Abstract
Laccase-2 is a highly conserved multicopper oxidase that functions in insect cuticle pigmentation and tanning. In many species, alternative splicing gives rise to two laccase-2 isoforms. A comparison of laccase-2 sequences from three orders of insects revealed eleven positions at which there are conserved differences between the A and B isoforms. Homology modeling suggested that these eleven residues are not part of the substrate binding pocket. To determine whether the isoforms have different kinetic properties, we compared the activity of laccase-2 isoforms from Tribolium castaneum and Anopheles gambiae. We partially purified the four laccases as recombinant enzymes and analyzed their ability to oxidize a range of laccase substrates. The predicted endogenous substrates tested were dopamine, N-acetyldopamine (NADA), N-β-alanyldopamine (NBAD) and dopa, which were detected in T. castaneum previously and in A. gambiae as part of this study. Two additional diphenols (catechol and hydroquinone) and one non-phenolic substrate (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid)) were also tested. We observed no major differences in substrate specificity between the A and B isoforms. Dopamine, NADA and NBAD were oxidized with catalytic efficiencies ranging from 51 – 550 min−1 mM−1. These results support the hypothesis that dopamine, NADA and NBAD are endogenous substrates for both isoforms of laccase-2. Catalytic efficiencies associated with dopa oxidation were low, ranging from 8 – 30 min−1 mM−1; in comparison, insect tyrosinase oxidized dopa with a catalytic efficiency of 201 min−1 mM−1. We found that dopa had the highest redox potential of the four endogenous substrates, and this property of dopa may explain its poor oxidation by laccase-2. We conclude that laccase-2 splice isoforms are likely to oxidize the same substrates in vivo, and additional experiments will be required to discover any isoform-specific functions.
Keywords: multicopper oxidase, laccase, substrate, insect, cuticle
1. Introduction
Laccase-2 is a highly conserved multicopper oxidase (Dittmer and Kanost, 2010). A single laccase-2 ortholog has been identified in each of the insect genomes analyzed to date, and the average amino acid identity between pairs of sequences is about 90 percent (Gorman et al., 2008). Several lines of research have demonstrated a role for laccase-2 in cuticle sclerotization and pigmentation. Laccase-2 is present in insect cuticles, and its expression correlates temporally and spatially with cuticle sclerotization and the formation of black cuticular markings (Arakane et al., 2005; Dittmer et al., 2009, 2004; Elias-Neto et al., 2010; Futahashi et al., 2011, 2010; Gorman et al., 2008; He et al., 2007; Niu et al., 2008; Yatsu and Asano, 2009). Laccase-2 knockdown results in decreased cuticle tanning in beetles, honey bees, stinkbugs and fruit flies (Arakane et al., 2005; Elias-Neto et al., 2010; Futahashi et al., 2011; Niu et al., 2008; Riedel et al., 2011). Finally, Laccase-2 oxidizes four o-diphenols that are known to be involved in cuticle pigmentation and/or sclerotization: dopamine, N-acetyldopamine (NADA), N-β-alanyldopamine (NBAD) and dopa (Dittmer et al., 2009; Thomas et al., 1989; Yamazaki, 1972; Yatsu and Asano, 2009).
Laccase-2 is synthesized by epithelial cells and secreted into new cuticle prior to the onset of sclerotization or pigmentation (Dittmer et al., 2009; Futahashi et al., 2010; Yatsu and Asano, 2009). Studies of cuticular laccases from two lepidopteran species suggest that laccase-2 from Manduca sexta is synthesized as an active enzyme, whereas laccase-2 from Bombyx mori is synthesized as a proenzyme and becomes activated via proteolytic cleavage (Dittmer et al., 2009; Yamazaki, 1989; Yatsu and Asano, 2009). It is unknown whether or not most laccase-2 orthologs require activation. Following deposition of laccase-2 in the cuticle, diphenols such as dopamine, NADA and NBAD are transported to the new cuticle where laccase-2 oxidizes them to generate semiquinones that react to form quinones; the quinones and quinone derivatives react with cuticle protein side chains, resulting in protein cross-linking and quinone tanning or undergo further reactions as part of the melanin synthesis pathway resulting in pigmentation (Andersen, 2010).
Most laccases consist of three cupredoxin-like domains and contain four copper ions that reside in a T1 copper site and a T2/T3 tricopper center (Zhukhlistova et al., 2008). The copper ions are coordinated by highly conserved residues in domains I and III, whereas the substrate binding pocket is formed from less conserved residues in domains II and III (Bertrand et al., 2002; Ferraroni et al., 2007; Kallio et al., 2011, 2009; Matera, 2008). A substrate binds near the T1 copper site and is oxidized by the transfer of an electron to the T1 copper (Zhukhlistova et al., 2008). Laccases oxidize a broad range of substrates, including polyphenols, methoxy-substituted phenols, aminophenols and phenylenediamines (Baldrian, 2006; Mayer and Staples, 2002; Sakurai and Kataoka, 2007). Substrate specificity is influenced by the size and shape of the substrate binding pocket, specific residues within the substrate binding pocket, and the difference in redox potential between the T1 copper and the substrate (Gupta et al., 2010; Kallio et al., 2011, 2009; Quintanar et al., 2007; Tadesse et al., 2008; Xu, 1996; Xu et al., 1996).
The substrate specificity of laccase-2 orthologs is not fully understood. Most of the studies of cuticular laccase activity were done prior to the identification of laccase-2 sequences; however, we assume that the laccases purified from the cuticles of fruit flies, blow flies, flesh flies and locusts were probably orthologs of laccase-2. Those cuticular laccases and laccase-2 from B. mori and M. sexta were found to oxidize a diverse set of substrates, including dopamine, NADA, NBAD, dopa, catechol and hydroquinone (Andersen, 1978; Barrett and Andersen, 1981; Barrett, 1987a, 1987b; Dittmer et al., 2009; Sugumaran, et al., 1992; Thomas et al., 1989; Yamazaki, 1972, 1969; Yatsu and Asano, 2009). Because cuticular laccases are difficult to purify, most of these studies were limited by the quantity and purity of the enzymes; therefore, with few exceptions, catalytic constants (kcat) were not determined. Michaelis constants (Km) mostly ranged from 0.2 – 8.7 mM (Andersen, 1978; Barrett and Andersen, 1981; Dittmer et al., 2009; Thomas et al., 1989; Yamazki, 1972). Without a measure of the turnover rate, it is difficult to draw strong conclusions about substrate specificity; nevertheless, data from several studies suggest that dopamine, NADA and NBAD are better laccase-2 substrates than dopa (Andersen, 1978; Arakane et al., 2009; Barrett and Andersen, 1981; Barrett, 1987a, 1987b; Sugumaran, et al., 1992; Thomas et al., 1989; Yamazaki, 1969). A comparison of endogenous and recombinant forms of M. sexta laccase-2 demonstrated that they oxidized NADA and NBAD with similar catalytic efficiencies (kcat/Km) (Dittmer et al., 2009). These results demonstrate that laccase-2 from M. sexta does not have a preference for NADA or NBAD and that recombinant forms of laccase-2 can have similar activity to the endogenous form.
Recently it was discovered that laccase-2 genes from several insect species encode alternatively spliced isoforms. Species with alternative exons include B. mori, M. sexta, Tribolium castaneum, Anopheles gambiae, Aedes aegypti and Drosophila melanogaster; species with no apparent alternative exons include Apis mellifera and Acyrthosiphon pisum (Gorman et al., 2008). A phylogenetic analysis demonstrated that the laccase-2A isoforms are highly conserved and form a well supported clade, whereas the laccase-2B isoforms are less conserved (Gorman et al., 2008). These data suggest that the A isoforms have a conserved function whereas the B isoforms may be more diverse in function. Temporal and spatial expression patterns of the two isoforms from T. castaneum and A. gambiae are consistent with a role in cuticle sclerotization and/or pigmentation, but so far there is no evidence that the B isoform is present in cuticle (Arakane et al., 2005; Gorman et al., 2008). Cuticular laccases purified from B. mori and M. sexta were shown to be A isoforms, and laccase-2A but not laccase-2B was detected in the cuticle of A. gambiae (Dittmer et al., 2009; He et al., 2007; Yatsu and Asano, 2009). These data suggest that laccase-2B may not function in cuticle sclerotization or pigmentation. On the other hand, RNAi mediated knockdown of laccase-2 in T. castaneum demonstrated that both isoforms are required for cuticle tanning (Arakane et al., 2005). Knockdown of the T. castaneum A isoform resulted in reduced cuticle tanning, knockdown of the B isoform resulted in delayed tanning, and knockdown of both isoforms resulted in extremely reduced tanning (Arakane et al., 2005).
Laccase-2A isoforms are undoubtedly involved in cuticle tanning, but the limited information about laccase-2B isoforms does not lead to an obvious prediction of biochemical function. One possibility is that both laccase-2A and laccase-2B participate in cuticle tanning, but that they have different substrate specificity; for example, the B isoform may be better than the A isoform at oxidizing dopamine or dopa (which are substrates in the melanin synthesis pathway), while the A isoform may be better at oxidizing NADA and NBAD (which are substrates in sclerotization pathways). To test the hypothesis that laccase-2 isoforms have different substrate specificities, we compared laccase-2A and laccase-2B sequences from three species of insects: B. mori (a lepidopteran species), T. castaneum (a coleopteran species) and A. gambiae (a dipteran species). This analysis revealed eleven positions at which there are conserved differences between the A and B isoforms, and homology modeling suggested that these residues are not present in the substrate binding pocket; however, the A. gambiae isoforms were predicted to have a non-conservative difference at one position in the substrate binding pocket, suggesting a possible difference in substrate preference. To test our hypothesis more directly, we compared the activity of isoforms from T. castaneum (TcLac2A and TcLac2B) and A. gambiae (AgMCO2A and AgMCO2B) by purifying the four enzymes as recombinant proteins and analyzing their ability to oxidize a range of laccase substrates. We observed no major differences in substrate specificity between the A and B isoforms, and we conclude that laccase-2 splice isoforms are likely to oxidize the same substrates in vivo.
2. Materials and Methods
2.1 Detection of catecholamines in A. gambiae
Three developmental stages of A. gambiae were collected and kept frozen at −80°C until they were analyzed. Fifty adult males and 50 adult females (sugar fed) and 100 fourth instar larvae were weighed and homogenized in 500 μl of 0.1 M perchloric acid. The homogenates were centrifuged at 13,000 × g for 10 min at 8°C. Ten μl of each sample was loaded onto a 5 μm Luna C18 (2) RP column (150 × 4.6 mm). The chromatography was accomplished using an isocratic mobile phase consisting of 26 % acetonitrile in phosphate buffer, pH 2.85, containing 0.1 % SDS at a flow rate of 0.5 ml/min on a Hewlett Packard HPLC fitted with UV/VIS and electrochemical detectors to monitor compound peaks. The electrochemical detector was set a +700 mV oxidative potential with a full scale response of 500 nA that had been previously determined to be optimal conditions for catecholamine determination. Data were collected and analyzed using Chemstation Software version 8.0.
2.2 Sequence analysis
Clustal W (Chenna et al., 2003) was used to align the predicted amino acid sequences of TcLac2A (GenBank ID: AY884061.2), TcLac2B (GenBank ID: AY884062.2, with two amino acid differences, as described in section 2.4), AgMCO2A (GenBank ID: AY943928.1), AgMCO2B (GenBank ID: AY943929.1), BmLac2A (GenBank ID: EU093074.1) and BmLac2B (GenBank ID: BK006378.1). Signal sequences were predicted by Signal P (Bendtsen et al., 2004). Cysteine rich regions were defined as previously described (Dittmer et al., 2004). Boundaries of the putative cupredoxin-like domains were estimated by aligning laccase-2 sequences with the sequence of a fungal laccase, Trametes versicolor laccaseIIIb (TvLacIIIb, PDB ID: 1KYA), which has a solved crystal structure, and then using SCOP (Murzin et al., 1995) to define the boundaries of the cupredoxin-like domains of TvLacIIIb (Figure S1).
2.3 Homology modeling
Clustal W (Chenna et al., 2003) was used to align the predicted amino acid sequences of AgMCO2A and AgMCO2B with the sequence of TvLacIIIb. Only the cupredoxin-like domains could be aligned, and within this region, the sequence identity between AgMCO2A or AgMCO2B and TvLacIIIb was approximately 30 % (Figure S1). SWISS-MODEL (Arnold et al., 2006; Kiefer et al., 2009; Peitsch, 1995) was used to construct homology models of AgMCO2A and AgMCO2B using TvLacIIIb (PDB ID: 1KYA) as the template. The PyMOL Molecular Graphics System, Version 1.3, (Schrödinger, LLC) was used to view the homology models and to highlight residues of interest.
2.4 Purification of recombinant laccase-2 isoforms
Four laccase-2 isoforms were expressed using a baculovirus expression system (Bac-to-Bac, Invitrogen). Three of the full length cDNAs matched sequences deposited in GenBank: TcLac2A (AY884061.2), AgMCO2A (AY943928.1) and AgMCO2B (AY943929.1). The sequences of TcLac2A and TcLac2B (AY884062.2) differ at two positions in the amino-terminal part of the protein (i.e., amino-terminal to the alternative exons); therefore, mutagenesis was used to change two codons in the TcLac2B cDNA to match the TcLac2A (and genome) sequence. These amino acid changes made were at residue 93 (Thr to Ala) and residue 182 (Gly to Asp). The cDNAs were cloned into pFastBac1, and the DNA sequences were verified to be correct. Recombinant baculoviruses were generated for each of the four isoforms. Plaque assays were used to determine titers of amplified virus stocks.
For expression, 2 liters of Sf9 cells (2 × 106 cells/ml Sf-900 II serum free medium supplemented with 0.1 mM copper sulfate) were infected with baculovirus at a multiplicity of infection of 2, and cells were incubated at 28 °C with shaking for 48 hours. Cells were removed by two centrifugation steps (500 × g for 10 min). Two protease inhibitors, 10 μM E64 and 0.5 mM p-aminobenzamidine, were added to the conditioned medium to reduce degradation of the recombinant proteins.
Partial purification of laccase-2 isoforms was accomplished by binding glycosylated proteins in the cell culture medium to concanavalin-A-Sepharose followed by eluting for at least 16 hours with 0.5 M methyl-α-D-mannopyranoside in 20 mM Tris-HCl, 0.5 M NaCl, 0.5 mM p-aminobenzamidine, pH 7.4. Eluted proteins were dialyzed against 20 mM Tris-HCl, 0.5 mM p-aminobenzamidine, pH 8.0 (A isoforms) or pH 8.2 (B isoforms). The recombinant proteins were further purified by loading the protein onto a Q-Sepharose column (TcLac2A, TcLac2B, and AgMCO2B) or a High-Q column (AgMCO2A) and eluting with a linear gradient of NaCl in 20 mM Tris, pH 8.0 (A isoforms) or pH 8.2 (B isoforms). AgMCO2A was further purified by pooling fractions from the High-Q column and running them on a Sephacryl S-100 HR column equilibrated with 20 mM Tris, 150 mM NaCl, pH 7.8. Fractions containing a high concentration of recombinant protein and a low concentration of contaminating proteins were pooled, glycerol was added to 50 %, and the partially purified enzymes were stored at −20 °C. The final concentrations were 250 ng/μl TcLac2A in 10 mM Tris, 125 mM NaCl, 50 % glycerol, pH 8.0; 100 ng/μl TcLac2B in 10 mM Tris, 120 mM NaCl, 50 % glycerol, pH 8.2; 75 ng/μl AgMCO2A in 10 mM Tris, 75 mM NaCl, 50 % glycerol, pH 7.8; and 34 ng/μl AgMCO2B in 10 mM Tris, 90 mM NaCl, 50 % glycerol, pH 8.2. From 2 liters of cell culture, we purified 1.4 mg of TcLac2A, 0.60 mg TcLac2B, and 0.75 mg AgMCO2A; from 4 liters of cell culture, we purified 0.41 mg AgMCO2B.
To determine whether any of the contaminating proteins in the enzyme preparations had laccase activity, we used an expression and purification strategy similar to the one we used to purify the laccase-2 isoforms but started with a cell culture that was infected with an “empty” baculovirus instead of one containing a laccase cDNA. Proteins eluted from concanavalin-A-Sepharose were dialyzed against 20 mM Tris, 0.5 mM p-aminobenzamidine, pH 8.2, and loaded onto a Q-Sepharose column. The column was washed with 20 mM Tris, pH 8.2, and then eluted with 20 mM Tris, 200 mM NaCl, pH 8.0 and 20 mM Tris, 1.5 M NaCl, pH 8.0. The column fractions were tested for laccase activity (see section 2.8) using 2 mM ABTS, 1 mM dopamine and 1 mM hydroquinone as substrates. No product formation was detected; therefore, we conclude that the contaminating proteins had no detectable laccase activity.
2.5 Estimation of enzyme concentration
The concentration of laccase-2 isoforms was estimated by performing SDS-PAGE analysis of at least two dilutions of each enzyme and several dilutions of bovine serum albumin (BSA). The gels were stained with Coomassie blue, and the band intensities of the recombinant proteins and the BSA standards were compared.
2.6 Amino-terminal sequencing
Purified recombinant enzymes were subjected to SDS-PAGE and transferred to a PVDF membrane. Proteins were lightly stained with Coomassie R, and protein bands were excised from the membrane. Edman protein sequencing was done by Dr. Kathleen Schegg at the Nevada Proteomics Center. An ABI 492 Procise sequencer was used to determine the first five residues of each purified enzyme.
2.7 Immunoblot analysis
Thirty-five ng of recombinant laccase-2 was subjected to reducing SDS-PAGE followed by protein transfer to nitrocellulose. Recombinant proteins were immunodetected using a 1:1,000 dilution of polyclonal antiserum generated against M. sexta laccase-2 (Dittmer et al., 2009).
2.8 Laccase activity assays
The laccase substrates used were N-acetyldopamine (NADA), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (ABTS), catechol, L-dopa, dopamine hydrochloride, and hydroquinone (all purchased from Sigma-Aldrich), and Nβ-alanyldopamine hydrochloride (NBAD), which was provided by the National Institute of Mental Health’s Chemical Synthesis and Drug Supply Program.
Reactions to determine enzyme activity were made by mixing 0.5 μg enzyme with substrate in a total volume of 200 μl and detecting product formation with a microplate spectrophotometer by observing the change in absorbance over time. To account for autoxidation of substrates, reactions with no enzyme were included, and the slopes of these “blank” reactions were subtracted from the slopes of the enzyme-containing reactions. The molar extinction coefficients (in M−1 cm−1) of the products of interest were: dopaminochrome, ε475 = 3,058 (Baez et al, 1997); NADA quinone and NBAD quinone, ε390 = 1,100 (Thomas et al., 1989); dopachrome, ε475 = 3,600 (Thomas et al., 1989); o-benzoquinone, ε450 = 2,211 (Eggert et al., 1996); p-benzoquinone ε248 = 17,252 (Eggert et al., 1996); and ABTS cation ε414 = 36,000 (Eggert et al., 1996).
The reactions used to determine the pH optima of each recombinant enzyme contained 0.5 μg enzyme and 0.5 mM substrate in citrate-phosphate buffer, pH 3.0 – 7.5. The buffers were made by mixing 0.1 M citric acid and 0.2 M sodium phosphate dibasic to generate buffers with the desired pH values. Reactions at higher pH values were excluded from analysis because of excessive autoxidation of many of the substrates. Assays were done in triplicate.
The reactions used to determine the kinetic properties of each recombinant enzyme contained 0.5 μg enzyme and substrate ranging from 10 μM to 6, 8, 20 or 50 mM (depending on substrate solubility and availability). The choice of buffer was dependent on the optimum pH of the enzyme-substrate combination. Reactions at pH 4.5 – 5.5 were buffered by 0.1 M sodium acetate; reactions at 6.0 – 6.5 were buffered by 0.1 M Mes; and reactions at 7.0 – 7.5 were buffered by 0.1 M sodium phosphate. Assays were done in triplicate.
Kinetic curves were made by plotting the activity of laccase-2 isoforms (in mOD min−1) versus substrate concentration. The data were fit to the Michaelis-Menten equation by non-linear regression using GraphPad Prism. The kinetic constants Vmax (in mOD min−1) and Km were estimated from the fitted data. The rate of product formation was estimated with the equation A = L C ε (where A is Vmax in OD min−1, L = the pathlength (0.5 cm), and ε = the molar extinction coefficient in M−1 cm−1). The kinetic constant kcat was calculated by dividing Vmax (in nM min−1) by the enzyme concentration (nM).
2.9 Purification of prophenoloxidase and phenoloxidase activity assays
Purification of prophenoloxidase from the hemolymph of M. sexta was done as described previously (Gorman et al., 2007). Reactions to estimate enzyme activity contained 1 μg prophenoloxidase, dopa at 0.4, 0.6, 1, 2, 4, 6 or 9 mM, and 0.1 % cetylpyridinium (CPC) in 0.1 M Mes, pH 6.0. Reaction volumes were 200 μl. CPC was added to activate prophenoloxidase (CPC causes a conformational change in prophenoloxidase leading to activation [Hall et al., 1995]). Formation of dopachrome was detected at 475 nm. Calculations of kinetic constants were done as described in section 2.8.
2.10 Cyclic voltammetry
Cyclic voltammetric experiments were carried out on a CHI400A potentiostat (CH Instruments, TX) with a three-electrode setup similar to a previous study (Kramer et al., 1983). A 3-mm diameter glassy carbon disk electrode was used as the working electrode, which was polished with an alumina paste on a clean micro-cloth prior to the experiment. A coiled Pt wire was used as the counter electrode, and a Ag/AgCl (saturated KCl) electrode was used as the reference electrode. The electrochemical studies were performed in 20% ethanol mixed with phosphate buffered saline at pH 7.0, and a concentration of 10 mM of the substrate was used. The solution was deoxygenated with nitrogen prior to the addition of the substrate. Cyclic voltammetric experiments for each substrate were carried out in triplicate with a range of −0.8 to +1.2 V [vs. Ag/AgCl (saturated KCl)] for NADA, NBAD and dopamine; and −0.8 to +0.8 V for dopa. Consistent results were obtained.
3. Results
3.1 Identification of catecholamines in A. gambiae
Reverse phase chromatography coupled with electrochemical detection was used to identify four catecholamines in whole body extracts of A. gambiae (Table 1). Dopamine, NADA, NBAD and dopa were detected in fourth instar larvae, adult females and adult males. Dopa was the predominant catecholamine in larvae; dopa and NBAD were the most abundant catecholamines in adults. These catecholamines have been identified in extracts of T. castaneum (Arakane et al., 2009; Kramer et al., 1984; Roseland et al., 1987), and they are known to be present in insect cuticle (Hopkins and Kramer, 1992; Kramer et al., 2001); therefore, we decided to test these four catecholamines as laccase-2 substrates.
Table 1.
Catecholamines detected in Anopheles gambiae
| dopamine (μmole/g) | NADA (μmole/g) | NBAD (μmole/g) | dopa (μmole/g) | |
|---|---|---|---|---|
| larvae | 1.20 ± 0.03 | 1.25 ± 0.10 | 0.95 ± 0.03 | 42.76 ± 0.35 |
| adult females | 0.70 ± 0.20 | 3.00 ± 0.05 | 14.00 ± 0.40 | 18.90 ± 0.45 |
| adult males | 0.98 ± 0.01 | 3.01 ± 0.10 | 57.85 ± 1.61 | 7.29 ± 0.33 |
Values are mean ± standard deviation (n = 3).
3.2 Identification of isoform-specific amino acids
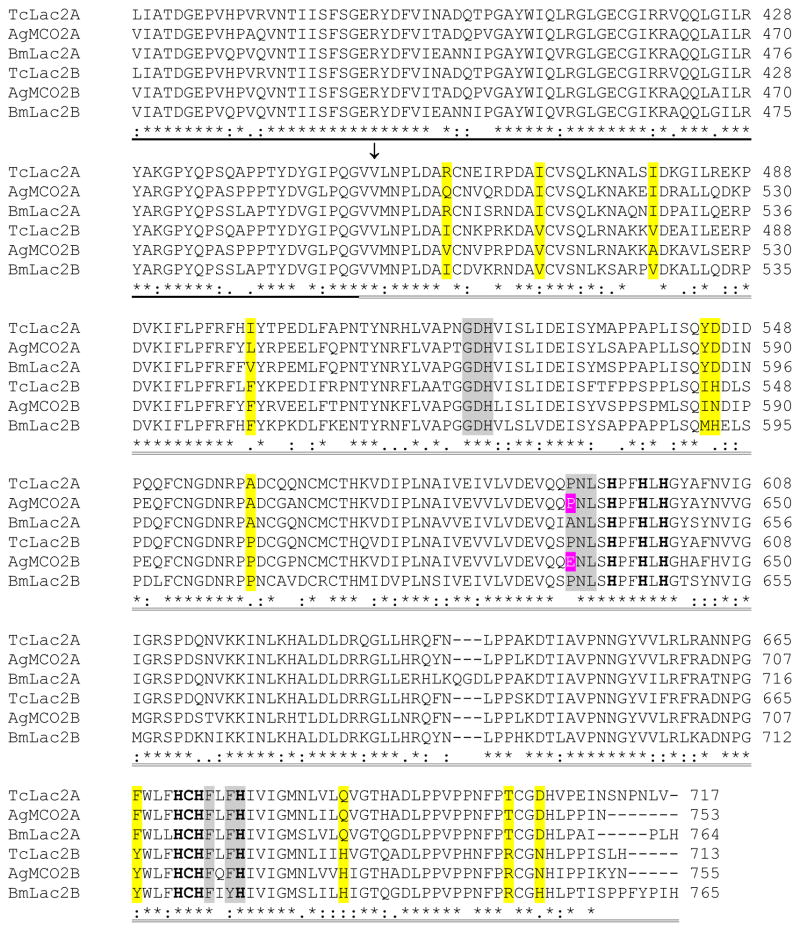
Insect laccase-2 sequences can be divided into six regions: a signal peptide, a nonconserved amino-terminal region, a cysteine rich region, and three cupredoxin-like domains (I, II and III) (Figure 1). Alternative splicing of laccase-2 isoforms results in proteins that are identical in sequence except for the third cupredoxin-like domain. A comparison of sequences from three orders of insects revealed eleven positions at which there are conserved differences between the A and B isoforms (Figure 1). Homology modeling of AgMCO2A and AgMCO2B suggests that these eleven residues are not part of the substrate binding pocket (Figure 2 and data not shown); therefore, our homology models support a prediction that the conserved differences between A and B isoforms would not affect substrate specificity.
Figure 1.
Alignment of the predicted amino acid sequences of laccase-2 isoforms from T. castaneum, A. gambiae, and B. mori. Predicted signal peptides are in italicized text. Amino-terminal sequences of purified recombinant enzymes are highlighted in black. A conserved cysteine-rich region is underlined. The three cupredoxin-like domains are indicated by dashed underlining (I), bold underlining (II), and double underlining (III). (The putative boundaries of the cupredoxin-like domains were based on the crystal structure of TvLacIIIb [Bertrand et al., 2002]). An arrow points to the first residue of the alternatively spliced exons. The 10 histidines and 1 cysteine that are predicted to bind copper are in bold text. Conserved differences between the A and B isoforms are highlighted in yellow. Residues in domain III that are predicted to be part of the substrate binding pocket are highlighted in magenta (residue 633 in AgMCO2) or gray.
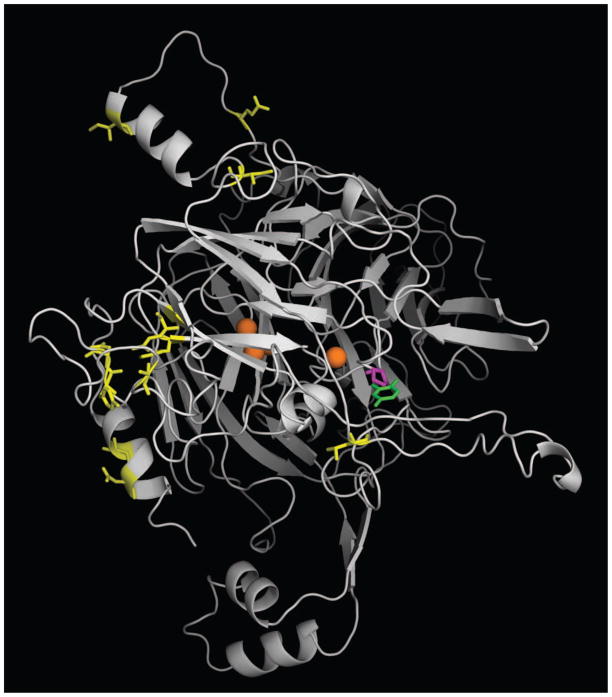
Figure 2.
Homology model of AgMCO2A. The crystal structure of T. versicolor laccaseIIIb complexed with a laccase substrate, 2,5-xylidine, was used as a template for generating a homology model of AgMCO2A. Coppers are shown as orange spheres. 2,5-xylidine is shown in green. Residues corresponding to conserved differences between A and B isoforms (Figure 1) are shown in yellow. Residue 633, which is a proline in AgMCO2A and a glutamine in AgMCO2B, is shown in magenta. Note that positions corresponding to conserved differences are outside of the predicted substrate binding pocket, but residue 633 is within the predicted substrate binding pocket.
Homology modeling of AgMCO2A was used to predict which residues in domain III contribute to the substrate binding pocket, and a sequence aligment was used to identify the corresponding residues in the other laccase-2 sequences (Figures 1 and 2). The T. castaneum and B. mori laccase-2 isoforms did not have significant differences at these positions. The A. gambiae isoforms had one noteable difference: residue 633 is occupied by a proline (a neutral, hydrophobic residue) in AgMCO2A and a glutamic acid (an acidic, polar residue) in AgMCO2B (Figures 1 and 2). This difference in the predicted substrate binding pocket of AgMCO2 isoforms suggested that these enzymes may have a difference in substrate specificity. In particular, the glutamic acid in AgMCO2A might interact with positively charged substrates such as dopamine and NBAD leading to improved oxidation of those substrates compared with oxidation of the similar but neutral substrate, NADA.
3.3 Purification of recombinant laccase-2 isoforms
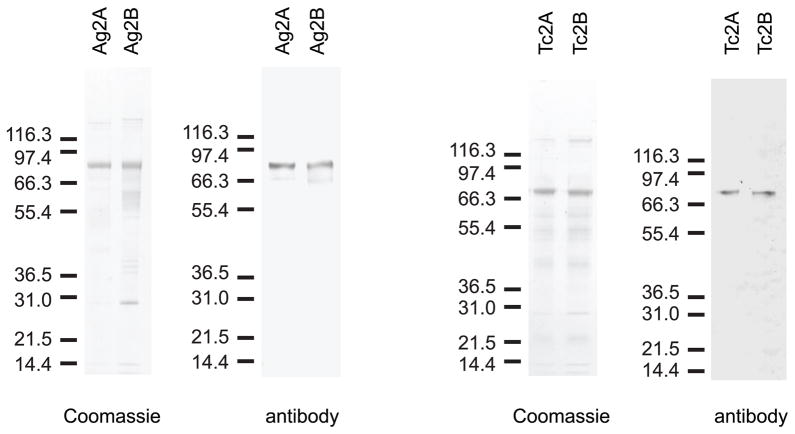
TcLac2A, TcLac2B, AgMCO2A and AgMCO2B were expressed in an insect cell culture system and partially purified using lectin affinity and ion exchange chromatography (Figure 3). We purified 1.4 mg of TcLac2A, 0.60 mg TcLac2B, 0.75 mg AgMCO2A and 0.41 mg AgMCO2B from 2–4 liters of cell culture, and we verified the identity of the major protein in each sample by immunoblot analysis (Figure 3) and amino-terminal sequencing (Figure 1). The estimated mass of each protein was consistent with the predicted mass (89 kDa estimated versus 81 kDa predicted for AgMCO2A and AgMCO2B, 77 kDa estimated versus 77 kDa predicted for TcLac2A and TcLac2B). Low expression, proteolytic degradation and enzyme loss during buffer exchange and concentration steps contributed to low yields and prevented us from purifying the enzymes to homogeneity. To test for possible laccase activity of the contaminating proteins, we used the expression and purification strategy that was used to purify the laccase-2 isoforms but we started with a cell culture that was infected with an “empty” baculovirus instead of one containing a laccase cDNA. The protein fractions (which should contain the contaminants present in the laccase-2 preparations) were tested for activity using dopamine, hydroquinone and ABTS as the substrate. These assays detected no product formation (data not shown); therefore, we concluded that the proteins contaminating the laccase-2 preparations had no detectable laccase activity.
Figure 3.
SDS-PAGE and immunoblot analysis of purified laccase-2 isoforms. Coomassie staining was used to detect 300 ng of partially purified enzyme. Laccase-2 bands were verified by immunoblot analysis.
3.4 Determination of pH profiles
The optimal pH range for each of the laccase-2 isoforms and five substrates was determined (Table 2, Figure S2 and data not shown). pH values greater than 7.5 were not analyzed because alkaline conditions caused considerable autoxidation of all substrates except ABTS. The optimal pH ranges for TcLac2A and TcLac2B were similar. In contrast, the pH optima of AgMCO2A were higher than those of AgMCO2B, especially with dopamine or dopa as the substrate. An optimal pH for each isoform - substrate combination was used in the kinetic analysis described in section 3.5.
Table 2.
pH optima for laccse-2 isoforms
| AgMCO2A | AgMCO2B | TcLac2A | TcLac2B | |
|---|---|---|---|---|
| dopamine | 7.5 | 5.5 – 6.0 | 5.5 – 7.5 | 6.0 |
| NADA | 5.5 – 6.5 | 5.0 | 5.0 – 6.0 | 5.5 – 6.0 |
| NBAD | 5.5 – 6.5 | 5.0 – 5.5 | 5.5 | 5.5 |
| dopa | 7.5 | 5.5 | 7.0 – 7.5 | 6.0 – 6.5 |
| ABTS | 4.5 | 4.5 | 4.5 | 4.5 |
3.5 Kinetic analysis
Kinetic curves were made by plotting activity of laccase-2 isoforms versus substrate concentration. The data were fit to the Michaelis-Menten equation by non-linear regression and the kinetic constants kcat (a measure of the catalytic rate) and Km (a measure of the affinity of the enzyme for the substrate) were estimated (Table 3 and Figures S3, S4 and S5). TcLac2B had higher kcat values than TcLac2A for all substrates tested and a higher Km value for all substrates except NADA; the net effect of these differences was that TcLac2A and TcLac2B had similar catalytic efficiencies (kcat/Km) for each substrate. The kinetic properties of AgMCO2A and AgMCO2B were similar for each substrate. These results demonstrate that the alternatively spliced isoforms of laccase-2 have similar substrate preferences.
Table 3.
Kinetic constants for laccase-2 isoforms
| Substrate | Enzyme | pH | kcat (min−1) | Km (mM) | kcat/Km (min−1 mM−1) |
|---|---|---|---|---|---|
| dopamine | AgMCO2A | 7.5 | 48 | 0.5 | 96 |
| AgMCO2B | 5.5 | 63 | 0.3 | 210 | |
| TcLac2A | 5.5 | 41 | 0.8 | 51 | |
| TcLac2B | 6.0 | 450 | 2 | 225 | |
| NADA | AgMCO2A | 5.5 | 92 | 0.3 | 307 |
| AgMCO2B | 5.0 | 127 | 0.7 | 181 | |
| TcLac2A | 5.5 | 147 | 0.7 | 210 | |
| TcLac2B | 5.5 | 330 | 0.6 | 550 | |
| NBAD | AgMCO2A | 5.5 | 219 | 1 | 219 |
| AgMCO2B | 5.5 | 381 | 2 | 190 | |
| TcLac2A | 5.5 | 165 | 1 | 165 | |
| TcLac2B | 5.5 | 1127 | 4 | 282 | |
| dopa | AgMCO2A | 7.5 | 61 | 2 | 30 |
| AgMCO2B | 5.5 | 34 | 4 | 8 | |
| TcLac2A | 7.0 | 29 | 3 | 10 | |
| TcLac2B | 6.5 | 147 | 13 | 11 | |
| catechola | AgMCO2A | 5.5 | 144 | 5 | 29 |
| AgMCO2B | 5.5 | 179 | 4 | 45 | |
| TcLac2A | 5.5 | 242 | 6 | 40 | |
| TcLac2B | 5.5 | 648 | 12 | 54 | |
| hydroquinonea | AgMCO2A | 5.5 | 229 | 2 | 114 |
| AgMCO2B | 5.5 | 252 | 0.8 | 315 | |
| TcLac2A | 5.5 | 213 | 1 | 213 | |
| TcLac2B | 5.5 | 552 | 2 | 276 | |
| ABTS | AgMCO2A | 4.5 | 340 | 13 | 26 |
| AgMCO2B | 4.5 | 192 | 5 | 38 | |
| TcMCO2A | 4.5 | 36 | 2 | 18 | |
| TcMCO2B | 4.5 | 85 | 13 | 7 |
pH optima not determined
We were interested in which of the four catecholamines detected in T. castaneum and A. gambiae might be natural substrates of laccase-2 isoforms. Dopamine, NADA and NBAD were oxidized with catalytic efficiencies ranging from 51 – 550 min−1 mM−1. These results support the hypothesis that dopamine, NADA and NBAD are endogenous substrates for both isoforms of laccase-2. In constrast, catalytic efficiencies associated with dopa oxidation were low, ranging from 8 – 30 min−1 mM−1. Dopa is a known substrate of insect tyrosinase (phenoloxidase); therefore, we thought it would be useful to compare the catalytic efficiency of dopa oxidation by phenoloxidase and laccase-2. We failed to find published estimates of the catalytic efficiencies of insect phenoloxidases, so we analyzed the activity of phenoloxidase from M. sexta hemolymph (Gorman et al., 2007). We determined that phenoloxidase oxidized dopa with a kcat of 361 min−1, a Km of 2 mM and a catalytic efficiency of 201 min−1 mM−1; therefore, the catalytic efficiency of dopa oxidation by phenoloxidase was 7 – 25 times greater than the catalytic efficiency of any of the laccase-2 isoforms.
3.6 Redox potentials of endogenous substrates
An inverse correlation between the redox potential of a laccase substrate and the catalytic efficiency of oxidizing that substrate has been observed (Tadesse et al., 2008; Xu, 1996; Xu et al., 1996). A previous study demonstrated that dopamine, NADA and NBAD have similar redox potentials (Kramer et al., 1983), but dopa was not tested. Therefore, we wondered whether dopa has a higher redox potential than that of the other three endogenous substrates. We used cyclic voltammetry to determine the redox potentials of all four substrates to allow a direct comparison (as summarized in Table 4). Dopa indeed showed drastically different redox properties from the other three substrates (Figure S6). The cyclic voltammograms of dopamine, NADA and NBAD presented very similar shapes, with a pair of well-defined redox peaks. The characteristic electrode potential E1/2, i.e., the midpoint between the oxidation peak potential Epa and reduction peak potential Epc, was 0.30 V for dopamine, 0.26 V for NADA, and 0.24 V for NBAD. In contrast, the cyclic voltammogram of dopa showed two pairs of redox peaks (E and E′). Even the pair of weak redox peaks at lower potential had an E1/2 higher than that of dopamine (0.34 V vs 0.30 V), and the E′1/2 of the stronger pair was even higher (>0.67 V), with an oxidation peak slightly beyond our scan range. This property of dopa may explain its poor oxidation by laccase-2.
Table 4.
Redox potentials of four endogenous substrates
| Substrate | Epa (V) | Epc (V) | E′pa (V) | E′pc (V) | E1/2 (V) | E′1/2 (V) |
|---|---|---|---|---|---|---|
| dopamine | 0.48 | 0.12 | 0.30 | |||
| NADA | 0.44 | 0.08 | 0.26 | |||
| NBAD | 0.39 | 0.08 | 0.24 | |||
| dopa | >0.80 | 0.53 | 0.47 | 0.20 | 0.67 | 0.34 |
Note: All potential are relative to a Ag/AgCl (saturated KCl) reference electrode.
Epa stands for the anodic peak potential.
Epc stands for the cathodic peak potential.
E1/2 = (Epa + Epc)/2
4. Discussion
The goal of this study was to determine whether alternatively spliced isoforms of laccase-2 differ in substrate specificity. Based on sequence analysis and homology modeling, we predicted that laccase-2 isoforms would not exhibit conserved differences in substrate specificity. This prediction was confirmed by an analysis of the kinetic properties of the A and B isoforms of TcLac2 and AgMCO2. Differences in the catalytic efficiencies of TcLac2A and TcLac2B were less than three fold for all substrates except dopamine. With dopamine as the substrate, TcLac2B had a catalytic efficiency 4.4 times higher than that of TcLac2A, but we suspect that even a four fold difference is not likely to be biologically relevant. Differences in the catalytic efficiencies of A. gambiae isoforms were less than three fold for all substrates except dopa, and those values were so low as to suggest that dopa is not a natural substrate (see below). Homology modeling suggested that AgMCO2A might have a preference for positively charged dopamine and NBAD over neutral NADA, but the catalytic efficiencies associated with these three substrates were similar.
A previous phylogenetic analysis suggested that the A isoforms of laccase-2 have a highly conserved function (Gorman et al., 2008). A comparison of the catalytic efficiencies of laccase-2A from T. castaneum, A. gambiae and M. sexta are consistent with this prediction: the catalytic efficiencies of the three laccase-2A isoforms with NADA as the substrate are 210, 307, and 220 min−1 mM−1, and with NBAD as the substrate are 165, 219, and 103 min−1 mM−1 (Table 1 and Dittmer et al., 2009). These data support the hypothesis that TcLac2A and AgMCO2A (like MsLac2A) participate in cuticle tanning by oxidizing NADA and NBAD.
Several studies have suggested that cuticular laccases oxidize dopamine, NADA and NBAD better than dopa (Andersen, 1978; Arakane et al., 2009; Barrett and Andersen, 1981; Barrett, 1987a, 1987b; Sugumaran, et al., 1992; Thomas et al., 1989; Yamazaki, 1969). Our set of laccase-2 kinetic constants support these previous findings. In addition, we demonstrated that the catalytic efficiency of dopa oxidation by phenoloxidase was 7 – 25 times greater than the catalytic efficiency of any of the laccase-2 isoforms. Taken together, the results suggest that dopa is not a significant natural substrate of laccase-2. Our finding that dopa has a higher redox potential than the other three endogenous substrates provides a possible explanation for its low oxidation rate by laccase-2 and suggests that laccase-2 orthologs are probably in the low (340 – 490 mV) redox potential category of multicopper oxidases (Zhukhlistova et al., 2008).
Previous studies suggest that laccase-2A from B. mori is expressed as a proenzyme and is activated by proteolytic cleavage (Yamazaki, 1989; Yatsu and Asano, 2009), but that laccase-2A from M. sexta is constitutively active, and that removal of the amino-terminal region of the protein is not associated with increased activity (Dittmer et al., 2009). Our results strongly suggest that lacase-2 from T. castaneum and A. gambiae are expressed as constitutively active enzymes, and we predict, based on studies of laccase-2 from B. mori, M. sexta and Papilio xuthus, that TcLac2A and AgMCO2A activity is regulated by the availability of substrate in newly synthesized cuticle (Dittmer et al., 2009; Futahashi et al., 2010; Yatsu and Asano, 2009).
Although the laccase-2 isoforms that we tested had similar substrate specificities, there were two interesting differences in activity. Most notably, TcLac2B had higher kcat values than TcLac2A for all of the substrates tested and a higher Km value for all of the substrates except NADA. The net effect of these differences was that the two isoforms had similar catalytic efficiencies, but in the presence of high substrate concentration, TcLac2B was more active. The concentration of dopamine and NBAD in the cuticle of newly eclosed T. castaneum is approximately 0.5 nmoles mg−1 or roughly 0.5 mM substrate (Roseland et al., 1987), which is not high enough to favor the activity of TcLac2B; however, TcLac2B may be adapted to other physiological environments with possibly higher substrate concentrations. A second interesting isoform-specific difference in activity was that the pH optima of AgMCO2A were higher than those of AgMCO2B. The pH of newly formed mosquito cuticle is not known; therefore, it is difficult to assess the significance of this result, but it suggests that the A. gambiae isoforms may be adapted to environments with different pH ranges.
Our starting hypothesis for this project was that laccase-2 splice isoforms have different substrate preferences, but our results suggest that they probably oxidize the same substrates in vivo. An assessment of the existing information about laccase-2 isoforms suggests that perhaps they function in different tissues (laccase-2A in cuticle, laccase-2B in other tissues). At present, the only evidence for the participation of laccase-2B in cuticle tanning is that knockdown of TcLac2B leads to a slight delay or decrease in cuticle tanning (Arakane et al., 2005); however, the RT-PCR data from that study suggest that the isoform-specific knockdown was not absolute (i.e., knockdown of TcLac2B resulted in a slight knockdown of TcLac2A), and, as a result, unintentional knockdown of TcLac2A could have caused the observed cuticle phenotype. Unfortunately, little is known about the tissue-specificity of laccase-2 isoforms in any insect species, but we have some information about tissue-specific expression of laccase-2 isoforms in A. gambiae. AgMCO2B (but not AgMCO2A) is strongly upregulated in ovaries in response to a blood meal (Gorman et al., 2008), and AgMCO2B (but not AgMCO2A) is detectable in chorions by a proteomics method (Amenya, et al., 2010); therefore, we predict that AgMCO2B is expressed in the ovaries and transported to the chorion where it may oxidize diphenols such as dopamine as part of the chorion tanning process. Additional experiments that focus on tissue-specificity and isoform-specific phenotypes will help to elucidate isoform-specific functions of laccase-2.
Supplementary Material
Sequence alignment of AgMCO2 isofoms and TvLacIIIb. Predicted signal sequences of AgMCO2 isoforms are in italicized text. The three cupredoxin-like domains are indicated by dashed underlining (I), bold underlining (II), and double underlining (III). An arrow points to the first residue of the alternatively spliced exons. Residue differences between AgMCO2 isoforms are in red text. (See Figure 1 for additional features of AgMCO2A and AgMCO2B.)
pH profiles of laccase-2 activity with four diphenols as substrates. Assays were performed with 0.5 mM substrate in citrate-phosphate buffer. Data are expressed as mean ± standard deviation (n = 3).
Kinetic curves of laccase-2 activity with three endogenous substrates. Data are expressed as mean ± standard deviation (n = 3). Non-linear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Kinetic curves of laccase-2 and phenoloxidase with dopa as the substrate. Phenoloxidase was purified from M. sexta hemolymph. Data are expressed as mean ± standard deviation (n = 3 for laccase-2 isoforms and n = 2 for phenoloxidase). Nonlinear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Kinetic curves of laccase-2 activity with catechol, hydroquinone and ABTS as substrates. Data are expressed as mean ± standard deviation (n = 3). Non-linear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Cyclic voltammograms of dopamine, NADA, NBAD, and dopa.
Acknowledgments
We thank Karl Kramer, Ramaswamy Krishnamoorthi and Celeste Yang for helpful suggestions regarding this work. Detection of catecholamines in A. gambiae was done by Moses Okot-Kotber. Edman protein sequencing was done by Kathleen Schegg at the Nevada Proteomics Center, which is supported by NIH Grant Number P20 RR-016464 from the INBRE Program of the National Center for Research Resources. This work was supported by Grant Number R01AI070864 from the National Institute of Allergy and Infectious Diseases. This is contribution 12-190-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amenya DA, Chou W, Li J, Yan G, Gershon PD, James AA, Marinotti O. Proteomics reveals novel components of the Anopheles gambiae eggshell. J Insect Physiol. 2010;56:1414–1419. doi: 10.1016/j.jinsphys.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SO. Characterization of a trypsin-solubilized phenoloxidase from locust cuticle. Insect Biochem. 1978;8:143–148. [Google Scholar]
- Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem Mol Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc Natl Acad Sci USA. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y, Lomakin J, Beeman RW, Muthukrishnan S, Gehrke SH, Kanost MR, Kramer KJ. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J Biol Chem. 2009;284:16584–16594. doi: 10.1074/jbc.M901629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli K, Kopp J, Schwede T. The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyze the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian P. Fungal laccases - occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. doi: 10.1111/j.1574-4976.2005.00010.x. [DOI] [PubMed] [Google Scholar]
- Barrett FM. Phenoloxidases from larval cuticle of the sheep blowfly, Lucilia cuprina: characterization, developmental changes, and inhibition by antiphenoloxidase antibodies. Arch Insect Biochem Physiol. 1987a;5:99–1118. [Google Scholar]
- Barrett FM. Characterization of phenoloxidases from larval cuticle of Sarcophaga bullata and a comparison with cuticular enzymes from other species. Can J Zool. 1987b;65:1158–1166. [Google Scholar]
- Barrett FM, Andersen SO. Phenoloxidases in larval cuticle of the blowfly, Calliphora vicina. Insect Biochem. 1981;11:17–23. [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Bertrand T, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C. Crystal structure of a four-copper laccase complexed with an arylamine: insights into substrate recognition and correlation with kinetics. Biochemistry. 2002;41:7325–7333. doi: 10.1021/bi0201318. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Tadashi K, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer NT, Kanost MR. Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol. 2010;40:179–188. doi: 10.1016/j.ibmb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Dittmer NT, Suderman RJ, Jiang H, Zhu YC, Gorman MJ, Kramer KJ, Kanost MR. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol. 2004;34:29–41. doi: 10.1016/j.ibmb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Dittmer NT, Gorman MJ, Kanost MR. Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2009;39:596–606. doi: 10.1016/j.ibmb.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert C, Temp U, Eriksson KEL. The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol. 1996;62:1151–1158. doi: 10.1128/aem.62.4.1151-1158.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias-Neto M, Soares MPM, Simoes ZLP, Hartfelder K, Bitondi MMG. Developmental characterization, function and regulation of a Laccase2 encoding gene in the honey bee, Apis mellifera (Hymenoptera, Apinae) Insect Biochem Mol Biol. 2010;40:241–251. doi: 10.1016/j.ibmb.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ferraroni M, Myasoedova NM, Schmatchenko V, Leontievsky AA, Golovleva LA, Scozzafava A, Briganti F. Crystal structure of a blue laccase from Lentinus tigrinus: evidences for intermediates in the molecular oxygen reductive splitting by multicopper oxidases. BMC Struct Biol. 2007;7:60. doi: 10.1186/1472-6807-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futahaski R, Banno Y, Fujiwara H. Caterpillar color patterns are determined by a two-phase melanin gene prepatterning process: new evidence from tan and laccase2. Evol Dev. 2010;12:157–167. doi: 10.1111/j.1525-142X.2010.00401.x. [DOI] [PubMed] [Google Scholar]
- Futahashi R, Tanaka K, Matsuura Y, Tanahashi M, Kikuchi Y, Fukatsu T. Laccase2 is required for cuticular pigmentation in stinkbugs. Insect Biochem Mol Biol. 2011;41:191–196. doi: 10.1016/j.ibmb.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, An C, Kanost MR. Characterization of tyrosine hydroxylase from Manduca sexta. Insect Biochem Mol Biol. 2007;37:1327–1337. doi: 10.1016/j.ibmb.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MF, Dittmer NT, Marshall JL, Kanost MR. Characterization of the multicopper oxidase gene family in Anopheles gambiae. Insect Biochem Mol Biol. 2008;38:817–824. doi: 10.1016/j.ibmb.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Lee NS, Farinas ET. Laboratory evolution of laccase for substrate specificity. J Mol Catal B: Enzym. 2010;62:230–234. [Google Scholar]
- Hall M, Scott T, Sugumaran M, Soderhall K, Law JH. Proenzyme of Manduca sexta phenol oxidase: purification, activation, substrate specificity of the active enzyme, and molecular cloning. Proc Natl Acad Sci USA. 1995;92:7764–7768. doi: 10.1073/pnas.92.17.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Botelho JMC, McNall RJ, Belozerov V, Dunn WA, Mize T, Orlando R, Willis JH. Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem Mol Biol. 2007;37:135–146. doi: 10.1016/j.ibmb.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Hopkins TL, Kramer KJ. Insect cuticle sclerotization. Annu Rev Entomol. 1992;37:273–302. [Google Scholar]
- Kallio JP, Auer S, Janis J, Andberg M, Kruus K, Rouvinen J, Koivula A, Hakulinen N. Structure-function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds. J Mol Biol. 2009;392:895–909. doi: 10.1016/j.jmb.2009.06.053. [DOI] [PubMed] [Google Scholar]
- Kallio JP, Gaparetti C, Andberg M, Boer H, Koivula A, Kruus K, Rouvinen J, Hakulinen N. Crystal structure of an ascomycete fungal laccase from Thielavia arenaria - common structural features of asco-laccases. FEBS J. 2011;278:2283–2295. doi: 10.1111/j.1742-4658.2011.08146.x. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer KJ, Nuntnarumit C, Aso Y, Hawley MD, Hopkins TL. Electrochemical and enzymatic oxidation of catecholamines involved in sclerotization and melanization of insect cuticle. Insect Biochem. 1983;13:475–479. [Google Scholar]
- Kramer KJ, Morgan TD, Hopkins TL, Roseland CR, Aso Y, Beeman RW, Lookhart GL. Catecholamines and β-alanine in the red flour beetle, Tribolium castaneum: roles in cuticle sclerotization and melanization. Insect Biochem. 1984;14:293–298. [Google Scholar]
- Kramer KJ, Kanost MR, Hopkins TL, Jiang H, Zhu YC, Xu R, Kerwin JL, Turecek F. Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron. 2001;57:385–392. [Google Scholar]
- Matera I, Gullotto A, Tilli S, Ferraroni M, Scozzafava A, Briganti F. Crystal structure of the blue multicopper oxidase from the white-rot fungus Tramete trogii complexed with p-toluate. Inorg Chim Acta. 2008;361:4129–4137. [Google Scholar]
- Mayer AM, Staples RC. Laccase: new functions for an old enzyme. Phytochemistry. 2002;60:551–565. doi: 10.1016/s0031-9422(02)00171-1. [DOI] [PubMed] [Google Scholar]
- Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. J Mol Biol. 1995;247:536–540. doi: 10.1006/jmbi.1995.0159. [DOI] [PubMed] [Google Scholar]
- Niu BL, Shen WF, Liu Y, Weng HB, He LH, Mu JJ, Wu ZL, Jiang P, Tao YZ, Meng ZQ. Cloning and RNAi-mediated functional characterization of MaLac2 of the pine sawyer, Monochamus alternatus. Insect Mol Biol. 2008;17:303–312. doi: 10.1111/j.1365-2583.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- Peitsch MC. Protein modeling by e-mail. Bio/Technology. 1995;13:658–660. [Google Scholar]
- Quintanar L, Stoj C, Taylor AB, Hart PJ, Kosman DJ, Solomon EI. Shall we dance? How a multicopper oxidase chooses its electron transfer partner. Acc Chem Res. 2007;40:445–452. doi: 10.1021/ar600051a. [DOI] [PubMed] [Google Scholar]
- Riedel F, Vorkel D, Eaton S. Megalin-dependent Yellow endocytosis restricts melanization in the Drosophila cuticle. Development. 2011;138:149–158. doi: 10.1242/dev.056309. [DOI] [PubMed] [Google Scholar]
- Roseland CR, Kramer KJ, Hopkins TL. Cuticular strength and pigmentation of rust-red and black strains of Tribolium castaneum. Insect Biochem. 1987;17:21–28. [Google Scholar]
- Sakurai T, Kataoka K. Basic and applied features of multicopper oxidases, CueO, bilirubin oxidase, and laccase. Chem Rec. 2007;7:220–229. doi: 10.1002/tcr.20125. [DOI] [PubMed] [Google Scholar]
- Sugumaran M, Giglio L, Kundzicz H, Saul S, Semensi V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch Insect Biochem Physiol. 1992;19:271–283. doi: 10.1002/arch.940190406. [DOI] [PubMed] [Google Scholar]
- Tadesse MA, D’Annibale A, Galli C, Gentili P, Sergi F. An assessment of the relative contributions of redox and steric issues to laccase specificity towards putative substrates. Org Biomol Chem. 2008;6:868–878. doi: 10.1039/b716002j. [DOI] [PubMed] [Google Scholar]
- Thomas BR, Yonekura M, Morgan TD, Czapla TH, Hopkins TL, Kramer KJ. A trypsin-solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989;19:611–622. [Google Scholar]
- Xu F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: correlation between activity and redox potentials as well as halide inhibition. Biochemistry. 1996;35:7608–7614. doi: 10.1021/bi952971a. [DOI] [PubMed] [Google Scholar]
- Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim Biophys Acta. 1996;1292:303–311. doi: 10.1016/0167-4838(95)00210-3. [DOI] [PubMed] [Google Scholar]
- Yamazaki HI. The cuticular phenoloxidase in Drosophila virilis. J Insect Physiol. 1969;15:2203–2211. [Google Scholar]
- Yamazaki HI. Cuticular phenoloxidase from the silkworm, Bombyx mori: properties, solubilization, and purification. Insect Biochem. 1972;2:431–444. [Google Scholar]
- Yamazaki HI. Laccase-type phenoloxidase in the cuticle of the silkworm, Bombyx mori. Res J. 1989;5:1–10. (Proceedings of the Department of General Education of Atomi Gakuen Women’s University) [Google Scholar]
- Yatsu J, Asano T. Cuticle laccase of the silkworm, Bombyx mori: purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem Mol Biol. 2009;39:254–262. doi: 10.1016/j.ibmb.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Zhukhlistova NE, Zhukova YN, Lyashenko AV, Zaitsev VN, Mikhailov AM. Three-dimensional organization of three-domain copper oxidases: a review. Crystallogr Rep. 2008;53:92–109. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence alignment of AgMCO2 isofoms and TvLacIIIb. Predicted signal sequences of AgMCO2 isoforms are in italicized text. The three cupredoxin-like domains are indicated by dashed underlining (I), bold underlining (II), and double underlining (III). An arrow points to the first residue of the alternatively spliced exons. Residue differences between AgMCO2 isoforms are in red text. (See Figure 1 for additional features of AgMCO2A and AgMCO2B.)
pH profiles of laccase-2 activity with four diphenols as substrates. Assays were performed with 0.5 mM substrate in citrate-phosphate buffer. Data are expressed as mean ± standard deviation (n = 3).
Kinetic curves of laccase-2 activity with three endogenous substrates. Data are expressed as mean ± standard deviation (n = 3). Non-linear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Kinetic curves of laccase-2 and phenoloxidase with dopa as the substrate. Phenoloxidase was purified from M. sexta hemolymph. Data are expressed as mean ± standard deviation (n = 3 for laccase-2 isoforms and n = 2 for phenoloxidase). Nonlinear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Kinetic curves of laccase-2 activity with catechol, hydroquinone and ABTS as substrates. Data are expressed as mean ± standard deviation (n = 3). Non-linear regression was used to fit the data to the Michaelis-Menten equation (dotted lines).
Cyclic voltammograms of dopamine, NADA, NBAD, and dopa.