Abstract
Tubulin glutamylation is a post-translational modification (PTM) occurring predominantly on ciliary axonemal tubulin and has been suggested to be important for ciliary function 1,2. However, its relationship to disorders of the primary cilium, termed ‘ciliopathies’, has not been explored. Here, in Joubert syndrome (JBTS) 3, we identify the JBTS15 locus and the responsible gene as CEP41, encoding a centrosomal protein of 41 KDa 4. We show that CEP41 is localized to the basal body/primary cilium, and regulates the ciliary entry of TTLL6, an evolutionarily conserved polyglutamylase enzyme 5. Depletion of CEP41 causes ciliopathy-related phenotypes in zebrafish and mouse, and induces cilia axonemal glutamylation defects. Our data identify loss of CEP41 as a cause of JBTS ciliopathy and highlight involvement of tubulin PTM in pathogenesis of the ciliopathy spectrum.
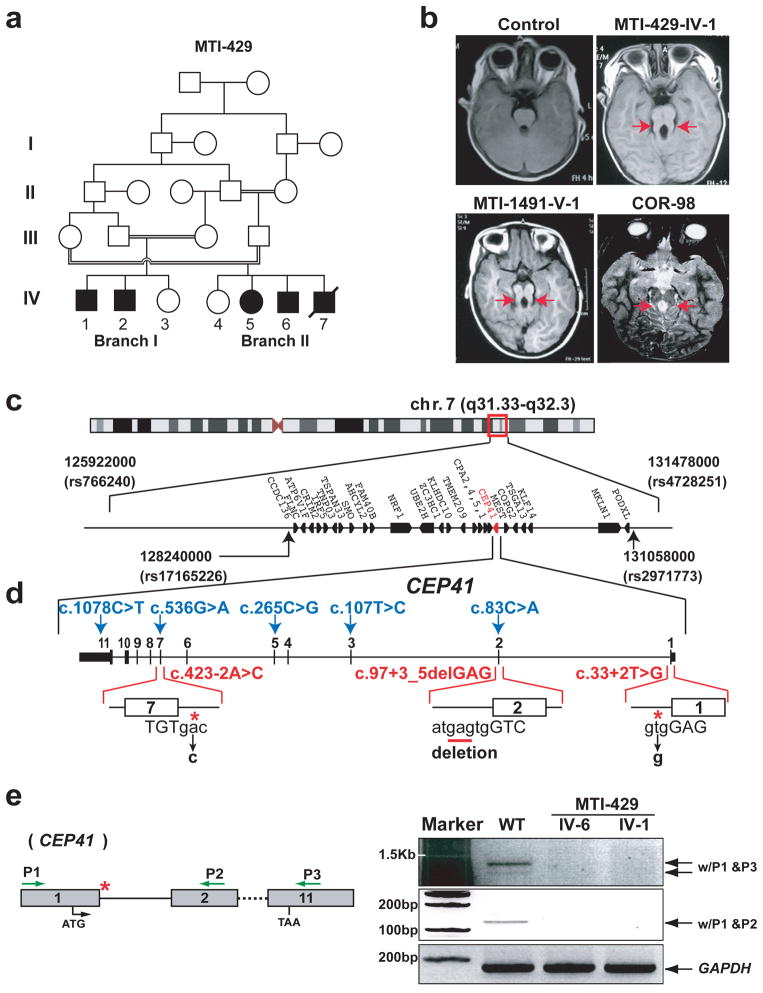
Joubert syndrome (OMIM 213300) is characterized by cerebellar hypoplasia, and neurological features including ataxia, psychomotor delay and oculomotor apraxia with a pathognomonic “molar tooth sign” on brain imaging. JBTS is frequently accompanied by various multiorgan signs and symptoms including retinal dystrophy, nephronophthisis, liver fibrosis and polydactyly, conditions associated with disorders of the ciliopathy spectrum of diseases that include Meckel-Gruber Syndrome (MKS), Bardet-Biedl Syndrome (BBS) and Nephronophthisis (NPHP). Though several causative genes have been found for these disorders, they account for less than 50% of cases 6,7. We recruited a consanguineous two-branch Egyptian family (MTI-429) with five affected members (Fig. 1a–b, Table 1). We excluded linkage to previously identified JBTS loci using a panel of highly informative markers. Analysis of the family using whole genome Illumina 5K SNP Linkage chip Ver. IV scan identified a 5 Mbp region of linkage on chromosome 7q31.33–32.3, with a peak multipoint LOD score of 3.71, thus defining the JBTS15 locus. Haplotype analysis suggested a candidate interval between rs766240 and rs4728251 delineating the peak of highest significance (Supplementary Fig. 1a–b).
Figure 1.
Identification of mutations in CEP41 in affected individuals linked to the JBTS15 locus. (a) Pedigree MTI-429 shows double first cousin marriage with five affected offspring. (b) Axial brain MRI images from patients with CEP41 mutations in each MTI-429, MTI-1491 (T1-weighted) and COR-98 (T2-weighted) family, showing the ‘molar tooth sign’ (red arrows). (c) JBTS15 is 5.5 Mbp located at Chr. 7q31.33–32.3 (red box) defined by rs766240 and rs4728251. Further narrowing of the interval to 2.8 Mbp based upon denser SNP scan to encompass rs17165226 and rs2971773 (black arrows). (d) CEP41 genomic organization, depicting locations of identified base changes including homozygous (red) splice mutations and heterozygous (blue) missense or nonsense mutations. Capital letters: exon sequences, small letters: intron sequences, asterisks: point mutations, underline: deletion. (e) RT-PCR confirmation of the splicing defect of CEP41 in MTI-429 patient fibroblasts. Both CEP41 mutant cells failed to produce CEP41 mRNA, compared with WT. GAPDH is control. Green arrows: positions of primers, asterisk: region of splice mutation in MTI-429.
Table 1.
Abbreviations. AG: Ambiguous genitalia, B: Bilateral, F: Female, GHD: Growth hormone deficiency, M: Male, MP: Micropenis, N: No, N/A: Not available/Not applicable, NPHP: Nephronophthisis, OMA: oculomotor arpaxia, PDSV: potentially deleterious sequence variant, UCD: Urinary concentration defect, U: Unilateral, Y: Yes
| Demographic Information | ||||||||
|---|---|---|---|---|---|---|---|---|
| Family ID | MTI-429-IV-1 | MTI-429-IV-2 | MTI-429-IV-5 | MTI-429-IV-6 | MTI-429-IV-7 | MTI-1491-V-2 | MTI-1491-V-3 | COR-98 |
| Country of orgin | Egypt | Egypt | Egypt | Egypt | Egypt | Egypt | Egypt | Portugal |
| Patient (sex) | M | M | F | M | M | F | F | M |
| Death | N | N | N | N | died at 7 days | N | N | N |
| Documented Consanguinity | Y | Y | Y | Y | Y | Y | Y | Y |
| Neurological signs | ||||||||
| Hypotonia/Ataxia | Y | Y | Y | Y | Y | Y | Y | Y |
| Psychomotor Delay | Y | Y | Y | Y | N/A | Y | Y | Y |
| Mental Retardation | mild | borderline | borderline | borderline | N/A | Y | Y | Y |
| OMA | Y | Y | N | N | N/A | Y | Y | N |
| Breathing Abnormalities | N | N | N | N | Y | Y | Y | N |
| Head Circumference | 50%ile | 50%ile | 50%ile | 50%ile | 75%ile | 50%ile | 50%ile | N/A |
| Ocular Signs | ||||||||
| Retinopathy | N | N | N | N | U | N | N | Y |
| Other abnormalities | B ptosis | U ptosis and leukoma | U ptosis | N | N | U ptosis, squint, leukoma | B squint and leukoma | N |
| Coloboma | N | N | N | N | U | N | N | N |
| Renal signs | ||||||||
| NPHP/UCD | N | N | N | N | U | N | N | N |
| Kidney ultrasound | N | N | N | N | N | N | N | N |
| Other organs | ||||||||
| Liver abnormalities | N | N | N | N | M | N | N | N |
| Polydactyly | N | N | U postaxial | U postaxial | B postaxial | N | U postaxial | Y |
| Other abnormalities | GHD, MP | GHD, MP | N | MP | AG, MP, hypoplastic scrotum | N | N | N |
| MRI reading | ||||||||
| MTI | Y | Y | Y | Y | Y | Y | Y | Y |
| Other abnormalities | N | N | N | N | N | N | N | N |
| CEP41 mutation analysis | ||||||||
| exon(s) | 1 | 2 | 7 | |||||
| Hetero/Homozygous | Homozygous | Homozygous in JS | Homozygous | |||||
| Nucleotide change | c.33+2T>G | c.97+3_5delGAG | c.423-2A>C | |||||
| Amino acid change | Splice | Splice | Splice | |||||
| Amino acid consequence | Premature stop | Premature stop | Predicted Premature stop | |||||
| Comprehensive JSRD Geneti Screening | ||||||||
| Linkage | Linkage chr7 (q31.33–q32.3) | Linkage negative to AHI1, TMEM67-, TMEM138/216- | Linkage negative to AHI1, TMEM67-, TMEM138/216- | |||||
| Additional gene with PDSV | None | None | CEP290 | |||||
| exon | 31 | |||||||
| Hetero/Homozygous | Heterozygous | |||||||
| Nucleotide change | c.3626C>G | |||||||
| Amino acid change | p.1209S>C | |||||||
| Amino acid consequence | Neutral to neutral | |||||||
| In NCBI SNP Database | No | |||||||
In order to further narrow the interval, we re-analyzed MTI-429 with the denser Affymetrix 250K NspI SNP array by applying a linkage-free IBD model 8. The combination of the 5K SNP and 250K SNP linkage analyses generated a 2.8 Mbp IBD interval between rs17165226 and rs2971773 containing 26 genes (Fig. 1c). Direct sequence analyses of candidate genes within the interval led to the identification of a homozygous c.33+2T>G base change from reference sequence NM_018718, which was predicted to abolish the consensus splice donor site from exon 1 of the CEP41 gene (Fig. 1d, Supplementary Fig. 2a). To confirm a mutation-specific splicing defect, we evaluated CEP41 transcripts from MTI-429 primary patient fibroblasts (MTI-429-IV-1 and -IV-6). The RT-PCR result showed an absence of mature CEP41 mRNA products in both affected patient cells, likely attributed to nonsense mediated decay (Fig. 1e).
We next screened an additional 832 ciliopathy patients: 720 of JBTS and 112 of MKS (many of whom were excluded for mutations in known ciliopathy genes) by directly sequencing CEP41 and found two additional consanguineous families with homozygous mutations: c.97+3_5delGAG in an Egyptian JBTS family (MTI-1491) and c.423-2A>C in a Portuguese JBTS family (COR-98) (Fig. 1b–d, Supplementary Fig. 2a). These mutations were predicted to abolish the consensus splice donor site from exon 2 and the splice acceptor site from exon 7, respectively. Moreover, we confirmed that the mutation in MTI-1491 led to skipping of exon 2, thereby generating a premature stop in exon 3 (Supplementary Fig. 2b). Interestingly, in addition to the JBTS patients, the MTI-1491 family included one individual that was consistent with a phenotype of BBS and lacked the pathognomonic “molar tooth sign”, and this patient was heterozygous for the c.97+3_5delGAG mutation (Supplementary Fig. 2b–d), suggesting CEP41 may modify other ciliopathy conditions. From our cohort screen, we further identified heterozygous CEP41 mutations (c.83C>A, c.107T>C, c.265C>G, c.536G>A, c.1078C>T), which altered highly conserved amino acid residues among vertebrates or led to a premature stop codon from five different families (Fig. 1d, Supplementary Fig. 2e, Supplementary Table 1). Each of these CEP41-mutated patients was additionally sequenced at the known JBTS genes, and in four there was an additional heterozygous potentially deleterious variant. It was notable that all homozygous mutations in CEP41 were splice site mutations and identified only in JBTS patients while heterozygous variants were present in several ciliopathies including BBS and MKS. Our findings suggest that constitutive disruptions of CEP41 result in JBTS, but that it may also serve as a modifier in the broader class of ciliopathies.
The CEP41 gene has been poorly characterized except for its expression analysis in human organs including brain, testis and kidney 9. CEP41 encodes for Centrosomal Protein 41 KDa, predicted to contain two coiled-coils and a rhodanese-like domain (RHOD), which is structurally related to the catalytic subunit of the Cdc25 class of phosphatases 10. However, we found that it lacks phosphatase activity in the in vitro para-Nitrophenylphosphate (pNPP) phosphatase assay (not shown). The RHOD domain therefore may be an enzymatically inactive version like some described RHOD domains in other proteins 10 functioning in protein interactions.
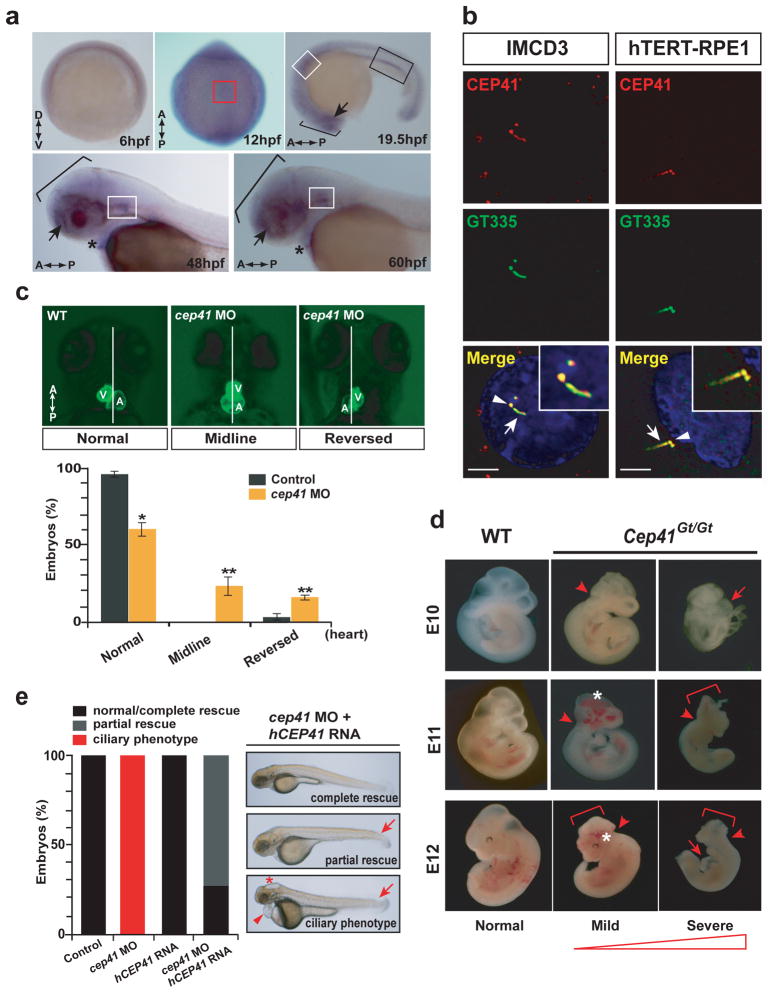
We next examined CEP41 gene expression at the mRNA level and CEP41 protein subcellular localization. In zebrafish, cep41 was expressed in the various ciliary organs including Kupffer’s vesicle (KV), ear and heart as well as brain and kidney, regions predominantly affected in JBTS (Fig. 2a, Supplementary Fig. 3). In several ciliated cell lines such as mouse inner medullary collecting duct (IMCD3) cells and human retinal pigment epithelial (hTERT-RPE1) cells, endogenous CEP41 was predominantly noted at the centrioles and cilia (Fig. 2b). The cilia-associated expression/localization of CEP41 prompted us to assess a possible role in cilia-related function. Accordingly, we performed knockdown experiments using translation blocking morpholino anti-sense oligonucleotides (MOs) in zebrafish. In cep41 MOs-injected embryos (morphants), we observed peripheral heart edema and tail defects along with ciliopathy-related phenotypes including hydrocephalus, abnormal ear otolith formation and smaller eyes 11–14 (Supplementary Fig. 4a–b). We further found that injection of cep41 MOs induced a decrease in production of the protein with a dose-dependent phenotypic severity in the embryos (Supplementary Fig. 4b–c).
Figure 2.
CEP41 is expressed in ciliated tissues and its loss recapitulates ciliopathy-related phenotypes in zebrafish and mouse. (a) Zebrafish cep41 mRNA is expressed ubiquitously at gastrulation stages (6 hpf), but at later stages, it is specifically expressed in ciliated organs: Kupffer’s vesicle (red box), inner ear (white boxes), brain (brackets), eyes (arrows), pronephric duct (black box), and heart (asterisks). A, anterior; D, dorsal; P, posterior; V, ventral. (b) CEP41 is predominantly localized to the basal body (arrowheads) and primary cilium (arrows) in ciliated IMCD3 and hTERT-RPE1 cells. Insets: co-localization of CEP41 with GT335, a marker of basal bodies and cilia. Scale bar 5 μm. (c) Knockdown of cep41 by injection of morpholino oligonucleotide (MO) causes heart asymmetry defects in Tg (myl7:egfp) zebrafish embryos. The cep41 morphants show either loss of asymmetry (midline) or inversion of V/A asymmetry (reversed) at 72hpf. *P < 0.01, **P < 0.001. Error bars: s.e.m. A, atrium; L, left; R, right; V, ventricle. (d) Murine Cep41 gene-trap shows altered embryonic morphogenesis. Range of phenotypes of Cep41Gt/Gt embryos includes mild malformed hindbrain (arrowheads), exencephaly (brackets), hemorrhage in the head (asterisk), dilated pericardial sac (arrows), failure to rotate and lethality at E10–12. (e) Injection of human CEP41 RNA into cep41 morphants rescued ciliary phenotypes of pericardial edema (arrowhead), hydrocephalus (asterisk) and curved tail (arrow), completely or partially.
Cilia of KV, a structure that corresponds to the mammalian embryonic node, are essential to mediate lateral asymmetry 15. Accordingly, the defect of left/right (L/R) asymmetry in the heart, a well-established ciliopathy phenotype in mammals, is also shown in zebrafish 16–18. To examine whether cep41 depletion results in the phenotype, we injected cep41 MOs into Tg(myl7:egfp) zebrafish, a myocardium-specific transgenic reporter line, and found heart asymmetry defects such as inversion or failure to develop asymmetry of the ventricle and atrium (Fig. 2c). We also generated a Cep41 knockout mouse line using a genetrap strategy (Supplementary Fig. 5a–c) and characterized its phenotype at E10–13. The homozygous Cep41Gt/Gt embryos showed a range of phenotypes: malformed hindbrain, exencephaly, brain hemorrhage, dilated pericardial sac and lethality as well as unexpected normal development in some homozygous mutants (Fig. 2d, Supplementary Fig. 5d–e, Supplementary Table 2). Although exencephaly, dilated heart and embryonic lethality suggest possible ciliary roles of Cep41 in mouse 16,19,20, the phenotypic variability including normal development suggests the presence of extragenic phenotypic modifiers. We next investigated a genetic rescue using human CEP41 in zebrafish cep41 morphants, and found partial rescue of the cilia-associated morphant phenotype (Fig. 2e). The data suggest a potential evolutionarily conserved role of CEP41 in ciliary function.
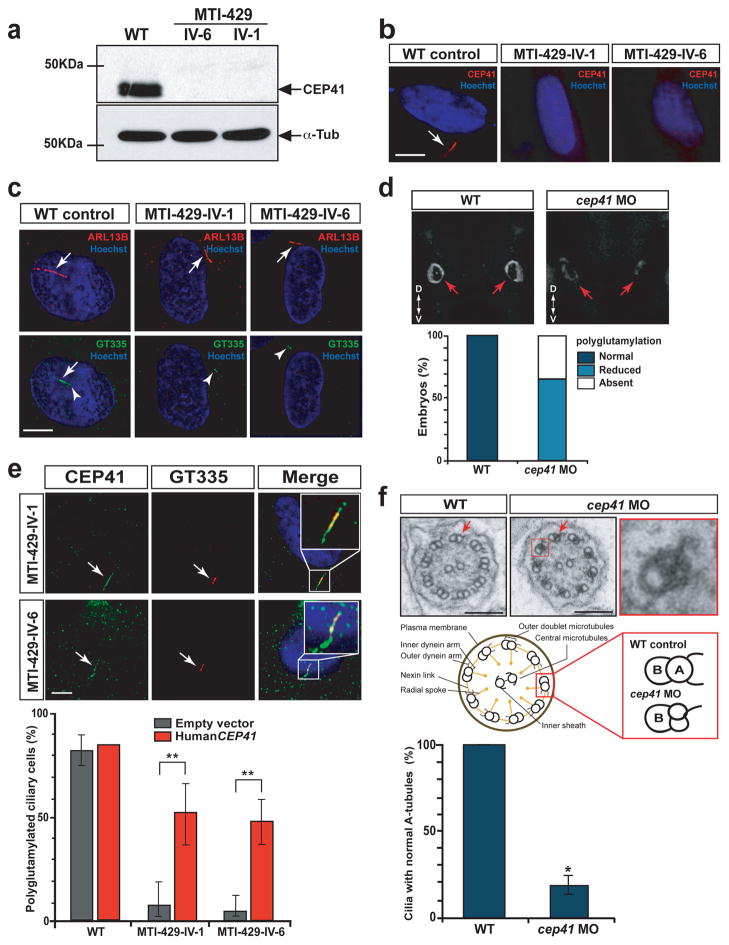
In order to explore possible roles of CEP41, we examined primary cultured fibroblasts of patients (MTI-429-IV-1 and -IV-6). We first tested whether CEP41 mutant cells were devoid of CEP41 protein. Consistent with RT-PCR results (Fig. 1e), neither patient fibroblast line tested produced detectable CEP41, suggesting that these mutant cells are nearly null for CEP41 (Fig. 3a–b). In order to examine the effect of CEP41 loss on cilia assembly, we induced ciliogenesis using serum starvation-mediated cell cycle arrest in confluent cells and visualized cilia by co-immunostaining using anti-ARL13B (cilia marker) 21 and GT335 (centrioles/cilia marker) 22 antibodies. In control fibroblasts, cilia were evident in 70 % of the total stained cells by 48 hr and 72 hr and nearly all co-stained with both markers (Fig. 3c, Supplementary Fig. 6). However, in the CEP41 mutant fibroblasts, cilia were stained positively with ARL13B but not with GT335 (Fig. 3c). We quantified this effect and found that, whereas the percent of ARL13B-positive ciliated cells in the mutant fibroblasts was approximately equal to control fibroblasts, the percentage of GT335-positive ciliated cells was dramatically reduced in mutant fibroblasts (Supplementary Fig. 6). The GT335 antibody was originally generated to recognize the glutamylated forms (both mono- and polyglutamylation) of tubulin 23. Therefore, the data suggest a potential role of CEP41 in regulating tubulin glutamyl posttranslational modifications (PTMs).
Figure 3.
CEP41 is required for tubulin glutamylation at the ciliary axoneme. (a) Absent CEP41 protein in CEP41 mutant patient cells. (b) Ciliary localization of endogenous CEP41 in human fibroblasts and loss of the protein in CEP41 mutant cilia. Scale bar 5 μm. (c) WT cells have both GT335-positive basal bodies (arrowheads) and cilia (arrows, marked by ARL13B), while mutant cells show staining of GT335 only at the basal bodies. Scale bar 5 μm. (d) Depletion of cep41 causes glutamylation defects in zebrafish olfactory placode cilia (red arrows). Images of GT335-stainined WT and cep41 morphant embryos were taken in anterior view (head is up and tail is down, D, dorsal; V, ventral), quantified below. (e) Exogenous CEP41 expression restores ciliary axoneme glutamylation in CEP41 mutant cells. Arrows: primary cilia stained for CEP41 and GT335. Insets: merged images at higher power, quantified below. Scale bar 5 μm. **P < 0.001; error bars = s.e.m. (f) Ultrastructural analysis of the pronephric ciliary axoneme at 72 hpf zebrafish embryos. Compared to WT, cep41 morphants have A-tubule specific defects in the outer doublet microtubules. Arrows: A-tubules and one of nine outer doublet microtubules is magnified in the red box. The numbers of cilia, categorized in normal and abnormal A-tubules according to the schematic, were counted in both WT embryos and cep41 morphants (n = 3 embryos, >20 cilia per animal), quantified below. Scale bar 100 nm. **P < 0.01; error bars = s.e.m.
Microtubules are the major structural scaffolds of the ciliary axoneme, composing the 9+0 or 9+2 arrangement, and undergo several PTMs including acetylation, detyrosination, glycylation and glutamylation (Supplementary Fig. 7). We therefore tested the effect of CEP41 deficiency on these tubulin PTMs and found no significant defects other than glutamylation in CEP41 mutant cells (Supplementary Fig. 8). In addition, the result of immunostaining using PolyE antibody (specific for polyglutamylated tubulins) in mutant fibroblasts suggested that CEP41 might function in regulating both tubulin mono- and polyglutamylation (Supplementary Fig. 9). Concurrently, we observed an effect of cep41 deficiency on tubulin glutamylation as well as mildly reduced glycylation of the placode cilia in zebrafish (Fig. 3d, Supplementary Fig. 10). Forced expression of exogenous CEP41 in the mutant fibroblasts remarkably increased the percentage of cells displaying glutamylated cilia (Fig. 3e), suggesting that glutamylation of the cilium is dependent on the expression of CEP41. Furthermore, we found that cell lines from patients with mutations in other JBTS genes including TMEM216 and INPP5E displayed no such glutamylation defect (not shown), suggesting the phenotype of tubulin glutamylation phenotype is not a non-specific consequence of ciliopathy mutation. Together these data suggest that CEP41 is required for ciliary glutamylation, but not necessary for initial cilia assembly.
Recent studies have shown that defective tubulin PTM is associated with altered ciliary axonemal structure 24–26. Accordingly, we investigated whether the CEP41-dysfunctional cilia exhibiting glutamylation defects gave rise to abnormal axonemal structure. Transmission electron microscopy (TEM) analysis demonstrated that the depletion of cep41 resulted in apparent structural defects in zebrafish renal cilia: specifically A tubules of the outer doublet microtubules of the axoneme were collapsed and/or duplicated (Fig. 3f, Supplementary Fig. 11). Previous studies have suggested that ciliary structural disruption affects ciliary motility 27,28, thus we examined the effect on the cilia of KV and kidney in zebrafish cep41 morphants and found disabled motility of both cilia (Supplementary Fig. 12, Supplementary Mov. 1–6). Our data suggest that CEP41 is involved in ciliary structural formation and motility by playing an essential role in tubulin glutamylation at the cilium.
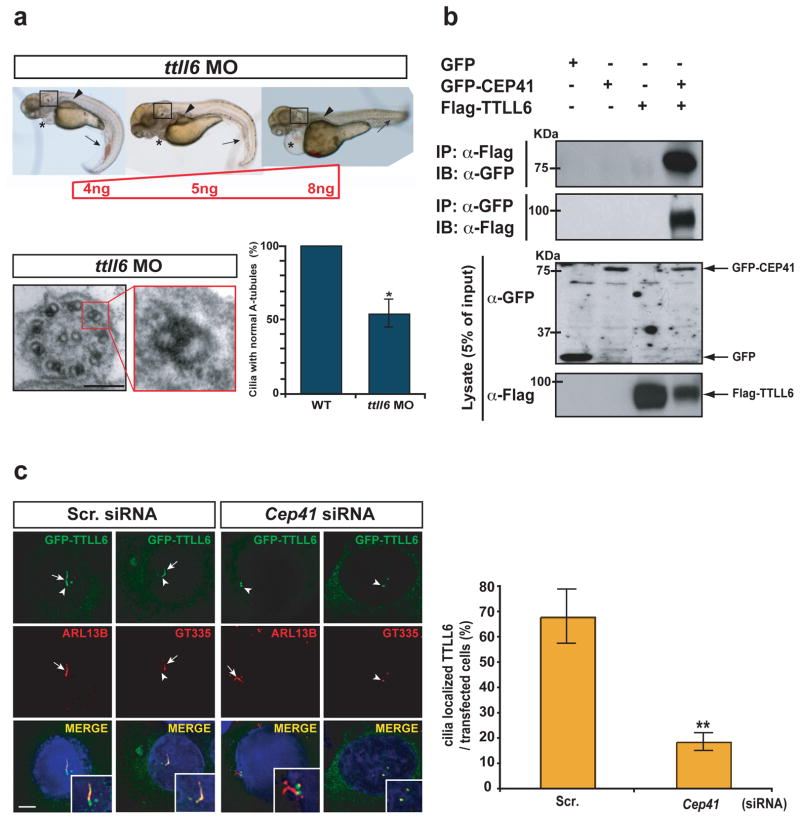
We next investigated how CEP41 modulates microtubule PTM at the ciliary axoneme. We noted that only microtubules of ciliary axonemes failed glutamylation, whereas those of centrioles were properly modified in CEP41 mutant patient cells. (Fig. 3c). Furthermore, the lack of a Tubulin Tyrosine Ligase (TTL) domain in CEP41, requisite for enzyme activity 5, implied that it is unlikely to serve as a glutamylase. The main enzymes mediating tubulin glutamylation are members of the conserved TTL-like (TTLL) family 5. Among several identified TTLL factors, we found consistently strong localization of TTLL6 at the basal body (the organizing structure at the base of cilium, derived from a mother centriole) and the cilium (Supplementary Fig. 13). In addition, previous studies have suggested that TTLL6 may be involved in ciliary function in several organisms including zebrafish 5,24,29. We therefore examined the effects of ttll6 deficiency using a ttll6 translational blocking MO 30 in zebrafish and observed ciliopathy-related morphological phenotypes, similar to although less severe than what we observed following cep41 knockdown (Fig. 4a). Additionally, ttll6 morphants showed A-tubule axonemal defects, similar to those of cep41 morphants (Fig. 4a), besides diverse axonemal structural defects, which were consistent with a previous report 30 (not shown). These data prompted us to test a possible functional relationship between CEP41 and TTLL6 by pair-wise co-immunoprecipitation (coIP), and found that the two proteins were part of a complex (Fig. 4b). Given evidence suggesting a candidate regulator associated with the transport of a polyglutamylase between the basal body and the cilium 29, we investigated a possible role for CEP41 in TTLL6 localization. Following efficient knockdown of Cep41 in IMCD cells using siRNA (Supplementary Fig. 14), we found localization of TTLL6 restricted mainly to the basal bodies, suggesting a block of entry of TTLL6 into the cilium (Fig. 4c). These data suggest that CEP41 functions in tubulin glutamylation by mediating transport of TTLL6 between the basal body and cilium.
Figure 4.
CEP41 interacts with TTLL6 and is required for localizing TTLL6 to the cilium. (a) Morpholino knockdown of zebrafish ttll6 associates with ciliary phenotypes such as curved tail (arrows), abnormal number/orientation of ear otolith (boxes), cystic kidney (arrowheads) and peripheral cardiac edema (asterisks) at different dosages indicated and show A-tubule specific defects in the outer doublet microtubules. The numbers of defective cilia were counted in both WT embryos and ttll6 morphants (n = 3 embryos, >20 cilia per animal), quantified below. (b) GFP-CEP41 was immunoprecipitated with anti-Flag antibody recognizing Flag-tagged TTLL6 from whole-cell extract (WCE), compared with GFP-empty vector. In the reciprocal co-IP experiment with anti-GFP antibody, the interaction between CEP41 and TTLL6 was confirmed. (c) Disturbed localization of TTLL6 to the cilium following Cep41 siRNA co-transfection with GFP-TTLL6 into IMCD3 cells and immunostained with either GT335 or ARL13B antibody. Arrows: cilia; Arrowheads: basal bodies. Cells expressing ciliary localized TTLL6 were counted only in siRNA-transfected cells quantified in graph. Scale bar 5 μm. *P< 0.01, **P < 0.001. Error bars = s.e.m.
Our finding of CEP41 mutations in JBTS patients provides the first evidence directly linking defective tubulin glutamylation at the cilium to a cause of ciliopathies. Consistent with previous studies implicating tubulin PTMs in cilia function 23,24,31,32, our data suggest CEP41-mediated ciliary glutamylation is essential for axonemal formation. Moreover, we found that CEP41 is required for transport of TTLL6 to modulate tubulin glutamylation, although it remains to be determined how these molecules co-function. Most likely, these proteins enter through the ciliary diffusion barrier at the transition zone 33,34 and are then transported along ciliary microtubules by intraflagellar transport (IFT) motor proteins. Thus examining whether CEP41 and TTLL6 form a complex with other factors at these locations will follow as a future study. Because tubulin glycylation is partially affected in cep41 morphants and the phenotype of cep41 morphants is more severe than ttll6 morphants, it is likely that CEP41 modulates other TTLL protein family members 5,30. Evidence for this possibility has been described in recent studies suggesting involvement of both tubulin- glycylation and glutamylation, each regulated by different TTLL-family members, in maintaining ciliary structure and motility 30,35–37.
METHODS
Research subjects
MTI-429 family and additional families were recruited worldwide based upon the presence of at least one individual with a neuroradiographically proven ‘molar tooth sign’ associated with any JBTS or related disorder (JSRD) phenotype. Whenever possible, patients underwent a full diagnostic protocol as previously reported 38 and a standardized clinical questionnaire was obtained to assess extent of multi-organ involvement. We used standard methods to isolate genomic DNA from peripheral blood of the affected and unaffected family members after obtaining informed consent from all participating. Human subject research was approved by the Ethics Boards of Leeds (East), CASA Sollievo della Sofferenza Hospital/CSS-Mendel Institute, Hôpital Necker-Enfants Malades, Human subjects divisions at the University of Washington, University of Michigan Institutional Review Board and Human Research Protection Program, University of California, San Diego.
Genome-wide screen and fine mapping
A 5K whole genome linkage SNP-scan was performed with family MTI-429 using the Illumina Linkage IVb mapping panel 39, and analyzed with easyLinkage-Plus software 40, which runs Allegro version 1.2c in a PC Windows interface to calculate multipoint LOD scores. Parameters were set to autosomal recessive with full penetrance, and disease allele frequency of 0.001. Fine mapping on the pedigree was performed with the Affymetrix 250K Nsp1 SNP array, and data were searched for common shared homozygous intervals from all affected family members using a custom script implemented in Mathematica (B. Merriman, unpublished). This script identifies all homozygous intervals longer than 2 Mbp for which there are no more than 1 % heterozygous calls (to permit potential genotyping errors).
Mutation screening
Mutational screening of CEP41 was performed by direct sequencing of the 11 coding exons and the adjacent intronic junctions in patients. PCR products obtained were treated with Exonuclease I (EXO) (Fermentas) and shrimp alkaline phosphatase (SAP) (Promega), and both strands were sequenced using a BigDye terminator cycle sequencing kit with an ABI3100 automated sequencer (Applied Biosystems). The primers and optimized PCR conditions used are depicted (Supplementary Table 3). Segregation of the identified mutations was investigated in all available family members. All identified mutations in CEP41 were not encountered in 96 ethnically matched controls (188 chromosomes) upon direct sequencing.
Bioinformatics
Genetic location is according to the March 2006 Human Genome Browser build hg18. The ciliary proteome was searched using web-based tools 41,42. Protein sequence conservation was determined using ClustalW multiple amino acid sequence alignment (see URL section).
Cloning
Full-length human CEP41 was cloned into the TOPO blunt vector, and then shuttled into EGFP- and HA- containing vectors. Human and zebrafish cep41 open reading frame were amplified by RT-PCR and cloned into the pCS2+ vector in order to synthesize RNA for injection into zebrafish embryos. Mouse Cep41 open reading frame was amplified and cloned into the GST-, EGFP-, and FLAG-conjugated vectors for biochemical assays.
Generation of Cep41 mutant mice
SIGTR ES cell line (AW0157), derived from 129P2/OlaHsd mice carrying a gene trap insertion 43 in the intron 1 of Cep41, was obtained from the European Conditional Mouse Mutagenesis Program (EUCOMM) and injected into C57BL/6 recipient blastocysts at the Mouse Biology Program, UC Davis. High-percentage chimeras (≥ 70 %) were bred to C57BL/6 for germline transmission. Gene-trapped mice were isolated by PCR for β-galactosidase-neomycin fusion gene and genotyped by Southern blot analysis hybridizing a ~10 kb (wild-type allele) and a ~8 kb (gene-trapped allele) product (Supplementary Fig. 5).
Zebrafish experiments
AB wild type zebrafish strains were used for in situ hybridization carried out by standard protocols using a DIG-labeled sense and anti-sense RNA probe for cep41. To knockdown zebrafish cep41, a translational blocking morpholino antisense oligonucleotide (MO, Gene Tools Inc) was used: 5′-CATCTTCCAGCAGCAGAGCTTCGGC-3′, diluted to appropriate concentrations in deionized sterile water, and injected into one-two cell stage embryos, obtained from natural spawning of zebrafish lines. To rescue the phenotypes in MO-injected embryos (morphants), RNA transcribed in vitro with the SP6 mMessage mMachine kit (Ambion) was co-injected. For characterization of ciliary defects in zebrafish embryos, the morphological phenotype of either cep41 or ttll6 morphants were observed until 5 days post-fertilization (dpf) and quantified under bright-field microscopy based upon previously established criteria 44. For western blot analysis at 1–2 dpf zebrafish control embryos and cep41 morphants, about 50 embryos with each genotype were deyolked and the embryo lysates were extracted with RIPA buffer. For immunostaining with GT335, Ac-Tub and PolyG Abs in whole-mount zebrafish embryos, 3 dpf control embryos and cep41 morphants were fixed in Dent’s fixative (80 % MeOH: 20 % DMSO) at 4 °C overnight and incubated with GT335 Ab (1:400), Ac-Tub (1:400) and PolyG (1:300) as primary and with anti-goat-mouse 594 (1:1000) as secondary in diluted blocking solution (10 % normal goat serum: 0.5 % Tween 20 in PBS).
Cell culture and transfection
IMCD3, hTERT-RPE1, human fibroblast, and human embryonic kidney (HEK293) cells were grown in appropriate DMEM or MEM media supplemented with 10–20 % fetal bovine serum (FBS) at 37 °C in 5 % CO2. Healthy human female and male control fibroblast cells were obtained from ATCC, and patient fibroblasts from skin biopsies were propagated in culture (≤ 5 passage number). Human fibroblast cells were transfected using the Basic Nucleofector Kit for Primary Mammalian Fibroblasts (Lonza). Other cells were transfected at 60–80 % confluency with plasmids or siRNAs using Lipofectamine 2000 (Invitrogen). The transfected cells were incubated for 24–72 hr with FBS or without FBS, dependent on the experimental purpose.
Fluorescence microscopy and transmission electron microscopy (TEM)
Images of immunofluorescent stained cells were obtained on a Deltavision RT Deconvolution microscope (Olympus IX70), under the same parameters for each experiment. Taken images were edited and analyzed using Adobe Photoshop CS. For electron microscopy, a standard protocol 45 was used except for one modification that tannic acid was included in the fixative to enhance the final contrast of the images. Formvar-coated slot grids (Electron microscopy Sciences) were used for sections (60–70 nm) to maximize visibility of the tissue and cross section performed to observe cilia axonemal structure.
Live imaging of zebrafish embryos
Zebrafish embryos (12 hpf for cilia in the KV and 2.5 dpf for the renal cilia) were transferred with embryo media to glass bottom culture dishes (MatTek), and 2.5 dpf embryos were anesthetized in tricaine solution (~0.016 mg/ml). Images were acquired for 30 sec – 2 min using a Perkin Elmer UltraView Vox Spinning Disk Confocal with EMCCD Hamamatsu 14 bit 1K × 1K camera, and edited with Volocity imaging software (Perkin Elmer).
Immunofluorescence and biochemical assay
For immunofluorescence, cells were fixed in 100 % methanol at −20 °C for 10 min. Primary antibodies used for immunofluorescence are: rabbit anti-CEP41, raised in rabbit to a purified bacterially expressed protein of GST-fused CEP41, (PRF&L, PA), mouse anti-acetylated-tubulin (Sigma), mouse GT335 Ab (gift from C. Janke), rabbit PolyE (gift from M. Gorovsky) and rabbit anti-ARL13B (gift from T. Caspary). We used Alexa 488- or Alexa 594-conjugated secondary antibodies (Molecular Probes) and Hoechst 33342 nuclear dye. All antibodies were diluted in 4 % normal donkey serum in PBS, primary antibodies were incubated for overnight at 4 °C and secondary antibodies were applied for 1 hr at room temperature. For immunoblotting, cells were extracted with RIPA lysis buffer 3 days after transfection and boiled with SDS sample buffer. Same concentrations of lysates were loaded for paired experiments. Primary antibodies used for western blotting were: rabbit anti-GFP (Roche), mouse anti-Flag (Sigma), and mouse anti-alpha-Tubulin (Sigma). Bound antibodies were detected using horseradish peroxidase-conjugated secondary antibodies (Pierce). For co-immunoprecipitation (co-IP), whole cell extracts (WCE) were prepared from confluent HEK293 cells transiently transfected with 14 μg plasmid DNA in 10 cm tissue culture dishes. WCE supernatants were processed for IP experiments by using 2 μg primary antibodies and protein A/G mixed agarose beads (Pierce).
Statistical analysis
The χ2 staticstic was computed manually with P value assigned for 1 degree of freedom in the characterization of mouse embryonic phenotype (Supplementary Table 2). For other studies, Student’s two-tailed non-paired t-tests were carried out to determine the statistical significance of differences between samples. P < 0.05 was considered statistically significant for all tests.
URLs
Human Genome Browser, http://www.genome.ucsc.edu; ClustalW, http://www.ebi.ac.uk/Tools/msa/clustalw2/.
Supplementary Material
Acknowledgments
We thank the Marshfield Clinic Research Foundation, Center for Inherited Disease Research (supported by the US National Institutes of Health and National Heart, Lung, and Blood Institute) for genotyping support. We thank the International JSRD Study Group, E. Bertini, and the French Society of Foetal Pathology for patient referrals, J. Meerloo at the UCSD Microscopy Core (P30NS047101), T. Meerloo, Y. Jones and M. Farquhar and the CMM Electron Microscopy Core Facility at UCSD, S. Wirth and B. Willis for mutant mouse generation, C. Janke at the Institute Curie Research Center for GT335 antibody, TTLLs plasmids and technical advice, I. Drummond and N. Pathak at the Massachusetts General Hospital for ttll6 MO, M. Gorovsky at the Univ. of Rochester for polyE and polyG antibodies, S. Audollent at the Hôpital Necker-Enfants Malades for technical help, B. Sotak, N. Akizu, A. Crawford, V. Cantagrel, and E-J. Choi for stimulating scientific discussion and comments. This work was supported by the US National Institutes of Health R01NS048453 and R01NS052455 (J.G.G.), R01DK068306 (F.H.), R01NS064077 (D.A.D.) the American Heart Association 09POST2250641 (J.E.L.), the Italian Ministry of Health (Ricerca Finalizzata Malattie Rare and Ricerca Corrente 2011), the Telethon Foundation Italy GGP08145, and the Pierfranco and Luisa Mariani Foundation (E.M.V.), Grants-in-Aid from MEXT and JSPS, #23117517 and #23570209 (K.I.), the BDF Newlife, the Medical Research Council (G0700073) and the Sir Jules Thorn Charitable Trust 09/JTA (C.A.J.), l’Agence National pour la Recherche ANR 07-MRAR-Fetalciliopathies (T.A.-B.), Simons Foundation Autism Research Initiative (J.G.G.) and the Howard Hughes Medical Institute (F.H. and J.G.G.).
Footnotes
AUTHOR CONTRIBUTIONS
J.E.L. M.S.Z. and J.G.G. designed the study and experiments with substantial contributions from B.M. and S.F.N. helped fine mapping, J.L.S., S.L.B., J.O., F.B., M.I., A.M.S., T.A.-B., C.V.L., I.A.G., A.C., F.H., C.A.J., D.A.D., and E.M.V. performed genetic screening, and J.E.L., J.L.S., J.S., J.O., F.B., M.I., T.A.-B., I.A.G., D.A.D., C.M.L., and J.H.L. performed mutation analysis. M.S.Z., S.E.M., H.R.R., I.R., I.P.C., E.B. and E.M.V. identified and recruited patients, K.I. and M.S. shared critical reagents, and J.S. helped genotyping of mutant mice. J.E.L. performed microscopy, biochemical assays, zebrafish and mouse experiments. J.E.L. and J.G.G. interpreted the data and wrote the manuscript.
References
- 1.Ikegami K, Sato S, Nakamura K, Ostrowski LE, Setou M. Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry. Proc Natl Acad Sci U S A. 2010;107:10490–5. doi: 10.1073/pnas.1002128107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kubo T, Yanagisawa HA, Yagi T, Hirono M, Kamiya R. Tubulin polyglutamylation regulates axonemal motility by modulating activities of inner-arm dyneins. Curr Biol. 2010;20:441–5. doi: 10.1016/j.cub.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 3.Parisi MA. Clinical and molecular features of Joubert syndrome and related disorders. Am J Med Genet C Semin Med Genet. 2009;151C:326–40. doi: 10.1002/ajmg.c.30229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen JS, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–4. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 5.van Dijk J, et al. A targeted multienzyme mechanism for selective microtubule polyglutamylation. Mol Cell. 2007;26:437–48. doi: 10.1016/j.molcel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Valente EM, Brancati F, Dallapiccola B. Genotypes and phenotypes of Joubert syndrome and related disorders. Eur J Med Genet. 2008;51:1–23. doi: 10.1016/j.ejmg.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–71. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 8.Lander ES, Botstein D. Homozygosity mapping: a way to map human recessive traits with the DNA of inbred children. Science. 1987;236:1567–70. doi: 10.1126/science.2884728. [DOI] [PubMed] [Google Scholar]
- 9.Yamada T, et al. The gene TSGA14, adjacent to the imprinted gene MEST, escapes genomic imprinting. Gene. 2002;288:57–63. doi: 10.1016/s0378-1119(02)00428-6. [DOI] [PubMed] [Google Scholar]
- 10.Bordo D, Bork P. The rhodanese/Cdc25 phosphatase superfamily. Sequence-structure-function relations. EMBO Rep. 2002;3:741–6. doi: 10.1093/embo-reports/kvf150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beales PL, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 12.Gorden NT, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83:559–71. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colantonio JR, et al. The dynein regulatory complex is required for ciliary motility and otolith biogenesis in the inner ear. Nature. 2009;457:205–9. doi: 10.1038/nature07520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna H, et al. A common allele in RPGRIP1L is a modifier of retinal degeneration in ciliopathies. Nat Genet. 2009;41:739–45. doi: 10.1038/ng.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–60. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- 16.Nonaka S, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 17.McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–92. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 18.Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–90. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Ross AJ, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–40. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 20.Hoover AN, et al. C2cd3 is required for cilia formation and Hedgehog signaling in mouse. Development. 2008;135:4049–58. doi: 10.1242/dev.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–78. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Jurczyk A, et al. Pericentrin forms a complex with intraflagellar transport proteins and polycystin-2 and is required for primary cilia assembly. J Cell Biol. 2004;166:637–43. doi: 10.1083/jcb.200405023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Million K, et al. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J Cell Sci. 1999;112 (Pt 23):4357–66. doi: 10.1242/jcs.112.23.4357. [DOI] [PubMed] [Google Scholar]
- 24.Suryavanshi S, et al. Tubulin glutamylation regulates ciliary motility by altering inner dynein arm activity. Curr Biol. 2010;20:435–40. doi: 10.1016/j.cub.2009.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogel P, Hansen G, Fontenot G, Read R. Tubulin tyrosine ligase-like 1 deficiency results in chronic rhinosinusitis and abnormal development of spermatid flagella in mice. Vet Pathol. 2010;47:703–12. doi: 10.1177/0300985810363485. [DOI] [PubMed] [Google Scholar]
- 26.Wloga D, et al. Hyperglutamylation of tubulin can either stabilize or destabilize microtubules in the same cell. Eukaryot Cell. 2010;9:184–93. doi: 10.1128/EC.00176-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker-Heck A, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet. 2011;43:79–84. doi: 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605–16. doi: 10.1016/j.mod.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Pathak N, Obara T, Mangos S, Liu Y, Drummond IA. The zebrafish fleer gene encodes an essential regulator of cilia tubulin polyglutamylation. Mol Biol Cell. 2007;18:4353–64. doi: 10.1091/mbc.E07-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pathak N, Austin CA, Drummond IA. Tubulin tyrosine ligase-like genes ttll3 and ttll6 maintain zebrafish cilia structure and motility. J Biol Chem. 2011;286:11685–95. doi: 10.1074/jbc.M110.209817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaertig J, Wloga D. Ciliary tubulin and its post-translational modifications. Curr Top Dev Biol. 2008;85:83–113. doi: 10.1016/S0070-2153(08)00804-1. [DOI] [PubMed] [Google Scholar]
- 32.Shida T, Cueva JG, Xu Z, Goodman MB, Nachury MV. The major alpha-tubulin K40 acetyltransferase alphaTAT1 promotes rapid ciliogenesis and efficient mechanosensation. Proc Natl Acad Sci U S A. 2010;107:21517–22. doi: 10.1073/pnas.1013728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SK, et al. Planar cell polarity acts through septins to control collective cell movement and ciliogenesis. Science. 2010;329:1337–40. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams CL, et al. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol. 2011;192:1023–41. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogowski K, et al. Evolutionary divergence of enzymatic mechanisms for posttranslational polyglycylation. Cell. 2009;137:1076–87. doi: 10.1016/j.cell.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Wloga D, et al. TTLL3 Is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell. 2009;16:867–76. doi: 10.1016/j.devcel.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Bulinski JC. Tubulin posttranslational modifications: a Pushmi-Pullyu at work? Dev Cell. 2009;16:773–4. doi: 10.1016/j.devcel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 38.Valente EM, et al. Distinguishing the four genetic causes of Jouberts syndrome-related disorders. Ann Neurol. 2005;57:513–9. doi: 10.1002/ana.20422. [DOI] [PubMed] [Google Scholar]
- 39.Murray SS, et al. A highly informative SNP linkage panel for human genetic studies. Nat Methods. 2004;1:113–7. doi: 10.1038/nmeth712. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann K, Lindner TH. easyLINKAGE-Plus--automated linkage analyses using large-scale SNP data. Bioinformatics. 2005;21:3565–7. doi: 10.1093/bioinformatics/bti571. [DOI] [PubMed] [Google Scholar]
- 41.Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Gherman A, Davis EE, Katsanis N. The ciliary proteome database: an integrated community resource for the genetic and functional dissection of cilia. Nat Genet. 2006;38:961–2. doi: 10.1038/ng0906-961. [DOI] [PubMed] [Google Scholar]
- 43.Schnutgen F, et al. Genomewide production of multipurpose alleles for the functional analysis of the mouse genome. Proc Natl Acad Sci U S A. 2005;102:7221–6. doi: 10.1073/pnas.0502273102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valente EM, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–25. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–9. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.