Abstract
Background
Scientific evidence supports an association between environmental exposures and cancer. However, a reliable estimate for the proportion of cancers attributable to environmental factors is currently unavailable. This may be related to the varying definitions of the term “environment.” The current review aims to determine how the reporting of the definition of the environment and of the estimates of environmentally attributable risks have changed over the past 50 years.
Methods
A systematic literature search was performed to retrieve all relevant publications relating to the environment and cancer from January 1960 to December 2010 using PubMed, EMBASE, Scopus, and Web of Science. Definitions of the environment and environmentally attributable risks for cancer were extracted from each relevant publication.
Results
The search resulted in 261 relevant publications. We found vast discrepancies in the definition of the environment, ranging from broad (including lifestyle factors, occupational exposures, pollutants, and other non-genetic factors) to narrow (including air, water, and soil pollutants). Reported environmentally attributable risk estimates ranged from 1–100%.
Conclusions
Our findings emphasize the discrepancies in reporting environmental causation of cancer and the limits of inference in interpreting environmentally attributable risk estimates. Rather than achieving consensus on a single definition for the environment, we suggest the focus be on achieving transparency for any environmentally attributable risks.
Keywords: cancer, environmental exposures, environmental health risks, epidemiology, public health
1. Introduction
Substantial scientific evidence exists to support the association between the environment and cancer (Tomatis and Bartsch, 1990; Boffetta, 2006; Clapp et al., 2007). Early links between environmental exposures and cancer date back to 1761 when John Hill discovered the association between snuff and nasal cancer (Hill, 1761); and in 1775 when Sir Percival Pott observed a relationship between chimney sweeping and scrotal cancer (Pott, 1775). Centuries later, researchers continue to demonstrate and quantify this relationship, through migrant, familial, and correlational studies, as well as identification of geographical variation in cancer incidence (Higginson and Muir, 1977; Parkin, 1992; Verkasalo et al., 1999; Lichtenstein et al., 2000). From these epidemiological studies, estimates of the proportion of cancer attributable to the environment have been derived. These estimates, often referred to as environmentally attributable risk (EAR), represent the proportion of cancers that would be eliminated if environmental factors were reduced to their lowest level (Smith et al., 1999). The general formula used to calculate an environmentally attributable risk is
where in this case, p is the proportion of the population exposed to the environmental factor, and RR is the causal risk ratio or the proportionate increase in average risk among the exposed due to the environmental exposure.
The most cited attributable risk estimate comes from John Higginson, who declared that 80–90% of all cancers are due to environmental exposures (Higginson, 1967). More recently, Doll and Peto stated that environmental factors cause only 1–3% of cancers. They also concluded that 75–80% of all cancers are ‘avoidable’ (Doll and Peto, 1981). Furthermore, in the Harvard Report on Cancer Prevention (1996) it is estimated that about 2% of cancer deaths are attributable to environmental pollution. There are clear inconsistencies in these estimates, which may be related to the varying definitions of the term “environment.” Therefore, it is difficult to place a reliable estimate on the proportion of cancers attributable to environment factors.
Previous studies have attempted to illustrate this concept by broadly reviewing definitions of the environment and their relation to environmentally attributable risk estimates (Thomas, 1978; Rushton, 2003; Boffetta et al., 2007; Saracci and Vineis, 2007). In a review by Boffetta et al. (2007), the authors note that the term “environment” is frequently used in the broad sense to include all non-genetic factors, and in the narrow sense to include only air, water, soil and food pollutants. Boffetta et al. concluded that the term environment should be abandoned and instead replaced with the terms “non-genetic” and “pollutants.” Saracci and Vineis (2007) refute this idea, and insist on keeping the term environment, while urging researchers to clearly report what components of the environment their risk estimate include.
In the current review, we built upon these prior studies and conducted a systematic literature review on both the definition of the environment and environmentally attributable risk estimates for cancer. The findings from the current review help describe the limits of interpreting environmentally attributable risk estimates, particularly in relation to cancer. Moreover, from the literature search results, we hope to uncover any potential temporal trends in estimates of the proportion of cancer attributable to the environment and to determine if these trends were related to changes in the definition of the term “environment.” Due to the ongoing controversies of the impact of the environment on human health, the current review has potential public health implications for interpreting past research, guiding future study, and informing policy-makers.
2. Methods
2.1 Data sources
We systematically searched electronic databases, including PubMed, EMBASE, Scopus, and Web of Science, for publications from January 1960 to December 2010 containing definitions of the environment and environmentally attributable risk estimates. Google Scholar was searched as well to capture any publications found in environmental journals, which might not appear in medical databases. In addition, reference lists of all publications were examined manually to identify additional relevant publications. Relevant websites were searched for documents or grey literature containing definitions of the environment and/or environmentally attributable risk estimates. Such websites include the World Health Organization, the International Agency for Research on Cancer, the National Cancer Institute, the National Institute for Environmental Health Sciences, the American Cancer Society, and the United Nations Environment Program. The titles and abstracts of all records were screened and if considered potentially relevant, a full-text copy was obtained.
2.2 Inclusion criteria
To be included in our analysis, documents had to be published from January 1960 to December 2010, involve human subjects, be cancer related (though not focused on particular cancer types) and written in English. Publications were excluded if they focused on only one type of cancer and were not related to the environment and cancer for the purpose of this analysis. Definitions and environmentally attributable risk estimates were extracted from each included publication and categorized as either “broad” or “narrow” (Boffetta and Nyberg, 2003). Definitions were categorized as narrow when they included only pollutants found in the air, water, food, and soil. All more expansive definitions were categorized as broad, for example those that may also have included diet, lifestyle, health-behavior, psychosocial, or occupational exposures etc. Publications reporting cancer incidence were separated from those reporting cancer mortality or disability adjusted life years.
3. Results
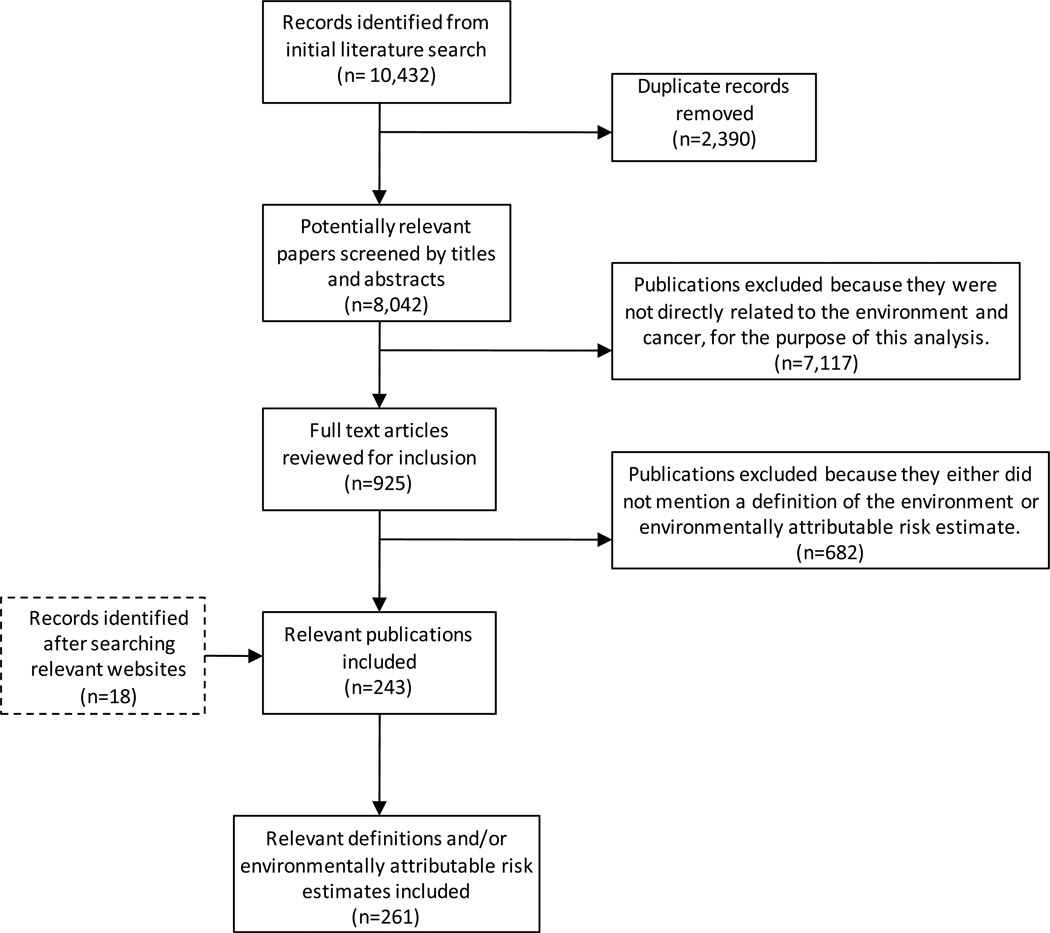
The search returned 10,432 potentially relevant publications. We retrieved and excluded 2,390 duplicate publications. Subsequently, the remaining 8,042 publications were screened by reviewing titles and abstracts. We excluded 7,117 publications that were not related to the environment and cancer for the purpose of this analysis. Of the 925 remaining full-text publications, 681 were excluded because they either did not mention a definition of the environment or an environmentally attributable risk estimate. An additional 17 records were identified from the internet search, which were considered to be relevant. Therefore, 261 publications were included in our final analysis (Figure 1). All publications documented either a definition of the environment or an environmentally attributable risk estimate (See Supplemental Material).
Figure 1. Publication identification and selection process.
Methodology and results from the literature analysis of cancer and the environment research articles. Databases used for the literature search were PubMed, EMBASE, Scopus, and Web of Science.
There were vast discrepancies in both the definition of the environment and environmentally attributable risk estimates, with the latter ranging from 1 to 100%. Among the publications reporting an estimate, 91% reported incidence as an outcome. The earliest environmentally attributable risk estimate came from Higginson and Oettle in 1960, where they suggested that 70–80% of cancers are due to environmental factors (Higginson and Oettle, 1960). The most recent estimate was from the Agency for Toxic Substances & Disease Registry, which reported that about two-thirds of all cancers are linked to environmental causes (Agency for Toxic Substances & Disease Registry, 2010). A substantial proportion of the publications analyzed for this review cited Higginson’s 80–90% estimate when mentioning their definition of the environment.
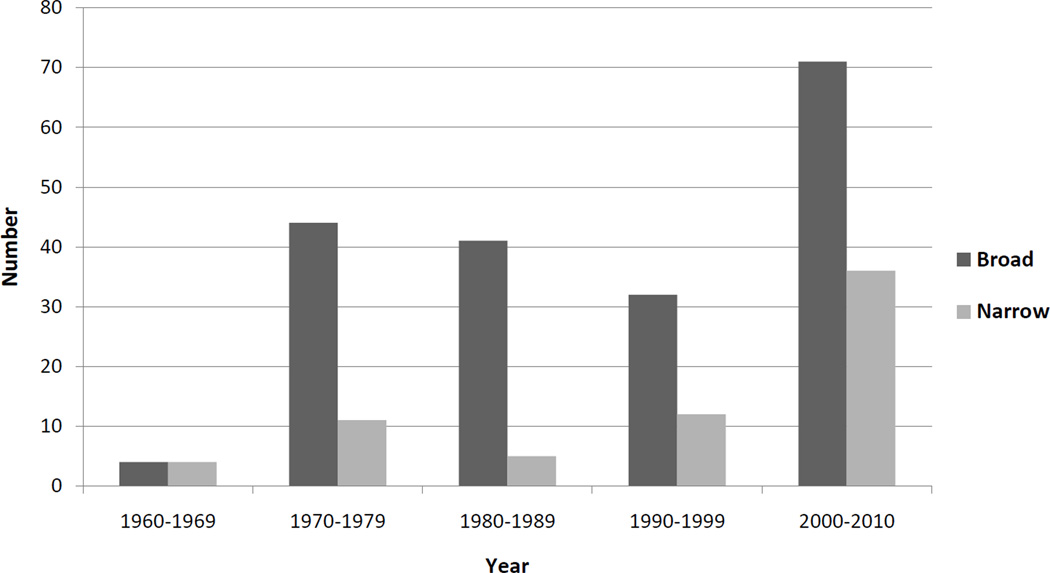
Of the publications reporting cancer incidence, 74.8% (n=119) referred to the environment in the broadest sense (including lifestyle factors, occupational exposures, pollutants, and other non-genetic factors), while 25.2% (n=40) referred to the environment in a more narrow sense (only air, water, food, and soil pollutants). Among the 8% of manuscripts reporting cancer mortality as the outcome there was an equal distribution between publications reporting narrow (n=7) and broad definitions (n=7). One publication reported cancer outcomes as disability-adjusted life years (DALYs). The numbers of publications providing definitions and environmentally attributable risk estimates somewhat increased over time (Figure 2).
Figure 2. Number of publications referring to the environment in the broad or narrow sense by ten-year period.
Definitions were categorized as narrow when they included only pollutants found in the air, water, food, soil. All more expansive definitions were categorized as broad, for example those that may also have included diet, lifestyle, health-behavior, psychosocial, or occupational exposures etc.
While examining the “narrow” definition of the environment some environmental pollutants emerged as the most common air, water, and soil pollutants among those classified as “Group 1” carcinogens (sufficient evidence of carcinogenicity in humans) by the International Agency for Research on Cancer (IARC) (IARC, 2011). Air pollutants included asbestos, environmental tobacco smoke, indoor emissions from household combustion of coal, and radon. Water pollutants included inorganic arsenic, cadmium, chromium-VI, pentachlorobiphenyl, and radium (EPA, 2009). Soil pollutants included petroleum hydrocarbons and solvents (ex: benzene), pesticides (ex: formaldehyde), and heavy metals (ex: nickel).
Among the reported environmental definitions, three categorization patterns emerged. The most commonly reported approach categorized the environment into either the narrow sense (air, water, food, and soil pollutants) or the broad sense (including diet, lifestyle, occupational exposures, and infections). The next most common approach was to describe the environment using as many as three categories consisting of personal behaviors (lifestyle), involuntary exposures (air, water, food, and soil pollutants), and occupational exposures. Finally, one publication divided the environment into the external environment (air, water, food, and soil chemicals/pollutants), behavioral and social factors (personal choices, tobacco, and alcohol consumption) or dietary factors and components relating to physical activity.
4. Discussion
Our findings reveal wide inconsistencies in both environmentally attributable risk estimates and definitions of the environment over the last 50 years. As expected, publications defining the environment in the narrow sense (air, water, food, and soil pollutants) tended to have smaller attributable risk estimates, whereas publications referring to the environment in the broadest sense (including lifestyle factors, occupational exposures, and pollutants) had consistently larger risk estimates. The majority of publications referred to the environment in the broadest sense, although a trend was observed where the proportion of publications using the narrow definition appeared to be increasing.
The findings from the current review emphasize the caution researchers must use when interpreting environmentally attributable risk estimates, particularly in relation to cancer. These results also contribute to the existing body of knowledge on the vast range of interpretations for the term “environment.” Recent reviews have urged researchers to clearly define what concepts of the environment they are referring to when reporting environmentally attributable risk estimates (Belpomme, 2007; Saracci and Vineis, 2007). The need for a clear definition of the environment was consistently observed throughout the conduct of the current review, as it was often difficult, and perhaps meaningless, to interpret an environmentally attributable risk estimate without a clear understanding of the researcher’s interpretation of the “environment.” Boffetta et al. (2007) emphasizes this fundamental concept by stating that “such ambiguous terminology should be avoided and caution should be exercised in providing and interpreting estimates of attributable and avoidable numbers of cases.” Thus, unless careful definitions for environmentally attributable risk estimates are provided to and understood by the reader, the intended purpose of the reported risk estimate is often undermined by public confusion.
An attempt to identify definitional differences in order to explain corresponding differences in risk estimates has been conducted (Saracci and Vineis, 2007). It was only when authors explicitly stated what factors they included in their definition of the environment could the appropriate comparisons be made. Saracci and Vineis (2007) compared Prüss-Üstün and Corvalan’s (World Health Organization) (2006) 19% environmentally attributable risk estimate with Boffetta et al.’s (2007) 1–3% estimate. The researchers were able to compare Prüss-Üstün and Corvalan’s definition (including eight classes of environmental agents) to Boffetta et al.’s (air, water, soil, or food pollutants) since each publication explicitly reported what components of the environment were included in their definition. From their analysis, the authors conclude that “population attributable proportions of a disease are summary indexes attractive for their simplicity but subject to severe limitations.”
Inconsistency in the use of language to describe risk estimates was observed frequently in the current review. Authors reported cancer outcomes as cancer cases, incidence, prevalence, or deaths. Each of these concepts convey different connotations, further complicating the task of comparing attributable risk estimates. Furthermore, the use of the terms “influenced,” “due to,” “caused by,” “associated with,” “attributed to,” “linked to” and “determined by” were all used interchangeably to describe risk estimates; however each have different implications. In addition, many authors refer to the environment in the broadest sense, when in reality they are strictly referring to pollutants.
The current review has several limitations. The literature search focused on publications defining the environment in relation to cancer, thus the main databases utilized were medical in nature. It is therefore possible that we may have discovered additional relevant definitions if we broadened our search to include other sources, such as environmental databases. Using Google Scholar and manually scanning all reference lists of our included publications did not indicate that it is likely any records relating to the current topic were omitted. Furthermore, separating the definition of the environment into narrow and broad categories may have put contrived restrictions on the term “environment.” Nonetheless, we felt that it was an adequate representation for the purpose of the current analysis, as it allowed us to systematically and graphically assess the different definitions. Cancer is a diverse and complex set of diseases with a multifactorial etiology. Environmental exposures also act on this complexity and have differential effects on the diversity of cancer subtypes. In the current review we excluded studies that were limited to relationships between specific environmental exposures and specific cancer subtypes as adding too much reporting complexity with minimal additional information. This decision may slightly underestimate the total environmentally attributable risk estimate, but does not affect our conclusions.
Despite these limitations, the current review emphasizes a few key points. Several publications cited Higginson’s 80–90% estimate when referring to their definition of the environment (Higginson, 1967). Caution must be exercised when interpreting this estimate. To explain, Higginson’s environmentally attributable risk estimate stemmed from his and Oettle’s early research in the 1950’s on an African cohort residing in a rural setting. Thus, his results should be generalized more broadly with caution. Authors, nonetheless, consistently apply his estimate on an international scale. Doll and Peto’s risk estimate is another widely quoted estimate in reference to the environmental etiology of cancer (Doll and Peto, 1981; Colditz, 2009). Their estimate, however, was directed strictly towards the United States, yet is again frequently reported on a global level.
A fundamental concern found in the reviewed publications was the misclassification and underestimation of risk when investigating environmental exposures (Vineis, 2004; Vineis, 2004; Wild, 2005; Vineis and Berwick, 2006). The role of the environment is frequently underestimated, particularly in relation to gene-environment interactions, due to the low sensitivity of environmental exposure estimates (Brunekreef, 2008). Misclassification is often apparent since environmental exposures are constantly fluctuating, both in the internal and external environment (Rappaport and Smith, 2010). Moreover, many exposures and their corresponding effects are measured at low levels, but have a ubiquitous presence in the environment, thus making their true etiological contribution difficult to interpret (Wild, 2009). Consequently, in order to reduce this common misclassification issue in measuring environmental exposures, Chris Wild coined the term “exposome” which measures all external sources of radiation, psycho-social stress, lifestyle, infections, drugs, diet and pollution and the resulting internal chemical environment (Wild, 2005; Wild, 2009; Rappaport and Smith, 2010). The exposome may offer a way to reduce exposure misclassification and more accurately measure what proportion of cancers are attributable to environmental exposures.
In consideration of possible misclassification and both underestimation and overestimation of environmental exposures, environmentally attributable risks should be used with caution. The term “attributable” implies causation between environmental exposures and cancer cases, yet often this causation is difficult to explicitly establish. It is a rare case that a single environmental agent is necessary or sufficient, let alone necessary and sufficient, for explaining cancer. In the strictest, though not necessarily the most useful, sense all disease is attributable to the environment given that the human genetic code must interact in some way with what surrounds it to develop any phenotypic outcome, including cancers. Interactions among different exposures as well as between exposures and genes are in effect. When one adds to these issues the emerging scientific data from epigenetic mechanisms, explicitly attributing risk is further complicated.
Another way of examining what portion of cancer risk is attributable to the environment involves asking what portion is not attributable to the environment. As definitions of what constitutes the environment become more and more inclusive they approach a possibly oversimplified, but still useful concept where the environmentally attributable risk = 1 – the genetically attributable risk. Irrespective of how one accounts for epigenetic changes in this concept, it is interesting to note that despite dramatic increases in genetic knowledge that began with a draft sequenced human genome in 2000 through today, the estimates of the genetic contribution to cancer incidence have not much changed. Vogelstein and Kinzler (1998) provided in 1998 an estimate that high-penetrance genes accounted for less than 5% of cancers. Few changes have occurred since then (Vineis, 2004), in fact it has been reported that only three new high-penetrance cancer susceptibility genes had been discovered between 2001 and 2007 with few expected in the future (Eisinger and Horsman, 2007). Additionally, from 2007 through 2011 genome-wide association studies have suggested no high-penetrance susceptibility alleles and taken in total genome-wide association study findings continue to explain only a small amount of the heritability of cancer. This all suggests there is a small fixed portion of risk attributable to genetics leaving the large remainder of risk attributable to epigenetic and environmental factors. During this same time period human activity has continued to substantially change the types of environmental exposures we experience and how they are distributed (for example Climate change, ubiquity of endocrine disrupters, shifting tobacco-smoking demographics, changing food environment, the Deepwater Horizon oil spill etc).
Taken together these observations suggest that the numerous definitions of the environment we observe are simply dividing up the same large and relatively fixed portion of the environmentally attributable “risk pie” in different ways, often appropriately reflecting a changing environment. Even Doll acknowledged that the “pie” includes many overlapping and interacting slices and the true proportions may total hundreds of percents (Doll, 1998). This also suggests that time and energy devoted to establishing a consensus on the environmentally attributable cancer risk may not be well-spent. Rather, the best way forward may prove to be a clear disclosure of the conceptual model and perspective used by the observer that is strongly tethered to the environmentally attributable risk estimate they have provided.
5. Conclusions
The question of estimating the environmentally attributable risk for cancer is deceptive as such estimates vary dramatically by the definition of the environment used. We suggest that establishing a consensus for a single standard definition may not be an achievable or a worthy goal. In the end, the best way forward with respect to scientists working in this arena, policy makers using their reports, and the public affected by their actions, may be transparency, to accurately describe the perspective used along with the estimate provided.
Highlights.
>We report definitions of the environment and environmentally attributable risk estimates over time. > We categorized definitions as broad or narrow, and assessed their relation to environmentally attributable risk estimates. > Instead of achieving consensus on a single definition, the focus should be on achieving transparency for any environmentally attributable risks.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agency for Toxic Substances & Disease Registry. What you Need to Know. What You Can Do. What causes cancer? Atlanta, Georgia: 2010. [accessed December 13, 2010]. http://www.atsdr.cdc.gov/risk/cancer/cancer-causes.html. [Google Scholar]
- Belpomme D. Cancer and the environment: Facts, figures, methods and misinterpretations. Biomedicine & Pharmacotherapy. 2007;61(10):611–613. [Google Scholar]
- Boffetta P. Human cancer from environmental pollutants: The epidemiological evidence. Mutation Research - Genetic Toxicology and Environmental Mutagenesis. 2006;608(2):157–162. doi: 10.1016/j.mrgentox.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Boffetta P, et al. 'Environment' in cancer causation and etiological fraction: Limitations and ambiguities. Carcinogenesis. 2007;28(5):913–915. doi: 10.1093/carcin/bgm034. [DOI] [PubMed] [Google Scholar]
- Boffetta P, Nyberg F. Contribution of environmental factors to cancer risk. British Medical Bulletin. 2003;68:71–94. doi: 10.1093/bmp/ldg023. [DOI] [PubMed] [Google Scholar]
- Brunekreef B. Environmental epidemiology and risk assessment. Toxicol Lett. 2008;180(2):118–122. doi: 10.1016/j.toxlet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- Clapp RW, et al. Environmental and occupational causes of cancer: A call to act on what we know. Biomedicine and Pharmacotherapy. 2007;61(10):631–639. doi: 10.1016/j.biopha.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Colditz GA. Cancer and the Environment: The American Cancer Society Prevention Priorities. Ca-a Cancer Journal for Clinicians. 2009;59(6):341–342. doi: 10.3322/caac.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R. Epidemiological evidence of the effects of behaviour and the environment on the risk of human cancer. Recent Results Cancer Res. 1998;154:3–21. doi: 10.1007/978-3-642-46870-4_1. [DOI] [PubMed] [Google Scholar]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–1308. [PubMed] [Google Scholar]
- Eisinger F, Horsman D. Hereditary cancer gene hunting. a phase of declining success? Med Hypotheses. 2007;69(3):690–692. doi: 10.1016/j.mehy.2007.01.050. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency (EPA) [accessed October 5, 2011];National Drinking Water Regulations. 2009 http://water.epa.gov/drink/contaminants/upload/mcl-2.pdf.
- Harvard Center for Cancer Prevention. Harvard Report on Cancer Prevention. Volume 1: Causes of human cancer. Cancer Causes Control. 1996;7 Suppl 1:S3–S59. doi: 10.1007/BF02352719. [DOI] [PubMed] [Google Scholar]
- Higginson J. Environment and cancer. Practitioner. 1967;198(187):621–630. [PubMed] [Google Scholar]
- Higginson J, Muir CS. Determination of the importance of environmental factors in human cancer: the role of epidemiology. Bull Cancer. 1977;64(3):365–384. [PubMed] [Google Scholar]
- Higginson J, Oettle AG. Cancer incidence in the Bantu and 'Cape Colored' races of South Africa: report of a cancer survey in the Transvaal (1953–55) J Natl Cancer Inst. 1960;24:589–671. doi: 10.1093/jnci/24.3.589. [DOI] [PubMed] [Google Scholar]
- Hill J. In: Cautions against the immoderate use of snuff. Baldwin R, Jackson J, editors. London, UK: 1761. [Google Scholar]
- International Agency for Research on Cancer (IARC) [accessed October 5, 2011];Agents Classified by the IARC Monographs. 2011 Volumes 1–102 http://monographs.iarc.fr/ENG/Classification/ClassificationsGroupOrder.pdf.
- Lichtenstein P, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- Parkin DM. Studies of cancer in migrant populations: methods and interpretation. Rev Epidemiol Sante Publique. 1992;40(6):410–424. [PubMed] [Google Scholar]
- Pott P. Chirurgical observations relative to the cataract, the polypus of the nose, the cancer of the scrotum, the different kinds of ruptures, and the mortification of the toes and feet. 1775 (Reprinted in Natl Cancer Inst Monogr 10: 7–13; 1963). [Google Scholar]
- Pruss-Ustun A, Corvalan C. Preventing disease through healthy environments. Towards an estimate of the environmental burden of disease. World Health Organization; 2006. [Google Scholar]
- Rappaport SM, Smith MT. Epidemiology. Environment and disease risks. Science. 2010;330(6003):460–461. doi: 10.1126/science.1192603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton L. How much does the environment contribute to cancer? Occupational and Environmental Medicine. 2003;60(2):150–156. doi: 10.1136/oem.60.2.150. +180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saracci R, Vineis P. Disease proportions attributable to environment. Environ Health. 2007;6:38. doi: 10.1186/1476-069X-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, et al. How much global ill health is attributable to environmental factors? Epidemiology. 1999;10(5):573–584. [PubMed] [Google Scholar]
- Thomas HF. Cancer and the Environment. Environmental Health. 1978 October;:232–233. [Google Scholar]
- Tomatis L, Bartsch H. The contribution of experimental studies to risk assessment of carcinogenic agents in humans. Exp Pathol. 1990;40(4):251–266. doi: 10.1016/s0232-1513(11)80309-9. [DOI] [PubMed] [Google Scholar]
- Verkasalo PK, et al. Genetic predisposition, environment and cancer incidence: A nationwide twin study in Finland 1976–1995. International Journal of Cancer. 1999;83(6):743–749. doi: 10.1002/(sici)1097-0215(19991210)83:6<743::aid-ijc8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Vineis P. Individual susceptibility to carcinogens. Oncogene. 2004;23(38):6477–6483. doi: 10.1038/sj.onc.1207897. [DOI] [PubMed] [Google Scholar]
- Vineis P. A self-fulfilling prophecy: are we underestimating the role of the environment in gene-environment interaction research? Int J Epidemiol. 2004;33(5):945–946. doi: 10.1093/ije/dyh277. [DOI] [PubMed] [Google Scholar]
- Vineis P, Berwick M. The population dynamics of cancer: a Darwinian perspective. Int J Epidemiol. 2006;35(5):1151–1159. doi: 10.1093/ije/dyl185. [DOI] [PubMed] [Google Scholar]
- Vogelstein, Kinzler . The genetic basis of human cancer. New York: McGraw-Hill, Health Professions Division; 1998. [Google Scholar]
- Wild CP. Complementing the genome with an 'exposome': the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- Wild CP. Environmental exposure measurement in cancer epidemiology. Mutagenesis. 2009;24(2):117–125. doi: 10.1093/mutage/gen061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.