Abstract
Ribonucleotide reductase (RNR) activity requires an electron donor, which in bacteria, yeast, and plants is usually either reduced thioredoxin (Trx) or reduced glutaredoxin (Grx). Mice lacking glutathione reductase are viable and, although mice lacking thioredoxin reductase 1 (TrxR1) are embryonic-lethal, several studies have shown that mouse cells lacking the txnrd1 gene, encoding TrxR1, can proliferate normally. To better understand the in vivo electron donor requirements for mammalian RNR, we here investigated whether replication of TrxR1-deficient hepatocytes in mouse livers employed either an alternative source of Trx-reducing activity or, instead, solely relied upon the glutathione (GSH) pathway. Neither normal nor genetically TrxR1-deficient livers expressed substantial levels of mRNA splice-forms encoding cytosolic variants of TrxR2, and the TrxR1-deficient livers showed severely diminished total TrxR activity, making it unlikely that any alternative TrxR enzyme activities complemented the genetic TrxR1 deficiency. To test whether the GSH pathway was required for replication, GSH levels were depleted by administration of buthionine sulfoximine (BSO) to juvenile mice. In controls not receiving BSO, replicative indexes were similar in hepatocytes having either two, one, or no functional alleles of txnrd1. Following BSO treatment, hepatocytes containing either two or one copies of this gene were also normal. However, hepatocytes completely lacking a functional txnrd1 gene exhibited severely reduced replicative indexes after GSH depletion. We conclude that hepatocyte proliferation in vivo requires either GSH or at least one functional allele of txnrd1, demonstrating that either the GSH- or TrxR1-dependent redox pathway can independently support hepatocyte proliferation during liver growth.
Keywords: DNA replication, glutathione, thioredoxin reductase, hepatocyte, mouse
DNA replication depends on the activity of ribonucleotide reductase (RNR)1 to make DNA precursors [1]. RNR catalyzes the conversion of ribonucleoside diphosphates into deoxyribonucleoside diphosphates by a reaction that requires an electron donor system [1–4]. Early studies on Escherichia coli established that either thioredoxin (Trx) or glutaredoxin (Grx) could serve as the terminal electron-donor for RNR, and either the Trx- or the glutathione- (GSH-) pathways could hence support bacterial genome replication [5–8]. Either the Trx- or the GSH-pathway can also support S phase replication in yeast or plants; however, in these eukaryotic organisms, ablation of the GSH-pathway has no effect on replication, whereas ablation of the Trx-pathway results in slow-growth phenotypes [9–12]. Thus, in yeast and plants the Trx-pathway seems to be primarily active in supporting RNR activity; the GSH-pathway is only marginally effective. Interestingly, in C. elegans, the only essential role of TrxR1 seems to be in assistance of the molting process, i.e. shedding of old cuticle in a process requiring both TrxR1 and glutathione reductase (Gsr), with no evidence for an essential role of TrxR1 in support of cell replication and thus in support of RNR activity [13]. These examples illustrate that different eukaryotes favor different redox pathways to support RNR. However, the relative in vivo contributions of these electron donor systems to RNR activity in mammalian systems have yet to be determined.
GSH- and Trx-pathways are each nearly ubiquitous in biology. In the GSH pathway, electrons flow from NADPH to oxidized glutathione disulfide (GSSG) via Gsr. In a separate reaction, the reduced GSH can reduce oxidized Grx isoenzymes, serving the roles of general GSH-dependent protein disulfide reductases [6, 14]. In the Trx pathway, electrons flow from NADPH to Trx in a single reaction catalyzed by thioredoxin reductase (TrxR), with Trx isoenzymes subsequently reducing a wide range of protein disulfide substrates [6, 15]. Although the relative roles of the GSH- and Trx-systems in supporting in vivo genome replication in mammalian systems has not previously been investigated, the catalytic mechanisms by which mammalian GSH- and Trx-systems support RNR activities in vitro differ qualitatively both from each other and from those found with bacterial RNR [16, 17]. Early genetic studies in mice suggested that the Trx pathway played a more essential role than did the GSH pathway in mammals. Mice genetically lacking Gsr protein are viable, fertile, and overtly normal [18]. Conversely, homozygous disruption of either the txn1 gene encoding cytosolic Trx1 (also called Txn1) [19], the txn2 gene encoding mitochondrial Trx2 (also called Txn2) [20], the txnrd1 gene encoding cytosolic TrxR1 (also called Txnrd1 or TR1) [21, 22], or the txnrd2 gene encoding mitochondrial TrxR2 (also called Txnrd2 or TR3) [23] in all cases, independently resulted in embryonic lethality. Nevertheless, uncertainties about the mechanistic causes of embryonic failure in the Trx-system mutants make it unclear why these pathways might be essential.
In mammals, DNA replication is largely restricted to developmental growth of pre-differentiated cells, although some differentiated immune and epithelial cells, germ and stem cells, cancers, and mammalian cell cultures actively proliferate. A classic study based on immuno-co-localization of the S phase-specific RNR subunit R1 (also called M1) and Trx1 in proliferative tissues of rats concluded that Trx1 is not likely the primary physiological electron donor for RNR in mammals [24]. Consistent with this, mice homozygous for disruptions of the txnrd1 gene survive until embryonic day 8.5 (E8.5) [22] or E10.5 [21], by which time proliferation has yielded thousands of txnrd1 deficient cells. In addition, knock-down of TrxR1 activity in mouse cell cultures does not disrupt proliferation [25, 26] and lymphomas lacking TrxR1 can exhibit normal proliferation [27]. One system that has proven particularly useful for studying mammalian replication in vivo has been in mouse hepatocytes, an endoderm-derived cell type that constitutes roughly 95% of the mass of adult liver [28]. Hepatocytes are one of few normal differentiated cell types that can proliferate [29]. During developmental liver growth from embryonic day 14 (E14) to postnatal day 56 (P56), liver increases >300-fold in mass; it has been estimated that proliferation of differentiated hepatocytes accounts for at least 1/3 of this growth [30]. By using a conditional mutagenesis system in which the txnrd1 gene is only disrupted in differentiated hepatocytes [22, 31, 32], we showed that mice lacking hepatocyte txnrd1 are overtly normal [31] and exhibit normal liver growth and normal developmental and regenerative hepatocyte proliferation [30]. In combination, all of these studies indicate that TrxR1 is not required for proliferation, per se, in mammalian cells. However, it remains unclear what provides electrons to RNR for replication in the absence of TrxR1.
Organellar compartmentalization in eukaryotes differentially affects the GSH and Trx systems. Most eukaryotic genomes possess a single gsr gene, the protein product of which is responsible for reduction of GSSG to GSH throughout the cell. By contrast, separate genes typically encode cytosolic- versus mitochondrial-isoforms of TrxR. Despite this, in plants the TrxR isoforms are largely functionally redundant. Thus, ablation of either TrxR gene in Arabidopsis is generally complemented by relocalization of a subset of the other protein isoform to the deficient subcellular compartment [9, 10]. Whereas similar re-distribution of TrxR protein isoforms between mitochondria and cytosol has not yet been shown to occur naturally in mammalian systems, several expressed sequence tags (ESTs) have been described representing alternatively initiated and spliced mRNAs that might encode versions of the normally mitochondrial TrxR2 protein lacking the N-terminal mitochondrial transit-peptide [33]. In vitro, synthetic mimics of these hypothetical TrxR2 isoforms readily reduce Trx1 and, when expressed in mammalian cell cultures, these proteins accumulate in the cytosol, not in mitochondria [33]. As such, it was plausible that cytosolic variants of TrxR2 might reduce Trx1 in TrxR1-deficient cells, and thus could provide the source of electrons for the replication-supporting activity of RNR.
Another possible source of electrons for RNR in Txnrd1-deficient mammalian cells could come from the GSH pathway. Biochemical studies showed that Grx purified from calf thymus will reduce RNR from the same source [34]. More recently it was shown that a GSH pathway reconstituted entirely from recombinant mammalian proteins is able to support mammalian RNR activity in vitro [16]. However, neither the GSH system nor a TrxR2-driven Trx system has previously been shown to support mammalian cell replication in vivo [17]. Therefore, we here investigated the contributions of these two pathways in supporting developmental proliferation of TrxR1-deficient hepatocytes in mice. Our results suggest that TrxR2 does not support replication of hepatocytes lacking TrxR1, whereas GSH is essential for their replication. Moreover, upon depletion of GSH, only one allele of txnrd1 is required to support hepatocyte growth. These findings illustrate that, in mouse liver, the GSH- and TrxR1-dependent pathways constitute complementary systems of supporting RNR. Either pathway alone is sufficient, and at least one of the two is required, for hepatocyte DNA replication.
Experimental procedures
Materials
Except as indicated, reagents were from Sigma.
Mouse lines, care conditions, and procedures
All animal procedures meet the International Guiding Principles for Biomedical Research Involving Animals and were approved by the Montana State University Institutional Animal Care and Use Committee under one or more of the following approved protocol numbers: 28-7, 2009-7, 2009-32; 2010-7, 2010-22, and 2010-37. In this paper, genetic loci are italicized and multiple loci are separated by a semi-colon. For known loci, allelic quality follows as a superscript, with each allele at a locus separated by a slash. Unmapped transgenes are italicized and followed by a superscript numeral to indicate whether they are homozygous (2) or hemizygous present (1). The conditional- and null-alleles of the txnrd1 gene (txnrd1cond and txnrd1null, respectively) were described previously [22]. These lines were extensively back-crossed onto C57Bl/6J [22] and are maintained by either homozygous sib-matings (txnrd1cond) or heterozygous back-crosses to C57Bl/6J (txnrd1null). C57Bl/6J mice or mice bearing the “albCre” transgene (B6.Cg-Tg(Alb-cre)21Mgn/J) [35] were purchased from Jackson Labs (stock numbers 000664 and 003574, respectively). All genotypes were determined by PCR on genomic DNA using previously reported primers [31]. Mice were maintained with a 14:10-hour light:dark cycle under sterile conditions in forced HEPA-filtered-air caging systems (Tecniplast) containing sterilized bedding and enrichment materials. Sterilized feed (PicoLab 5058, which contains selenium as a natural component of the grains and is supplemented to 0.33 p.p.m. with sodium selenite by the manufacturer) and autoclaved water were unrestricted.
Although txnrd1null/null mice are embryonic-lethal [21, 22], use of the txnrd1cond allele allows us to achieve normal development by restricting conversion to the txnrd1null/null genotype to specific times or cell types [30, 31]. The albCre transgene [35] is activated coincident with differentiation of hepatocytes from prehepatocyte cell types and results in functional conversion of Cre-responsive reporter genes in most or all hepatocytes as early as E15 [32, 36]. In this study albCre-expressing juvenile mice (P23–30, weighing 15–20 g) of the indicated txnrd1 genotypes were used, which represents a stage having active proliferative growth of livers [28, 30] in which hepatocyte lineages had activated functional Cre expression ~4 weeks earlier [32, 36].
Where indicated, a single inoculation of 0.5 ml of either saline or saline containing 100 mg/ml freshly dissolved BSO (MP Biomedicals) was administered I.P. [37, 38]. Bromodeoxyuridine (BrdU) was administered in sterile saline as an I.P. inoculation at a dose of 2.5 μmol/animal [30]. For harvests, mice were asphyxiated with carbon dioxide and perfused by cardiac injection/portal drainage with 3–10 ml sterile saline to remove blood from the liver. Livers were divided into three pieces. One was snap-frozen in liquid nitrogen for enzyme assays; one was used fresh to isolate RNA; and one was fixed in 10% neutral-buffered formalin and embedded in paraffin for histology and immunostaining.
RNA preparation and mRNA analyses
Fresh liver samples (~50 mg) were homogenized by sonication for 10 s in ~20-volumes of Tri-reagent (Ambion) and total RNA was purified as recommended by the manufacturer. First-strand cDNAs were synthesized from 5 μg of total RNA using oligo(dT)-primers and M-MuLV reverse transcriptase (New England Biolabs). General reaction conditions for RT-PCR, semi-quantitative RT-PCR, and qPCR were performed as described previously using the conditions specified in figure legends [22, 31, 39]. For qPCR, relative specific product in each reaction was determined by SYBR Green (Invitrogen) fluorescence using the relative standard curve method on a MyIQ PCR System (BioRad) using endogenous β-actin mRNA signals as internal controls. Primer sequences were as follows (all 5′-to-3′, “F-” for forward and “R-” for reverse): β-actin, exon 5-F-gctgtctggtggtaccaccatgta, exon 6-R-atctgctggaaggtggacagtgag; TrxR1, exon 2-F-tatactagtgctggtcttggattttgtcac, exon 3-R-tatgaattccagccatagttgcgcgagtctttcag, exon 6-R-atagaattccaaggcgacataggatgcac; TrxR2, exon 1a-F-tatgaattctgaccgcctgaggtccccggaccatgg, exon 1b-F-tatgaattcgttgaaacagagacatgttctggag, exon 1c-F-tatgaattcaggacaggcatcagcaatggaag, exon 2-F-tatgaattcggcagcagagctttgatctcttgg, exon 2-R-tatggatccagcttccttggcacaagctaggc, exon 4-R-tatggatccagcctgatgcatcagcttcttg.
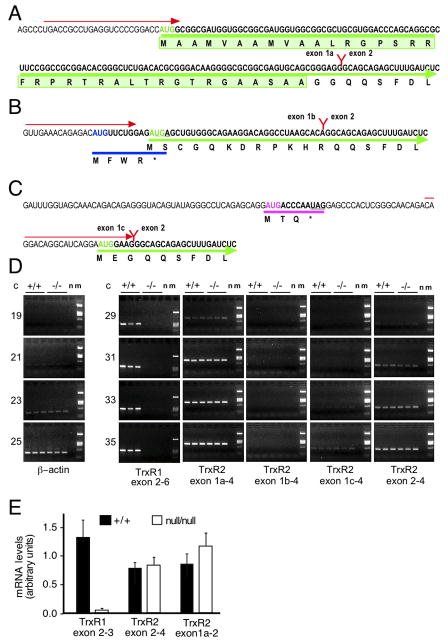
Different nomenclatures exist for mouse txnrd2 gene structure. Exon designations used here are consistent with NCBI (http://www.ncbi.nlm.nih.gov), MGI (http://www.informatics.jax.org), VEGA (http://vega.sanger.ac.uk/index.html), and Ensemble (http://uswest.ensembl.org) for the major (mitochondrial isoform-encoding) mRNA splice-variant issued from the gene, with transcription initiating at the first base of exon 1 and the remaining exons of this mRNA spliceform numbered consecutively through exon 18. Since two other alternative promoters/first exons have been subsequently reported for this gene [33], each alternative first exon is numbered 1 followed by a letter corresponding to the order they appear on the gene, as detailed in Fig. 1A. Alternative first exon 1a encodes the mitochondrial signal peptide and initiates the message encoding the familiar mitochondrial TrxR2 protein.
Fig. 1. Expression of TrxR1 mRNA and alternative splice-forms of TrxR2 mRNA in livers of normal and txnrd1cond/null;albCre1 mice.
(A) Structure of mitochondrial splice-form of TrxR2 mRNA. mRNA sequence is shown in black with the first AUG in green font. The heavy green line under the sequence initiating at this AUG is the N-terminal protein-coding region, with the a.a. sequence indicated below. The light green highlighted box is the 39-a.a. mitochondrial transit peptide. The exon 1a/2 splice-junction is noted with a red “Y”, and is labeled. The red arrow above the mRNA sequence indicates the sequence and orientation of the exon 1a-specific primer used for RT-PCR and qPCR. (B) Structure of alternatively initiated/spliced exon 1b-containing transcript. Symbols are as above. In addition, the first AUG, which is in the +1 reading frame from TrxR2, is blue, as is the heavy line denoting the 4-a.a. ORF this initiates. The stop codon for this ORF, which overlaps the second AUG, is underlined. (C) Structure of alternatively initiated/spliced exon 1c-containing transcript. The first AUG, which is in the +2 reading frame from TrxR2, is lavender, as is the heavy line denoting the 3-a.a. ORF this initiates. (D) Semi-quantitative RT-PCR analysis of mRNA levels for β-actin, TrxR1, and TrxR2 mRNA isoforms in mouse livers. Total RNA was prepared from three wild-type (+/+) and three mutant (−/−) P28 mice and oligo(dT)-primed cDNAs were prepared. Semi-quantitative RT-PCR was performed using the primers indicated below images and the number of PCR cycles indicated in columns labeled “C”. Images show ethidium bromide-stained gels. All six samples were analyzed with each primer set; the hepatocyte genotypes are indicated above images. Other abbreviations: n, no-template control; m, molecular size markers. (E) Expression of mRNAs encoding TrxR1 and TrxR2 were quantified by qPCR performed on equivalent amounts of oligo(dT)-primed first-strand cDNAs from wild-type (hepatocytes txnrd1+/+) and txnrd1cond/null;albCre1 (hepatocytes txnrd1null/null) mouse livers. Data were normalized to qPCR signals for β-actin mRNA in each sample. Bars represent mean and S.E.M. from three mice of each genotype. Below each column of gel images, the cDNA region spanned by the PCR reactions is indicated.
Enzyme assays
Snap-frozen liver samples were thawed on ice and cut in two pieces (~0.1 g per piece). One piece was used for measuring TrxR or Trx activity; the other one was used for GSH + GSSG quantification. For measuring TrxR or Trx activity, samples were homogenized in 800 μl ice-cold buffer containing 50 mM Tris-Cl, pH, 7.5, 5 mM EDTA, 0.15 M NaCl, 1% Triton-X 100, and fresh protease inhibitors (complete EDTA-free, Roche). For measuring total GSH + GSSG, samples were homogenized in 800 μl ice-cold 10 mM HCl. After homogenization, non-soluble debris was removed by centrifugation (16,000 × g at 4 °C for 35 min) and protein content was determined on supernatants using Bradford reagent (BioRad).
Total TrxR activity was determined according to the end-point Trx1-dependent insulin reduction assay [40], modified for microtiter plates. Briefly, lysate (50 μg protein) was incubated with 20 μM recombinant human Trx (kindly provided by Dr. Arne Holmgren, Karolinska Institutet) in the presence of 314 μM insulin, 1.3 mM NADPH (AppliChem), 84 mM HEPES, pH 7.6, and 12.5 mM EDTA in a total volume of 50 μl. After 40 min incubation at 37°C, the reaction was stopped by adding 200 μl 7.2 M guanidine-HCl (Acros) containing 1 mM DTNB. Background references contained all components, but the reaction was stopped immediately. Absorbance was recorded at 412 nm using a VersaMax microplate reader (Molecular Devices). Total Trx activity was measured similarly to TrxR activity except that lysates (25 μg protein) were supplemented with 0.56 μM recombinant rat TrxR (19 U/mg; kindly provided by Dr. Jianqiang Xu, Karolinska Institutet) instead of Trx [40]. Measurements of total GSH + GSSG were performed by the Gsr-DTNB recycling assay as described recently [41], except that we used 10 μl of supernatant for each assay. Quantification was based on comparison to a GSH standard curve; the total concentrations of GSH + GSSG in samples were normalized to protein content as above.
Immunostaining for proliferative or replicative indexes
Processing, embedding, sectioning, and immunostaining for proliferative cell nuclear antigen (PCNA), phosphohistone H3 (PHH3), or BrdU incorporation were performed as described previously [30]. Primary antibodies used were mouse-anti-PCNA (AbCam #A1029), rabbit-anti-PHH3 (Cell Signaling Technologies #9701), and mouse-anti-BrdU-DNA (Sigma #B2531). Horseradish peroxidase conjugated secondary antibodies were goat-anti-mouse (Pierce #31430) or goat-anti-rabbit (GIBCO #13859.012). All immunostaining was performed as per the suppliers’ recommendations and our previously reported protocols [30, 31].
Histological data collection and statistical analyses
Short incubations with BrdU (here 1 h) label nuclei that are in the process of active DNA replication. PCNA labeling, which marks all cells that are in or have been in S phase recently [30], showed small (~2-fold range) animal-to-animal variation that was independent of treatment group or genotype (Fig. 3A–F; data not shown; and reference [30]). To reduce variance arising from such stochastic influences, we here measured the fraction of proliferative hepatocyte nuclei that were actively synthesizing DNA under each condition rather than the fraction of total liver hepatocyte nuclei that were synthesizing DNA, as detailed in the Results section and in the figure legends. To do this, we used the PHH3 labeling index, which reflects the percent of cells in M phase and will be unaffected by short-term (here 4–5 h) disruption of DNA synthesis, as the denominator to represent normalize the BrdU-labeling index. In each case, 30 arbitrary full fields-of-view from BrdU- or PHH3-stained liver sections from each animal were photographed using the 20X objective, representing ~500 hepatocyte nuclei per field or 15,000 hepatocyte nuclei per animal (Supplemental Figs 1A, B). BrdU- or PHH3 positive nuclei were enumerated on these to get the labeling index for each animal (Supplemental Figs S1C, D). Statistical analyses used the mean and S.E.M. of the four biological replicates for each treatment group and Student’s two-tailed T-tests with n = 4.
Fig. 3. Requirements for TrxR1 or glutathione for replication in proliferative hepatocytes in juvenile livers.
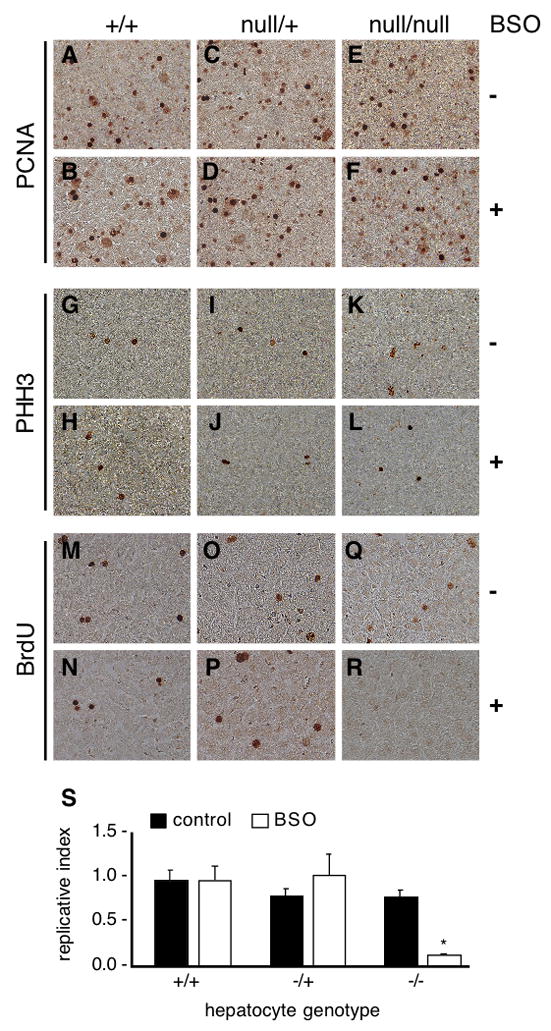
Juvenile (P23–25) wild-type (hepatocytes txnrd1+/+), txnrd1cond/+;albCre1 (hepatocytes txnrd1null/+), and txnrd1cond/null;albCre1 (hepatocytes txnrd1null/null) mice received an I.P. inoculation with saline alone or 50 mg BSO in sterile saline. Four hours later, mice received an I.P. inoculation with 2.5 μmol of BrdU in sterile saline. One hour later, mice were sacrificed and livers were harvested for immunohistological analyses. Global proliferative indexes were assessed by PCNA-staining (Panels A–F) and by analysis of the M phase index by PHH3-staining (Panels G–L). None of the conditions tested affected the percent of proliferative cells in liver (Panels A–L), verifying that the conditions used did not have a major impact on cell survival or general cell physiology within the time-frame of the experiment. Replication was assessed by staining for BrdU (Panels M–R). Images from one animal under each condition are shown to illustrate the methodology that was used and further details are presented in Supplemental Fig. S1. The experiment was repeated on four mice for each condition. For each animal, ~15,000 hepatocyte nuclei (30 fields with ~500 hepatocyte nuclei in each) were assayed. To determine the fraction of proliferative hepatocytes that were able to sustain DNA replication under each condition (“replicative index”), the number of BrdU-labeled hepatocyte nuclei per 15,000 total hepatocyte nuclei was divided by the number of PHH3-labeled nuclei per 15,000 total hepatocyte nuclei for each liver. Quantitative data is presented in Panel S as the mean + S.E.M. for the four animals in each condition. The asterisk indicates the only significantly deviant condition, txnrd1cond/null;albCre1 animals treated with BSO, which was significantly lower than all five other conditions (P < 0.05, Student’s T-test).
Results
Relative expression of transcripts encoding TrxR2 protein isoforms in normal and TrxR1-deficient livers
S phase RNR is strictly cytosolic [42, 43], and thiol redox-equivalents do not directly shuttle between the cytosol and the mitochondria [16, 17]. Moreover, a previous report showed that TrxR2 mRNA is roughly 5-fold less abundant than TrxR1 mRNA in normal mouse liver [44]. Nevertheless, ESTs have been reported that suggest the txnrd2 gene can issue cytosolic isoforms of the normally mitochondrial TrxR2 protein [33], which theoretically could allow TrxR2 to contribute electrons for S phase RNR activity. Mouse, rat, and human txnrd2 genes each have three known alternative first exons that can be spliced onto exon 2 [33]. The most upstream first exon (exon 1a) encodes a mitochondrial transit-peptide, resulting in the familiar mitochondrial TrxR2 protein (Fig. 1A), whereas transcription initiation at either of the two putative downstream first exons (exons 1b or 1c) might generate mRNAs encoding cytosolic versions of TrxR2 (Figs 1B,C). Although cytosolic accumulation of endogenous TrxR2 has not yet been shown to occur in vivo, translation of synthetic mimics of full-length TrxR2 open reading frames having initiation-optimized versions of either exon 1b or 1c in transfected cells results in cytosolic accumulation of TrxR2 protein [33]. Also, recombinant versions of these hypothetical proteins possess potent Trx1-reducing activity in vitro [33]. To investigate whether cytosolic TrxR2 protein issued from alternative TrxR2 mRNA splice-forms might complement the hepatocyte TrxR1-deficiency in txnrd1cond/null;albCre1 mice and support replicative RNR activity, we measured levels of the alternative TrxR2 mRNA splice-forms and of TrxR1 mRNA in normal and TrxR1-deficient juvenile livers. In our analyses, we only detected a substantial level of the exon 1a-containing variant of TrxR2 and there was no difference or switch in levels of the three TrxR2 mRNA splice-forms upon TrxR1 deletion (Figs 1D,E). As reported previously [22, 31], disruption of the txnrd1 gene had furthermore no effect on levels of total TrxR2 mRNA (Figs 1D,E). Based on these results, we consider it highly unlikely that cytosolic isoforms of TrxR2 protein functionally replace TrxR1 in txnrd1null/null hepatocytes.
Levels of TrxR and Trx activities in normal and hepatocytic txnrd1-deficient livers
Based on the relative TrxR1 and TrxR2 mRNA levels, we predicted that hepatic TrxR activity would be substantially diminished in livers having hepatocyte-specific disruption of the txnrd1 gene. Lysates from txnrd1cond/null;albCre1 (hepatocytes txnrd1null/null) and wild-type (hepatocytes txnrd1+/+) livers were compared and the results indeed showed that disruption of the txnrd1 gene in hepatocytes reduced total TrxR activity to ~25% that of wild-type controls (Fig. 2A). Residual TrxR activity in the homozygous mutant samples likely arose from non-hepatocyte cell-types of the liver, in which one txnrd1 allele would remain functional (txnrd1cond/null), and from TrxR2 present in mitochondria. Neither of these sources can provide electrons to the hepatocyte S phase RNR, located in the cytosol, for DNA replication [16, 45]. Importantly, lysates contained TrxR1 from all liver cell types in addition to hepatocytes, yet total TrxR activity in the homozygous hepatocytic txnrd1-deficient livers was substantially diminished (Fig 2A, black bars). Trx activities were not significantly affected by the txnrd1 status, although there was a slight tendency for increased Trx activity in the txnrd1null/null livers (Fig. 2B, black bars). These results verified that there was no substantial source of alternative Trx-reductase activity that could complement the TrxR1 deficiency in txnrd1null/null hepatocytes.
Fig. 2. Effects of BSO treatment of juvenile mice on levels of components of the Trx- and GSH-pathways in livers.
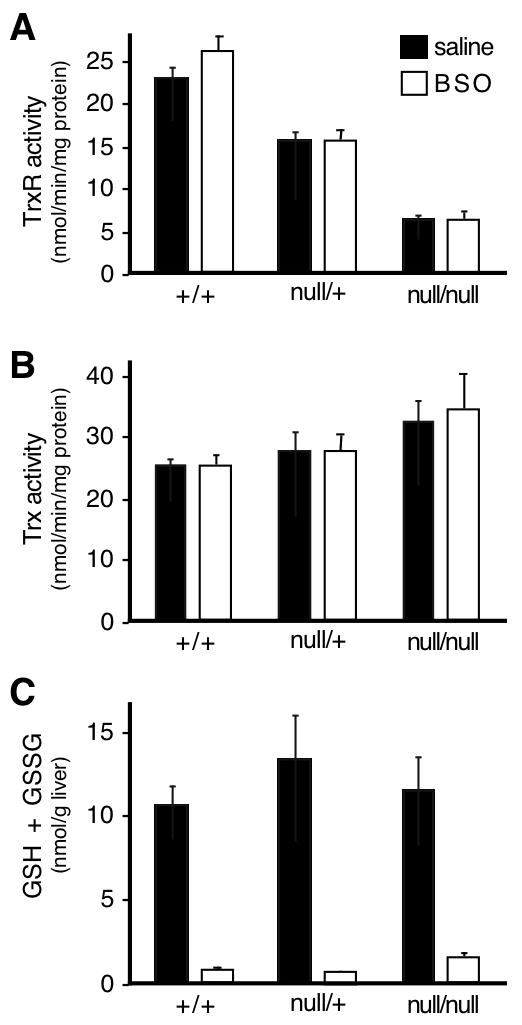
Juvenile (P23–25) wild-type (hepatocytes txnrd1+/+), txnrd1cond/+;albCre1 (hepatocytes txnrd1null/+), and txnrd1cond/null;albCre1 (hepatocytes txnrd1null/null) mice received an I.P. inoculation with saline alone (black bars) or 50 mg of BSO in sterile saline (open bars). Five hours later, mice were sacrificed and livers were perfused with saline and harvested for enzymatic analyses. Bars represent mean + S.E.M. for four mice in each condition. (A) Effects of BSO treatment on total TrxR activity. (B) Effects of BSO treatment on Trx levels. (C) Effects of BSO treatment on GSH + GSSG levels.
Effects of GSH depletion on hepatocyte replication
Previous data showed that Gsr mRNA expression was not increased in response to ablation of TrxR1 [22, 31] but that mRNA encoding the modifier subunit of glutamate-cysteine ligase (Gclm), a critical step in glutathione biosynthesis, was induced by ablation of TrxR1 [22, 31]. Despite the up-regulation of Gclm mRNA levels, we here found that total steady-state levels of glutathione in wild-type, heterozygous-, and homozygous-mutant livers were not altered upon ablation of TrxR1 (Fig. 2C, black bars). Nevertheless, it remained possible that the normally non-essential Gsr-dependent pathways, in the absence of compensatory up-regulation, were sufficiently robust to support homeostasis and replication of TrxR1-deficient hepatocytes. Therefore, we tested whether GSH was necessary for replication in txnrd1null/null hepatocytes. For this, we treated the mice with the drug buthionine sulfoximine (BSO), which inhibits GSH biosynthesis [38] and results in rapid depletion of liver glutathione stores [37]. Juvenile (P28) wild-type, heterozygous-, or homozygous-mutant mice were given a single inoculation of BSO or mock-treated with saline and, 4 hours later, all mice received BrdU, with livers harvested 1-hour after the BrdU inoculation. Enzyme assays showed that the BSO treatment reduced hepatic glutathione levels 8- to 20-fold as compared to mock-treated controls in livers of all three genotypes (Fig. 2C, compare black and white bars for each genotype), but had no significant effect on the total TrxR or Trx activity levels (Figs. 2A,B, compare black and white bars for each genotype).
Paraffin sections of each liver sample were immunostained for PCNA, a marker found from early G1 through mitosis of proliferative cells, or for PHH3, an M phase-specific marker, as measures of the proportions of input proliferative cells (Fig. 3, panels A–L) [30]. Sections of the same samples were also stained for BrdU incorporation to determine whether proliferative hepatocytes were able to continue DNA replication under each condition (Fig. 3, panels M-R, and Supplemental Fig. S1). Results showed similar levels of hepatocyte replication under all conditions except for the txnrd1cond/null;albCre1 mice treated with BSO, in which very few hepatocytes remained replicative (Fig. 3R and Supplemental Fig. S1, panels C, D). Quantitative analysis of the proportion of proliferative hepatocyte nuclei under each condition that could replicate DNA revealed that neither disruption of one nor both copies of the txnrd1 gene alone, nor depletion of GSH stores in cells containing two or one functional copy of the txnrd1 gene, had a significant effect on DNA replication (Fig. 3, panel S). Continued DNA replication following BSO treatment in all samples in which hepatocytes contained at least one functional copy of the txnrd1 gene indicated that, under the conditions used, BSO was non-toxic and GSH was superfluous for replication in hepatocytes having a functional TrxR1 system. Depletion of GSH in the txnrd1cond/null;albCre1 mice, however, inhibited the replication in > 90% of the proliferative hepatocyte nuclei (Fig. 3, panel S). We thus conclude that normal replication in mouse hepatocytes requires either one functional copy of the txnrd1 gene or a functional GSH system.
Discussion
Here we tested whether hepatocyte proliferation in vivo depended upon an alternative source of TrxR activity or on the GSH pathway. Our results revealed that TrxR1-deficient hepatocytes are severely deficient in total Trx-reductase activity and that, although Gsr is non-essential in mice [18] and GSH was not required for replication of hepatocytes lacking one copy of the txnrd1 gene (Fig. 3, panels P and S), GSH was critical for replication of txnrd1null/null hepatocytes in mice (Fig. 3, panels R and S). These results are, to our knowledge, the first showing that hepatocyte replication in vivo requires either at least one allele of trnrd1 or sufficient GSH levels, thereby indicating that in these cells, the GSH- and txnrd1-dependent systems are mutually complementary. Thus, either system alone is sufficient, and at least one of the two systems is required, for S phase DNA replication.
In considering the interplay between the GSH- and Trx-pathways, it is interesting to note that depletion of txnrd1 leads to induction of the Nrf2 oxidative stress-response pathway [31], which upregulates components of the Trx pathway as well as many GSH-dependent enzyme systems [46–50]. Importantly, our results reveal that in mouse hepatocytes, preservation of either the GSH- or txnrd1-dependent system is sufficient to support viability and replication. However, it should be noted that different cell- or tissue-types can have highly divergent profiles of their redox pathways in mammals [50–52]. It is uncertain how well the current results for mouse hepatocytes will reflect requirements for other mammalian cell types. For example, a recent study showed that, whereas mouse lymphomas lacking TrxR1 grow and progress, these tumors are highly susceptible to treatment with BSO [27]. Tumor cells, in particular those of aggressive cancers, exhibit enhanced oxidative stress and, in fact, frequently over-express TrxR1 [53]. This oxidative stress might increase demands on the two major endogenous antioxidant systems, the GSH- and the Trx-pathways, for detoxification and repair. It was therefore suggested that the enhanced susceptibility of TrxR1-deficient lymphomas to BSO treatment might reflect this critical need for protection against oxidative damage [27]. The results we report here suggest an alternative explanation for the susceptibility of TrxR1-deficient cancers to BSO treatment. Thus, by analogy to mouse hepatocytes in the current study, it is possible that mouse lymphoma cells also cannot replicate their DNA in the absence of both TrxR1 and GSH. It would be interesting to test whether BSO treatment of TrxR1-deficient tumors results in a rapid block to replication, as one might expect from inhibiting RNR activity, or a protracted death of the cancer cells, as one might expect from accumulation of oxidative damage.
The embryonic lethality of mice lacking any of the major components of either the cytosolic or the mitochondrial Trx-system remains mechanistically unexplained (see Introduction). Disruption of TrxR1 gives the least severe of these phenotypes, with embryos surviving past E8.5 or E10.5, depending on allelic design [21, 22]. The extensive proliferation that these embryos exhibit prior to failure, along with more recent studies on proliferation of TrxR1-deficient cells bearing either of these two defective alleles [27, 30], makes it unlikely that replicative defects underlie the failure of TrxR1-deficeint embryos. The present study, as well as at least one classic study [24], point to competence of the Grx pathway in supporting proliferative growth in mammalian systems. Still, the GSH pathway is not essential, as mice lacking Gsr develop normally [18], and both txnrd1-wild-type and – heterozygous hepatocytes depleted of GSH replicate their DNA (Fig. 3S). Our results however reveal that, upon depletion of GSH, TrxR1 does become necessary for support of hepatocyte proliferation. This highlights the complexity and complementarity of these redox pathways. More detailed studies on all possible roles for TrxR1 in embryonic development, including for example in signaling, gene regulation, apoptosis, redox modulation, repair, or other processes, will be needed to understand why TrxR1 is critical for normal embryonic development. Based on the present study, we conclude that TrxR1 and GSH are two mutually complementary components, one of which is required for support of hepatocyte replication in mice.
Supplementary Material
Highlights.
Mouse hepatocytes genetically lacking TrxR1 exhibit normal proliferation rates.
Cytosolic variants of TrxR2 do not functionally replace TrxR1 in txnrd1-null livers.
Wild-type hepatocytes lacking GSH proliferate normally.
Hepatocytes lacking both TrxR1 and GSH exhibit severely reduced replication.
S-phase DNA replication in hepatocytes requires either TrxR1 or GSH.
Acknowledgments
The authors thank J.A. Kundert and E.A. Talago at Montana State University for technical support, A. Holmgren and J. Xu at the Karolinska Institutet for generously providing reagents, and G.F. Merrill at Oregon State University for discussions and assistance with the manuscript. This work was funded by grants from the U.S. National Institutes of Health National Cancer Institute and National Institute on Aging, and by an appointment from the Montana Agricultural Experiment Station to EES. Infrastructure support was provided by a COBRE grant to Montana State University. TAM was supported by a visiting summer Research Experiences for Undergraduates fellowship from the NSF. ESJA and SE received support from the Swedish Research Council (Medicine), the Swedish Cancer Society and Karolinska Institutet. None of these funding agencies played any role in study design; in the collection, analysis, or interpretation or data; in the writing of this report; or in the decision to submit this paper for publication.
Footnotes
Abbreviations used in this paper. a.a., amino acid; Alb, serum albumin mRNA or protein; albCre, a Cre transgene driven by the serum albumin gene promoter/enhancer; BrdU, bromodeoxyuridine; BSO, buthionine sulfoximine; C-, carboxyl-; Cre, bacteriophage P1 cyclization recombinase optimized for activity in mammalian cells; Cys, cysteine; dNTP, deoxyribonucleoside triphosphate; DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid) or Ellman’s reagent; EDTA, ethyldiaminetetraacetic acid; EST, expressed sequence tag; FAD, flavin-adeninedinucleotide; Grx, glutaredoxin; GSH, reduced glutathione; GSSG, oxidized glutathione; Gsr, glutathione reductase; HEPES, N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid); I.P., intraperitoneal; loxP, Cre-recognition sites; mRNA, messenger RNA; N-, amino-; P, postnatal; PCNA, proliferative cell nuclear antigen; PCR, polymerase chain reaction; PHH3, phosphohistone H3; qPCR, quantitative (“real-time”) RT-PCR; RNR, ribonucleotide reductase; RT-PCR, reverse transcriptase-mediated PCR; Sec, selenocysteine; S.E.M., standard error of the mean; Trx, thioredoxin; TrxR1 thioredoxin reductase 1 mRNA or protein; TrxR2, thioredoxin reductase 2 mRNA or protein; txnrd1, thioredoxin reductase 1 gene; txnrd2, thioredoxin reductase 2 gene; UTR, untranslated region.
The authors have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–58. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- 2.Atkin CL, et al. Iron and free radical in ribonucleotide reductase. Exchange of iron and Mossbauer spectroscopy of the protein B2 subunit of the Escherichia coli enzyme. J Biol Chem. 1973;248(21):7464–72. [PubMed] [Google Scholar]
- 3.Brown NC, et al. Nonheme iron as a cofactor in ribonucleotide reductase from E. coli. Biochem Biophys Res Commun. 1968;30(5):522–7. doi: 10.1016/0006-291x(68)90083-1. [DOI] [PubMed] [Google Scholar]
- 4.Stubbe JA. Mechanism of B12-dependent ribonucleotide reductase. Mol Cell Biochem. 1983;50(1):25–45. doi: 10.1007/BF00225278. [DOI] [PubMed] [Google Scholar]
- 5.Laurent TC, Moore EC, Reichard P. Enzymatic Synthesis Of Deoxyribonucleotides. Iv. Isolation And Characterization Of Thioredoxin, The Hydrogen Donor From Escherichia Coli B. J Biol Chem. 1964;239:3436–44. [PubMed] [Google Scholar]
- 6.Holmgren A. The function of thioredoxin and glutathione in deoxyribonucleic acid synthesis. Biochem Soc Trans. 1977;5(3):611–2. doi: 10.1042/bst0050611. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren A. Glutathione-dependent synthesis of deoxyribonucleotides. Characterization of the enzymatic mechanism of Escherichia coli glutaredoxin. J Biol Chem. 1979;254(9):3672–8. [PubMed] [Google Scholar]
- 8.Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A. 1976;73(7):2275–9. doi: 10.1073/pnas.73.7.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweat TA, Wolpert TJ. Thioredoxin h5 is required for victorin sensitivity mediated by a CC-NBS-LRR gene in Arabidopsis. Plant Cell. 2007;19(2):673–87. doi: 10.1105/tpc.106.047563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichheld JP, et al. Inactivation of thioredoxin reductases reveals a complex interplay between thioredoxin and glutathione pathways in Arabidopsis development. Plant Cell. 2007;19(6):1851–65. doi: 10.1105/tpc.107.050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koc A, et al. Thioredoxin is required for deoxyribonucleotide pool maintenance during S phase. J Biol Chem. 2006;281(22):15058–63. doi: 10.1074/jbc.M601968200. [DOI] [PubMed] [Google Scholar]
- 12.Muller EG. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991;266(14):9194–202. [PubMed] [Google Scholar]
- 13.Stenvall J, et al. Selenoprotein TRXR-1 and GSR-1 are essential for removal of old cuticle during molting in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2011;108(3):1064–9. doi: 10.1073/pnas.1006328108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sagemark J, et al. Redox properties and evolution of human glutaredoxins. Proteins. 2007;68(4):879–92. doi: 10.1002/prot.21416. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2000;2(4):811–20. doi: 10.1089/ars.2000.2.4-811. [DOI] [PubMed] [Google Scholar]
- 16.Avval FZ, Holmgren A. Molecular mechanisms of thioredoxin and glutaredoxin as hydrogen donors for Mammalian s phase ribonucleotide reductase. J Biol Chem. 2009;284(13):8233–40. doi: 10.1074/jbc.M809338200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren A, Sengupta R. The use of thiols by ribonucleotide reductase. Free Radic Biol Med. 2010;49(11):1617–28. doi: 10.1016/j.freeradbiomed.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Rogers LK, et al. Analyses of glutathione reductase hypomorphic mice indicate a genetic knockout. Toxicol Sci. 2004;82(2):367–73. doi: 10.1093/toxsci/kfh268. [DOI] [PubMed] [Google Scholar]
- 19.Matsui M, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol. 1996;178(1):179–85. doi: 10.1006/dbio.1996.0208. [DOI] [PubMed] [Google Scholar]
- 20.Nonn L, et al. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23(3):916–22. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakupoglu C, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25(5):1980–8. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bondareva AA, et al. Effects of thioredoxin reductase-1 deletion on embryogenesis and transcriptome. Free Radic Biol Med. 2007;43(6):911–23. doi: 10.1016/j.freeradbiomed.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conrad M, et al. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24(21):9414–23. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansson HA, et al. Different cellular distribution of thioredoxin and subunit M1 of ribonucleotide reductase in rat tissues. Exp Cell Res. 1986;163(2):363–9. doi: 10.1016/0014-4827(86)90067-4. [DOI] [PubMed] [Google Scholar]
- 25.Yoo MH, et al. Targeting thioredoxin reductase 1 reduction in cancer cells inhibits self-sufficient growth and DNA replication. PLoS ONE. 2007;2(10):e1112. doi: 10.1371/journal.pone.0001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo MH, et al. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem. 2006;281(19):13005–8. doi: 10.1074/jbc.C600012200. [DOI] [PubMed] [Google Scholar]
- 27.Mandal PK, et al. Loss of thioredoxin reductase 1 renders tumors highly susceptible to pharmacologic glutathione deprivation. Cancer Res. 2010;70(22):9505–14. doi: 10.1158/0008-5472.CAN-10-1509. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt EE, Schibler U. Cell size regulation, a mechanism that controls cellular RNA accumulation: consequences on regulation of the ubiquitous transcription factors Oct1 and NF-Y and the liver-enriched transcription factor DBP. J Cell Biol. 1995;128(4):467–83. doi: 10.1083/jcb.128.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melchiorri C, et al. Ploidy and nuclearity of rat hepatocytes after compensatory regeneration or mitogen-induced liver growth. Carcinogenesis. 1993;14(9):1825–30. doi: 10.1093/carcin/14.9.1825. [DOI] [PubMed] [Google Scholar]
- 30.Rollins MF, et al. Hepatocytes lacking thioredoxin reductase 1 have normal replicative potential during development and regeneration. J Cell Sci. 2010;123(Pt 14):2402–12. doi: 10.1242/jcs.068106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suvorova ES, et al. Cytoprotective Nrf2 pathway is induced in chronically txnrd 1-deficient hepatocytes. PLoS One. 2009;4(7):e6158. doi: 10.1371/journal.pone.0006158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weisend CM, et al. Cre activity in fetal albCre mouse hepatocytes: Utility for developmental studies. Genesis. 2009;47(12):789–92. doi: 10.1002/dvg.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turanov AA, Su D, Gladyshev VN. Characterization of alternative cytosolic forms and cellular targets of mouse mitochondrial thioredoxin reductase. J Biol Chem. 2006;281(32):22953–63. doi: 10.1074/jbc.M604326200. [DOI] [PubMed] [Google Scholar]
- 34.Luthman M, et al. Glutathione-dependent hydrogen donor system for calf thymus ribonucleoside-diphosphate reductase. Proc Natl Acad Sci U S A. 1979;76(5):2158–62. doi: 10.1073/pnas.76.5.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Postic C, et al. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274(1):305–15. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- 36.Iverson SV, et al. Contributions of new hepatocyte lineages to liver growth, maintenance, and regeneration in mice. Hepatology. 2011 doi: 10.1002/hep.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffith OW, Meister A. Origin and turnover of mitochondrial glutathione. Proc Natl Acad Sci U S A. 1985;82(14):4668–72. doi: 10.1073/pnas.82.14.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Williamson JM, Boettcher B, Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci U S A. 1982;79(20):6246–9. doi: 10.1073/pnas.79.20.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt EE, et al. Fundamental cellular processes do not require vertebrate-specific sequences within the TATA-binding protein. J Biol Chem. 2003;278(8):6168–74. doi: 10.1074/jbc.M211205200. [DOI] [PubMed] [Google Scholar]
- 40.Arner ES, Zhong L, Holmgren A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 1999;300:226–39. doi: 10.1016/s0076-6879(99)00129-9. [DOI] [PubMed] [Google Scholar]
- 41.Eriksson SE, et al. High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic Biol Med. 2009;47(11):1661–71. doi: 10.1016/j.freeradbiomed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Newport J, Dasso M. On the coupling between DNA replication and mitosis. J Cell Sci Suppl. 1989;12:149–60. doi: 10.1242/jcs.1989.supplement_12.13. [DOI] [PubMed] [Google Scholar]
- 43.Pontarin G, et al. p53R2-dependent ribonucleotide reduction provides deoxyribonucleotides in quiescent human fibroblasts in the absence of induced DNA damage. J Biol Chem. 2007;282(23):16820–8. doi: 10.1074/jbc.M701310200. [DOI] [PubMed] [Google Scholar]
- 44.Jurado J, et al. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J Biol Chem. 2003;278(46):45546–54. doi: 10.1074/jbc.M307866200. [DOI] [PubMed] [Google Scholar]
- 45.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267(20):6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 46.Li J, et al. Role of Nrf2-dependent ARE-driven antioxidant pathway in neuroprotection. Methods Mol Biol. 2007;399:67–78. doi: 10.1007/978-1-59745-504-6_6. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen T, Sherratt PJ, Pickett CB. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol. 2003;43:233–60. doi: 10.1146/annurev.pharmtox.43.100901.140229. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi A, Ohta T, Yamamoto M. Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods Enzymol. 2004;378:273–86. doi: 10.1016/S0076-6879(04)78021-0. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen T, Nioi P, Pickett CB. The nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284(20):13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arner ES. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790(6):495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 51.Dammeyer P, Arner ES. Human Protein Atlas of redox systems - what can be learnt? Biochim Biophys Acta. 2011;1810(1):111–38. doi: 10.1016/j.bbagen.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Godoy JR, et al. Redox atlas of the mouse. Immunohistochemical detection of glutaredoxin-, peroxiredoxin-, and thioredoxin-family proteins in various tissues of the laboratory mouse. Biochim Biophys Acta. 2011;1810(1):2–92. doi: 10.1016/j.bbagen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 53.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16(6):420–6. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.