Abstract
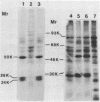
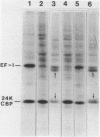
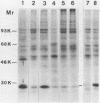
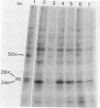
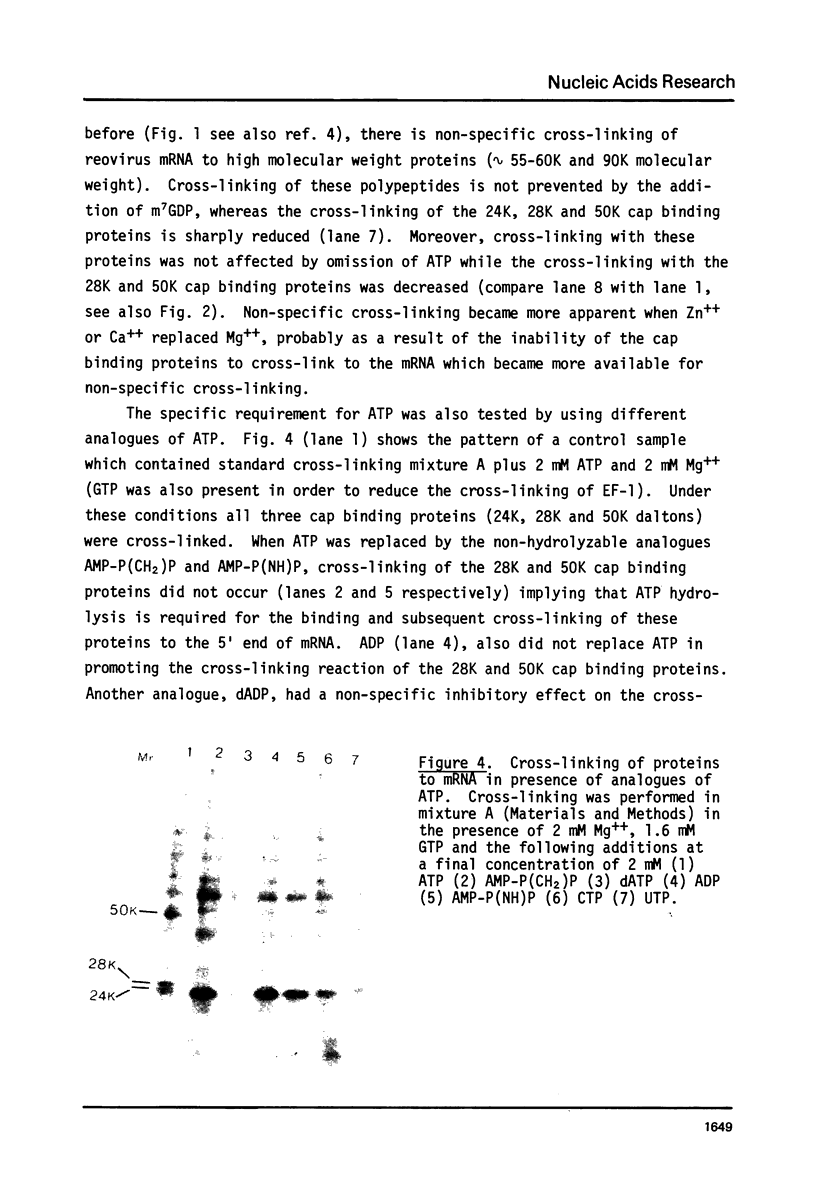
Two proteins of apparent molecular weights of 28,000 and 50,000 daltons were shown to recognize and cross-link specifically to the 5' cap end of oxidized reovirus mRNA. Cross-linking of these proteins to mRNA was ATP/Mg++ dependent, in sharp contrast to cross-linking of a 24K cap binding protein which was purified and characterized previously (Sonenberg, N., Rupprecht, K.M., Hecht, S.M. and Shatkin, A.J. (1979) Proc. Natl. Acad. Sci, USA 76, 4345-4349). Non-hydrolyzable analogues of ATP as well as other nucleotides did not substitute for ATP in the cross-linking reaction and Mg++ was significantly preferred over other divalent cations in cross-linking of the 28K and 50K dalton proteins. A model involving the function of the latter proteins in recognition and unwinding of the 5' end structure of capped eukaryotic mRNAs is suggested.
Full text
PDF



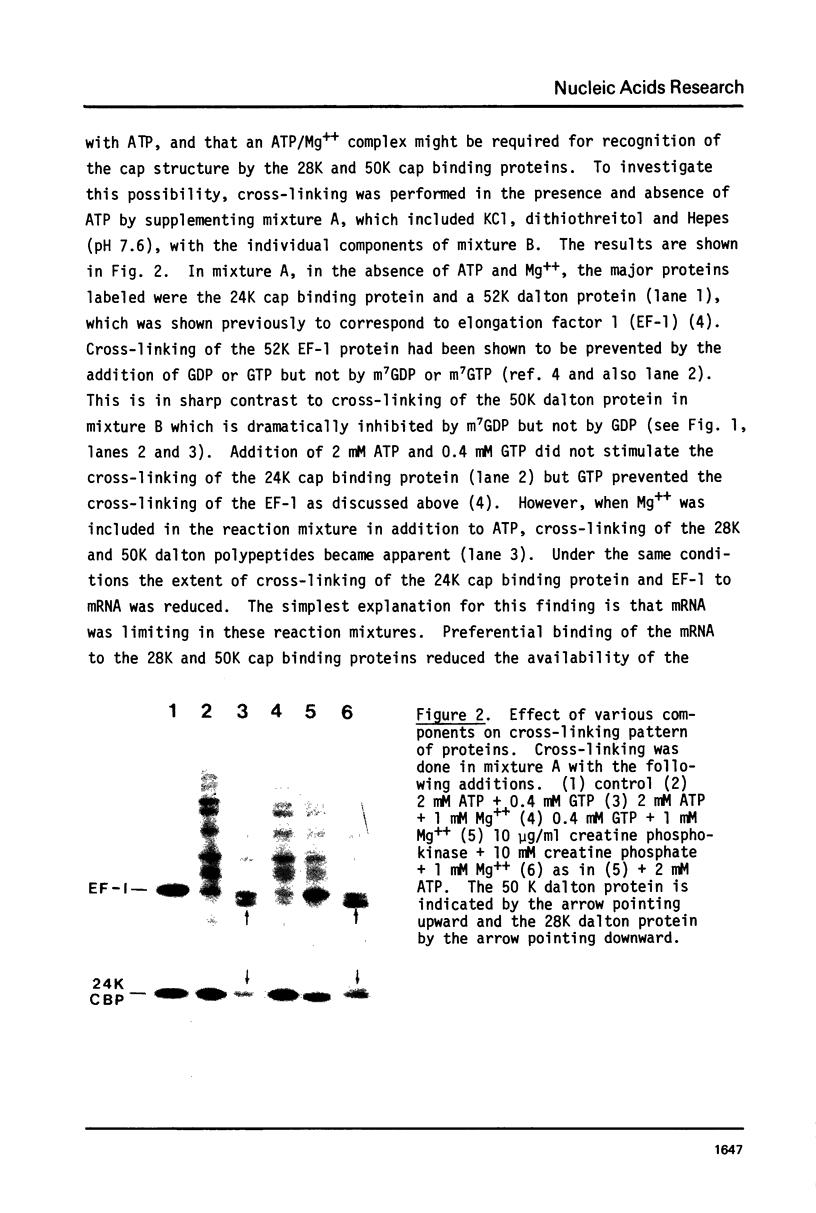
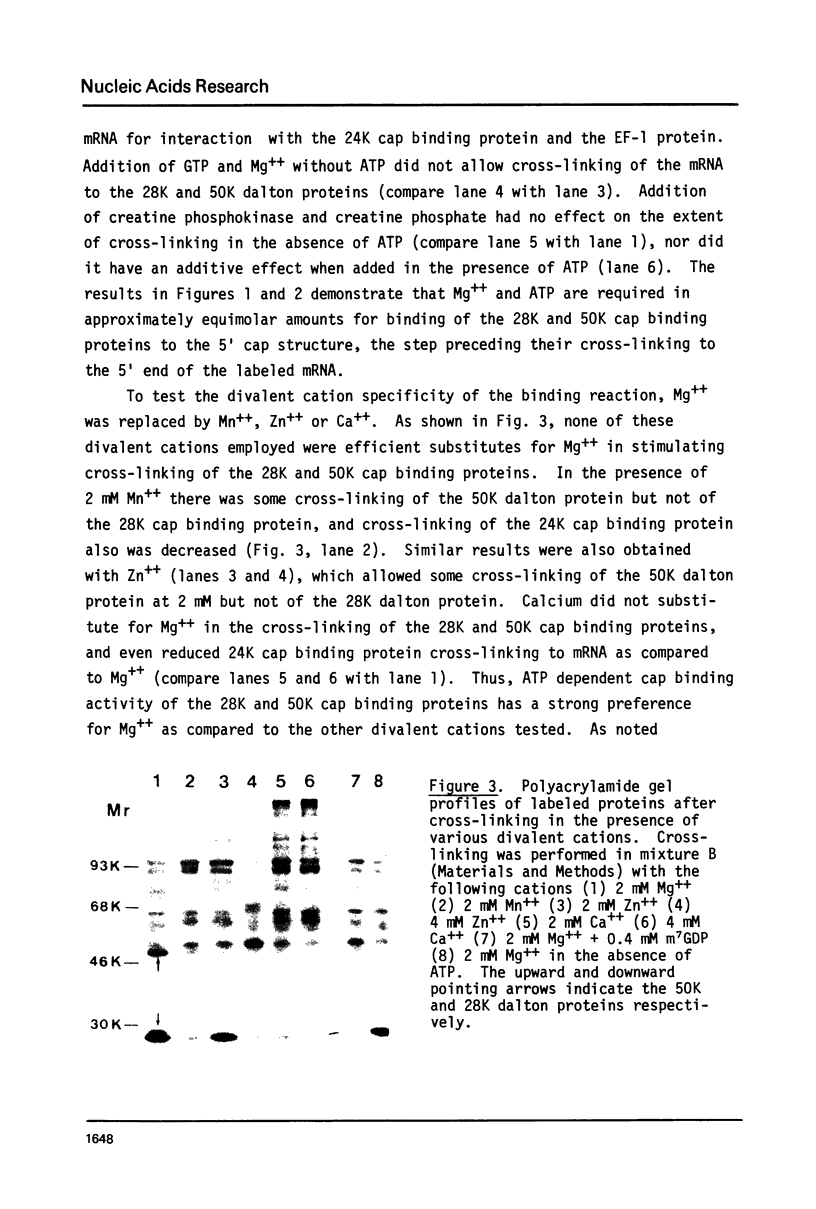







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frisby D., Eaton M., Fellner P. Absence of 5' terminal capping in encephalomyocarditis virus RNA. Nucleic Acids Res. 1976 Oct;3(10):2771–2787. doi: 10.1093/nar/3.10.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., LaFiandra A., Shatkin A. J. 5'-Terminal structure and mRNA stability. Nature. 1977 Mar 17;266(5599):235–239. doi: 10.1038/266235a0. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Control of protein synthesis in extracts from poliovirus-infected cells. I. mRNA discrimination by crude initiation factors. J Virol. 1978 May;26(2):510–521. doi: 10.1128/jvi.26.2.510-521.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan J., Ilan J. Unwinding protein specific for mRNA translation fractionated together with rabbit reticulocyte initiation factor 3 complex. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2325–2329. doi: 10.1073/pnas.74.6.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Goldstein E., Penman S. Poliovirus-induced inhibition of polypeptide initiation in vitro on native polyribosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1834–1838. doi: 10.1073/pnas.73.6.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Influence of mRNA secondary structure on binding and migration of 40S ribosomal subunits. Cell. 1980 Jan;19(1):79–90. doi: 10.1016/0092-8674(80)90390-6. [DOI] [PubMed] [Google Scholar]
- Marcus A. Tobacco mosaic virus ribonucleic acid-dependent amino acid incorporation in a wheat embryo system in vitro. Analysis of the rate-limiting reaction. J Biol Chem. 1970 Mar 10;245(5):955–961. [PubMed] [Google Scholar]
- Moss B. Utilization of the guanylyltransferase and methyltransferases of vaccinia virus to modify and identify the 5'-terminals of heterologous RNA species. Biochem Biophys Res Commun. 1977 Jan 24;74(2):374–383. doi: 10.1016/0006-291x(77)90314-x. [DOI] [PubMed] [Google Scholar]
- Mumby M., Traugh J. A. Dephosphorylation of translational initiation factors and 40S ribosomal subunits by phosphoprotein phosphatases from rabbit reticulocytes. Biochemistry. 1979 Oct 16;18(21):4548–4556. doi: 10.1021/bi00588a015. [DOI] [PubMed] [Google Scholar]
- Muthukrishnan S., Morgan M., Banerjee A. K., Shatkin A. J. Influence of 5'-terminal m7G and 2'--O-methylated residues on messenger ribonucleic acid binding to ribosomes. Biochemistry. 1976 Dec 28;15(26):5761–5768. doi: 10.1021/bi00671a012. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Trachsel H., Leong K., Baltimore D. Inhibition of translation by poliovirus: inactivation of a specific initiation factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2732–2736. doi: 10.1073/pnas.75.6.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkin A. J. Capping of eucaryotic mRNAs. Cell. 1976 Dec;9(4 Pt 2):645–653. doi: 10.1016/0092-8674(76)90128-8. [DOI] [PubMed] [Google Scholar]
- Sonenberg N., Morgan M. A., Merrick W. C., Shatkin A. J. A polypeptide in eukaryotic initiation factors that crosslinks specifically to the 5'-terminal cap in mRNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4843–4847. doi: 10.1073/pnas.75.10.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Rupprecht K. M., Hecht S. M., Shatkin A. J. Eukaryotic mRNA cap binding protein: purification by affinity chromatography on sepharose-coupled m7GDP. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4345–4349. doi: 10.1073/pnas.76.9.4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J. Reovirus mRNA can be covalently crosslinked via the 5' cap to proteins in initiation complexes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4288–4292. doi: 10.1073/pnas.74.10.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Shatkin A. J., Ricciardi R. P., Rubin M., Goodman R. M. Analysis of terminal structures of RNA from potato virus X. Nucleic Acids Res. 1978 Jul;5(7):2501–2512. doi: 10.1093/nar/5.7.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N., Trachsel H., Hecht S., Shatkin A. J. Differential stimulation of capped mRNA translation in vitro by cap binding protein. Nature. 1980 May 29;285(5763):331–333. doi: 10.1038/285331a0. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Erni B., Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis. II. The assembly of the initiation complex with purified initiation factors. J Mol Biol. 1977 Nov;116(4):755–767. doi: 10.1016/0022-2836(77)90269-8. [DOI] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer E., Chang A. Y., Clark J. M., Jr, Reichmann M. E. Sequence studies of satellite tobacco necrosis virus RNA. Isolation and characterization of a 5'-terminal trinucleotide. J Mol Biol. 1968 Nov 28;38(1):59–73. doi: 10.1016/0022-2836(68)90128-9. [DOI] [PubMed] [Google Scholar]