Abstract
Background
Nearly 35% of men treated for prostate cancer (PrCA) will experience biochemically defined recurrence, noted by a rise in PSA, within ten years of definitive therapy. Diet, physical activity, and stress reduction may affect tumor promotion and disease progression.
Methods
A randomized trial of an intensive diet, physical activity, and meditation intervention was conducted in men with rising post-treatment PSA after definitive treatment for PrCA. Intention-to-treat methods were used to compare usual care to the intervention in 47 men with complete data. Signal detection methods were used to identify dietary factors associated with PSA change.
Results
The intervention and control groups did not differ statistically on any demographic or disease-related factor. Although the intervention group experienced decreases of 39% in intakes of saturated fatty acid (SFA as percent of total calories) (p<0.0001) and 12% in total energy intake (218 kcal/day p<0.05)], no difference in PSA change was observed by intervention status. Signal detection methods indicated that in men increasing their consumption of fruit, 56% experienced no rise in PSA (vs. 29% in men who did not increase their fruit intake). Among men who increased fruit and fiber intakes, PSA increased in 83% of participants who also increased saturated fatty acid intake (vs. 44% in participants who decreased or maintained saturated fatty acid intake).
Conclusion
Results are discussed in the context of conventional treatment strategies that were more aggressive when this study was being conducted in the mid-2000s. Positive health changes in a number of lifestyle parameters were observed with the intervention, and both increased fruit and reduced saturated fat intakes were associated with maintaining PSA levels in men with biochemically recurrent disease.
Keywords: prostate cancer, diet, physical activity, stress reduction
INTRODUCTION
Prostate cancer (PrCA) is the most commonly occurring cancer, excluding skin cancer, in males in Western populations (1). In the U.S., one man in six will be diagnosed with invasive PrCA in his lifetime (2). Generally, patients who present with PrCA are treated with either radical prostatectomy or radiation therapy (3). Though not a particularly virulent cancer (its mortality:incidence ratio, 0.17, is the lowest of any common malignancy (4, 5)), incidence and mortality are much higher in Black men than their White counterparts (5, 6). Overall, approximately 35% of men diagnosed and treated for PrCA will have a biochemically defined recurrence, marked by a detectable prostate specific antigen (PSA) elevation, within ten years of definitive local therapy (7). Unfortunately, over a third of those individuals with PSA elevations will go on to develop metastatic disease within the subsequent five years (7). Recent (i.e., published after this study was conducted) findings from well-designed cohort studies showing that mortality rates are as high or higher with intensive screening (8, 9) underline the need to identify means for improving survival in men with biochemically recurrent disease (i.e., those who show the first evidence of metastasis).
No curative therapy exists for metastatic PrCA(10), although medical and surgical androgen ablation can produce responses in most patients, with an average duration of favorable response in the range of 18-24 months (11-13). Androgen ablation, however, produces side effects whose severity has motivated a search for novel adjunctive strategies that could retard tumor progression and postpone the use of such therapy (14-16).
Dramatic international variations exist in age-adjusted cancer incidence and mortality rates (1, 17). Large (three- to nine-fold) and rapid increases in incidence and mortality rates (compared to approximate host-country rates) are evident by the second generation after migration (18-20). These ecological observations implicate environmental, rather than genetic, factors in accounting for most of these differences. Epidemiologic and laboratory studies further suggest that among environmental influences dietary factors constitute the most important modifiable risk factors in determining aggressiveness of tumors (21-27). Likewise, using criteria to assess diet - cancer relationships (28) suggests it is “probable” that lack of physical activity (PA) is associated with PrCA (29). While there is conflicting interpretation of this evidence (30), it is known that PA has a variety of health benefits, including enhancing emotional (e.g., depression, anxiety) and physical functioning following diagnosis and treatment (31). Also, it is known that PA can affect endogenous androgens and sex hormone binding globulin (SHBG), a plausible biologic mechanism for reducing risk of cancer (32). The intersection of the effects of diet and PA on prostate cancer recurrence may be mediated in part through a change in relative weight. Obesity is positively associated with aggressive PrCA (33), and a recent review suggests that increased weight is a risk factor for biochemical recurrence and disease progression (34).
Because of their potential for reducing risk of recurrence, lifestyle interventions are of interest to many cancer patients following their diagnosis and treatment (35). This intervention trial builds on results from an earlier study in which we found that the strongest relationships existed between decreases in PSA change and increases in dietary fiber intake [a good indicator of dietary change (Spearman’s r = −0.73, p = 0.02)] and in the number of minutes of exercise [Spearman’s r = −0.60, p = 0.04 (10)]. The setting for this diet and PA intervention trial was South Carolina, an area of high prostate cancer incidence in which Black residents experience the highest mortality rate of the disease in the world (5, 36-38).
METHODS
Participants
Men eligible to participate were required to have had a histologically confirmed diagnosis of adenocarcinoma of the prostate, for which they were treated by radical prostatectomy or radiation therapy as a primary therapy. In addition, each man had to experience a rise in serum PSA concentration after achieving a post-surgery nadir (usually at or close to zero). The definition for biochemical recurrence based on PSA has been debated in the literature (7, 39, 40) At the time of this study, the commonly used community standard for judging radical prostatectomy treatment failure was based on 3 successive PSA rises at 2- to 3-month intervals, rising to a level of 1.5ng/mL. In addition, men had no other malignancy in the past 5 years; were not taking thyroid medication, antibiotics, diuretics or steroids; were able to read at a sixth-grade level; spoke English as their first language; were compos mentis; and were willing to be randomized to the treatment or usual care control (with an option to take the treatment after their sixth month participating as a control). Each man was required to enter the trial with a partner who had to fulfill all of these additional eligibility criteria. Other exclusions for participants included receiving post-operative hormone therapy for PrCA; having a diagnosis or symptoms of cardiovascular, pulmonary, Crohn’s disease or active ulcerative colitis or metabolic disease; experiencing a weight loss in excess of five pounds in the previous 3 months; regularly consuming more than two alcoholic drinks per day; planning to take hormone supplements, or fish oil or other supplements rich in omega-3 fatty acids; or having been diagnosed with post traumatic stress disorder (PTSD). A total of 60 men, each with a partner of his choice, were recruited into the study. Randomization was blocked by age (± 5 years) and race (Black / White). All recruitment and data collection procedures were approved by the Institutional Review Boards of Palmetto Health and the University of South Carolina.
Setting
Participants for this study were drawn primarily from the major urology practices in Richland (67%) and Lexington Counties (9%), which comprise the most densely populated center of the Columbia, South Carolina Standardized Metropolitan Statistical area (also known as the “Midlands”), as well as from Orangeburg (6%), Newberry (6%), Kershaw (6%), Sumter (4%), and Fairfield (2%) counties. The intervention was conducted under the auspices of the South Carolina Cancer Prevention and Control Program at various locations in the Columbia area. All clinical data were collected in the facilities of the Cancer Prevention and Control Program in Columbia.
Intervention
Participants were randomized to either the intervention or control group. The intervention consisted of an individual session in which dietary and PA goals were discussed and set as well as twelve 2.5-hour group sessions conducted weekly over the first three months of involvement. Monthly group booster sessions and progress calls by instructors to each participant and partner continued for 3 months thereafter. Participants were given daily “homework” assignments that consisted of cooking, PA, and stress reduction activities. There were a total of six 6-month intervention cycles initiated in June 2004 and continued over 28 months through October 2006.
The decision to combine diet, PA, and stress reduction was based on the observation that interventions that include multiple behavioral techniques are associated with the largest health behavior change (41-44). The diet portion of the intervention was focused on food-related goals including decreasing meat and dairy consumption while increasing consumption of whole grains, soybeans and soybean products, other beans, and vegetables. As with previous studies, the diet included some ingredients familiar to participants and others that were new and different (10, 45, 46). The components are consistent with a prudent, if ambitious, interpretation of current dietary goals, incorporating foods that have been shown or are thought to influence PrCA progression such as soy, carotenoids, and various spices (28, 47, 48). Cooking techniques and condiments were used to create a sense of familiarity and adventure (as opposed to the feeling of deprivation that diets often engender). We have used similar diets in other studies in breast (46) and prostate cancer (10) patients, and in hyperlipidemic subjects (45, 49).
The objective of the exercise training protocol was to improve fitness and general well-being by working with participants to identify an activity that they enjoyed. Initial PA goals were determined by the participant’s physical functioning, consistent with past interventions with cancer patients (50). To maximize safety, men were instructed to increase their exercise duration and intensity gradually. The intervention aimed to have the participant attain CDC/ACSM recommendations of ≥30 minutes of moderate intensity exercise on ≥5 days per week, a goal consistent with the level of leisure-time activity currently recommended for attaining health benefits (51). The 45-minute training sessions, which were completed as part of the “homework” assignment, included stretching (5 minutes), warm-up (3-5 minutes), and 30 minutes of the actual exercise (e.g., brisk walking), followed by a cool-down period of “active” recovery (e.g., walking at an easy pace).
The intervention strategy was based on the premise that successful lifestyle change relies on the participant developing a self-awareness of the mind/body connection and internalizing the relationship between healthy lifestyle habits and the experience of well-being. It integrated the essential features of Mindfulness Based Stress Reduction (MBSR) into all aspects of the intervention. As with our previous work (10), we combined elements of MBSR with delivery of this intervention, a strategy that also may contribute to self-regulation of other physiologic processes (52). We have had a long history of integrating work in mindfulness with cancer treatment (10, 53-56).
Control Condition
Participants randomized to the control group underwent the same assessment protocols as those randomized to intervention. No attempt was made to limit control participants’ use of psychosocial care available to PrCA patients in the community or to national educational or supportive resources. All men and partners assigned to control were offered the opportunity to take the intervention, free of charge, at the next available cycle following their participation as a control for six months.
Measures
Data Collected Only at Baseline (i.e., enrollment, prior to randomization)
Clinical, pathological, and histological data were abstracted from the participant’s medical record. Demographic and health-related data, including participants’ prior experience in meditation or other spiritual practices, were obtained. Response sets previously found to bias self-reported diet and PA include social desirability and social approval were measured using the Marlowe-Crowne Social Desirability (MCSD) Scale (57, 58) and the Martin-Larsen Approval Motivation (MLAM) Scale (59).
Data Collected at Baseline, 3 and 6 Months
Dietary intake was assessed using 24-hour recall interviews (24HR), the method that we (60-62) and others (63, 64) have found to produce the lowest overall error and that is least prone to measurement biases (65). Three randomly selected days (2 weekdays and 1 weekend day) were queried at each of the three time points during a 2-week sampling window. Physical activity was assessed using the Community Health Activities Model Program for Seniors (CHAMPS) Physical Activity Questionnaire and PA levels and household activity levels (66). The values are expressed in metabolic equivalents (METs), with one MET being the equivalent of resting metabolic energy expenditure (67). Anthropometric measurements that were collected included the following: standing height (cm), weight (kg), waist and hip circumferences (cm), and elbow diameter (cm). These measures were chosen to capture changes in overall/regional adiposity (68, 69). In addition, bioelectric impedance (BIA) measures were obtained using the RJL Quantum X to estimate changes in % body fat and lean body mass (kg) using an appropriate prediction equation (70).
PSA was measured in serum obtained via venipuncture by trained phlebotomists at each of the three times (baseline, 3-months, and 6-months). A 5 mL vial of blood was collected from each male participant (not partners) for testing by Quest® Laboratories.
Statistical Methods
Descriptive statistics were computed for all variables using frequencies or means (± standard deviation) as appropriate. Baseline differences between intervention and control participants were assessed using Chi Square for categorical variables and a t-test for continuous variables. Formal intention-to-treat analyses were conducted using a repeated measures mixed-model analysis of variance (ANOVA) to test the overall intervention effect on PSA levels and on intervention-related anthropometric, dietary, and PA factors (71) using Proc Mixed in SAS® (72). In these models, an intervention condition (intervention vs. control) and an intervention-by-time (baseline, 3-month and 6-month) interaction term were fit as fixed factors, while participant was fit as the repeated factor. Results are presented as least squares means and its standard error; differences between intervention and control at each time point were assessed with a t-test. A separate model was fit for each dependent variable. Prior to fitting a model, each outcome variable was assessed for normality. Subsequently, PSA was log transformed, while total and moderate-vigorous PA (MVPA, in METS/d) and fruit servings per day were square-root transformed to adjust for positive skew in the data. The influence of outliers was evaluated and subsequently, we reanalyzed the PSA data without 2 values, and found that although the means changed slightly, the overall interpretation did not change. Results are presented for the entire sample of 47 men. All analyses were conducted using SAS® software (version 9.1; SAS Institute, Inc. Cary, NC).
Often in randomized trials post hoc analyses provide insights into future research that cannot be gleaned from classical intention-to-treat analyses. In this study post hoc analyses entailed testing specific components of the diet and PA interventions on PSA change using signal detection methods (i.e., Decision Tree Analyses). All analyses were performed using Answer Tree v. 3.1 (SPSS, Chicago, IL). The change in PSA was dichotomized to 1) decrease or no change and 2) an increase. Predictors include PA (total and moderate-vigorous physical activity), and diet [total calories, fat and specific categories of fatty acids as a percent of calories (fat %), fiber, fruit and vegetable intake. The Chi-square Automatic Interaction Detection (CHAID) method was used to develop the classification trees. As the sample size is small, and the power to detect significant associations is relatively low, in the model investigating all predictors together, the significance level was set at alpha=0.20 to minimize missing potentially important associations. Tree branches were developed using a parent node ≥10, child node ≥5. For the purposes of the CHAID analysis, the effective sample size was n=46 for all participants with paired baseline and 3-month data. Analyses were conducted based on both 3-month and 6-month PSA data.
RESULTS
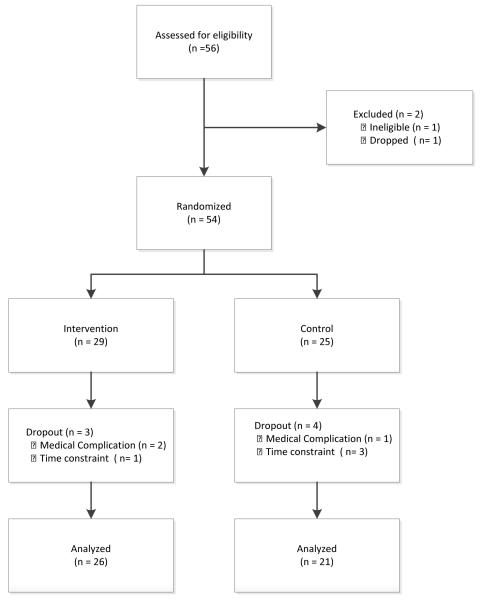
A total of 60 eligible men were recruited to participate in the study. Because recruitment was slow at the beginning and it was important to begin the first intervention cycle in order to do a trial run of the intervention and satisfy participant demand, the first four men and their partners were assigned to the intervention. Data from these individuals are excluded from analyses presented here. In Figure 1, the total participants assessed for eligibility excludes these initial four recruits. Two participants withdrew before randomization. Of the remaining 54 participants who were successfully randomized, complete data were available for univariate analyses on a total of 47 men with rising post-treatment PSA (Figure 1). The other 7 participants dropped due to time constraints (n=4) or complications associated with recurrence (n=3).
Figure 1.
Consort Diagram showing participant recruitment, screening, randomization, and retention
The intervention and control groups did not differ statistically on any demographic, anthropometric, lifestyle or medical characteristics (Table 1). Similarly, there were no significant differences at baseline in any dietary or PA factor. Prior to entering the study roughly half received both a prostatectomy and radiation as treatment; a third had radiation only, while the remainder had only a prostatectomy.
Table 1.
Baseline characteristics of study participants by randomization status (n=47)
| Intervention (n=26) | Control (n=21) | P | |
|---|---|---|---|
| Age, y* | 69.7 ± 8.8 | 71.1 ± 8.1 | 0.55 |
| Height, cm* | 173.0 ± 6.6 | 173.1 ± 5.4 | 0.96 |
| Weight, kg* | 86.4 ± 14.8 | 88.1 ± 16.8 | 0.71 |
| BMI, kg/m2* | 28.9 ± 4.9 | 29.4 ± 5.6 | 0.73 |
| Fat mass (lb)* † | 67.3 ± 18.3 | 68.4 ± 22.7 | 0.86 |
| Fat-free mass (lb)* † | 122.7 ± 18.8 | 125.4 ± 17.0 | 0.62 |
| Percent fat-free mass*† | 64.9 ± 5.3 | 65.5 ± 5.5 | 0.74 |
| Waist:Hip ratio* | 0.93 ± 0.11 | 0.94 ± 0.06 | 0.62 |
| Race‡ | 0.63 | ||
| White/ European American | 19 (73.1%) | 14 (66.7%) | |
| Black/ African American | 7 (26.9%) | 7 (33.3%) | |
| Education‡ | 0.21 | ||
| High school grad or less | 5 (19.2%) | 7 (33.3%) | |
| High school and some college | 11 (42.3%) | 4 (19.1%) | |
| College grad or more | 10 (38.5%) | 10 (47.6%) | |
| Marital status‡ | 0.27§ | ||
| Widowed, divorced, or single | 6 (23.1%) | 2 (9.5%) | |
| Married or with partner | 20 (76.9%) | 19 (90.5%) | |
| Employment‡ | 0.91§ | ||
| Yes, Full time | 4 (15.4%) | 5 (23.8%) | |
| Yes, Part time | 4 (15.4%) | 3 (14.3%) | |
| No | 18 (69.2%) | 13 (61.9%) | |
| Smoking status‡ | 0.82§ | ||
| Never | 9 (36.0%) | 8 (40.0%) | |
| Current | 3 (12.0%) | 1 (5.0%) | |
| Former | 13 (52.0%) | 11 (55.0%) | |
| Gleason score‡ | 0.77§ | ||
| Missing | 5 (19.2%) | 6 (28.6%) | |
| Well differentiated (<5) | 1 (3.9%) | 0 (0.0%) | |
| Moderately differentiated (5-6) | 6 (23.1%) | 6 (28.6%) | |
| Poorly differentiated (≥7) | 14 (53.9%) | 9 (42.9%) | |
| Type of treatment‡ | 0.85§ | ||
| Prostatectomy | 3 (11.5%) | 4 (19.1%) | |
| Prostatectomy & radiation | 13 (50.0%) | 10 (47.6%) | |
| Radiation only | 10 (38.5%) | 7 (33.3%) |
Continuous variables are presented as the mean ± SD; P values are based on Student’s t-test.
Computed from bioelectric impedance (BIA) measures, as described in text .
Categorical variables are presented as the frequency and %;
P values are based on ChiSquare or Fisher’s Exact tests as noted with §.
At 3 months, intervention participants, relative to controls, experienced significant decreases in energy, total fat, saturated and monounsaturated fatty acid intakes, and an increase in dietary fiber (Table 2). Although we expected to see significant increases in fruit and vegetable intakes as a result of the intervention, the changes at 3 months were modest, and of similar magnitude in both intervention and control participants. The effect on saturated fat intake persisted to 6 months (3 months post intervention); however, the changes we observed in the other dietary factors began to recede back towards pre-intervention levels. In addition, there were significant increases in both total PA and moderate-vigorous PA in the intervention group at 3 months. Commensurate with the decrease in energy intake and an increase in energy expenditure in the intervention participants was a BMI [body mass intake = weight(kg)/height(m)2] decrease of 0.5 kg/m2, a change whose direction would be expected but whose magnitude is smaller than expectation based on our previous research (10, 46).
Table 2. Baseline, Three-Months, and Six-Month Measurements by Randomization Status for Diet, Physical Activity, PSA and BMI
| Variable/Randomization Condition |
Baseline Means (95% CI) |
3 Months Means (95% CI) |
6 Months Means (95% CI) |
p- value* |
|---|---|---|---|---|
|
Total energy intake (kcal/d)† |
||||
| Control | 1587 (1393 - 1782) | 1797 (1603 -1992) | 1685 (1491 -1880) | 0.01 |
| Intervention | 1784 (1610 - 1959) | 1566 (1391 -1741) | 1631 (1457 - 1806) | |
| (.08)‡ | ||||
| Total fat (%en) † § | ||||
| Control | 32.7 (29.7 – 35.7) | 33.6 (30.7 – 36.6) | 32.8 (29.9 – 35.8) | 0.02 |
| Intervention | 31.7 (29.0 -34.4) | 26.1 (23.4 – 28.8) | 29.3 (26.6 – 32.0) | |
| (<0.001)† | ||||
| Saturated Fat (%en) † § | ||||
| Control | 9.8 (8.6 – 11.0) | 10.2 (9.0 – 11.4) | 10.1 (8.9 – 11.3) | <.0001 |
| Intervention | 9.9 (8.8 – 10.9) | 6.0 (5.0 – 7.1) | 6.9 (5.8 – 8.0) | |
| (<0.0001)† | (0.0001)† | |||
|
Monounsaturated Fat
(%en) † § |
||||
| Control | 12.5 (11.1 – 14.0) | 12.8 (11.4 – 14.3) | 12.8 (11.3 – 14.2) | 0.14 |
| Intervention | 12.0 (10.7 – 13.3) | 10.1 (8.8 – 11.4) | 11.7 (10.4 – 13.0) | |
| (0.007)† | ||||
|
Polyunsaturated
Fat(%en) † § |
||||
| Control | 7.6 (6.4 – 8.7) | 7.7 (6.5 – 8.8) | 7.1 (6.0 – 8.3) | 0.14 |
| Intervention | 7.2 (6.2 – 8.2) | 7.8 (6.8 – 8.8) | 8.4 (7.4 – 9.4) | |
| ω-3 Fatty acids (g/d) † ¶ | ||||
| Control | 1.5 (0.9 – 2.1) | 1.8 (1.2 – 2.3) | 1.5 (0.9 – 2.1) | 0.40 |
| Intervention | 1.9 (1.4 – 2.4) | 2.2 (1.7 – 2.7) | 2.4 (1.9 – 2.9) | |
| (0.02) | ||||
| Fiber (g) † | ||||
| Control | 15.5 (11.6 – 19.4) | 17.6 (13.7 – 21.5) | 17.5 (13.6 – 21.4) | 0.09 |
| Intervention | 17.3 (13.8 – 20.8) | 23.9 (20.4 – 27.4) | 21.1 (17.6 – 24.6) | |
| (0.02)† | ||||
| Fruit (srv/d) † | ||||
| Control | 1.3 (0.7 – 2.0) | 2.0 (1.2 – 2.9) | 1.4 (0.8 – 2.1) | 0.28 |
| Intervention | 1.4 (0.8 – 2.1) | 1.7 (1.1 – 2.4) | 2.0 (1.4 – 2.9) | |
| Vegetable (srv/d) † | ||||
| Control | 2.8 (1.9 – 3.7) | 3.5 (2.6 – 4.4) | 2.7 (1.9 – 3.7) | 0.87 |
| Intervention | 3.5 (2.7 – 4.3) | 4.0 (3.2 – 4.8) | 3.1 (2.3 – 4.0) | |
|
Total Physical Activity
(METS/week) ∥ |
||||
| Control | 32.8 (21.8 - 46.0) | 29.3 (19.0 – 41.8) | 38.4 (26.4 –52.6) | 0.08 |
| Intervention | 44.4 (32.7 – 57.8) | 53.6 (40.8 – 68.1) | 42.6 (31.1 – 56.0) | |
| (0.009) | ||||
|
Moderate & Vigorous
PA (METS/week) ∥ |
||||
| Control | 20.2 (11.6 – 31.1) | 16.0 (8.5 – 25.8) | 26.9 (16.8 – 39.4) | 0.02 |
| Intervention | 28.2 (18.8 – 39.5) | 36.8 (26.0 – 49.3) | 27.1 (17.8 – 38.3) | |
| (0.006)† | ||||
| BMI (kg/m2) | ||||
| Control | 29.4 (27.1 – 31.7) | 29.5 (27.2 – 31.8) | 29.2 (26.9 – 31.5) | 0.11 |
| Intervention | 28.9 (26.8 – 30.9) | 28.3 (26.3 – 30.4) | 28.5 (26.4 – 30.5) | |
| PSA (ng/ml) ** | ||||
| Control | 0.71 (0.33 – 1.54) | 0.77 (0.36 – 1.68) | 0.78 (0.36 – 1.70) | 0.45 |
| Intervention | 0.87 (0.43 – 1.74) | 1.09 (0.54 – 2.18) | 0.84 (0.42 – 1.68) |
interaction between time and randomization condition
derived from three 24-hour dietary recall interviews (24HR) at each of three measurement points, including baseline
comparison between the intervention and control at the specific time interval
expressed as a percentage of total energy intake
the sum of all omega-three fatty acids as derived from 24HR
obtained from the CHAMPS (66), the values are expressed in metabolic equivalents (METS), with one MET = the equivalent of resting metabolic energy expenditure. Values were square root transformed for analysis, and then back transformed for presentation
log transformed and then back transformed for presentation
Using a simple intention-to-treat repeat measures analysis, no difference in PSA was observed by intervention status. Approximately ½ (48% overall; 50% in intervention group participants) of men experienced a decrease or no change in PSA at 3 months, a high percentage given that most men who have relapsed biochemically will continue to experience an inexorable increase in PSA (consistent with metastatic disease) (73-75). As noted above, intervention participants experienced a decrease in BMI at 3 months, but this was not statistically significant relative to control participants whose BMI remained relatively stable (p=0.11). Overall, BMI appeared to be positively correlated with PSA (ρ =0.27), but did not reach statistical significance (p=0.07).
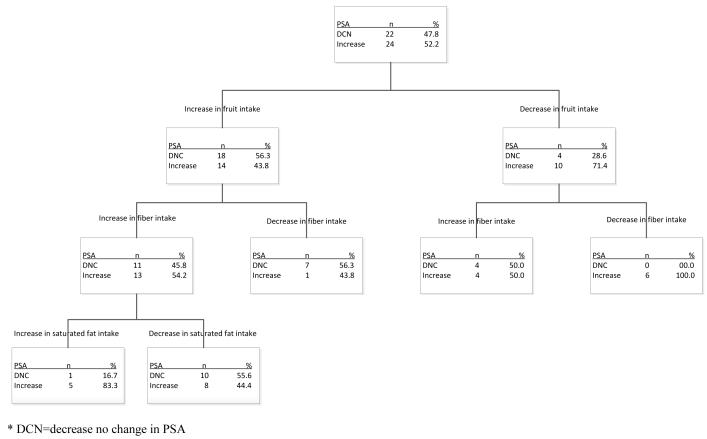
Although the intervention was not observed to affect PSA levels, there was suggestive evidence that diet and PA were changed and that many participants did not experience the expected post-treatment rise in PSA concentrations. To examine which factors may have played a role in blunting a PSA rise, we conducted an exploratory classification tree analysis in all participants with paired baseline and 3-month PSA measures using the CHAID method and evaluated 3-month change in dietary and PA factors as potential predictors of PSA change at 3 months. All change scores were dichotomized as either a decrease or no change (DNC) vs. an increase. An increase in fruit intake was associated with a DNC in PSA in 56% of participants, while a DNC in fruit intake was associated with a 29% DNC in PSA (Figure 2). The next branch of the tree is based on dietary fiber intake. In participants with increased fruit intake and an increase in fiber intake, 46% had DNC in PSA, while 56% of participants with an increase in fruit but DNC in fiber experienced a DNC in PSA. All participants with a DNC in both fruit and fiber experienced an increase in PSA. In the third and final branch of the tree PSA increased in 83% of participants with increased fruit and fiber intakes who also increased saturated fatty acid intake vs. 44% in participants who decreased or maintained saturated fatty acid intake. Results based on the 6-month data, to which 44 men contributed (1 was lost to follow up and 2 went on hormone therapy; i.e., Lupron®) were virtually identical. In the right branch of the tree fiber is still the best predictor and the groups are identical except for the 1 subject who is lost. On the left branch, where fruit intake increases, the first and only split is for vegetable intake (not counting French fries). For those with an increase in vegetable intake, 87% had no change in PSA, while for those with no increase in vegetable intake only 60% had no change in PSA (p=0.10).
Figure 2.
Results of signal detection modeling for PSA change* at 3 months
* DCN=decrease no change in PSA
DISCUSSION
While no change was observed in PSA levels over the intervention period using intention-to-treat analyses, positive health changes in a number of lifestyle parameters were observed with the intervention. Furthermore, longer-term effects on PSA rise cannot be ruled out. Commonly used community standard for judging radical prostatectomy treatment failure is based on 3 successive PSA rises at 2- to 3-month intervals (12, 14), rising to a level of 1.5ng/mL. During the period of this study, practice in the Midlands Region of South Carolina and stringent interpretation of HIPAA laws ruled out our having access to men whose PSA levels had risen above this level (76). Because of the more aggressive treatment of men with rising PSA in South Carolina vs. Massachusetts, where our previous study was conducted, men in this study had somewhat lower PSA levels than those of men in the Massachusetts study (10). Medical practice in Massachusetts, which has one of the highest rates of managed care in the United States (94%), is very different than in South Carolina, which has the lowest rates (43%) (77).The tendency to treat PrCA more aggressively placed a constraint that restricted our ability to observe an effect across a broadly relevant range of both outcomes and exposures. Future research will need to better account for this; perhaps working with clinical colleagues to relax this constraint or even focusing on men who have failed previous treatment or who choose this approach in favor of more conventional treatment options, which provide no survival benefit in any event (34, 78). Findings that were published more recently (i.e., in 2009) showing that mortality rates are as high or higher with intensive screening, and by inference, more aggressive treatment (8, 9) should create a climate more conducive to the conduct of studies such as this. Furthermore, hospitals, internal review boards and physician practices better understand the partial waiver component of HIPAA, which should contribute to making recruitment easier in the future.
Despite no significant short-term change in PSA rise in men with biochemical evidence of disease progression, it is reasonable to expect that the changes associated with the intervention will improve the quality of life of men exposed to the intervention and may translate into substantial health benefits with long-term adherence to the intervention recommendations, as demonstrated in one longer-term low fat/high fiber/soy supplement intervention in men with prostate cancer (79). To date, the majority of short-term diet and lifestyle interventions in PrCA have focused either on intensive interventions during the time period between diagnosis and surgery (80, 81), or on the feasibility of conducting dietary interventions in men with PrCA without reporting effects on PSA (82)or without including a comparison control group in the design (83, 84). Others have focused on specific components of the diet (e.g., rye and bran (85); flaxseed (86); soy Maskarinec, 2006 #9643}; and tomatoes (87),with varied results (88). Similar to our results, a recent randomized controlled trial in men receiving androgen suppression therapy found no effect of a diet and PA intervention on PSA levels, but significant beneficial changes in health behaviors in those men in the intervention arm (89).
Results from the signal detection method used to conduct post hoc analyses on 3-month data suggested a number of factors that might influence the process of PrCA metastasis. Most of these are related to diet; e.g., there was a suggestion that both decreased saturated fatty acid intake and increased fiber and fruit intake were associated with absence of a rise in PSA. Results from the 6-month data, which were a bit less robust due to the loss of 3 participants, were virtually identical (with vegetable intake substituting for fiber). Future research should focus on:
Examining the effect of specific dietary components; answering questions regarding how they would work synergistically with one another (but also keeping in mind that the individual components tend to be highly inter-correlated).
Addressing the effect of PA on PSA change and possible interactions between PA and diet.
Focusing on changes in fitness.
Critical examination of the mindfulness-based meditation component – both in terms of measurement as well as modeling data in relation to the other components of the intervention.
Issues regarding how such an intensive intervention could fail to produce a measurable effect on PSA change, especially in light of positive results obtained in previous work (10),might also consider:
Insensitivity of PSA as a marker of metastatic disease progression (90, 91). It is well known that PSA is a crude screening test, but is more effective in circumstances where the prostate gland is missing entirely (hence the reliance on PSA doubling time or velocity in following men post-definitive therapy). Still, it is hardly equivalent to actually visualizing, or measuring in some other objective way, metastatic disease.
Time needed to change PSA may vary considerably across patients and may not be closely associated with virulence, though doubling time or velocity is widely used based on the assumption that the two concepts are linked (90, 92).
Unusual favorable changes in the control group, which are also consistent with the effect of diet on PSA. Perhaps this is an example of the “MRFIT effect,” wherein men in the control group are subject to secular trends in improving aspects of lifestyle (93). This change could also result from compensatory rivalry although this should be ruled out with a wait list control. It also must be remembered that though PrCA is usually an indolent cancer (3, 94) many men and their partners will perceive it as a life-threatening illness (95). While this may increase motivation in the intervention group, participants randomized to control are also likely to be subject to this influence.
Results based on signal detection analyses that indicated a beneficial effect of increasing fruit and fiber intake and decreasing intake of saturated fatty acids.
Differences in effect by race or educational status. Table 1 shows over 74% of our study population had at least some college education. In several of our previous studies we have found that participants who have less formal education are more compliant and derive greater health benefits than those with more schooling (45, 49). That previous work was conducted in hyperlipidemic individuals; so the effect of education may be quite different than in individuals having a condition that is more imminently life threatening.
SUMMARY and CONCLUSIONS
While there was no large difference in PSA change between men randomized to intervention vs. control, there were significant changes in dietary intake consistent with planned intervention effects; i.e., fruit and vegetable intake increased and both total fat and saturated fatty acid intake decreased. There also was a suggestion of an effect of BMI in increasing PSA. Post hoc signal detection analyses were consistent with the hypothesized effects of increased fruit and fiber intake and decreased saturated fat intake on PSA change. Future studies should focus on practical alternatives to PSA as a marker of metastatic disease progression, longer-term studies in order to observe change, and differences in intervention effects by race or educational status.
AKNOWLEDGEMENTS
The research upon which this publication is based was conducted with funding from the US Department of Defense Army Award DAMD 17-03-1-0139. We also acknowledge additional support by a grant from the National Cancer Institute, Center to Reduce Cancer Health Disparities (Community Networks Program) to the South Carolina Cancer Disparities Community Network (SCCDCN) [1U54 CA153461-01 Hébert, JR (PI)] and an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute to JR Hébert (K05 CA136975).
We wish to acknowledge the contribution of: Elizabeth A. Fallon, who worked on the PA aspects of the study as a postdoctoral fellow; Jamie Ritchie, who contributed to the signal detection analyses; Philip Cavicchia, who assisted with data analyses; Lynn Bridges, who assisted with patient recruitment, and Wendy B. McKenzie and Madeline Broderick who functioned as project coordinators in the early phases of the work.
Abbreviations
- PrCA
Prostate cancer
- PSA
prostate specific antigen
- SFA
saturated fatty acid
- SHBG
sex hormone binding globulin
- PTSD
post traumatic stress disorder
- MBSR
Mindfulness Based Stress Reduction
- MCSD
Marlowe-Crowne Social Desirability Scale
- MLAM
Martin-Larsen Approval Motivation
- CHAMPS
Community Health Activities Model Program for Seniors
- METs
metabolic equivalents
- BIA
bioelectric impedance
- ANOVA
analysis of variance
- CHAID
Chi-square Automatic Interaction Detection
Footnotes
CONFLICT of INTEREST None of the authors has a conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hebert JR, Hurley TG, Olendzki B, Ma Y, Teas J, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J Natl Cancer Inst. 1998;90:1637–47. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 2.Pienta KJ. Aetiology, epidemiology, and prevention of carcinoma of the prostate. In: Walsh PC, editor. Campbell’s Urology. 7th ed Saunders; Philadelphia: 1998. pp. 2489–96. [Google Scholar]
- 3.Klotz LH. Active surveillance with selective delayed intervention: walking the line between overtreatment for indolent disease and undertreatment for aggressive disease. Can J Urol. 2005;1:53–7. [PubMed] [Google Scholar]
- 4.United States Cancer Statistics: 1999-2005 Incidence and Mortality Web-based Report. 2009 US DHHS/CDC/NIH-NCI. (Accessed at www.cdc.gov/cancer/npcr/uscs.)
- 5.Hebert JR, Daguise VG, Hurley DM, Wilkerson RC, Mosley C, Adams SA, Puett R, Burch JB, Steck SE, Bolick-Aldrich S. Mapping cancer mortality-to-incidence ratios to illustrate racial and gender disparities in a high-risk population. Cancer. 2009;115:2539–52. doi: 10.1002/cncr.24270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daguise VG, Burch JB, Horner MJD, Mosley C, Hofseth LJ, Wargovich MJ, Lloyd SC, Hebert JR. Colorectal cancer disparities in South Carolina: Descriptive epidemiology, screening, special programs, and future direction. J South Carolina Med Assoc. 2006;102:212–20. [PubMed] [Google Scholar]
- 7.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 8.Schroder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Maattanen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A. Investigators E. Screening and prostate-cancer mortality in a randomized European study. New England Journal of Medicine. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [see comment] [DOI] [PubMed] [Google Scholar]
- 9.Andriole GL, Grubb RL, 3rd, Buys SS, Chia D, Church TR, Fouad MN, Gelmann EP, Kvale PA, Reding DJ, Weissfeld JL, Yokochi LA, Crawford ED, O’Brien B, Clapp JD, Rathmell JM, Riley TL, Hayes RB, Kramer BS, Izmirlian G, Miller AB, Pinsky PF, Prorok PC, Gohagan JK, Berg CD, Team PP. Mortality results from a randomized prostate-cancer screening trial. New England Journal of Medicine. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saxe GA, Hebert JR, Carmody JF, Kabat-Zinn J, Rosenzweig PH, Jarzobski D, Reed GW, Blute RD. Can diet, in conjunction with stress reduction, affect the rate of increase in prostate specific antigen after biochemical recurrence of prostate cancer? J Urol. 2001;166:2202–7. [PubMed] [Google Scholar]
- 11.Vassilikos EJ, Yu H, Trachtenberg J, Nam RK, Narod SA, Bromberg IL, Diamandis EP. Relapse and cure rates of prostate cancer patients after radical prostatectomy and 5 years of follow-up. Clin Biochemistry. 2000;33:115–23. doi: 10.1016/s0009-9120(99)00099-5. [DOI] [PubMed] [Google Scholar]
- 12.Hardie C, Parker C, Norman A, Eeles R, Horwich A, Huddart R, Dearnaley D. Early outcomes of active surveillance for localized prostate cancer. BJU Int. 2005;95:956–60. doi: 10.1111/j.1464-410X.2005.05446.x. [DOI] [PubMed] [Google Scholar]
- 13.Newling DW. The management of hormone refractory prostate cancer. Eur Urol. 1996;29:69–74. doi: 10.1159/000473843. [DOI] [PubMed] [Google Scholar]
- 14.Duchesne GM, Millar JL, Moraga V, Rosenthal M, Royce P, Snow R. What to do for prostate cancer patients with a rising PSA?--A survey of Australian practice. Int J Radiation Oncol, Biol, Phys. 2003;55:986–91. doi: 10.1016/s0360-3016(02)04213-x. [comment] [DOI] [PubMed] [Google Scholar]
- 15.Ornstein DK, Colberg JW, Virgo KS, Chan D, Johnson ET, Oh J, Johnson FE. Evaluation and management of men whose radical prostatectomies failed: results of an international survey. Urology. 1998;52:1047–54. doi: 10.1016/s0090-4295(98)00403-8. [DOI] [PubMed] [Google Scholar]
- 16.Taylor N, Kelly JF, Kuban DA, Babaian RJ, Pisters LL, Pollack A. Adjuvant and salvage radiotherapy after radical prostatectomy for prostate cancer. Int J Radiation Oncol, Biol, Phys. 2003;56:755–63. doi: 10.1016/s0360-3016(03)00069-5. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong B, Doll R. Environmental factors and cancer incidence and mortality in different countries with special references to dietary practices. Int J Cancer. 1975;15:617–31. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- 18.Heber D, Fair WR, Ornish D. Nutrition and prostate cancer: CaPCure Nutrition Project Monograph. 2nd Edition CaPCure; Santa Monica, CA: 1998. [Google Scholar]
- 19.Shimuzu H, Ross RK, Bernstein L, Yatani R, Henderson BE, Mack TM. Cancers of the prostate and breast among Japanese and white immigrants to Los Angeles County. Br J Cancer. 1991;63:963–6. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muir CS, Nectoux J, Staszewski J. The epidemiology of prostatic cancer: geographical distributions and time-trends. Acta Oncol. 1991;30:133–40. doi: 10.3109/02841869109092336. [DOI] [PubMed] [Google Scholar]
- 21.Clinton SK, Palmer SS, Spriggs CE, Visek WJ. Growth of Dunning transplantable prostate adenocarcinomas in rats fed diets with various fat contents. J Nutr. 1988;118:908–14. doi: 10.1093/jn/118.7.908. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KK, Noble RC. Dietary fat in relation to hormonal induction of mammary and prostate carcinoma in Nb rats. Carcinogenesis. 1987;81:851–3. doi: 10.1093/carcin/8.6.851. [DOI] [PubMed] [Google Scholar]
- 23.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumours. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 24.Harvei S, Bjerve KS, Tretli S, Jellum E, Robsahm TE, Vatten L. Prediagnostic level of fatty acids in serum phospholipids: omega-3 and omega-6 fatty acids and the risk of prostate cancer. Int J Cancer. 1997;71:545–51. doi: 10.1002/(sici)1097-0215(19970516)71:4<545::aid-ijc7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 25.Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO. Natural history of early, localized prostate cancer. JAMA. 2004;291:2713–9. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
- 26.Lehrer S, Diamond EJ, Stagger S, Stone NN, Stock RG. Increased serum insulin associated with increased risk of prostate cancer recurrence. Prostate. 2002;50:1–3. doi: 10.1002/pros.10026. [DOI] [PubMed] [Google Scholar]
- 27.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, Willett WD. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–9. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 28.Food, Nutrition and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research; Washington, DC: 1997. [DOI] [PubMed] [Google Scholar]
- 29.Friedenreich CM. Physical activity and cancer prevention: From observational to intervention research. Cancer Epidemiol Biomark Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 30.Byers T, Nestle M, McTiernan A, Doyle C, Currie-Williams A, Gansler T, Thun M. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2002;52:92–119. doi: 10.3322/canjclin.52.2.92. [DOI] [PubMed] [Google Scholar]
- 31.U.S. Department of Health and Human Services . Physical Activity and Health: A Report of the Surgeon General. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; Atlanta, GA: 1996. [Google Scholar]
- 32.Oliveria SA, Lee IM. Is exercise beneficial in the prevention of prostate cancer? Sports Med. 1997;23:271–8. doi: 10.2165/00007256-199723050-00001. [DOI] [PubMed] [Google Scholar]
- 33.Su LJ, Arab L, Steck SE, Fontham ET, Schroeder JC, Bensen JT, Mohler JL. Obesity and prostate cancer aggressiveness among African and Caucasian Americans in a population-based study. Cancer Epidemiol Biomarkers Prev. 2011;20:844–53. doi: 10.1158/1055-9965.EPI-10-0684. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Moyad MA. Dietary intervention in the management of prostate cancer. Curr Opinion Urol. 2007;17:168–74. doi: 10.1097/MOU.0b013e3280eb10fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–84. [PubMed] [Google Scholar]
- 36.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK. SEER Cancer Statistics Review, 1975-2002. National Cancer Institute; Bethesda, MD: 2005. [Google Scholar]
- 37.United States Cancer Statistics: 1999-2003 Incidence and Mortality Web-based Report. 2006 US DHHS/CDC/NIH-NCI. ( Accessed at www.cdc.gov/cancer/npcr/uscs.)
- 38.Drake BF, Keane TE, Mosley CM, Adams SA, Elder KT, Modayil MV, Ureda JR, Hebert JR. Prostate cancer disparities in South Carolina: Early detection, special programs, and descriptive epidemiology. J South Carolina Med Assoc. 2006;102:241–9. [PubMed] [Google Scholar]
- 39.Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–78. doi: 10.1038/nrc2351. [DOI] [PubMed] [Google Scholar]
- 40.Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ, Lilja H, Scardino PT. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 41.NHLBI Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1997;158:1855–67. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 42.Svendsen K, Kuller L, Martin M, Ockene J. Effects of passive smoking in the Multiple Risk Factor Intervention Trial. Am J Epidemiol. 1987;126:783–95. doi: 10.1093/oxfordjournals.aje.a114715. [DOI] [PubMed] [Google Scholar]
- 43.Lee CD, Sui X, Hooker SP, Hebert JR, Blair SN. Combined Impact of Lifestyle Factors on Cancer Mortality in Men. Ann Epidemiol. 2011 doi: 10.1016/j.annepidem.2011.04.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson KE, Sorensen G, Pearson M, Hebert JR, Gottlieb BR, McCormick MC. Design of an intervention addressing multiple levels of influence on dietary and activity patterns of low-income, postpartum women. Health Educ Research. 2002;17:531–40. doi: 10.1093/her/17.5.531. [DOI] [PubMed] [Google Scholar]
- 45.Hebert JR, Ebbeling CB, Ockene IS, Ma Y, Rider L, Merriam PA, Ockene JK, Saperia GM. A dietitian-delivered group nutrition program leads to reductions in dietary fat, serum cholesterol, and body weight: findings from the Worcester Area Trial for Counseling in Hyperlipidemia (WATCH) J Am Diet Assoc. 1999;99:544–52. doi: 10.1016/s0002-8223(99)00136-4. [DOI] [PubMed] [Google Scholar]
- 46.Hebert JR, Ebbeling CB, Hurley TG, Ma Y, Clemow L, Olendzki BC, Saal N, Ockene JK. Change in women’s diet and body mass following intensive intervention in early-stage breast cancer. J Am Diet Assoc. 2001;101:421–31. doi: 10.1016/S0002-8223(01)00109-2. [DOI] [PubMed] [Google Scholar]
- 47.U.S. Department of Health and Human Services . The Surgeon General’s Report on Nutrition and Health. U.S.G.P.O.; Washington DC: 1988. DHHS (PHS Publ No. 88-50210) [Google Scholar]
- 48.National Research Council . Report of the Committee on Diet and Health, Food and Nutrition Board. National Academy Press; Washington, DC: 1989. [Google Scholar]
- 49.Ockene IS, Hebert JR, Ockene JK, Saperia GM, Stanek E, Nicolosi R, Merriam PA, Hurley TG. Effect of physician-delivered nutrition counseling training and an office support system on saturated fat intake, weight, and serum lipid measurements in a hyperlipidemic population: the Worcester-Area Trial for Counseling in Hyperlipidemia (WATCH) Arch Intern Med. 1999;159:725–31. doi: 10.1001/archinte.159.7.725. [DOI] [PubMed] [Google Scholar]
- 50.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21:171–9. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 51.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 52.Coker KH. Meditation and prostate cancer: Integrating a mind/body intervention with traditional therapies. Seminars in Urologic Oncology. 1999;17:111–8. [PubMed] [Google Scholar]
- 53.Kabat-Zinn J, Massion AO, Hebert JR, Rosenbaum E. Meditation. In: Holland JC, editor. Psychooncology. Oxford University Press; New York: 1998. pp. 767–79. [Google Scholar]
- 54.Clemow L, Hebert JR, Massion AO, Fowke J, Druker S, Kabat-Zinn J. A meditation-based stress reduction intervention for younger women with breast cancer. Annals Behav Med. 1997;19:77. [Google Scholar]
- 55.Kabat-Zinn J, Massion AO, Hebert JR, Rosenbaum E. Meditation. In: Tagliaferri M, Cohen I, Tripathy D, editors. Breast Cancer: Beyond Convention. Pocket Books; New York: 2002. [Google Scholar]
- 56.Massion AO, Teas J, Hebert JR, Wertheimer MD, Kabat-Zinn J. Meditation, melatonin, and breast/prostate cancer: hypothesis and preliminary data. Medical Hypotheses. 1995;44:39–46. doi: 10.1016/0306-9877(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 57.Marlowe D, Crowne DP. Social desirability and response to perceived situational demands. J Consult Clin Psychol. 1961;25:109–15. doi: 10.1037/h0041627. [DOI] [PubMed] [Google Scholar]
- 58.Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Clin Psychol. 1960;24:349–54. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- 59.Martin HJ. A revised measure of approval motivation and its relationship to social desirability. J Pers Assess. 1984;48:508–16. doi: 10.1207/s15327752jpa4805_10. [DOI] [PubMed] [Google Scholar]
- 60.Hebert JR, Hurley TG, Chiraboga DE, Barone J. A comparison of selected nutrient intakes derived from three diet assessment methods used in a low-fat maintenance trial. Public Health Nutr. 1998;1:207–14. doi: 10.1079/phn19980032. [DOI] [PubMed] [Google Scholar]
- 61.Hebert JR, Hurley TG, Cavicchia P, Ma Y, Magner RP, Olendzki BC, Merriam PA, Ockene IS, Nebeling L. Response to Dr. Arab et al on “Number of 24-hour diet recalls needed to estimate energy intake”. Ann Epidemiol. 2010;20:87–8. doi: 10.1016/j.annepidem.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y, Olendzki BC, Pagoto SL, Hurley TG, Magner RP, Ockene IS, Schneider KL, Merriam PA, Hebert JR. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19:553–9. doi: 10.1016/j.annepidem.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buzzard IM, Faucett CL, Jeffery RW, McBane L, McGovern P, Baxter JS, Shapiro AC, Blackburn GL, Chlebowski RT, Elashoff RM, Wynder EL. Monitoring dietary change in a low-fat diet intervention study: advantages of using 24-hour dietary recalls vs food records. J Am Diet Assoc. 1996;96:574–9. doi: 10.1016/S0002-8223(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 64.Posner BM, Martin-Munley SS, Smigelski C, Cupples LA, Cobb JL, Schaefer E, Miller DR, D’Agostino RB. Comparison of techniques for estimating nutrient intake: The Framingham Study. Epidemiology. 1992;3:171–7. doi: 10.1097/00001648-199203000-00016. [DOI] [PubMed] [Google Scholar]
- 65.Hebert JR, Ebbeling CB, Matthews CE, Ma Y, Clemow L, Hurley TG, Druker S. Systematic errors in middle-aged women’s estimates of energy intake: Comparing three self-report measures to total energy expenditure from doubly labeled water. Ann Epidemiol. 2002;12:577–86. doi: 10.1016/s1047-2797(01)00297-6. [DOI] [PubMed] [Google Scholar]
- 66.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS Physical Activity Questionnaire for Older Adults: Outcomes for interventions. Med Sci Sport Exer. 2001;33:126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 67.Ainsworth B, Haskell W, Whitt M, Irwin M, Swartz A, Strath S, O’Brien W, Schmitz K. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2001;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 68.Durnin JVGA, Womersley J. Body fat assessed from total body density and its estimation from skinfold thickness: measurements on 481 men and women aged from 16 to 72 years. Br J Nutr. 1974;32:77–92. doi: 10.1079/bjn19740060. [DOI] [PubMed] [Google Scholar]
- 69.VanItallie T. When the frame is part of the picture. Am J Public Health. 1985;75:1054–5. doi: 10.2105/ajph.75.9.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segal KR, Van Loan M, Fitzgerald PI, Hodgdon JA, Van Itallie TB. Lean body mass estimation by bioelectrical impedance analysis: a four-site cross-validation study. Am J Clin Nutr. 1988;47:7–14. doi: 10.1093/ajcn/47.1.7. [DOI] [PubMed] [Google Scholar]
- 71.Brown H, Prescott R. Applied Mixed Models in Medicine. John Wiley & Sons, LTD; West Sussex: 1999. [Google Scholar]
- 72.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for Mixed Models. Cary: 1996. [Google Scholar]
- 73.Sandler HM, Dunn RL, McLaughlin PW, Hayman JA, Sullivan MA, Taylor JM. Overall survival after prostate-specific-antigen-detected recurrence following conformal radiation therapy. Int J Radiation Oncol, Biol, Phys. 2000;48:629–33. doi: 10.1016/s0360-3016(00)00717-3. [DOI] [PubMed] [Google Scholar]
- 74.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–53. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 75.Narain V, Cher ML, Wood DP., Jr. Prostate cancer diagnosis, staging and survival. Cancer & Metastasis Reviews. 2002;21:17–27. doi: 10.1023/a:1020104004588. [DOI] [PubMed] [Google Scholar]
- 76.Heiney SP, Adams SA, Cunningham JE, McKenzie W, Harmon B, Hebert JR, Modayil M. Subject recruitment for cancer control studies in an adverse environment. Cancer nursing. 2006;29:291–9. doi: 10.1097/00002820-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 77.Managed Care Penetration by State. Modesto, CA: 2011. [Google Scholar]
- 78.Carter HB, Ferrucci L, Kettermann A, Landis P, Wright EJ, Epstein JI, Trock BJ, Metter EJ. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521–7. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Z, Aronson WJ, Arteaga JR, Hong K, Thames G, Henning SM, Liu W, Elashoff R, Ashley JM, Heber D. Feasibility of a low-fat/high-fiber diet intervention with soy supplementation in prostate cancer patients after prostatectomy. Eur J Clin Nutr. 2008;62:526–36. doi: 10.1038/sj.ejcn.1602743. [DOI] [PubMed] [Google Scholar]
- 80.Heymach JV, Shackleford TJ, Tran HT, Yoo SY, Do KA, Wergin M, Saintigny P, Vollmer RT, Polascik TJ, Snyder DC, Ruffin MT, Yan S, Dewhirst MW, Kunnamakkara AB, Aggarwal BB, Demark-Wahnefried W. Effect of low-fat diet on plasma levels of NF-{kappa}B-regulated inflammatory cytokines and angiogenic factors in men with prostate cancer. Cancer Prev Res. 2011 doi: 10.1158/1940-6207.CAPR-10-0136. (Epub Ahead of Print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin DW, Neuhouser ML, Schenk JM, Coleman IM, Hawley S, Gifford D, Hung H, Knudsen BS, Nelson PS, Kristal AR. Low-fat, low-glycemic load diet and gene expression in human prostate epithelium: a feasibility study of using cDNA microarrays to assess the response to dietary intervention in target tissues. Cancer Epidemiol Biomarkers Prev. 2007;16:2150–4. doi: 10.1158/1055-9965.EPI-07-0154. [DOI] [PubMed] [Google Scholar]
- 82.Parsons JK, Newman V, Mohler JL, Pierce JP, Paskett E, Marshall J. The Men’s Eating and Living (MEAL) study: a Cancer and Leukemia Group B pilot trial of dietary intervention for the treatment of prostate cancer. Urology. 2008;72:633–7. doi: 10.1016/j.urology.2007.11.050. [DOI] [PubMed] [Google Scholar]
- 83.Parsons JK, Newman VA, Mohler JL, Pierce JP, Flatt S, Messer K, Marshall J. Dietary intervention after definitive therapy for localized prostate cancer: results from a pilot study. Can J Urol. 2009;16:4648–54. [PubMed] [Google Scholar]
- 84.Saxe GA, Major JM, Nguyen JY, Freeman KM, Downs TM, Salem CE. Potential attenuation of disease progression in recurrent prostate cancer with plant-based diet and stress reduction. Integr Cancer Ther. 2006;5:206–13. doi: 10.1177/1534735406292042. [DOI] [PubMed] [Google Scholar]
- 85.Landberg R, Andersson SO, Zhang JX, Johansson JE, Stenman UH, Adlercreutz H, Kamal-Eldin A, Aman P, Hallmans G. Rye whole grain and bran intake compared with refined wheat decreases urinary C-peptide, plasma insulin, and prostate specific antigen in men with prostate cancer. J Nutr. 2010;140:2180–6. doi: 10.3945/jn.110.127688. [DOI] [PubMed] [Google Scholar]
- 86.Demark-Wahnefried W, Robertson CN, Walther PJ, Polascik TJ, Paulson DF, Vollmer RT. Pilot study to explore effects of low-fat, flaxseed-supplemented diet on proliferation of benign prostatic epithelium and prostate-specific antigen. Urology. 2004;63:900–4. doi: 10.1016/j.urology.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 87.Grainger EM, Schwartz SJ, Wang S, Unlu NZ, Boileau TW, Ferketich AK, Monk JP, Gong MC, Bahnson RR, DeGroff VL, Clinton SK. A combination of tomato and soy products for men with recurring prostate cancer and rising prostate specific antigen. Nutr Cancer. 2008;60:145–54. doi: 10.1080/01635580701621338. [DOI] [PubMed] [Google Scholar]
- 88.Van Patten CL, de Boer JG, Guns ES Tomlinson. Diet and dietary supplement intervention trials for the prevention of prostate cancer recurrence: a review of the randomized controlled trial evidence. J Urol. 2008;180:2314–21. doi: 10.1016/j.juro.2008.08.078. discussion 721-2. [DOI] [PubMed] [Google Scholar]
- 89.Bourke L, Doll H, Crank H, Daley A, Rosario D, Saxton JM. Lifestyle intervention in men with advanced prostate cancer receiving androgen suppression therapy: a feasibility study. Cancer Epidemiol Biomarkers Prev. 2011;20:647–57. doi: 10.1158/1055-9965.EPI-10-1143. [DOI] [PubMed] [Google Scholar]
- 90.D’Amico AV. Prostate-specific antigen (PSA) and PSA velocity: competitors or collaborators in the prediction of curable and clinically significant prostate cancer. J Clin Oncol. 2008;26:823–4. doi: 10.1200/JCO.2007.15.1902. [DOI] [PubMed] [Google Scholar]
- 91.Farwell WR, Linder JA, Jha AK. Trends in prostate-specific antigen testing from 1995 through 2004. Arch Int Med. 2007;167:2497–502. doi: 10.1001/archinte.167.22.2497. [DOI] [PubMed] [Google Scholar]
- 92.Garnick MB, Fair WR. Prostate cancer: emerging concepts. Part I. Ann Intern Med. 1996;125:118–25. doi: 10.7326/0003-4819-125-2-199607150-00008. [DOI] [PubMed] [Google Scholar]
- 93.Eberly LE, Neaton JD, Thomas AJ, Yu D. Multiple Risk Factor Intervention Trial Research G. Multiple-stage screening and mortality in the Multiple Risk Factor Intervention Trial. Clin Trials. 2004;1:148–61. doi: 10.1191/1740774504cn018oa. [DOI] [PubMed] [Google Scholar]
- 94.Byun W, Sui X, Hebert JR, Church TS, Lee IM, Matthews CE, Blair SN. Cardiorespiratory fitness and risk of prostate cancer: findings from the Aerobics Center Longitudinal Study. Cancer Epidemiol. 2011;35:59–65. doi: 10.1016/j.canep.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Friedman DB, Corwin SJ, Dominick GM, Rose ID. African American men’s understanding and perceptions about prostate cancer: why multiple dimensions of health literacy are important in cancer communication. J Community Health. 2009;34:449–60. doi: 10.1007/s10900-009-9167-3. [DOI] [PubMed] [Google Scholar]