Abstract
Purpose
To examine whether family history of unprovoked seizures is associated with behavioral disorders in epilepsy probands, thereby supporting the hypothesis of shared underlying genetic susceptibility to these disorders.
Methods
We conducted an analysis of the 308 probands with childhood onset epilepsy from the Connecticut Study of Epilepsy with information on first degree family history of unprovoked seizures and of febrile seizures whose parents completed the Child Behavior Checklist (CBCL) at the 9-year follow-up. Clinical cut-offs for CBCL problem and DSM-Oriented scales were examined. The association between first degree family history of unprovoked seizure and behavioral disorders was assessed separately in uncomplicated and complicated epilepsy and separately for first degree family history of febrile seizures. A subanalysis, accounting for the tendency for behavioral disorders to run in families, adjusted for siblings with the same disorder as the proband. Prevalence ratios were used to describe the associations.
Key findings
In probands with uncomplicated epilepsy, first degree family history of unprovoked seizure was significantly associated with clinical cut-offs for Total Problems and Internalizing Disorders. Among Internalizing Disorders, clinical cut-offs for Withdrawn/Depressed, and DSM-Oriented scales for Affective Disorder and Anxiety Disorder were significantly associated with family history of unprovoked seizures. Clinical cut-offs for Aggressive Behavior and Delinquent Behavior, and DSM-Oriented scales for Conduct Disorder and Oppositional Defiant Disorder were significantly associated with family history of unprovoked seizure. Adjustment for siblings with the same disorder revealed significant associations for the relationship between first degree family history of unprovoked seizure and Total Problems and Agressive Behavior in probands with uncomplicated epilepsy; marginally significant results were seen for Internalizing Disorder, Withdrawn/Depressed and Anxiety Disorder.
There was no association between family history of unprovoked seizure and behavioral problems in probands with complicated epilepsy. First degree family history of febrile seizure was not associated with behavioral problems in probands with uncomplicated or in those with complicated epilepsy.
Significance
Increased occurrence of behavioral disorders in probands with uncomplicated epilepsy and first degree family history of unprovoked seizure suggests familial clustering of these disorders. This supports the idea that behavioral disorders may be another manifestation of the underlying pathophysiology involved in epilepsy or closely related to it.
Keywords: epilepsy, psychiatric disorders, family history, epidemiology
Psychiatric disorders are associated with epilepsy. When time order is examined Attention Deficit Hyperactivity Disorder (ADHD),(Austin et al., 2001; Hesdorffer et al., 2004) major depression, (Forsgren et al., 1996; Hesdorffer et al., 2000; Hesdorffer et al., 2005; Nilsson et al., 2003) bipolar disorder (Hesdorffer, et al. 2005), and attempted suicide (Hesdorffer, et al. 2005) are associated with an increased risk for the later development of epilepsy in children and adults. Among people with epilepsy, neuropsychiatric disorders present at the initial diagnosis were associated with a decreased chance of seizure remission (Hitiris et al., 2007; Petrovski et al., 2010). Other studies have shown that a history of psychiatric disorders before epilepsy surgery were associated with a poorer chance of post-surgical remission (Guarnieri et al., 2009; Kanner et al., 2009) These data suggest that psychiatric disorders may lower seizure threshold, leading to an increased risk for developing epilepsy and to continued seizures in epilepsy.
Several explanations may be posited for the comorbidity of psychiatric disorders and epilepsy (Kanner, 2003). Though unlikely, one possibility is that psychiatric disorders directly cause epilepsy, in the way that diabetes causes diabetic retinopathy. A second possibility, which is the focus of this investigation, is that there may be a common underlying predisposition to both epilepsy and psychiatric disorders. We tested the hypothesis that epilepsy and behavioral disorders cluster in families. We evaluated this by examining whether a first degree family history of unprovoked seizures was associated with an increased prevalence of behavioral disorders in probands with epilepsy. We separately examined whether a first degree family history of febrile seizures was associated with an increased prevalence of behavioral disorders in probands with epilepsy.
METHODS
Study sample
Briefly, probands with incident epilepsy in childhood were recruited from the offices of pediatric neurologists, pediatricians and adult neurologists throughout the state of Connecticut from 1993–1997 (Berg et al., 1999). Parents were interviewed after the initial diagnosis and contacted every 3–4 months to determine the occurrence of any new seizures. Seizures, etiology, and syndromes were classified based on information available at initial diagnosis and updated as new information became available. Eight to nine years after study entry, probands were invited to participate in a reassessment protocol which included a parental questionnaire. Of the 613 probands in the original cohort, 502 (89.1%) completed questionnaires, 94.7% of those actively followed at the nine-year assessment. Additionally, 285 sibling controls were recruited and the same parental questionnaire was administered.
Epilepsy categories
The designation of uncomplicated epilepsy required a normal neurological exam, no underlying structural or metabolic neurological condition or insult, and an FSIQ ≥ 80 (Berg et al., 2010; Berg et al., 2011). Epilepsy was considered “complicated” in subjects with FSIQ<80, an abnormal exam, or a structural metabolic cause. This is consistent with the use of these terms by other investigators (Davies et al., 2003).
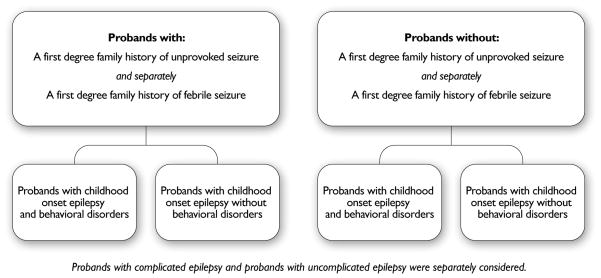
We restricted the analysis of childhood onset epilepsy to the 308 probands with information on first degree family history of unprovoked seizures and of febrile seizures whose parents completed the Child Behavior Checklist (CBCL) at the time of the 9-year follow-up (Figure 1A and B). Subanalysis was restricted to 114 probands with uncomplicated epilepsy whose siblings were similarly assessed.
Figure 1.
Study design for the examination of the association between first degree family history of unprovoked seizure, first degree family history of febrile seizure and behavioral disorders in probands with epilepsy
Descriptive data
Several descriptive factors were examined, including gender, age at onset, age at the 9-year interview, ever in 5-year remission, and type of epilepsy. Age at onset was categorized as 1 month through 1 year, 2 years through 4 years 4 years, 5 years through 9 years and ≥ 10 years. Age at the nine year interview was categorized as <10 years, 10 through 14 years, and 15 through 19 years.
Behavioral disorders
Behavioral disorders were assessed using the Child Behavior Checklist (CBCL) (Achenbach & Rescorla, 2001), which was completed by parents for included probands and for siblings, when available. We examined the clinical cut-offs for Total Problems, Internalizing Disorders, Externalizing Disorders, Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints, Thought Problems, Attention Problems, Rule-Breaking Behavior, Aggressive Behavior and Delinquent Behavior based upon the developer’s published norms. In addition, we considered the six Diagnostic and Statistical Manual Of Mental Disorders (DSM)-oriented scales for Affective Disorder, Anxiety Disorder, Attention-Deficit Hyperactivity Disorder, Conduct Disorder, and Oppositional Defiant Disorder.
First degree family history of unprovoked seizures and of febrile seizures was assessed in parents and siblings of probands during the baseline interview and at a 5-year follow-up interview. We evaluated first degree family history of unprovoked seizure separately from first degree family history of febrile seizures.
Bivariate analyses were conducted using Chi-square and Fisher’s exact tests and t-tests. Crude and adjusted prevalence ratios were calculated using multiple logistic regression with a binomial response function for modeling prevalence ratios instead of odds ratios, using proc genmod. When models did not converge, a Poisson response function was used. All analyses were conducted using SAS, v 9.2.
We examined the association between first degree family history of unprovoked seizure and each of the clinical cut-offs and DSM-Oriented scales for behavioral disorders separately for uncomplicated epilepsy and complicated epilepsy. Further analysis of statistically significant associations included adjustment for age at interview, gender, and ever in 5 year remission. Because behavioral disorders are familial (Hettema et al., 2001; Sullivan et al., 2000), for each significant association further analysis took into account the identical behavioral disorder in the sibling control. This analysis was conducted in the subset of probands whose parents had completed the CBCL for the proband and sibling.
Results
Among the 308 probands eligible for this analysis, 169 (54.9%) were boys. The median age at onset was 4.2 years (Interquartile range (IQR)=2.0–6.4 years), the median age at the time of the 9–year interview was 13.5 years (IQR=11.3–15.6). Epilepsy was characterized as uncomplicated in 213 (69.2%) and complicated in 95 (30.8%; Table 1). Family history of unprovoked seizure in first degree relatives was present in 24 probands with uncomplicated epilepsy (11.3%) and 9 probands with complicated epilepsy (9.5%; p=0.6). Family history of febrile seizure in first degree relatives was present in 21 probands with uncomplicated epilepsy (9.9%) and 8 probands with complicated epilepsy (8.4%; p=0.7). First degree family history of unprovoked seizures and of febrile seizures were not associated with age, gender, and ever having been in 5-year remission in either the complicated or uncomplicated epilepsy groups (Table 1 and ETable 1).
Table 1.
Demographics of children with childhood onset epilepsy by type of epilepsy and first degree family history of unprovoked seizure
| Factor | Uncomplicated epilepsy1 (N=213) | Complicated epilepsy2 (N=95) | ||||
|---|---|---|---|---|---|---|
| Family history of unprovoked seizure (N=24) | No family history of unprovoked seizure (N=189) | p-value | Family history of unprovoked seizure (N=9) | No family history of unprovoked seizure (N=86) | p-value | |
| Median age at onset (yrs) | 5.0 | 6.0 | 0.4 | 5.0 | 6.0 | 0.4 |
| Age at onset (yrs) | ||||||
| 0 through 1 | 4 (16.6%) | 41 (21.7%) | 2 (22.2%) | 30 (34.9%) | ||
| 2 through 4 | 10 (41.7%) | 60 (31.7%) | 4 (44.4%) | 33 (38.4%) | ||
| 5 through 9 | 10 (41.7%) | 88 (46.6%) | 2 (22.2%) | 23 (26.7%) | ||
| ≥10 | 0 (0%) | 0 (0%) | 0.63 | 1 (11.1%) | 0 (0%) | 0.13 |
| Age at interview | ||||||
| <10 Years | 3 (12.5%) | 24 (12.7%) | 1 (11.1%) | 19 (22.1%) | ||
| 10 through 14 Years | 14 (58.3%) | 94 (49.7%) | 6 (66.7%) | 54 (62.8%) | ||
| 15 through 19 Years | 7 (29.2%) | 71 (37.6%) | 2 (22.2%) | 13 (15.1%) | 0.73 | |
| ≥20 Years | 0 (0%) | 0 (0%) | 0.63 | 0 (0%) | 0 (0%) | |
| Gender | ||||||
| Male | 15 (55.2%) | 99 (51.8%) | 3 (33.3%) | 52 (60.5%) | ||
| Female | 9 (44.8%) | 90 (48.2%) | 0.43 | 6 (66.7%) | 34 (39.5%) | 0.23 |
| Ever 5 year remission | ||||||
| Yes | 16 (66.7%) | 141 (74.6%) | 5 (55.6%) | 37 (43.0%) | ||
| No | 8 (33.3%) | 48 (25.4%) | 0.53 | 4 (44.4%) | 49 (57.0%) | 0.53 |
| Type of epilepsy | ||||||
| BECTS+ | 2 (8.3%) | 44 (17.5%) | 0 (0%) | 2 (2.3%) | ||
| CAE | 4 (16.7%) | 38 (15.1%) | 0 (0%) | 7 (8.1%) | ||
| JAME | 2 (8.3%) | 18 (7.2%) | 0 (0%) | 0 (0%) | ||
| Other | 16 (66.7%) | 151 (60.2%) | 0.053 | 9 (100%) | 77 (89.5%) | 1.03 |
Uncomplicated: Subject has cognitive function consistent with IQ >80 and no identified structural brain lesion or similar condition to which the epilepsy can be attributed.;
Complicated: Subject has cognitive function level consistent with IQ<80 or there is a documented brain lesion or related condition to which the occurrence of epilepsy has been attributed;
Fisher’s Exact Test
In probands with uncomplicated epilepsy, family history of unprovoked seizure in first degree relatives was statistically significantly associated with clinical cut offs for Total Problems and Internalizing Disorders, but not with Externalizing Disorders (Table 2). In further examination of Internalizing Disorders, clinical cut-offs for Withdrawn/Depressed, and DSM-Oriented scales for Affective Disorder and Anxiety Disorder were significantly associated with family history of unprovoked seizures. Among the Externalizing Disorders, clinical cut-offs for Aggressive Behavior and Delinquent Behavior as well as DSM-Oriented scales for Conduct Disorder and Oppositional Defiant Disorder were also significantly associated with first degree family history of unprovoked seizure. In contrast, first degree family history of febrile seizure was not associated with any behavioral disorders in the uncomplicated group (ETable 2).
Table 2.
First degree family history of unprovoked seizure and risk for psychiatric disorders in probands with childhood onset epilepsy
| Behavioral disorder from CBCL | Uncomplicated epilepsy1 (N=213) | Complicated epilepsy2 (N=95) | ||||
|---|---|---|---|---|---|---|
| Family history of unprovoked seizure (N=24) | No family history of unprovoked seizure (N=189) | PR (95% CI) | Family history of unprovoked seizure (N=9) | No family history of unprovoked seizure (N=86) | PR (95% CI) | |
| Clinical cut off | ||||||
| Total problems | 7 (29.2%) | 20 (10.6%)p=0.018 | 2.8 (1.3–5.8) | 3 (33.3%) | 19 (22.0%)p=0.43 | 1.5 (0.6–4.1) |
| Internalizing disorders | 8 (33.3%) | 30 (15.9%)p=0.047 | 2.1 (1.09–4.0) | 2 (22.2%) | 17 (19.8%)p=1.0 | 1.2 (0.3–4.1) |
| Externalizing disorders | 5 (20.8%) | 21 (11.1%)p=0.19 | 1.9 (0.8–4.5) | 2 (22.2%) | 11 (12.8%)p=0.61 | 1.7 (0.5–6.6) |
| Anxious/Depressed | 3 (12.5%) | 12 (6.4%)p=0.39 | 2.0 (0.6–6.5) | 1 (11.1%) | 7 (8.1%)p=0.56 | 1.3 (0.2–9.9) |
| Withdrawn/Depressed | 4 (16.7%) | 9 (4.8%)p=0.044 | 3.5 (1.2–10.5) | 1 (11.1%) | 8 (9.3%)p=1.0 | 1.2 (0.2–8.5) |
| Somatic Complaints | 4 (16.7%) | 17 (9.0%)p=0.27 | 1.9 (0.7–5.1) | 0 (0%) | 7 (8.1%) p=1.0 | NA |
| Thought Problems | 2 (8.3%) | 11 (5.8%)p=0.64 | 1.4 (0.3–6.1) | 1 (11.1%) | 9 (10.5%) p=1.0 | 1.1 (0.2–7.5) |
| Attention Problems | 4 (16.7%) | 12 (6.4%)p=0.089 | 2.6 (0.9–7.5) | 0 (0%) | 17 (19.8%)p=0.35 | NA |
| Intrusive Problems | 2 (8.3%) | 13 (6.9%)p=0.68 | 1.2 (0.3–5.1) | 2 (22.2%) | 13 (15.1%) p=0.63 | 1.4 (0.4–5.5) |
| Aggressive Behavior | 4 (16.7%) | 6 (3.2%)p=0.017 | 5.3 (1.6–17.3) | 0 (0%) | 3 (3.5%) p=1.0 | NA |
| Delinquent Behavior | 4 (16.7%) | 10 (5.3%)p=0.057 | 3.2 (1.1–9.3) | 0 (0%) | 8 (9.3%) p=1.0 | NA |
| DSM diagnoses | ||||||
| Affective Disorder | 5 (20.8%) | 14 (7.4%)p=0.046 | 2.8 (1.1–7.1) | 1 (11.1%) | 13 (15.1%) p=1.0 | 0.7 (0.1–5.0) |
| Anxiety Disorder | 5 (20.8%) | 15 (7.9%)p=0.057 | 2.6 (1.1–6.6) | 1 (11.1%) | 8 (9.3%) p=1.0 | 1.2 (0.2–8.5) |
| ADHD3 | 1 (4.2%) | 9 (4.8%)p=1.0 | 0.9 (0.1–6.6) | 0 (0%) | 5 (5.8%) p=1.0 | NA |
| Conduct Disorder | 4 (16.7%) | 7 (3.7%)p=0.024 | 4.5 (1.4–14.3) | 0(0%) | 6 (7.0%) p=1.0 | NA |
| Oppositional Defiant Disorder | 5 (20.8%) | 10 (5.3%)p=0.016 | 3.9 (1.5–10.6) | 0 (0%) | 3 (3.5%) p=1.0 | NA |
Uncomplicated: Subject has cognitive function consistent with IQ >80 and no identified structural brain lesion or similar condition to which the epilepsy can be attributed.;
Complicated: Subject has cognitive function level consistent with IQ<80 or there is a documented brain lesion or related condition to which the occurrence of epilepsy has been attributed;
Attention Deficit Hyperactivity Disorder;
NA - Model did not converge
Associations between first degree family history of unprovoked seizure and behavioral disorders in probands remained statistically significant after adjustment for age at interview, gender and ever experiencing a 5 year remission (Table 3), except for Delinquent Behavior (Prevalence Ratio=2.9; 95% CI=0.93–8.9). Additional analyses of these associations were performed for probands stratified into groups for those with childhood absence epilepsy, juvenile absence epilepsy or juvenile myoclonic epilepsy (combined, N= 78) and for those with nonsyndromic epilepsy (N=167) (Table 3). Within both groups, prevalence ratios on the order of 2–3 were found for most CBCL clinical cut-offs; however, with the smaller subgroup sample sizes, only one was statistically significant (Withdrawn-Depressed in the nonsyndromic group).
Table 3.
First degree family history of unprovoked seizure and occurrence of psychiatric disorders in uncomplicated epilepsy, adjusted analysis
| PR (95% CI) Uncomplicated epilepsy (N=213) 1 |
PR (95% CI) Uncomplicated epilepsy2 (N=213) 2 |
PR (95% CI) Uncomplicated epilepsy with CAE, JME and JAE (N=78) 4 |
PR (95% CI) Uncomplicated epilepsy with non-syndromic epilepsy (N=167)3 |
PR (95% CI) Uncomplicated epilepsy with CBCL on siblings (N=114) 4 |
|
|---|---|---|---|---|---|
| CBCL clinical cut off | |||||
| Total problems | 3.0 (1.4–6.2) | 2.7 (1.3–6.0)A | 1.8 (0.2–13.2) | 2.8 (0.94–8.2) | 3.5 (1.4–8.8) |
| Internalizing Disorder | 2.4 (1.2–4.5) | 2.2 (1.2–4.3) | 3.0 (0.8–12.3) | 2.1 (0.8–5.6) | 2.2 (0.98–4.7) |
| Withdrawn/Depressed | 3.4 (1.1–10.2) | 3.5 (1.2–10.6) | NA(neither dist) | 5.0 (1.2–21.7) | 3.5 (0.95–12.9) |
| Aggressive Behavior | 6.0 (1.8–19.6) | 5.1 (1.5–17.4)A | NA(neither dist) | 3.3 (0.6–16.7) | 6.7 (1.03–44.3) |
| Delinquent Behavior | 3.3 (1.1–9.8) | 2.9 (0.93–8.9)A | NA(neither dist) | 2.1 (0.5–9.6) | NA(neither dist) |
| CBCL DSM disorders | |||||
| Affective Disorder | 2.9 (1.1–7.3) | 2.7 (1.1–6.9) | 2.9 (0.3–29.7) | 2.6 (0.8–9.2) | NA(neither dist) |
| Anxiety Disorder | 2.6 (1.03–6.7) | 2.7 (1.04–6.8) | 2.0 (0.22–18.8) | 2.6 (0.6–10.1) | 2.5 (0.9–7.5) |
| Conduct Disorder | 4.6 (1.4–14.8) | 4.1 (1.2–13.4)A | NA(neither dist) | 2.2 (0.5–10.2) | 3.2 (0.67–14.9) |
| Oppositional Defiant Disorder | 3.9 (1.5–10.6) | 3.8 (1.4–10.4) | NA(neither dist) | 2.4 (0.7–8.0) | NA(neither dist) |
adjusting for age at interview and gender;
adjusting for age at interview, gender and ever in 5 year remission;
adjusting for age at interview and gender;
adjusting for sibling history of the same disorder age at interview and gender. These estimates are all very unstable because either no sibling of one sibling control with first degree family history of unprovoked seizure has the same psychiatric disorder of the epilepsy proband. This also means that psychiatric disorder of the sibling likely has no effect on the associations observed.
Poisson distribution used because binomial would not converge
Among probands with complicated epilepsy, first degree family history of unprovoked seizure was not associated with clinical cut-offs or DSM oriented cut-offs for any of the behavioral disorders (Table 2). Similarly, there was no association between behavioral disorders and first degree family history of febrile seizures.
There were 114 probands with uncomplicated epilepsy whose parents completed a CBCL for the sibling control. Among the sibling controls, behavioral problems meeting the clinical cutoff occurred in 3 (2.6%) for Total Problems, 6 (5.3%) for Internalizing Problems, 1 (0.9%) for Withdrawn-Depressed, 2 (1.8%) for Aggressive Behavior, and 1 (0.9%) for Delinquent Behavior. There were no sibling controls who met clinical cut-offs for Externalizing Problems, Anxious/Depressed, Somatic Complaints, Thought Problems, Attention Problems, or Intrusive Problems. Among sibling controls, the DSM cut-offs occurred in 1 (0.9%) for Affective Disorder were met for 1 (0.9%), 4 (3.5%) for Anxiety Disorder, 2 (1.8%) for Conduct Disorder, and 2 (1.8%) for Oppositional Defiant Disorder. There were no sibling controls meeting criteria for ADHD.
In further adjustment for sibling history of the same behavioral disorder as the proband, family history of unprovoked seizure in first degree relatives was significantly associated with clinical cut-offs for Total Problems and Aggressive Behavior in uncomplicated epilepsy. Associations for Internalizing Disorder, Withdrawn/Depressed and the DSM cut-off for Anxiety Disorder, though virtually unchanged, were no longer statistically significant.
Discussion
We found evidence suggesting an association between a first degree family history of unprovoked seizure and the occurrence of behavioral disorders in probands with uncomplicated epilepsy which persisted after adjustment for age at interview, gender and ever having been in a 5-year remission. Further adjustment for a history of behavioral disorders in a subset of the probands with sibling controls indicated that there was still a statistically significant association between family history of epilepsy and the clinical cut-offs for Total Problems and Aggressive Behavior. By contrast, we observed no associations in probands with complicated epilepsy. Among probands with complicated epilepsy, there was limited statistical power as only 9 (9.5%) of these probands had a first degree family history of unprovoked seizure compared to 21.2% of those with uncomplicated epilepsy. This underscores both the lesser role of genetic factors in complicated epilepsy in contrast to uncomplicated epilepsy.
Our results support the hypothesis of a common underlying genetic predisposition for epilepsy and behavioral disorders in children with uncomplicated epilepsy. Discrepant findings by etiology of epilepsy may be explained by the known structural or metabolic insults which alone are sufficient to increase seizure risk in the children with complicated epilepsy and overwhelm any additional influence of genetic factors, especially in a sample of this size. These factors are not present in children with uncomplicated epilepsy in whom genetic or other environmental factors play a greater role in the development of epilepsy.
The presence of general psychopathology involving a wide range of disorders is suggested by the association between first degree family history of unprovoked seizure and the clinical cut-off for Total Problems in uncomplicated epilepsy even after adjustment for Total Problems in sibling controls. The specific association with Aggressive Behavior supports this notion as aggressive behavior is a symptom found in patients with a wide range of psychiatric disorders, including depression, generalized anxiety disorder, schizophrenia, bipolar disorder, post-traumatic stress disorder, obsessive compulsive disorder, and conduct disorder (Kohn & Asnis, 2003).
The approach we have used is similar to that in other studies of familial clustering of diverse disorders. For example, an increased familial occurrence of arousal parasomnias has been shown in families of probands with nocturnal frontal lobe epilepsy (Bisulli et al., 2011). To our knowledge, only one other study has examined the association between behavioral disorders and family history of epilepsy (Murray et al., 1994). These authors compared family members of Juvenile Myoclonic Epilepsy probands without psychiatric disorders to family members of acquired epilepsy probands without psychiatric disorders, and found a 3.6-fold increased prevalence of psychiatric disorders in family members of the Juvenile Myoclonic Epilepsy probands. The Family History Research Diagnostic Criteria instrument (Thompson et al., 1982) used to assess history of psychiatric disorders in family members and probands had a low sensitivity compared to the CBCL (Ferdinand, 2008). Our design is different, but our finding that Total Problems in uncomplicated epilepsy was associated with a first degree family history of unprovoked seizures after adjusting for sibling disorder is similar to that found in the Juvenile Myoclonic Epilepsy study. To our knowledge ours is the first study to examine this issue in a representative community-based sample with childhood onset epilepsy.
In contrast to our findings for unprovoked seizures, first degree family history of febrile seizures was not associated with behavioral disorders in probands with either uncomplicated epilepsy or complicated epilepsy. As best we can ascertain, only one study has examined and found any evidence of increased behavioral problems assessed using the CBCL in children with febrile seizures compared to controls (Kölfen et al., 1998). Although mean problem scores differed by only about 3–4 points between cases and controls, 22% of cases versus only 6% of controls scores in the borderline or clinical range. A large, national cohort reported no increase in behavioral disorders in children followed after a febrile seizure. Several studies have examined the association between febrile seizures in early childhood and later cognitive and school performance in childhood or adolescence (Ellenberg & Nelson, 1978; Knudsen et al., 1996; Verity et al., 1998) or early adulthood (Nørgaard et al., 2009) and all have reported no overall association with febrile seizures and later cognitive outcomes or school performance. Thus, there is little evidence that febrile seizures are associated with any increase in cognitive or behavioral difficulties, consistent with our findings.
We were unable to adjust for behavioral disorders in parents. The degree to which these may exist and not be reflected by behavioral disorders in the sibling control creates some potential for uncontrolled confounding in our study. That is, we may have been unable to completely remove the effect of a family history of behavioral disorder from the relationship between first degree family history of unprovoked seizure and behavioral disorders in uncomplicated epilepsy. However, given the familial nature of behavioral disorders and our ability to control for at least a sibling as an indicator of family history of behavioral disorders, unassessed parental behavioral disorders may not have substantially influenced our findings.
Our observation is consistent with the concept that epilepsy belongs to a spectrum of disorders (Jensen, 2011). The evidence of increased levels of behavioral disorders in probands with uncomplicated epilepsy and first degree family history of unprovoked seizure suggests that these disorders may be clustering within families. This supports the idea that behavioral disorders may be another manifestation of the underlying pathophysiology involved in epilepsy or closely related to it. That is, the behavioral comorbidities may be part of the expression of a presumed genetic epilepsy. This is consistent with our findings of behavioral problems in probands with uncomplicated epilepsy and family history of unprovoked seizures. We would also expect to find more behavioral problems in probands’ family members without epilepsy. In studies focusing on monogenic causes of epilepsy, we are learning about the polyphenic expression of individual genes, as is well illustrated by SLC2A1 which is associated with a broad array of different forms of epilepsy in extended families (Mullen et al., 2010).
A common predisposition to epilepsy and comorbidities share genetic susceptibility, then future studies of shared susceptibility should focus upon neurologically normal probands and psychiatric phenotypes. Next steps would also involve, assessing lifetime history of DSM diagnoses of psychiatric disorders in families of probands with epilepsy to further to elucidate these relationships. Findings from such investigations could potentially results in future recommendations for different classes of drugs to treat epilepsy depending on the presence of behavioral comorbidities in the family or in the child.
Supplementary Material
Acknowledgments
This study was funded by a grant from the National Institutes of Health, NINDS-NS-R37-31146. The authors confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
We are very grateful to all the physicians in Connecticut who have made it possible for us to recruit and follow their patients all these years. We also thank Eugene Shapiro who provided essential administrative help throughout and Drs. Susan Levy, Francine Testa, Shlomo Shinnar and Francis DiMario who participated in other phases of this study. This study was made possible by the generous help of the many families who have participated over the course of the last many years.
Footnotes
Disclosures reflect the past two years:
Dale Hesdorffer: Advisory board for Pfizer; travel funding from the International League Against Epilepsy
Rochelle Caplan: No disclosures
Anne T. Berg: Dr. Berg has received travel funding and honoraria from Eisai, the British Pediatric Neurological Association, and the Epilepsy Research Center (Melbourne); travel funding from UCB the American Epilepsy Society and the International League Against Epilepsy; awards from the American Epilepsy Society and British Pediatric Neurological Association; and consulting fees from Dow Agro Science.
References
- Achenbach T, Rescorla L. Manual for the ASEBA School-Age Forms and Profiles. University of Vermont, Research Center for Children, Youth and Families; Burlington, VT: 2001. [Google Scholar]
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107:115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51:676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Caplan R, Hesdorffer DC. Psychiatric and neurodevelopmental disorders in childhood-onset epilepsy. Epilepsy Behav. 2011;20:550–555. doi: 10.1016/j.yebeh.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999;40:445–452. doi: 10.1111/j.1528-1157.1999.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Bisulli F, Vignatelli L, Naldi I, Licchetta L, Provini F, Plazzi G, Di Vito L, Ferioli S, Montagna P, Tinuper P. Increased frequency of arousal parasomnias in families with nocturnal frontal lobe epilepsy: a common mechanism? Epilepsia. 2011;51:1852–1860. doi: 10.1111/j.1528-1167.2010.02581.x. [DOI] [PubMed] [Google Scholar]
- Davies S, Heyman I, Goodman R. A population survey of mental health problems in children with epilepsy. Dev Med Child Neurol. 2003;45:292–295. doi: 10.1017/s0012162203000550. [DOI] [PubMed] [Google Scholar]
- Ellenberg JH, Nelson KB. Febrile Seizures and Later Intellectual Performance. Arch Neurol. 1978;35:17–21. doi: 10.1001/archneur.1978.00500250021004. [DOI] [PubMed] [Google Scholar]
- Ferdinand RF. Validity of the CBCL/YSR DSM-IV scales Anxiety Problems and Affective Problems. J Anxiety Disord. 2008;22:126–134. doi: 10.1016/j.janxdis.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Forsgren L, Bucht G, Eriksson S, Bergmark L. Incidence and clinical characterization of unprovoked seizures in adults: a prospective population-based study. Epilepsia. 1996;37:224–229. doi: 10.1111/j.1528-1157.1996.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Guarnieri R, Walz R, Hallak JE, Coimbra E, de Almeida E, Cescato MP, Velasco TR, Alexandre V, Jr, Terra VC, Carlotti CG, Jr, Assirati JA, Jr, Sakamoto AC. Do psychiatric comorbidities predict postoperative seizure outcome in temporal lobe epilepsy surgery? Epilepsy Behav. 2009;14:529–534. doi: 10.1016/j.yebeh.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Annegers JF, Cascino G. Major depression is a risk factor for seizures in older adults. Ann Neurol. 2000;47:246–249. [PubMed] [Google Scholar]
- Hesdorffer DC, Hauser WA, Olafsson E, Ludvigsson P, Kjartansson O. Depression and suicide attempt as risk factors for incident unprovoked seizures. Ann Neurol. 2005;59:35–41. doi: 10.1002/ana.20685. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC, Ludvigsson P, Olafsson E, Gudmundsson G, Kjartansson O, Hauser WA. ADHD as a risk factor for incident unprovoked seizures and epilepsy in children. Arch Gen Psychiatry. 2004;61:731–736. doi: 10.1001/archpsyc.61.7.731. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Neale MC, Kendler KS. A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry. 2001;158:1568–1578. doi: 10.1176/appi.ajp.158.10.1568. [DOI] [PubMed] [Google Scholar]
- Hitiris N, Mohanraj R, Norrie J, Sills GJ, Brodie MJ. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007;75:192–196. doi: 10.1016/j.eplepsyres.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Jensen FE. Epilepsy as a spectrum disorder: Implications from novel clinical and basic neuroscience. Epilepsia. 2011;52:1–6. doi: 10.1111/j.1528-1167.2010.02904.x. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Byrne R, Chicharro A, Wuu J, Frey M. A lifetime psychiatric history predicts a worse seizure outcome following temporal lobectomy. Neurology. 2009;72:793–799. doi: 10.1212/01.wnl.0000343850.85763.9c. [DOI] [PubMed] [Google Scholar]
- Knudsen FU, Paerregaard A, Andersen R, Andresen J. Long term outcome of prophylaxis for febrile convulsions. Arch Dis Child. 1996;74:13–18. doi: 10.1136/adc.74.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn SR, Asnis GM. Aggression in Psychiatric disorders. In: Mattson MP, editor. Neurobiology of aggression: Understanding and preventing violence. Humana Press, Inc; Totowa: 2003. pp. 135–149. [Google Scholar]
- Kölfen W, Pehle K, Konig S. Is the long-term outcome of children following febrile convulsions favorable? Dev Med Child Neurol. 1998;40:667–671. doi: 10.1111/j.1469-8749.1998.tb12326.x. [DOI] [PubMed] [Google Scholar]
- Mullen SA, Suls A, De Jonghe P, Berkovic SF, Scheffer IE. Absence epilepsies with widely variable onset are a key feature of familial GLUT1 deficiency. Neurology. 2010;75:432–440. doi: 10.1212/WNL.0b013e3181eb58b4. [DOI] [PubMed] [Google Scholar]
- Murray RE, Abou-Khalil B, Griner L. Evidence for familial association of psychiatric disorders and epilepsy. Biol Psychiatry. 1994;36:428–429. doi: 10.1016/0006-3223(94)91218-1. [DOI] [PubMed] [Google Scholar]
- Nilsson FM, Kessing LV, Bolwig TG. On the increased risk of developing late-onset epilepsy for patients with major affective disorder. J Affect Disord. 2003;76:39–48. doi: 10.1016/s0165-0327(02)00061-7. [DOI] [PubMed] [Google Scholar]
- Nørgaard M, Ehrenstein V, Mahon BE, Nielsen GL, Rothman KJ, Sørensen HT. Febrile Seizures and Cognitive Function in Young Adult Life: A Prevalence Study in Danish Conscripts. J Pediatr. 2009;155:404–409. doi: 10.1016/j.jpeds.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Petrovski S, Szoeke CE, Jones NC, Salzberg MR, Sheffield LJ, Huggins RM, O’Brien TJ. Neuropsychiatric symptomatology predicts seizure recurrence in newly treated patients. Neurology. 2010;75:1015–1021. doi: 10.1212/WNL.0b013e3181f25b16. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Suls A, Dedeken P, Goffin K, Van Esch H, Dupont P, Cassiman D, Kempfle J, Wuttke TV, Weber Y, Lerche H, Afawi Z, Vandenberghe W, Korczyn AD, Berkovic SF, Ekstein D, Kivity S, Ryvlin P, Claes LR, Deprez L, Maljevic S, Vargas A, Van Dyck T, Goossens D, Del-Favero J, Van Laere K, De Jonghe P, Van Paesschen W. Paroxysmal exercise-induced dyskinesia and epilepsy is due to mutations in SLC2A1, encoding the glucose transporter GLUT1. Brain. 2008;131:1831–1844. doi: 10.1093/brain/awn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WD, Orvaschel H, Prusoff BA, Kidd KK. An evaluation of the family history method for ascertaining psychiatric disorders. Arch Gen Psychiatry. 1982;39:53–58. doi: 10.1001/archpsyc.1982.04290010031006. [DOI] [PubMed] [Google Scholar]
- Verity CM, Greenwood R, Golding J. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med. 1998;338:1723–1728. doi: 10.1056/NEJM199806113382403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.