMed] [Google Scholar]
- 11.Mendoza-Rodriguez CA, Gonzalez-Mariscal L, Cerbon M. Changes in the distribution of ZO-1, occludin, and claudins in the rat uterine epithelium during the estrous cycle. Cell Tissue Res. 2005;319:315–30. doi: 10.1007/s00441-004-1010-7. [DOI] [PubMed] [Google Scholar]
- 12.Tatum R, Zhang Y, Salleng K, et al. Renal salt wasting and chronic dehydration in claudin-7-deficient mice. Am J Physiol Renal Physiol. 2010;298:F24–34. doi: 10.1152/ajprenal.00450.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dussault AA, Pouliot M. Rapid and simple comparison of messenger RNA levels using real-time PCR. Biol Proced Online. 2006;8:1–10. doi: 10.1251/bpo114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox JG, Li X, Cahill RJ, et al. Hypertrophic gastropathy in Helicobacter felis-infected wild-type C57BL/6 mice and p53 hemizygous transgenic mice. Gastroenterology. 1996;110:155–66. doi: 10.1053/gast.1996.v110.pm8536852. [DOI] [PubMed] [Google Scholar]
- 15.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–6. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 16.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–88. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 17.Jupp J, Hillier K, Elliott DH, et al. Colonic expression of leukotriene-pathway enzymes in inflammatory bowel diseases. Inflamm Bowel Dis. 2007;13:537–46. doi: 10.1002/ibd.20094. [DOI] [PubMed] [Google Scholar]
- 18.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–29. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 19.Imai K, Yokohama Y, Nakanishi I, et al. Matrix metalloproteinase 7 (matrilysin) from human rectal carcinoma cells. Activation of the precursor, interaction with other matrix metalloproteinases and enzymic properties. J Biol Chem. 1995;270:6691–7. doi: 10.1074/jbc.270.12.6691. [DOI] [PubMed] [Google Scholar]
- 20.Pender SL, Tickle SP, Docherty AJ, et al. A major role for matrix metalloproteinases in T cell injury in the gut. J Immunol. 1997;158:1582–90. [PubMed] [Google Scholar]
- 21.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 10:712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 22.Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–36. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 23.Manicone AM, McGuire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford HC, Krishna US, Israel DA, et al. Helicobacter pylori strain-selective induction of matrix metalloproteinase-7 in vitro and within gastric mucosa. Gastroenterology. 2003;125:1125–36. doi: 10.1016/s0016-5085(03)01206-x. [DOI] [PubMed] [Google Scholar]
- 25.Bruewer M, Samarin S, Nusrat A. Inflammatory bowel disease and the apical junctional complex. Ann N Y Acad Sci. 2006;1072:242–52. doi: 10.1196/annals.1326.017. [DOI] [PubMed] [Google Scholar]
- 26.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 27.Smalley-Freed WG, Efimov A, Burnett PE, et al. p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest. 120:1824–35. doi: 10.1172/JCI41414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laukoetter MG, Nava P, Lee WY, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–76. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su L, Shen L, Clayburgh DR, et al. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology. 2009;136:551–63. doi: 10.1053/j.gastro.2008.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gow A, Southwood CM, Li JS, et al. CNS myelin and sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–59. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 31.Furuse M, Hata M, Furuse K, et al. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Biol. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nitta T, Hata M, Gotoh S, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.