Hair cells of the inner ear are not normally replaced during an animal's life, and must continually replace components of their various organelles1. Among these are the stereocilia, each with a core of several hundred actin filaments, that arise from their apical surfaces and that bear the mechanotransduction apparatus at their tips. Rzadzinska et al.2 studied actin turnover in stereocilia by transfecting neonatal rat hair cells in culture with a β-actin:GFP fusion, and found evidence that actin is replaced, from the top down, in 2-3 days. Overexpression of the actin-binding protein espin causes elongation of stereocilia within 12-24 hours, also suggesting rapid regulation of stereocilia lengths3. Similarly, the mechanosensory “tip links” are replaced in 5-10 hours after cleavage in chicken and mammalian hair cells4,5. In contrast, turnover in chick stereocilia in vivo is much slower6. It might be that only certain components of stereocilia turn over quickly, or that rapid turnover occurs only in neonatal animals, or only in culture, or only in response to a challenge like breakage or actin overexpression. To quantify protein turnover, we fed animals with a 15N-labelled precursor amino acid and used multi-isotope imaging mass spectrometry to measure incorporation of new protein. Surprisingly, in adult frogs and mice, in neonatal mice, in vivo and in vitro, the stereocilia were remarkably stable, incorporating newly synthesized protein at <10%/day. Only stereocilia tips had rapid turnover, and no treadmilling was observed. Other methods confirmed this: In hair cells expressing β-actin:GFP, we bleached fiducial lines across hair bundles, but they did not move in six days. When we stopped expression of β- or γ-actin with tamoxifen-inducible recombination, neither actin isoform left stereocilia, except at the tips. Thus rapid turnover in stereocilia occurs only at the tips, and not by a treadmilling process.
To understand protein turnover in the inner ear, we sought a method that could reveal new synthesis with high spatial resolution, in adult animals, in vivo, and without transfection of cells. We tagged L-leucine with the stable isotope 15N, fed frogs with 15N-leucine-enriched food for up to 32 days, and sacrificed by cardiac perfusion. As leucine is an essential amino acid, newly synthesized protein would contain 15N in nearly the same proportion as the food. To locate 15N-tagged protein, plastic sections of frog saccules were placed onto silicon wafers or electron microscopy grids for MIMS imaging. We detected nitrogen as the CN- ion, with the MIMS detectors set for the masses of 12C, 13C, 12C14N and 12C15N, and calculated the 15N/ 14N ratio at each location in a field7,8 (see Methods).
A mass-26 (12C14N) image of the saccular macula, representing total protein, shows the apical surface of a hair cell with its hair bundle (Fig. 1a, left). Lateral resolution approaches 30 nm, and depth resolution is ∼2 nm (ref 7). The mass-27 channel (12C15N; not shown) revealed newly-synthesized protein, and the mass-27/mass-26 ratio indicated percent incorporation. A ratio image (Fig. 1a, right; 32-d feeding) shows that the stereocilia incorporate new protein more slowly than the cell body. We quantified turnover by summing the mass-26 and mass-27 counts for all pixels in each region of interest (ROI) and calculated percent incorporation (Fig. 1c). Hair-cell cytoplasm and cuticular plates incorporated new protein at ∼0.5%/day. But stereocilia showed <0.3%/day, far lower than the 50%/day suggested for actin in mammalian cochlea2. One difference is that MIMS assesses total protein; perhaps actin turns over rapidly but most other proteins are very stable. However actin is 50-60% of total protein in stereocilia9,10 and would account for the majority of the MIMS signal.
Figure 1. Incorporation of 15N into frog saccular epithelium.
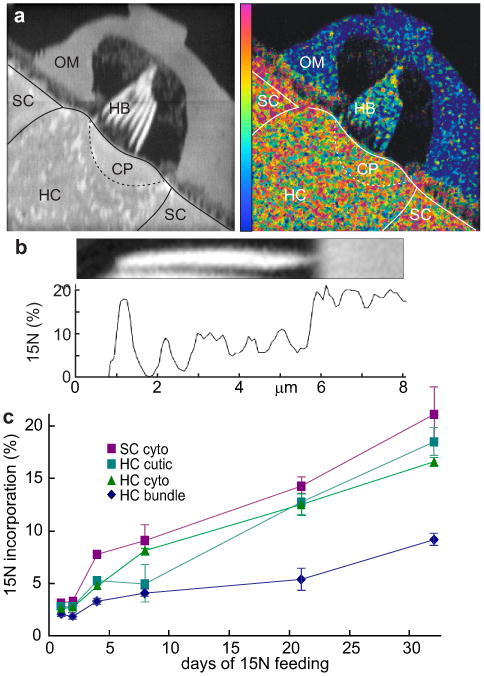
a, left, Mass-26 image for a hair cell (HC) and supporting cells (SC) at day 32. CP: cuticular plate, HB: hair bundle, OM: otolithic membrane. right, Image of mass27/mass26 ratio, revealing incorporation of 15N. Color scale represents 0-30% incorporation. b, top, Mass-26 image of the shortest stereocilium in a. bottom, Profile of incorporation along its length. c, Turnover for days 1, 2, 4, 8, 21 and 32. Mean ± SE; N was typically 8-10.
Surprisingly, incorporation appeared higher at the very tips of stereocilia (Fig. 1b). Incorporation at the tip almost equaled that of cytoplasm (Fig. 1c). In this bundle, incorporation at the tips of all stereocilia was 12.5%, compared to 6.1% in the shafts. We also measured incorporation in individual ROIs along the length of a stereocilium. Of 37 frog stereocilia, there was no correlation with height in 31 and positive correlation in just one, inconsistent with movement of label from top to bottom.
Protein turnover in bullfrog stereocilia was much slower than that inferred for mouse and rat hair cells2,11. Is turnover more rapid in mammals? We fed adult mice 15N-leucine and sacrificed after 1, 2, 8, 32, 56, or 150 days. In mouse vestibular system, 15N incorporation was faster in the cytoplasm of hair cells and supporting cells (Fig. 2a,b,e; ∼7%/day initially) but still slow in stereocilia (<3%/day initially). This was not a peculiarity of actin-based structures: intestinal-brush-border microvilli showed more incorporation than the cytoplasm, and much more than stereocilia (Fig. S2).
Figure 2. Incorporation of 15N into adult mouse hair cells.
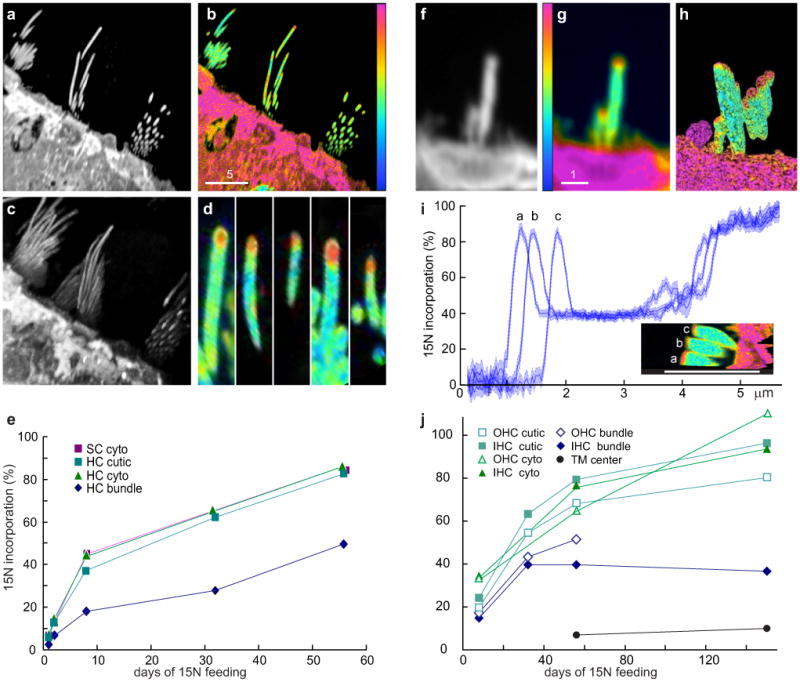
a, Mass-26 image of utricle; day 56. b, Mass 27/mass26 ratio: low incorporation in stereocilia. c, Projection of a 3D stack of a. d, Ratio image from c: high turnover at tips. e, Incorporation, days 1-56. SC-supporting cell; HC-hair cell; cutic-cuticular plate; cyto-cytoplasm. f, Cochlear inner hair cell; day 56; mass 26. g, Ratio image. h, 3D reconstruction from 2200 images. i, Incorporation along axis of stereocilia from h. Bar: plotted length. Line widths: 95% confidence. j, Incorporation after 8, 32, 56 or 150 days. Color scale 0-100%; scale bars in μm.
The MIMS instrument acquires repeated images, slowly etching the surface to create three-dimensional images with thousands of XY planes and Z resolution ∼10 nm (Fig. 2c,d; Supplementary Movies 1&2). In mouse utricle, incorporation was again much higher in the tips than elsewhere in stereocilia. Although tip labeling would be expected in the initial phase of treadmilling, the high-turnover region never extended much below the top micrometer, which apparently represents a fixed zone that does not move down by treadmilling.
Is turnover more rapid in cochlear than vestibular hair cells? Apparently not: at 8, 32, 56 and 150 days (Fig. 2f-j) cochlear stereocilia had slower incorporation of new protein (∼2% initially) than did cell bodies or cuticular plates (∼3.5% initially). Moreover, adult stereocilia apparently have a pool of protein that is replaced exceedingly slowly: 15N incorporation after 5 months was <60%.
A 3D image of cochlear hair bundles (Fig. 2h; Supplementary Movie 3) also showed high turnover in their distal micrometer. At 56 days, 15N incorporation was 40% along most of the length of individual stereocilia, but >80% at the tips (Fig. 2i). Except for the tips, we did not see a gradient of incorporation. In addition, the resolution of MIMS revealed higher turnover in the region of the cell membrane than in stereocilia cores (Fig. 2i, inset), perhaps reflecting rapid diffusion of some membrane proteins12.
Elsewhere in the cochlea, 15N incorporation varied considerably (Figure S1). Like the frog otolithic membrane, the mouse tectorial membrane was exceptionally stable, showing ∼10% incorporation in 5 months. Pillar cell shafts, containing stiff microtubule bundles, had just 23% incorporation. The reticular lamina, comprising the actin-rich cuticular plates of hair cells, terminal webs of Dieter's cells, and their zonulae adherens, showed low turnover. Thus, low turnover occurred in structures that convey sound to stereocilia.
Perhaps protein turnover is more rapid in neonatal mice. To test this, mice were moved at birth to surrogate mothers that had been fed 15N for 24-28 days to raise the 15N content of their milk; pups were nursed for 0-15 days before sacrifice. In a second group, mothers were fed 15N before and during pregnancy. At birth, pups were moved to control nurse mothers, and protein turnover was measured as 15N loss. Pups nursed by 15N mothers showed a normal rise in 15N in utricular cytoplasm (Fig. S3), with 30-50% incorporation in 4 days. In stereocilia, protein turnover was slower, with 25% in 4 days (Fig. S3b,g). A 3D rendering also showed low turnover in stereocilia, except at the tips (Fig. S3c).
A difficulty in interpretation is that about half the hair bundles in mouse utricle develop postnatally13, so incorporation of new protein in stereocilia might reflect development rather than turnover. Indeed, small bundles, presumably still developing, showed high incorporation (Fig. S4), and were therefore excluded from the analysis in Fig. S3g. Another difficulty is the assumption that tagged leucine is incorporated equally into proteins. Proteins with a lower abundance of leucine than the average (9.36%) would have a lower 15N/14N ratio, which might be interpreted as slow turnover. In fact, stereocilia proteins do have less leucine. The ten most abundant proteins of neonatal rat utricular stereocilia (P. Gillespie, personal communication), adjusted for abundance, have 8.53% leucine. This is not enough to account for low stereocilia incorporation, but we sought a different way to measure turnover.
We therefore measured the loss of 15N in pups born to 15N-fed mothers but nursed by controls, normalizing 15N levels in each structure to that at P0 (Fig. S3h). Consistent with some postnatal bundle development, small bundles had little or no label, but full-sized hair bundles retained 15N for many days, with 70% remaining at P4 (Fig. S3d-f, h; Fig. S4).
Perhaps turnover in stereocilia is more rapid in vitro. We cultured utricles from P0 pups and added 15N-leucine after one day. Over four more days, hair bundles showed relatively low 15N incorporation (∼8%/day vs. 12-16%/day in cytoplasm; Fig. S5).
Perhaps the fluorescence observed by Rzadzinska et al.2 represents newly synthesized β-actin:GFP that was but a small fraction of the actin in the core—in essence a tracer for treadmilling, the bulk of which was driven by recycling of actin monomers that are only slowly replaced. To assess treadmilling with an alternative to MIMS, and with steady rather than transient expression of a tracer, we constructed mouse lines expressing β-actin:GFP under the myosin-7a promoter (Fig. S6). Stereocilia were brightly fluorescent (Fig. 3). We dissected utricles at ages P0-P5 and cultured for one day. In a confocal microscope, we selected a field in which the epithelium was tilted sideways, and bleached the GFP in a 1-μm-wide line mostly perpendicular to the bundle axis, for 5-8 bundles (Fig. 3b,c). A 3D confocal stack was acquired, and the utricle placed back into culture. Two, four and six days later, each utricle was returned for imaging (Fig. 3d). The bleached line was sometimes obscured if the bundle axis changed in culture, but the 3D image could be used to reconstruct it. In all experiments, we saw no significant movement of the bleached line (Fig. 3f). On average, the line moved by 2±3% of the bundle height in six days (Fig. 3g).
Figure 3. Tracking treadmilling by bleaching GFP-tagged β-actin.
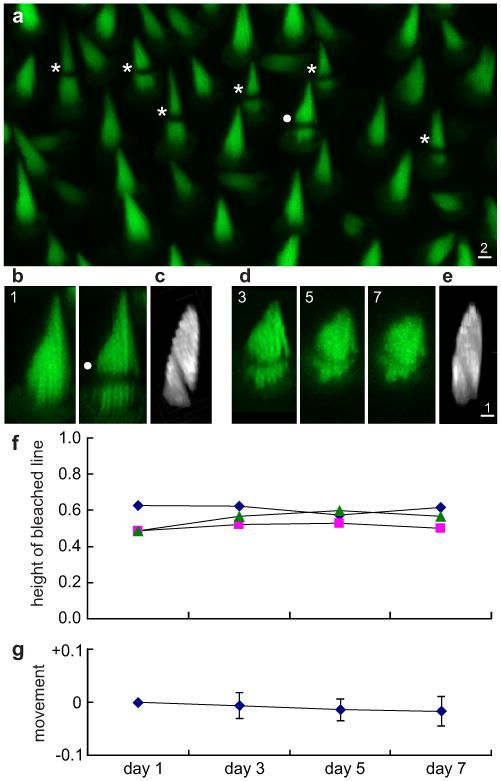
a, Neonatal utricular macula in culture, hair cells expressed β-actin:GFP. Bleached bundles indicated (*); scale in μm. b Projection of confocal planes before and after bleaching. c, 3D stack rotated to show oblique bleached line. d, Same bundle on days 3, 5, and 7. A shift in bundle orientation partially obscured the line. e, Rotation of the day-7 stack revealed the bleached line. f, Line positions for three bundles, as a proportion of bundle height. g, Average movement of bleached lines, as a proportion of bundle height. N=24; mean ± SE.
It is possible that our cultures were not healthy, and that treadmilling was impaired. However, in the same utricles, we observed the appearance and growth of new bundles. Moreover, both bleached and unbleached hair cells took up the fluorescent dye RH414, a marker for functional transduction (data not shown). Thus an independent method gave no evidence of treadmilling on a timescale of days.
Perhaps the GFP tag inhibited treadmilling in Myo7a∷β-actin:GFP mice. We therefore studied treadmilling by terminating β- or γ-actin expression and following the redistribution of actin with antibodies. Mice expressing a tamoxifen-inducible cre under the β-actin promoter were crossed with mice carrying either a floxed β- or γ-actin gene14. At three weeks of age, after bundles had developed, mice were given tamoxifen to promote excision. One, 3, 18 or 34 weeks later, we fixed and removed cochleas, and used antibodies specific for β- and γ-actin.
In control mice without tamoxifen, β- and γ-actin were uniformly distributed (Fig. 4a). If there is rapid treadmilling in adults, β-actin protein should be lost from stereocilia soon after excision blocked β-actin transcription. Similarly, γ-actin should be lost in mice with a floxed γ-actin gene. This occurred, but only for a 0.4-μm region at stereocilia tips (Fig. 4a,b). The rest of a stereocilium retained the deleted actin isoform, and the region of isoform loss did not progress towards stereocilia bases, even after 34 weeks.
Figure 4. Loss of actin from tips following knockout of actin genes.
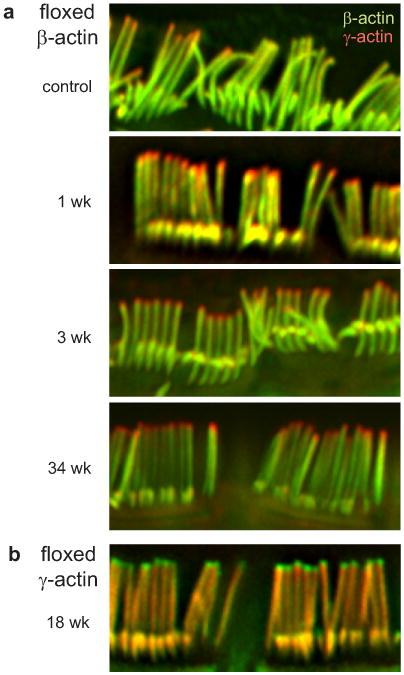
Adult (three-week) cochlear inner hair cells, labeled with antibodies to β-actin (green) or γ-actin (red). a, Floxed β-actin mouse crossed to Cagg-CreER, without tamoxifen (control), or 1, 3 or 34 weeks after tamoxifen. Loss of β-actin occurred only at tips of stereocilia. In control mice, a few hair cells displayed loss of β-actin at tips without tamoxifen, suggesting a leaky promoter. b, Floxed γ-actin mouse 18 weeks after tamoxifen. Loss of γ-actin occurred only at tips of stereocilia.
The MIMS method provides a new way to study protein stability in the inner ear7. MIMS is based on Schoenheimer's pioneering use of stable isotopes to reveal the dynamics of cellular components1, but adds imaging with exceptional spatial resolution. 15N incorporation can be calculated with an accuracy that depends only on acquisition time. It does not rely on tagging any one protein species, or transfecting a specific cell type.
In contrast to previous studies2, MIMS revealed unexpectedly low turnover of protein in hair-cell stereocilia. Incorporation of 15N in stereocilia occurred at <0.3% per day in frog, <2% per day in adult mouse, and <9% per day in neonatal mice, incompatible with treadmilling in 2-3 days. By bleaching fluorescently-labeled actin in bundles, we saw no significant movement of the actin. Finally, excision of the β- and γ-actin genes in mature mice showed loss of the excised actin from the stereocilia tips, but not a progressive loss from tip to base. We found no evidence for treadmilling of stereocilia cores, over weeks or even months. It is possible that the previously observed treadmilling was produced by overexpression of β-actin, if the exogenous actin significantly exceeds endogenous actin. Or—at least in the vestibular system—addition of actin to hair bundles developing postnatally might have confused interpretation.
Nevertheless, there is a conspicuously dynamic turnover of protein at the tips of stereocilia, in the distal 0.3-0.5 μm. This could represent turnover of transduction components, which can be replaced in hours when damaged4,5. It could represent movement of proteins to the tips by hair-cell myosins, most of which climb from bases to tips. Or it may reflect a rapid regulation of actin filaments, which can change length by ∼0.1 μm when tip links are cut2. If so, there might be both polymerization and depolymerization at the barbed end. Rapid but local regulation of filament length is not inconsistent with the limited growth of stereocilia induced by overexpression of espin3.
In long-lived vertebrates, the actin of stereocilia must be replaced. It may be that treadmilling does occur, but at a timescale far longer than studied here. It may instead be that individual actin filaments do not extend the length of the stereocilium--that free ends can accept or shed monomers and that unsynchronized treadmilling occurs on a submicroscopic scale. Unlike filopodia, stereocilia appear to develop once, elaborating an intricate staircase of heights that persists for the life of an animal; in most of their length, they then replace individual proteins slowly and without disrupting this exquisite structure.
Supplementary Material
Supplementary Movie 1: Three dimensional image for mass 26 (total protein) for four hair bundles of an adult mouse utricle. The imaging beam progressively etched the surface by 2-3 μm, continuously acquiring images that were reconstructed into a volume image. (AVI file; 16.4 Mb).
Supplementary Movie 2: Three dimensional image for one hair bundle of a mouse utricle showing 15N incorporation, from a neonatal mouse fed 15N for four days. The HSI scale is 0-100% incorporation in the first movie segment; then 0-45% in the second. The stereocilia have lower incorporation than do the cell bodies, but the tips of stereocilia can be seen to have higher incorporation than the stereocilia shafts. (AVI file; 21.0 Mb)
Supplementary Movie 3: Three dimensional image for one hair bundle of a mouse cochlea showing 15N incorporation, from a adult mouse fed 15N for 56 days. The HSI scale is 0-100% incorporation. The tips of stereocilia have higher incorporation than do the stereocilia shafts; in addition, the cell membrane has higher turnover than the actin cores. (AVI file; 21.5 Mb)
Acknowledgments
Development of the SIMS instrument was supported by ONERA, CNRS, Université Paris Sud and Cameca (France). We thank Drs. Edmund Mroz and Gilles Benichou for providing additional mouse cochlear samples, Louise Trakimas for histological assistance, Zeke Kaufman for MIMS processing, and Dr. Gilles Benichou for initial mouse samples. This work was supported by NIH grants P41RR14579, P41EB001974, R01DC00033, R01DC03463, R01DC04179, P41RR14579, P41EB001974, R37DK39773, R01EY12963, R01GM47214, and R01DK58762 and NSF/IBN grant IBN-998298 to CPL, by NIH grant R01DC02281 to DPC, and by NIH grants F32DC009539 to BJP and R01AR049899 to JME. The work was also helped in part by software funded by the NIH/NCRR Center for Integrative Biomedical Computing, 2P41 RR0112553-12 and the DOE SciDAC Visualization and Analytics Center for Enabling Technologies, DEFC0206ER25781. D-SZ is a Research Associate, VP was an Associate and DPC is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions: D-S.Z carried out the 15N experiments and preparation of tissue for MIMS imaging. V.P. and A.K.R conceived the photobleaching experiments; A.K.R and H.M.P. made the β-actin:GFP mouse and V.P. did the experiments. B.J.P. and J.M.E conceived the conditional actin deletion experiments; B.J.P made the mice and carried out the experiments. C.P.L. and D.P.C. conceived the MIMS study of hair cells. C.P.L. developed the MIMS method, oversaw the MIMS imaging with M.W., and analyzed the MIMS images with J.C.P. D.P.C. wrote the manuscript.
Author Information: Reprint and permissions information is available at www.nature.com/reprints.
The authors declare no competing financial interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
References
- 1.Schoenheimer R. The Dynamic State of Body Constituents. Harvard University Press; Cambridge, MA: 1942. [Google Scholar]
- 2.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, et al. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rzadzinska A, Schneider M, Noben-Trauth K, Bartles JR, et al. Balanced levels of espin are critical for stereociliary growth and length maintenance. Cell Motil Cytoskeleton. 2005;62:157–165. doi: 10.1002/cm.20094. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Yamoah EN, Gillespie PG. Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci U S A. 1996;93:15469–15474. doi: 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia S, Yang S, Guo W, He DZ. Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J Neurosci. 2009;29:15277–15285. doi: 10.1523/JNEUROSCI.3231-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickles JO, Billieux-Hawkins DA, Rouse GW. The incorporation and turnover of radiolabelled amino acids in developing stereocilia of the chick cochlea. Hear Res. 1996;101:45–54. doi: 10.1016/s0378-5955(96)00129-3. [DOI] [PubMed] [Google Scholar]
- 7.Lechene C, Hillion F, McMahon G, Benson D, et al. High-resolution quantitative imaging of mammalian and bacterial cells using stable isotope mass spectrometry. J Biol. 2006;5:20. doi: 10.1186/jbiol42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechene CP, Luyten Y, McMahon G, Distel DL. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science. 2007;317:1563–1566. doi: 10.1126/science.1145557. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd GMG, Barres BA, Corey DP. Proceedings of the National Academy of Sciences. Vol. 86. USA: 1989. “Bundle Blot” purification and initial protein characterization of hair-cell stereocilia; pp. 4973–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin JB, Streijger F, Beynon A, Peters T, et al. Hair bundles are specialized for ATP delivery via creatine kinase. Neuron. 2007;53:371–386. doi: 10.1016/j.neuron.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider ME, Belyantseva IA, Azevedo RB, Kachar B. Rapid renewal of auditory hair bundles. Nature. 2002;418:837–838. doi: 10.1038/418837a. [DOI] [PubMed] [Google Scholar]
- 12.Grati M, Schneider ME, Lipkow K, Strehler EE, et al. Rapid turnover of stereocilia membrane proteins: evidence from the trafficking and mobility of plasma membrane Ca2+-ATPase 2. J Neurosci. 2006;26:6386–6395. doi: 10.1523/JNEUROSCI.1215-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirkegaard M, Nyengaard JR. Stereological study of postnatal development in the mouse utricular macula. J Comp Neurol. 2005;492:132–144. doi: 10.1002/cne.20736. [DOI] [PubMed] [Google Scholar]
- 14.Perrin BJ, Sonnemann KJ, Ervasti JM. Beta-actin and gamma-actin are each dispensable for auditory hair cell development but required for stereocilia maintenance. PLoS Genet. 2010;6:e1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prosser HM, Rzadzinska AK, Steel KP, Bradley A. Mosaic complementation demonstrates a regulatory role for myosin VIIa in actin dynamics of stereocilia. Mol Cell Biol. 2008;28:1702–1712. doi: 10.1128/MCB.01282-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanft LM, Rybakova IN, Patel JR, Rafael-Fortney JA, et al. Cytoplasmic gammaactin contributes to a compensatory remodeling response in dystrophin-deficient muscle. Proc Natl Acad Sci U S A. 2006;103:5385–5390. doi: 10.1073/pnas.0600980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holt JR, Gillespie SK, Provance DW, Shah K, et al. A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell. 2002;108:371–381. doi: 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- 18.Perrin BJ, Sonnemann KJ, Ervasti JM. β-actin and γ-actin are each dispensable for auditory hair cell development but required for stereocilia maintenance. PLoS Genet. 2010;6:e1001158. doi: 10.1371/journal.pgen.1001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Movie 1: Three dimensional image for mass 26 (total protein) for four hair bundles of an adult mouse utricle. The imaging beam progressively etched the surface by 2-3 μm, continuously acquiring images that were reconstructed into a volume image. (AVI file; 16.4 Mb).
Supplementary Movie 2: Three dimensional image for one hair bundle of a mouse utricle showing 15N incorporation, from a neonatal mouse fed 15N for four days. The HSI scale is 0-100% incorporation in the first movie segment; then 0-45% in the second. The stereocilia have lower incorporation than do the cell bodies, but the tips of stereocilia can be seen to have higher incorporation than the stereocilia shafts. (AVI file; 21.0 Mb)
Supplementary Movie 3: Three dimensional image for one hair bundle of a mouse cochlea showing 15N incorporation, from a adult mouse fed 15N for 56 days. The HSI scale is 0-100% incorporation. The tips of stereocilia have higher incorporation than do the stereocilia shafts; in addition, the cell membrane has higher turnover than the actin cores. (AVI file; 21.5 Mb)


