Abstract
Objective
To examine prospectively associations between urinary phthalate metabolite concentrations and body size measures in children.
Methods
Urinary concentrations of nine phthalate metabolites: monoethyl (MEP); mono-n-butyl (MBP); mono-(3-carboxypropyl) (MCPP); monobenzyl (MBzP); mono-isobutyl (MiBP); mono-(2-ethylhexyl) (MEHP); mono-(2-ethyl-5-oxohexyl) (MEOHP); mono-(2-ethyl-5-carboxypentyl) (MECPP); and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP) and the molar sum of the low molecular-weight phthalate metabolites (low MWP: MEP, MBP and MiBP) and high molecular-weight phthalate metabolites (high MWP: MECPP, MEHHP, MEOHP, MEHP and MBzP) and of four di-(2-ethylhexyl) phthalate (DEHP) metabolites (ΣDEHP: MEHP, MEHHP, MEOHP, MECPP) and anthropometry, including body mass index and waist circumference were measured among 387 Hispanic and Black, New York City children who were between six and eight years at cohort enrollment (2004-2007). Relationships between baseline metabolite concentrations and body size characteristics obtained one year later were examined using multivariate-adjusted geometric means for each body size characteristic by continuous and categories of phthalate metabolite concentrations. Stratified analyses by body size (age/sex specific) were conducted.
Results
No significant associations are reported among all girls or boys. Dose response relationships were seen with monoethyl phthalate and the sum of low molecular-weight phthalates and body mass index and waist circumference among overweight children; for increasing monoethyl phthalate concentration quartiles among girls, adjusted mean body mass indexes were as follows: 21.3, 21.7, 23.8, 23.5 and adjusted mean waist circumference (cm) were as follows: 73.4, 73.5, 79.2, 78.8 (p-trend <0.001 for both).
Conclusion
In this prospective analysis we identified positive relationships between urinary concentrations of monoethyl phthalate and the sum of low molecular-weight phthalates and body size measures in overweight children. These are metabolites with concentrations above 1μM.
Keywords: body mass index, children, obesity, phthalates, waist circumference
Introduction
Childhood obesity is a leading public health problem in the United States (CDC 2010). In 2000, over 15% of children 6-19 years of age were obese, defined as body mass index (BMI) greater than the 95th percentile for age and sex based on Centers for Disease Control and Prevention (CDC) standardized growth charts. While poor diet and physical inactivity are thought to be the main contributors to the current obesity epidemic, recent attention is focusing on whether chemicals in the environment could play a role. Growing evidence suggests that exposure to endocrine disrupting chemicals, such as phthalates, may have an impact on obesity prevalence (Newbold et al. 2008). Obesity disproportionately affects low-income and minority children, and phthalate metabolites urinary concentrations in children and minorities are generally higher than those reported in the general population of U.S. children (CDC 2009).
Phthalates are industrial chemicals found in many consumer products, resulting in widespread exposure in non-occupational settings (Hauser and Calafat 2005). High molecular-weight phthalates are primarily used as plasticizers in the manufacture of flexible vinyl and may be found in certain consumer products, flooring, wall coverings, food processing materials and medical devices. Low molecular-weight phthalates are used in personal care products (e.g. perfumes, lotions and cosmetics) and in making varnishes and coatings. Phthalate exposure occurs mainly through ingestion, inhalation, dermal absorption, and parenteral routes. Phthalates are quickly metabolized and excreted in the urine and feces. In the 2005-2006 National Health and Nutrition Examination Survey (NHANES) data phthalate metabolites were detected in 100% of children and >97% in adults (CDC 2009).
Animal evidence and limited human data suggest that phthalates may be associated with measures of obesity. Several mechanisms have been proposed including anti-androgenic effects, inhibition of thyroid hormone action and activation of peroxisome proliferator-activated receptors (National Academy of Sciences 2008;Bility et al. 2004;Hurst and Waxman 2003;Meeker et al. 2007). While studies in both animals and humans demonstrate phthalates’ biological activity on hormone receptors in adipose tissues implying a potential effect on obesity (Bility et al. 2004;Hurst and Waxman 2003;Feige et al. 2007), few studies have investigated the direct association between phthalates and obesity-related biomarkers or body size measures. Studies of male rodents differed in their findings on di-(2-ethylhexyl) phthalate (DEHP) and body weight(Botelho et al. 2009;Dostal et al. 1987). Among adult women, positive correlations between DEHP metabolites and obesity-related biomarkers, including serum glucose and trigylcerides, were observed (Hines et al. 2009). Two NHANES cross-sectional studies reported associations of specific phthalate metabolites urinary concentrations with waist circumference and insulin resistance in adult males (Stahlhut et al. 2007) and associations with BMI and WC differed by age and sex (Hatch et al. 2008). In contrast, a cross-sectional study of Danish children observed inverse associations between the urinary concentrations of various phthalate metabolites and height (Boas et al. 2010).
Phthalate-related health outcomes in children are of particular concern because children are generally more vulnerable to environmental exposures due to their larger surface area per body weight as compared to adults (Snodgrass 1992) and the unique development that occurs during this time period. Given the increasing rates of obesity in children, the ubiquitous exposure to phthalates and the sparse information on anthropometric effects related to phthalates, we investigated longitudinal relationships between phthalate exposure and a comprehensive set of body size characteristics, measured one year later, among a population of ethnic minority children. Relationships between phthalates, assessed from the urinary concentrations of phthalate metabolites, and many of these body size characteristics have not been previously examined.
Materials and Methods
Growing Up Healthy is a prospective cohort study of 521 Hispanic and Black, New York City children enrolled between the ages of six and eight years (2004-2007). Recruitment was community-based and potential study participants were recruited at the Mount Sinai Medical Center Pediatric Clinic, local community health centers and local schools. Participants were eligible if they were healthy and free of diseases or conditions affecting metabolism or growth. At enrollment (baseline) a parent or guardian was interviewed in person, in either English or Spanish, about the child’s environmental exposures, physical activity, medical history, and demographics; children provided a casual spot urine sample; and approximately one year later, anthropometric measurements were obtained. Informed consent was obtained from a parent or guardian, and assent was obtained from all children. Institutional Review Boards of Mount Sinai School of Medicine and CDC approved this study.
Baseline urine samples were shipped to the National Center for Environmental Health at the CDC to be analyzed. Urinary concentrations were obtained for creatinine and nine phthalate metabolites: monoethyl (MEP); mono-n-butyl (MBP); mono-(3-carboxypropyl) (MCPP); monobenzyl (MBzP); mono-isobutyl (MiBP); mono-(2-ethylhexyl) (MEHP); mono-(2-ethyl-5-oxohexyl) (MEOHP); mono-(2-ethyl-5-carboxypentyl) (MECPP)); and mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP). Analytic methods have been published (Kato et al. 2005). Conjugated analytes are enzymatically hydrolyzed, and then concentrated and separated from other urine components by on-line solid phase extraction coupled to high performance liquid chromatography. Quantitation is achieved by isotope dilution tandem mass spectrometry. Limits of detection were calculated as three times the standard deviation of near-zero or blank quality control specimens. For all analytical methods, standard quality control and reagent blank samples were included in each analytical batch along with the unknown samples. Quality control samples were evaluated according to standard statistical probability rules (www.westgard.com). The CDC laboratory is certified by the Health Care Financing Administration to comply with the requirements set forth in the Clinical Laboratory Improvement Act of 1988 (CLIA ‘88) (Certification # 11D0668200) and is recertified biannually.
Interviewers were trained to measure weight, standing height, waist circumference and hip circumference, and body composition using a standard protocol adapted from NHANES (CDC 2005). Body size characteristics were: body mass index (BMI:weight in kilograms divided by squared height in cm), BMI z-score (calculated using CDC 2002 growth data), height and umbilical waist circumference.
Participation in various forms of physical activity was ascertained by questionnaire. Metabolic equivalent (MET) values assigned to each type of activity were used to convert reported moderate to vigorous activity to average annual metabolic hours per week (Ainsworth et al. 2000). Time spent in sedentary activities on the day prior to interview was defined as the sum of hours spent sleeping, watching television, playing video games, using the computer, attending school, doing homework, and reading.
Dietary recall interviews were collected using the Nutrition Data System for Research which produces measures of macro and micro-nutrients. Between two and four 24-hour dietary recalls collected during the year after baseline interview were averaged to calculate average daily total energy (kcal) intake.
Race (Black/non-Black) and ethnicity (Hispanic/non-Hispanic) were derived using parent report of race and information given about national origin of each child’s parents and grandparents. Socioeconomic status was represented by the highest attained education level of the primary caregiver (≤high school diploma vs. some college or greater).
Phthalate metabolite concentrations measured at baseline and anthropometric measurements taken one year later were available for 307 girls and 80 boys. Complete covariate information was not available for 16 girls and 4 boys and we removed those with dilute urine (creatinine <10mg/dL) (n=6); therefore, multivariate adjusted results are based on 361 children (285 girls and 76 boys).
Partial Spearman correlation coefficients (radj) adjusted for age at baseline visit were computed among the concentrations of all phthalate metabolites (creatinine adjusted and unadjusted) and all anthropometric measures.
Using linear regression models we examined associations between baseline phthalate metabolites and body size measured approximately one year later. Phthalate metabolites were included in the model as continuous natural log-transformed phthalate concentrations (ln μg/L) along with natural log transformed creatinine concentration as a covariate. In addition, we assessed dose-response relationships using two categorizations of phthalate metabolite concentrations: (1) quartiles based on individual creatinine-corrected metabolite concentrations (μg/gC) and (2) three groups based on micromolar concentrations (<1μM, 1-3μM and >3μM), roughly corresponding to biological doses used in experimental studies that find an effect (National Academy of Sciences 2008). Cutpoints were based on concentrations of phthalates divided by creatinine (g/L) to normalize urine dilution (μg/gC, uM/gC). These potentially biologically meaningful categories provide insight into the linear or non-linear associations, which often are seen in other studies with weak environmental agents. Confounding by potential covariates was assessed for the following variables: age at baseline; sex (when appropriate); hours of sedentary activity; day of week for reported sedentary activity (weekend/school day); MET hours, total caloric intake, race, ethnicity, family income, and parent’s education. These variables were chosen because they have been associated with either varying levels of phthalate concentrations or body size measures. Those that resulted in a change of more than 10% in the estimate of effect were retained as a covariate in the model. Final models were adjusted for: age at baseline; sex (when appropriate); sedentary activity; day of week for reported sedentary activity (weekend/school day); MET hours; total caloric intake; race; ethnicity; and parental education. Tests for trend in adjusted body size measures across phthalate metabolite categories were performed using the midpoints of each quantile range included as a continuous variable in the model.
We hypothesized that associations between the phthalate metabolite concentrations and body size characteristics differ by adiposity because obesity is associated with alterations in the endocrine system (Pasquali 2006). We tested for interactions by modeling continuous phthalate metabolite concentrations and dichotomized BMI percentiles (< and ≥85th percentile) as main effects along with their 2-way product (the product being the test of interaction). The Centers for Disease Control and Prevention defines overweight children as those with BMIs at or above the 85th percentile of their age and sex-specific BMI distribution (Ogden et al. 2008). We also created interaction terms using quantiles of phthalates. Due to small numbers, interactions were not tested among boys.
In addition to 9 individual phthalates, we combined the phthalate metabolites into molar sums that represent similar sources and similar biologic activity, low molecular-weight phthalate metabolites (low MWP: MEP, MBP and MiBP) and high molecular-weight phthalate metabolites (high MWP: MECPP, MEHHP, MEOHP, MEHP and MBzP) and the sum of four DEHP metabolites (ΣDEHP: MEHP, MEHHP, MEOHP, MECPP). We expressed DEHP sum and high MWP molar sum as MEHP (molecular weight 278) and the low MWP as MEP (molecular weight 194) so that units were the same as the other analytes (μg/L; (Wolff et al. 2008)).
Results
Demographic characteristics and anthropometric measurements are shown in Table 1. Age distributions among girls and boys were similar, mean 7.3 years, and follow-up was completed an average of 1.1 years later. The majority of the children were Hispanic (>70%), which is representative of the ethnic composition of East Harlem, New York (Olson et al. 2006). Family income was below $50,000 for 90% of the population, and more than half of primary caregivers had not completed high school. The prevalence of overweight and obese girls, 40.2% at baseline, is similar to that of the East Harlem population (Black and Macinko 2010), but is somewhat higher than among all NHANES 6-11 (2002) year old girls (32.6%). However, it was similar to minority children e.g., 40.1 % for non-Hispanic Blacks and 38.1% for Mexican-Americans (NHANES race-specific childhood obesity prevalence; (Ogden et al. 2008)). Other body size measures were also higher in our population than in the NHANES children (McDowell et al. 2005). For example, mean anthropometric measurements taken at the age of 7 years in Growing Up Healthy girls compared to NHANES girls, were, respectively, BMI: 17.9 vs. 16.6; height: 125.8 vs.124.4 cm; and waist circumference: 63.2 vs. 58.4 cm (data not shown). Similar differences were seen in boys.
Table 1. Demographic and anthropometric characteristics by gender among participants in Growing Up Healthy Study, East Harlem, New York City, 2004-2008.
| Characteristic | Girls (n=307) Mean ± Standard deviation (sd) or N (%) |
Boys (n=80) Mean ± sd or N (%) |
|---|---|---|
| Age in years at baseline | 7.34 ± 0.89 | 7.34 ± 0.89 |
| Age in years at Follow-Up 1 | 8.42 ± 0.93 | 8.43 ± 0.90 |
| Years to Follow-Up 1 | 1.08 ± 0.22 | 1.09 ± 0.21 |
| Range of years to Follow-Up 1 | 0.42 - 1.83 | 0.83 - 1.75 |
| Body Mass Index (BMI) a | 18.8 ± 4.2 | 19.4 ± 3.58 |
| BMI Percentile a | 67.7 ± 31.1 | 75.6 ± 27.7 |
| BMI z-score a | 0.66 ± 1.2 | 1.02 ± 1.1 |
| Height(cm) | 131.5 ± 8.3 | 130.7 ± 6.8 |
| Waist Circumference (cm) | 66.5 ± 10.94 | 67.5 ± 10.4 |
| Hispanic | ||
| Yes | 228 (74%) | 63 (79%) |
| No | 79 (26%) | 17 (21%) |
| African American | ||
| Yes | 115 (37%) | 25 (31%) |
| No | 192 (63%) | 55 (69%) |
| Parent Education b | ||
| ≤ High school | 177 (59%) | 56 (72%) |
| > High school | 124 (41%) | 22 (28%) |
| Hours spent doing sedentary activities yesterday c | 3.73 ± 2.64 | 3.97 ± 2.90 |
|
Metabolic Equivalent (MET) hours per week in scheduled activities d |
4.56 ± 11.86 | 4.20 ± 10.62 |
| Was yesterday a school day? e | ||
| Yes | 171 (56%) | 45 (56%) |
| No | 133 (44%) | 35 (44%) |
| Total caloric intake at baseline f | 1449.2 ± 408.6 | 1582.9 ± 530.9 |
| Season urine sample collected g | ||
| Summer | 72 (24%) | 14 (18%) |
| Other Season | 234 (76%) | 66 (82%) |
Body mass index: weight in kilograms divided by squared height in cm; BMI percentile: percentile ranking based on the 2000 CDC BMI-for-age sex-specific growth charts; BMI z-score: standardized score for BMI based on the 2000 CDC BMI-for-age sex-specific growth charts.
missing for 5 girls and 2 boys
missing for 5 girls
missing for 2 girls
missing for 4 girls
missing for 3 girls and 1 boy
missing for 1 girl
Phthalate metabolites were detected in the urine of virtually all children, >97% except for MEHP (81% in girls and 90% in boys). The concentration range of the individual metabolites differed so widely that quartiles did not represent the same exposures for different biomarkers. For example, the 95th percentile concentration in girls for MEP was 2075 μg/gC while for MCPP it was 25 μg/gC and 216 μg/gC for MBP. Therefore, we also examined concentration groups by creating cutpoints based on micromolar concentration (individual metabolite concentration (μg/L) divided by its molecular weight, resulting in umoles/L). This method avoided the limited dose range represented by quartiles for many biomarker analyses, exemplified by their median concentrations (Table 2; Supplemental Table 1). As shown in Table 2, for six of the phthalate metabolites, more than 90% of the children had analyte concentrations below 1μM, while MEP was the only metabolite where more than 10% of the children had concentrations greater than 3μM.
Table 2. Distribution of urinary phthalate metabolite concentrations1 by micromolar cutpoints by sex, Growing Up Healthy Study, New York City, 2004-2008.
| Girls (N=2992) |
Boys (N=80) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1μM | ≥1-<3μM | ≥3μM | Median (μM) |
Median (μg/g C) |
Median (ng/ml) |
<1μM | ≥1-<3μM | ≥3μM | Median (μM) |
Median (μg/g C) |
Median (ng/ml) |
|||||||
| Analyte3 | N | (%) | N | (%) | N | (%) | N | % | N | (%) | N | (%) | ||||||
| MEP | 157 | (53) | 84 | (28) | 58 | (19) | 0.91 | 177.7 | 168.4 | 46 | (58) | 21 | (26) | 13 | (16) | 0.78 | 152.0 | 191.2 |
| MBP | 286 | (96) | 11 | (4) | 2 | (0.1) | 0.28 | 62.7 | 47.2 | 76 | (95) | 4 | (5) | 0 | 0 | 0.33 | 74.0 | 68.6 |
| MiBP | 297 | (99) | 2 | (0.1) | 0 | 0.1 | 22.2 | 16.8 | 80 | (100) | 0 | 0 | 0 | 0.1 | 22.7 | 22 | ||
| MCPP | 299 | (100) | 0 | 0 | 0.02 | 5.1 | 4.2 | 80 | (100) | 0 | 0 | 0 | 0.02 | 5.5 | 5.3 | |||
| MBzP | 288 | (96) | 10 | (3) | 1 | 0.13 | 34.0 | 28.4 | 72 | (90) | 7 | (9) | 1 | (1) | 0.2 | 49.6 | 53.8 | |
| MEHP | 298 | (100) | 1 | 0 | 0.03 | 6.5 | 5.7 | 80 | (100) | 0 | 0 | 0 | 0.03 | 6.3 | 6.7 | |||
| MEOHP | 286 | (96) | 10 | (3) | 3 | (1) | 0.15 | 44.8 | 36.2 | 71 | (90) | 9 | (11) | 0 | 0 | 0.17 | 50.4 | 50.7 |
| MEHHP | 263 | (88) | 29 | (10) | 7 | (2) | 0.24 | 72.0 | 59.1 | 69 | (88) | 9 | (11) | 2 | (3) | 0.26 | 75.7 | 76.6 |
| MECPP | 246 | (82) | 42 | (14) | 11 | (4) | 0.37 | 114.2 | 97.8 | 66 | (83) | 11 | (14) | 3 | (4) | 0.37 | 114.6 | 126.9 |
| Low MWP | 91 | (30) | 132 | (44) | 76 | (25) | 1.5 | 294.0 | 259.2 | 27 | (34) | 37 | (46) | 16 | (20) | 1.3 | 253.2 | 304.3 |
| High MWP | 127 | (42) | 125 | (42) | 47 | (16) | 1.2 | 326.6 | 264.8 | 29 | (36) | 36 | (45) | 15 | (19) | 1.3 | 356.0 | 388.3 |
| ΣDEHP | 167 | (56) | 94 | (31) | 38 | (13) | 0.85 | 235.5 | 199.2 | 45 | (56) | 23 | (29) | 12 | (15) | 0.9 | 251.2 | 259.4 |
creatinine corrected. Median creatinine was 92 mg/dl (89mg/dl for girls and 9 7mg/dl for boys)
excluding 6 girls with creatinine 10 mg/dL
Phthalate metabolite acronyms (in decreasing order of molecular weight): MEP: monoethyl; MBP: mono-n-butyl; MiBP: mono-isobutyl; MCPP: mono-(3-carboxypropyl); MBzP :monobenzyl;; MEHP: mono-(2-ethylhexyl); MEOHP: mono-(2-ethyl-5-oxohexyl); MECPP: mono-(2-ethyl-5-carboxypentyl); and MEHHP: mono-(2-ethyl-5-hydroxyhexyl) phthalate;
Low MWP (low molecular weight phthalates) is the creatinine corrected molar sum of MEP, MBP, MiBP and MCPP, (expressed as MEP (molecular weight 194)).
High MWP (high molecular weight phthalates) is the creatinine corrected molar sum of MECPP, MEHHP, MEOHP, MEHP and MBzP (expressed as MEHP (molecular weight 278)).
ΣDEHP (sum di-(2-ethylhexyl) phthalate) is the creatinine corrected molar sum of DEHP metabolites: MECPP, MEHHP, MEOHP and MEHP (expressed as MEHP (molecular weight 278)).
We compared phthalate metabolite geometric means in our children with a subset of NHANES 2003-4 data, restricted to non-white children in the same age range (mean 7.4 years) (data not shown). For most metabolites, sex-specific concentrations in our population were slightly higher but of similar magnitude compared to those in NHANES e.g., median MBP was 60 μg/gC for girls in our sample and 48 μg/gC in NHANES.
The concentrations of phthalate metabolites were moderately or strongly correlated with each other (Spearman radj =0.3-0.98, see Supplemental Table 2). As expected, concentrations of phthalate metabolites from common parent compounds were most highly correlated (ex. MEHHP and MEOHP radj =0.98), and those with differing origins were less strongly correlated. Overall, MEP was the least correlated with other phthalate metabolites (Spearman radj = −0.003 to 0.26). Phthalate metabolite concentrations among children who were of normal weight did not differ significantly from those who were overweight or obese (Kruskal-Wallis test of medians).
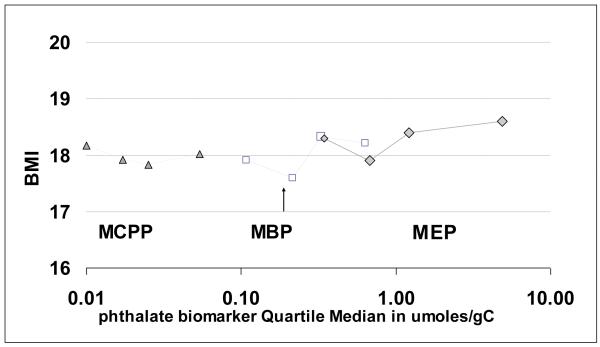
Examination of continuous measures of phthalate metabolite concentrations (natural log transformed) did not reveal positive associations with any of the anthropometric measures (Table 3; results for BMI, BMI-z score, waist-circumference and height shown). Inverse associations for MBP and MCPP with height were seen in both continuous models and models using quartiles of phthalate metabolites; for example, height decreased 1.56 cm for each ln unit change in MCPP concentration (only results of continuous models are shown). When sex-stratified analyses were conducted, the associations among phthalate metabolites and body size measures were generally similar; however some differences in direction and magnitude were observed (Supplemental Table 3). When analytes were examined as three micromolar groups, MCPP and MBP concentrations were all in the lowest quantile, with 75th percentile concentrations < 115μg/gC (median μM <0.30 or median <75 μg/gC) (Figure 1 and Table 2). Therefore we could not examine these (and other phthalates) using this categorization.
Table 3. Associations between log urinary phthalate metabolite concentrations and anthropometric outcomes, Growing Up Healthy Study, East Harlem, New York City, 2004-2008.
| (N=3611) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Body Mass Index (BMI)2 | BMI z-score3 | Waist Circumference (WC in cm) |
Height (Ht in cm) |
|||||
| Analyte4 | Change in BMI per ln unit change in ug/gC |
95% Confidence Interval |
Change in BMI z- score per ln unit change in ug/gC |
95% Confidence Interval |
Change in WC per ln unit change in ug/gC |
95% Confidence Interval |
Change in Ht per ln unit change in ug/gC |
95% Confidence Interval |
| MEP | 0.19 | (− 0.17 - 0.55) | −0.01 | (− 0.12 - 0.09) | 0.51 | (− 0.45 - 1.46) | −0.20 | (− 0.80 - 0.39) |
| MBP | 0.19 | (− 0.31 - 0.69) | 0.01 | (− 0.14 - 0.17) | 0.54 | (− 0.80 - 1.89) | −0.77 | (− 1.60 - 0.06) |
| MiBP | −0.27 | (− 0.73 - 0.18) | −0.10 | (− 0.12 - 0.09) | −0.62 | (− 1.84 - 0.61) | −0.26 | (− 1.03 - 0.50) |
| MCPP | −0.31 | (− 0.84 - 0.22) | −0.12 | (− 0.28 - 0.04) | −0.87 | (− 2.28 - 0.55) | −1.56 | (− 2.43 - -0.70) |
| MBzP | −0.50 | (− 1.51 - 0.51) | −0.04 | (− 0.16 - 0.07) | 0.12 | (− 0.91 - 1.14) | −0.33 | (− 0.97 - 0.30) |
| MEHP | −0.03 | (− 0.38 - 0.31) | 0.00 | (− 0.1 - 0.11) | 0.06 | (− 0.85 - 0.98) | −0.32 | (− 0.88 - 0.25) |
| MEOHP | −0.04 | (− 0.43 - 0.36) | 0.00 | (− 0.12 - 0.12) | −0.01 | (− 1.07 - 1.05) | −0.41 | (− 1.07 - 0.24) |
| MEHHP | 0.02 | (− 0.36 - 0.41) | 0.01 | (− 0.10 - 0.13) | 0.15 | (− 0.88 - 1.19) | −0.36 | (− 1.00 - 0.28) |
| MECPP | −0.05 | (− 0.49 - 0.38) | 0.00 | (− 0.13 - 0.13) | −0.12 | (− 1.28 - 1.05) | −0.63 | (− 1.35 - 0.09) |
| Low MWP | −0.03 | (− 0.49 - 0.43) | 0.01 | (− 0.13 - 0.14) | 0.02 | (− 1.22 - 1.25) | −0.67 | (− 1.43 - 0.10) |
| High MWP | 0.23 | (− 0.20 - 0.66) | −0.01 | (− 0.14 - 0.12) | 0.63 | (− 0.52 - 1.78) | −0.38 | (− 1.09 - 0.34) |
| SDEHP | −0.03 | (− 0.44 - 0.39) | 0.00 | (− 0.12 - 0.13) | 0.00 | (− 1.11 - 1.12) | −0.49 | (− 1.19 - 0.20) |
Estimates for change in anthropometric measure per unit change in ln-concentration (ug/gC) adjusted for creatinine, age, sex, sedentary hours, metabolic equivalent (MET) Hours, Hispanic ethnicity, caloric intake, season in which urine sample was collected (summer or not), parental education level (≤ or > high school).
GUH participants with all covariates and creatinine≥10mg/dL
BMI: Body mass index: weight in kilograms divided by squared height in cm
BMI z-score: standardized score for BMI based on the 2000 CDC BMI-for-age sex-specific growth charts.
Phthalate metabolite acronyms: MEP: monoethyl; MBP: mono-n-butyl; MiBP: mono-isobutyl; MCPP: mono-(3-carboxypropyl); MBzP :monobenzyl;; MEHP: mono-(2-ethylhexyl); MEOHP: mono-(2-ethyl-5-oxohexyl); MECPP: mono-(2-ethyl-5-carboxypentyl); and MEHHP: mono-(2-ethyl-5-hydroxyhexyl) phthalate;
Low MWP (low molecular weight phthalates) is the creatinine corrected molar sum of MEP, MBP, MiBP and MCPP, (expressed as MEP (molecular weight 194)).
High MWP (high molecular weight phthalates) is the creatinine corrected molar sum of MECPP, MEHHP, MEOHP, MEHP and MBzP (expressed as MEHP (molecular weight 278)).
ΣDEHP (sum di-(2-ethylhexyl) phthalate) is the creatinine corrected molar sum of DEHP metabolites: MECPP, MEHHP, MEOHP and MEHP (expressed as MEHP (molecular weight 278)).
Figure 1.
Body Mass Index (BMI) (adjusted means) in relation to urinary phthalate metabolite concentrations, by median (μm/gC) quartiles for three biomarkers MCPP: mono-(3-carboxypropyl) phthalate, MBP: mono-n-butyl phthalate and MEP: monoethyl phthalate in girls (N=299), Growing Up Healthy Study, East Harlem, New York City, 2004-2008.
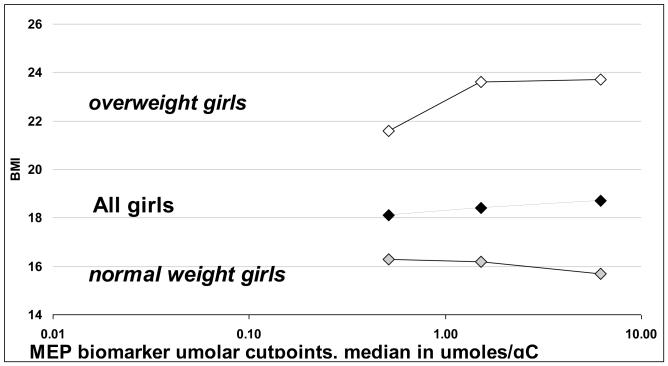
Among girls, there was a significant interaction (p<0.05) of BMI percentile (< and ≥85th percentile) with MEP and low MWP using all three types of analyses: continuous model, quartiles and micromolar groups (Table 4) for adjusted mean BMI and waist circumference one year after urine donation. Among overweight/obese girls (≥85th BMI percentile), MEP and low MWP exhibited dose response relationships with BMI. For example BMI increased 2.2 kg/m2 when comparing overweight girls in the uppermost quartile of MEP to those in the lowest quartile. (ptrend<0.0001), but a similarly large, significant change was not observed among all girls or normal-weight girls (Table 4, figure 2). Similar results were observed when these analyses were carried out among girls and boys combined (Supplemental Table 4); however, not all of the interactions remained significant. Additionally, we could not examine the associations among the boys only due to the small sample size
Table 4. Adjusted1 mean and 95% confidence interval for BMI and waist circumference among girls by analyte quartile and by micromolar concentration-based groups2 stratified by body size. Growing Up Healthy Study, East Harlem, New York City, 2004-2008.
| Monoethyl Phthalate (MEP) |
Body Mass Index (kg/m2) |
Waist Circumference (cm) |
|||||
|---|---|---|---|---|---|---|---|
| Quartile | Median (ug/gC) |
All | Normal Weight4 | Overweight5 | All | Normal Weight4 | Overweight5 |
| 1 | 67 | 18.3 (17.3-19.4) | 16.3 (15.4-17.1) | 21.3 (20.5-22.2) | 65.4 (62.7-68.2) | 59.9 (57.6-62.2) | 73.4 (71.0-75.7) |
| 2 | 131 | 17.9 (16.8-18.9) | 16.4 (15.6-17.1) | 21.7 (20.7-22.8) | 63.8 (60.9-66.7) | 60.1 (57.9-62.2) | 73.5 (70.7-76.4) |
| 3 | 235 | 18.4 (17.4-19.5) | 16.1 (15.4-16.9) | 23.8 (22.7-24.8) | 65.3 (62.5-68.0) | 59.3 (57.2-61.3) | 79.2 (76.3-82.0) |
| 4 | 948 | 18.6 (17.6-19.6) | 15.9 (15.1-16.6) | 23.5 (22.5-24.3) | 66.0 (63.4-68.5) | 58.7 (56.7-60.7) | 78.8 (76.3-81.3) |
|
|
|
|
|||||
| p-trend | 0.53 | 0.41 | <.0001 | 0.59 | 0.37 | <.0001 | |
| p-interaction | 0.03 | 0.002 | |||||
|
|
|
||||||
| Micromolar based-grouping |
Median (ug/gC) |
All | Normal Weight4 | Overweight5 | All | Normal Weight4 | Overweight5 |
|---|---|---|---|---|---|---|---|
| ≤1 μM | 100 | 18.1 (17.3-18.9) | 16.3 (15.7-16.9) | 21.6 (20.9-22.3) | 64.6 (62.5-66.7) | 59.9 (58.2-61.5) | 73.7 (71.8-75.5) |
| 1-3μM | 295 | 18.4 (17.5-19.4) | 16.2 (15.6-16.9) | 23.6 (22.7-24.6) | 65.5 (63.0-67.9) | 59.6 (57.7-61.4) | 79.2 (76.6-81.8) |
| >3μm | 1211 | 18.7 (17.6-19.9) | 15.7 (14.8-16.6) | 23.7 (22.6-24.8) | 66.1 (63.1-69.2) | 58.3 (55.9-60.8) | 78.9 (76.0-81.8) |
|
|
|
||||||
| p-trend | 0.33 | 0.26 | <.0001 | 0.38 | 0.28 | 0.002 | |
| p-interaction | 0.01 | 0.02 | |||||
|
|
|
||||||
| NHANES 6 | 18.1 | 15.9 | 22.9 | 63.6 | 57.0 | 77.1 | |
| Low Molecular-Weight Phthalate3 |
Body Mass Index (kg/m2) |
Waist Circumference (cm) |
|||||
|---|---|---|---|---|---|---|---|
| Quartile | Median (ug/gC) |
All | Normal Weight4 | Overweight5 | All | Normal Weight4 | Overweight5 |
| 1 | 124 | 18.1 (17.0-19.1) | 16.3 (15.5-17.2) | 21.4 (20.5-22.3) | 64.4 (61.7-67.2) | 60.1 (57.9-62.3) | 72.3 (70.2-75.3) |
| 2 | 218 | 18.3 (17.3-19.4) | 16.1 (15.2-17.0) | 22.1 (21.1-23.0) | 65.4 (62.5-68.3) | 58.9 (56.6-61.3) | 75.8 (73.4-78.3) |
| 3 | 365 | 18.3 (17.3-19.3) | 16.3 (15.6-17.1) | 23.5 (22.4-24.6) | 65.0 (62.3-67.7) | 60.1 (58.1-62.1) | 77.7 (74.7-80.7) |
| 4 | 1031 | 18.6 (17.6-19.5) | 15.9 (15.1-16.7) | 23.3 (22.4-24.3) | 65.8 (63.3-68.4) | 58.8 (56.7-60.8) | 78.4 (75.8-81.0) |
|
|
|
|
|||||
| p-trend | 0.51 | 0.52 | 0.005 | 0.50 | 0.57 | 0.008 | |
| p-interaction | 0.02 | 0.02 | |||||
|
|
|
||||||
| Micromolar based-grouping |
Median (ug/gC) |
All | Normal Weight4 | Overweight5 | All | Normal Weight4 | Overweight5 |
|---|---|---|---|---|---|---|---|
| ≤1 μM | 133 | 18.2 (17.2-19.2) | 16.6 (15.8-17.4) | 21.7 (20.7-22.4) | 65.1 (62.5-67.8) | 60.7 (58.7-62.9) | 74.0 (71.6-76.2) |
| 1-3μM | 307 | 18.2 (17.4-19.0) | 16.1 (15.5-16.7) | 22.8 (22.0-23.6) | 64.5 (62.4-66.7) | 59.3 (57.7-60.9) | 76.4 (74.2-78.5) |
| >3μm | 1105 | 18.7 (17.7-19.7) | 16.0 (15.1-16.7) | 23.7 (22.4-24.3) | 66.3 (63.7-69.0) | 58.7 (56.5-60.9) | 78.5 (76.0-81.1) |
|
|
|
||||||
| p-trend | 0.45 | 0.34 | 0.007 | 0.50 | 0.17 | 0.007 | |
| p-interaction | 0.004 | 0.003 | |||||
Adjusted for age, sedentary hours, metabolic equivalent (MET) hours, Hispanic ethnicity, season in which urine sample was collected (summer/other season), parental education level (< or ≥ high school), caloric intake.
note that 1μM MEP =194 μg/gCreatinine MEP
Low molecular-weight phthalate is the creatinine corrected molar sum of MEP: monoethyl; MBP: mono-n-butyl; MiBP: mono-isobutyl; MCPP: mono-(3-carboxypropyl); (expressed as MEP (molecular weight 194)).
Body Mass Index (BMI)< 85th percentile of age and sex-specific BMI percentile according to CDC 2000 growth data.
BMI > 85th percentile of age and sex-specific BMI percentile according to CDC 2000 growth data.
NHANES (2003-2004) restricted to Hispanic and Black girls age 7-9 years, N=63; Low molecular-weight phthalate not available
Figure 2.
Body Mass Index (BMI) (adjusted means) in relation to monethyl phthalate (MEP) urinary metabolite concentrations and stratified by BMI percentile: normal weight girls (<85th %) and overweight girls (≥85th%), Growing Up Healthy Study, East Harlem, New York City 2004-2008 (Girls, n=299). See Table 4 for adjusted means, p-interactions and p-trends.
Discussion
Our main findings were associations of MEP and low MWP with BMI and waist circumference among overweight children. For example, there was a 2 unit increase in BMI and a 5cm increase in waist circumference for both MEP and low MWP going from the from 1st (<1μm) to 3rd (>3μm) micromolar biomarker quantiles. This corresponds approximately to a tenfold increase in concentrations (median MEP 100μg/gC in <1μm quantile and 1211μg/gC in >3μm quantile). Of note, MEP was the highest concentration individual metabolite in our study. We did not observe associations between the other phthalates and body size among normal weight girls and boys. To our knowledge, this is the first longitudinal study to examine the association between phthalate exposure and anthropometric measures of children.
Our results offer interesting comparisons with those from two cross-sectional NHANES studies that examined the urinary concentrations of several phthalates and body size (Stahlhut et al. 2007;Hatch et al. 2008). Consistent with findings in adolescents and older women (Hatch et al. 2008), we found a positive association between MEP concentrations with both BMI and WC. Although our findings were not significant, the magnitude of associations were comparable to Hatch, who reported a difference of about 2 units in BMI for adolescent girls: 22.9 to 24.7 kg/m2 with a 2-3 μM change in MEP (quartile 1-4; 110-694 μg/gC). We observed a similar increase in our high BMI girls (21 to 23 kg/m2) for a 1-3 μM change. For waist circumference, they found an increase of about 4 cm (77.4 to 81.6 cm) with a 1-3 μM change in MEP (quartile 1-4); we also report about a 5 cm difference in our high BMI girls (see figure 1 and table 4). However, among the most comparable NHANES female age group (children ages 6-11 years), no phthalate metabolite-body size characteristic associations were observed in boys (N=329) or girls (N=327) (Hatch et al. 2008). We saw no significant associations in boys, whereas among older NHANES males, positive associations between the concentrations of some phthalate metabolites and body size measures were observed, both in adolescents and adults (Stahlhut et al. 2007;Hatch et al. 2008).
Our failure to see other similar relationships with those found in NHANES may be due to differences in the distribution of the exposure, assessed from the magnitude and range of the urinary concentrations, and body size measures resulting from the lack of similarity between the populations studied. Our sample had a narrow range of exposures (ex. MEP range in quartiles: 67-948 μg/gC) and outcome (ex. our percentage of girls in the overweight and obese categories compared to the general population 40.2% vs. 32.6%). Furthermore, associations seen in Stahlhut and Hatch were not monotonic increases. Reports of nonmonotonic (e.g., U-shaped) dose-response relationships, ultra-low dose effects and nonthreshold effects for endocrine disrupting chemicals continue to challenge some of the basic assumptions of toxicology and risk assessment (Hotchkiss et al. 2008). It is possible that these low level exposures could be spurious associations due to residual confounding (see below discussion).
With biomarkers of phthalates having limited range of exposure, population-based quartiles may not create a sufficient gradient as biomarker concentrations are low (95%<1 μM) (Table 2, Supplemental Table 1). The exposure range exhibited in our children may have been too low to elicit biologic effects. Population quartiles are assumed to represent increasing dose, when it is possible that there is no real biologic difference in concentration across the low quartiles. We created biologically relevant exposure categories based on molar concentrations to provide a possible parallel with biological doses in experimental studies. In our study and NHANES, few individuals have biomarker levels above 1 μM.
Among Danish children aged 4 to 9 years, the only associations observed were inverse phthalate-height among boys and girls regardless of the metabolite examined (Boas et al. 2010); urinary phthalate metabolite concentrations among the Danish were fairly similar to those reported for the NHANES children except that MEP was lower, (median MEP for girls was 36 μg/gC and boys 31 μg/gC whereas NHANES median MEP for girls was 137.7 μg/gC and 93.9 μg/gC for boys). We observed significant inverse height associations in girls for MBP and MCPP when using the log-linear model. The Danish study did not examine MCPP, a non specific metabolite of high molecular-weight phthalates and a minor metabolite of di-n-butyl phthalate. In our girls when we created cutpoints based on μM, there were no individuals with medium and high concentrations of MCPP or MBP. Inverse associations across a low concentration range may reflect increased random variation (noise) at the lower end of the detection range and may not be biologically meaningful. Another possibility is residual confounding by creatinine may underlie these inverse associations between body size measures; creatinine correction on adjustment of the urinary concentrations of the phthalate biomarkers may not be adequate or may introduce error. As a possible improved approach, we used the residual method to adjust for creatinine (Willett 1998). Results were similar to the main models implying that creatinine itself does not appear to account for these inverse associations (not shown). In girls, the inverse height relationships were observed primarily in normal-weight but not in overweight girls, possibly due to a general decline in muscularity and glomerular filtration rate (Engel et al. 2007). Excretion of creatinine is related to muscle mass, and thus in children strongly correlated with age and anthropometric measures such as height and weight (Skinner et al. 1996). Therefore, creatinine-corrected phthalate metabolite urinary concentrations would tend to decrease as age and body size increase, which could create a negative association between phthalates and anthropometric measures (Boas et al. 2010). Other investigators have utilized specific gravity to normalize urinary dilution; it was not available to us. However, specific gravity and creatinine are highly correlated, and thus similar relationships may hold (Carrieri et al. 2001).
The biological basis of increased body size with phthalate exposure rests most strongly on their anti-androgenic effects, which have been observed in both epidemiologic and animal research. The best evidence, on testicular and gonadal toxicity, suggests reduced testosterone levels (Parks et al. 2000;Pan et al. 2006). A possible mechanism supported by clinical and epidemiologic research may be the role of androgens in regulation of adipose tissue distribution (Mayes and Watson 2004). The observed association between MEP and increased body size among the overweight and obese girls is consistent with the idea that obesity is associated with alterations in androgen secretion, metabolism, and action (Pasquali 2006). Differences in hormonal makeup between the sexes (Wajchenberg 2000) support the findings that some of the effects of phthalates on body size differ among girls and boys. We could not examine boys in great detail as >50% of our boys were overweight/obese and our sample size was small. Other emerging mechanistic pathways that implicate phthalates and obesity include thyroid hormone (Meeker et al. 2007) and peroxisome proliferator-activated receptors (PPARs) (Bility et al. 2004;Feige et al. 2007). These hormonal pathways may affect adipocyte formation and/or function (Feige et al. 2007;Casals-Casas et al. 2008;Boberg et al. 2008).
The overweight/obese stratum-specific associations could be due to the presence of higher phthalate metabolite concentrations in larger children, if phthalate metabolism differed by body size or if greater exposure to phthalates occurred through increased dietary consumption. Mean MEP and ΣDEHP urinary concentrations did not differ by body size in our sample, although it is possible that phthalate metabolites in obese children lengthen exposure time. The absence of statistically significant associations for the majority of the phthalate metabolites may be due to a lack of power given the size of our study population; however, the small magnitude of the non-significant regression coefficients may also indicate that there is no association between body size and exposure to these particular analytes.
A strength of this study is the availability of detailed anthropometric measures specific to adiposity. BMI and WC have been studied much more extensively than any of the other body size measures and have been shown to be risk factors for cardiovascular diseases in adults and a means of identifying children susceptible to developing cardiovascular disease in adulthood (Freedman et al. 2007). However, body fat distribution, particularly an increase in abdominal adipose tissue, is associated more strongly with cardiovascular disease risk than is BMI (Goran and Gower 1999;Maffeis et al. 2003). Several studies in children have shown WC, a measure of visceral fat deposits, to be associated with cardiovascular disease risk independent of BMI (US Department of Health and Human Service 2000;Janssen et al. 2002). We found BMI, WC and percent body fat all to be highly correlated with each other (radj >.90). An additional strength is that we have longitudinal analyses as body size measures were obtained one year after phthalate exposure was assessed. The availability of a single spot urine could be considered a limitation because urinary phthalate metabolite concentrations are short-term biomarkers. However, sources of phthalate exposures are likely to be reasonably steady because patterns of use for personal care products, diet and other daily activities are relatively constant. In fact, we undertook this study only after showing that the temporal stability of urinary concentrations of phthalate metabolites was sufficient to categorize exposure level based on a single urine sample over at least a 6 month period (Teitelbaum et al. 2008). However, it may not represent exposure earlier in life during more susceptible periods.
We and others find associations of MEP and low MWP but not other metabolites with obesity, which raises several questions. MEP is not active in classic androgen insufficiency bioassays, while other phthalates including the MBP precursor DBP exhibit a range of activity (e.g., five-fold for testicular testosterone depletion). As noted in the NAS report (National Academy of Sciences 2008), MEP in epidemiologic research may be a surrogate for other coexisting phthalates; this is consistent in our data with the correlation of MEP and MBP (rS 0.23 for girls, 0.32 for boys). In our children, MEP and MBP represent 68% and 22% of low MWP among girls, but the range is wide (IQR 52-84% and 9.5-51%, respectively). Thus MBP, if more potent, might influence the association for both MEP and low MWP with obesity. This further raises the neglected area of mixed, multiple exposures, a problem that low MWP and high MWP molar sums do not solve. Other statistical methods, including factor analysis and principal components analysis, could be unduly influenced by MEP. Thus a more useful approach incorporating differential potency may be needed for phthalate exposures. This method would be similar to the toxic equivalent factors used for dioxin-like exposures or the functional toxicity suggested by McLachlan specifically for hormonally active agents (McLachlan 1993). Such factors do not yet exist for phthalates, and they might include more than one (National Academy of Sciences 2008). The approach has been examined in detail for androgen insufficiency in experimental data (National Academy of Sciences 2008). Finally, the associations of low MWP with obesity in our and other studies may also be due to differences in pharmacokinetics, distribution, and excretion of different phthalate monoesters. For example, MEP may represent more recent and MBP less recent exposure, and either metabolite or their sums may misclassify exposure. As discussed recently using DEHP metabolites, metabolites with longer or shorter elimination half-lives may underestimate or overestimate exposure over an interval (“near” vs. “distant”) (Lorber et al. 2007). Taking these factors into account, in addition to biologic potency and dilution correction (see (McLachlan 1993)), could improve risk estimates using exposure biomarkers.
Phthalates are increasingly being removed from products used by children (US Consumer Product Safety Commission 2010;The European Parliament and the Council of the European Union 2010) mainly DEHP in the U.S. and dibutyl phthalate in the European Union. Phthalates are found in common consumer products such as perfumes, medications, deodorants, nail polish, shampoos, hair sprays, and cosmetics as well as vinyl household products such as floor covering and shower curtains. A major question among health and environmental scientists is whether phthalates are safe enough for use in common products that are not tested for safety (Koo et al. 2002). In 2008, the FDA determined that there was insufficient evidence upon which to take regulatory action (US Food and Drug Administration 2010). The current evidence is not sufficient for attributing a causal effect for phthalates on increased body size, but the urinary concentrations of some phthalate metabolites among children and the epidemic of obesity may merit voluntary reductions in exposure where possible.
Supplementary Material
Highlights.
We examine the association between phthalate metabolites and body size measures in children.
No significant associations between phthalate metabolites and body size measures are reported among all girls or boys.
Among overweight children, dose response relationships for body mass index (BMI) and waist circumference were observed with monoethyl phthalate (MEP) and the molar sum of low molecular-weight phthalate metabolites.
Acknowledgments
The authors wish to thank the study investigators and staff involved in this research including Julie Britton, Ana Mejia, Arkeyris Richiez, Jessica Montana, Rochelle Osborne, Eunpa Chae, Senaka Peter, Lisa Boguski, and Joel Forman as well as Ella Samandar, Jim Preau and Jack Reidy at the Centers for Disease Control and Prevention (CDC) for the phthalate metabolites measures.
This research was supported by National Institute of Environmental Health Sciences (NIEHS)/National Cancer Institute ES012771; NIEHS ES12645; NIEHS/U.S. Environmental Protection Agency Children’s Center grants ES09584 and R827039; the Agency for Toxic Substances and Disease Registry ATSDR Grant #ATU 300014 the Pediatric Environmental Health Fellowship HD049311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimersthat apply to the journal pertain.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Conflict of interest: The authors declare no conflict of interest.
Reference List
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Jr., Schmitz KH, Emplaincourt PO, Jacobs DR, Jr., Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Bility MT, Thompson JT, McKee RH, David RM, Butala JH, Vanden Heuvel JP, Peters JM. Activation of mouse and human peroxisome proliferator-activated receptors (PPARs) by phthalate monoesters. Toxicol Sci. 2004;82:170–182. doi: 10.1093/toxsci/kfh253. [DOI] [PubMed] [Google Scholar]
- Black JL, Macinko J. The changing distribution and determinants of obesity in the neighborhoods of New York City, 2003-2007. Am J Epidemiol. 2010;171:765–775. doi: 10.1093/aje/kwp458. [DOI] [PubMed] [Google Scholar]
- oas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, Juul A, Main KM. Childhood Exposure to Phthalates - Associations with Thyroid Function, Insulin-like Growth Factor I (IGF-I) and Growth. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J, Metzdorff S, Wortziger R, Axelstad M, Brokken L, Vinggaard AM, Dalgaard M, Nellemann C. Impact of diisobutyl phthalate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology. 2008;250:75–81. doi: 10.1016/j.tox.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Botelho GG, Golin M, Bufalo AC, Morais RN, Dalsenter PR, Martino-Andrade AJ. Reproductive effects of di(2-ethylhexyl)phthalate in immature male rats and its relation to cholesterol, testosterone, and thyroxin levels. Arch Environ Contam Toxicol. 2009;57:777–784. doi: 10.1007/s00244-009-9317-8. [DOI] [PubMed] [Google Scholar]
- Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63–67. doi: 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- Casals-Casas C, Feige JN, Desvergne B. Interference of pollutants with PPARs: endocrine disruption meets metabolism. Int J Obes (Lond) 2008;32(Suppl 6):S53–S61. doi: 10.1038/ijo.2008.207. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . National Center for Health Statistics. The Third National Health and Nutrition Examination Survey (NHANES III 1988-94); National Center for Health Statistics, Bethesda, MD: 2005. Reference Manuals and Reports. [Google Scholar]
- Centers for Disease Control and Prevention Fourth National Report on Human Exposure to Environmental Chemicals. 2009 http://www.cdc.gov/exposurereport/
- Centers for Disease Control and Prevention National Center for Health Statistics. 2010 www.cdc.gov/nchs.
- Dostal LA, Jenkins WL, Schwetz BA. Hepatic peroxisome proliferation and hypolipidemic effects of di(2-ethylhexyl)phthalate in neonatal and adult rats. Toxicol Appl Pharmacol. 1987;87:81–90. doi: 10.1016/0041-008x(87)90086-x. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, Wetmur JG, Wolff MS. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Feige JN, Gelman L, Rossi D, Zoete V, Metivier R, Tudor C, Anghel SI, Grosdidier A, Lathion C, Engelborghs Y, Michielin O, Wahli W, Desvergne B. The endocrine disruptor monoethyl-hexyl-phthalate is a selective peroxisome proliferator-activated receptor gamma modulator that promotes adipogenesis. J Biol Chem. 2007;282:19152–19166. doi: 10.1074/jbc.M702724200. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Kahn HS, Mei Z, Grummer-Strawn LM, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and waist-to-height ratio to cardiovascular disease risk factors in children and adolescents: the Bogalusa Heart Study. Am J Clin Nutr. 2007;86:33–40. doi: 10.1093/ajcn/86.1.33. [DOI] [PubMed] [Google Scholar]
- Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70:149S–156S. doi: 10.1093/ajcn/70.1.149s. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Nelson JW, Qureshi MM, Weinberg J, Moore LL, Singer M, Webster TF. Association of urinary phthalate metabolite concentrations with body mass index and waist circumference: a cross-sectional study of NHANES data, 1999-2002. Environ Health. 2008;7:27. doi: 10.1186/1476-069X-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. doi: 10.1136/oem.2004.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ Health Perspect. 2009;117:86–92. doi: 10.1289/ehp.11610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AK, Rider CV, Blystone CR, Wilson VS, Hartig PC, Ankley GT, Foster PM, Gray CL, Gray LE. Fifteen years after “Wingspread”--environmental endocrine disrupters and human and wildlife health: where we are today and where we need to go. Toxicol Sci. 2008;105:235–259. doi: 10.1093/toxsci/kfn030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74:297–308. doi: 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr. 2002;75:683–688. doi: 10.1093/ajcn/75.4.683. [DOI] [PubMed] [Google Scholar]
- Kato K, Silva MJ, Needham LL, Calafat AM. Determination of 16 phthalate metabolites in urine using automated sample preparation and on-line preconcentration/high-performance liquid chromatography/tandem mass spectrometry. Anal Chem. 2005;77:2985–2991. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- Koo JW, Parham F, Kohn MC, Masten SA, Brock JW, Needham LL, Portier CJ. The association between biomarker-based exposure estimates for phthalates and demographic factors in a human reference population. Environ Health Perspect. 2002;110:405–410. doi: 10.1289/ehp.02110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M, Gibb H, Grant L, Pinto J, Pleil J, Cleverly D. Assessment of inhalation exposures and potential health risks to the general population that resulted from the collapse of the World Trade Center towers. Risk Anal. 2007;27:1203–1221. doi: 10.1111/j.1539-6924.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- Maffeis C, Corciulo N, Livieri C, Rabbone I, Trifiro G, Falorni A, Guerraggio L, Peverelli P, Cuccarolo G, Bergamaschi G, Di PM, Grezzani A. Waist circumference as a predictor of cardiovascular and metabolic risk factors in obese girls. Eur J Clin Nutr. 2003;57:566–572. doi: 10.1038/sj.ejcn.1601573. [DOI] [PubMed] [Google Scholar]
- Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev. 2004;5:197–216. doi: 10.1111/j.1467-789X.2004.00152.x. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Adv Data. 2005:1–5. [PubMed] [Google Scholar]
- McLachlan JA. Functional toxicology: a new approach to detect biologically active xenobiotics. Environ Health Perspect. 1993;101:386–387. doi: 10.1289/ehp.93101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academy of Sciences . Phthalates and Cumulative Risk Assessment The Task Ahead. National Academy Press; Washington, DC: 2008. National Academies Press. [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31:201–208. doi: 10.1111/j.1365-2605.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003-2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Olson E, Van Wye G, Kerker B, Thorpe L, Frieden T. Take Care East Harlem. Second Edition NYC Community Health Profiles; 2006. [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks LG, Ostby JS, Lambright CR, Abbott BD, Klinefelter GR, Barlow NJ, Gray LE., Jr. The plasticizer diethylhexyl phthalate induces malformations by decreasing fetal testosterone synthesis during sexual differentiation in the male rat. Toxicol Sci. 2000;58:339–349. doi: 10.1093/toxsci/58.2.339. [DOI] [PubMed] [Google Scholar]
- Pasquali R. Obesity and androgens: facts and perspectives. Fertil Steril. 2006;85:1319–1340. doi: 10.1016/j.fertnstert.2005.10.054. [DOI] [PubMed] [Google Scholar]
- Skinner AM, Addison GM, Price DA. Changes in the urinary excretion of creatinine, albumin and N-acetyl-beta-D-glucosaminidase with increasing age and maturity in healthy schoolchildren. Eur J Pediatr. 1996;155:596–602. doi: 10.1007/BF01957912. [DOI] [PubMed] [Google Scholar]
- Snodgrass W. Physiological and biochemical differences between children and adults as determinants of toxic exposure to environmental pollutants. In: Guzelian P, Henry C, Olin S, editors. Similarities and Differences between children and adults: implications for risk assessment. International Life Sciences Institute; Washington, DC: 1992. pp. 35–42. [Google Scholar]
- Stahlhut RW, van WE, Dye TD, Cook S, Swan SH. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ Health Perspect. 2007;115:876–882. doi: 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- The European Parliament and the Council of the European Union D12 . Directive 2005/84/EC. The European Parliament and the Council of the European Union; 2010. December 14, 2005. [Google Scholar]
- US Consumer Product Safety Commission Consumer Product Safety Improvement Act of 2008, H.R. 4040; 110th Congress, August 14, 2008. US Consumer Product Safety Commission; 2010. [Google Scholar]
- US Department of Health and Human Service . The practical guide-identification, evaluation and treatment of overweight and obesity in adults. 00-4084. National Institutes of Health; Bethesda, MD: 2000. [Google Scholar]
- US Food and Drug Administration . Phthalates and cosmetic Products. US Department of Health and Human Services; 2010. [Google Scholar]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21:697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- Willett . Nutritional Epidemiology. 2nd ed Oxford University Press; New York, NY: 1998. [Google Scholar]
- Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, Wetmur J, Calafat AM. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116:1092–1097. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.