Abstract
L–DOPA–induced dyskinesia, the rate–limiting side–effect in the therapy of Parkinson’s Disease, is mediated by activation of mTOR signaling in the striatum. We show that Rhes, a striatal–specific protein, binds to and activates mTOR. Moreover, Rhes deleted mice manifest reduced striatal mTOR signaling and diminished dyskinesia but maintain motor improvement upon L–DOPA treatment, implying therapeutic benefit for Rhes–binding drugs.
The beneficial effects of L–3,4–dihydroxyphenylalanine (L–DOPA) in the therapy of Parkinson’s Disease (PD) are markedly limited by the disabling dyskinetic effects of the drug. Recently, it was discovered that L–DOPA–induced dyskinesia is mediated by activation of mammalian target of rapamycin (mTOR) in the striatum1. The mTOR inhibitor rapamycin prevented dyskinesia but not the therapeutic effects on limb motion of L–DOPA. We previously reported that Ras homolog enriched in striatum (Rhes), a striatal–enriched small G–protein, accounts for the unique neuropathology of Huntington’s Disease (HD) by enhancing mutant huntingtin sumoylation and toxicity2. These findings predict that Rhes deletion should be neuroprotective as reported in HD striatal cultures3. Rhes also promotes cross–sumoylation of SUMO enzymes4. Rhes resembles Ras homolog enriched in brain (Rheb), the known physiologic activator of mTOR signaling. Here we show that Rhes directly binds and activates mTOR. Moreover, L–DOPA–induced mTOR activation and dyskinesia is substantially reduced in Rhes deleted mice, while beneficial motor effects are not altered. The mediation by Rhes of L–DOPA–induced dyskinesia implies therapeutic benefit for drugs that bind and inactivate Rhes.
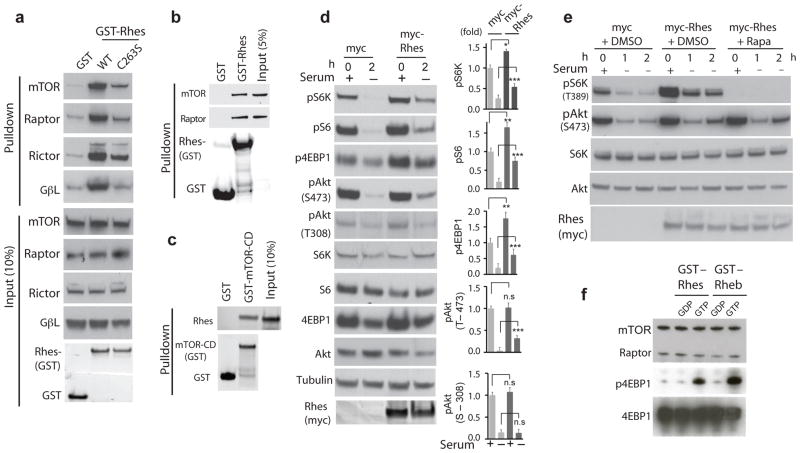
In striatal cell lines, striatal tissue and HEK293 cells Rhes binds mTOR (Figure 1a,b & Supplementary Figure 1, 2). The mTOR–associated proteins RAPTOR, RICTOR and GβL also coprecipitate with Rhes. Purified Rhes binds directly to purified mTOR –catalytic domain5 (Figure 1c). Binding is highly selective, with much less binding for Rheb and negligible binding for other G–proteins (Supplementary Figure 2a). The GTP binding activity of Rhes is required for interactions with the mTOR complex, as binding is greatly reduced in Rhes–S33N, which cannot bind GTP (Supplementary Figure 2b). Like other small G–proteins, Rhes is anchored to membranes by farnesylation6. Rhes–C263S, which is farnesylation–resistant, shows reduced binding to the mTOR complex (Figure 1a and Supplementary Figure 2b).
Figure 1.
Rhes binds and activates mTOR. Endogenous mTOR, Raptor or Rictor and Gβl binding with overexpressed GST, GST–Rhes (WT or C263S) in striatal cells (a). Endogenous mTOR or Raptor binding with recombinant GST or GST–Rhes WT in striatal tissue (b). Purified Rhes binds directly to purified mTOR–CD (catalytic domain) (c). Rhes increases phosphorylation of S6K (Thr389), S6 (Ser240/244), 4EBP1 (Ser65) and Akt (Thr473) in serum containing and deprived (2h) striatal cells (d). Rapamycin blocks Rhes-induced phosphorylation of S6K (Thr389) but not Akt (Thr473) in HEK293 cells (e). Rhes activates mTOR directly in vitro (f). All data are expressed as means ± s.d. * p < 0.05, **p< 0.01, *** p < 0.001 compared to myc controls. Full–length blots are presented in Supplementary Figures 4–9.
Rhes activates signaling by mTOR Complex 1 (mTORC1), which is comprised of mTOR, RAPTOR, and GβL (Figure 1d). Thus, overexpression of Rhes stimulates phosphorylation of the mTORC1 targets S6–kinase, 4EBP1 and S6, but does not activate phosphorylation of Akt at Thr308, which is upstream of mTOR. A direct influence of Rhes on mTOR is evident from in vitro experiments wherein Rhes and Rheb similarly augment phosphorylation of 4EBP1 (Figure 1f). Rhes appears also to influence mTORC2, as it binds to RICTOR (Figure 1a & Supplementary Figure 2), which is selectively associated with the mTORC2 complex, and elicits some increase in phosphorylation of Akt at Ser473, a target of mTORC2 (Figure 1e). Moreover, the decline of pAkt–Ser473 upon removal of serum is much less with Rhes than with vehicle. Whereas actions of Rhes upon mTORC1 signaling are abolished by rapamycin (Figure 1d), influences on pAkt–Ser473 resist rapamycin treatment, consistent with the known rapamycin resistance of mTORC2.
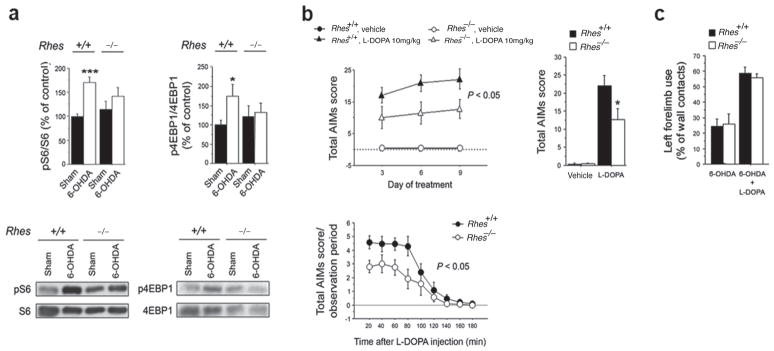
As reported previously1, L–DOPA treatment of mice with unilateral 6–hydroxydopamine (6-OHDA) striatal lesions markedly augments striatal mTOR signaling. This increase is abolished in Rhes deleted mice treated with L–DOPA (Figure 2a). Treatment of unilaterally lesioned mice for 3, 6 or 9 days with 10 mg/kg L–DOPA elicits pronounced dyskinesia (Figure 2b). Dyskinesia following 10 mg/kg L–DOPA is markedly reduced in Rhes knockout mice (Figure 2b). The dyskinetic influences of L–DOPA are similar at 20 – 60 min following injection and then decline markedly by two hours. The rate of decline is similar in wild–type and Rhes knockout mice, suggesting that Rhes deletion does not simply alter L–DOPA turnover (Figure 2b). These observations establish that Rhes mediates both mTOR activation and L–DOPA induced dyskinesia in the striatum. Striatal levels of Rheb, which also activates mTOR, are unaltered in Rhes deleted striatum (Supplementary Figure. 3a).
Figure 2.
Effect of Rhes deletion on mTOR striatal signaling and dyskinesia in unilaterally 6–OHDA–lesioned mice. (a) Phosphorylation levels of S6 and 4EBP1 at Ser240/244 and Ser65 residues, respectively, in the striatum of Rhes+/+ and Rhes−/− mice lesioned with 6–OHDA (n = 10–12) Full–length blots are presented in Supplementary Figure 10. (b) Temporal evolution of abnormal involuntary movements (AIMs) in lesioned Rhes+/+ and Rhes−/− animals during chronic treatment with 10 mg/kg L– DOPA (Rhes+/+, n = 12; Rhes−/−, n = 10). The mice AIMs are also separately shown following L–DOPA treatment at day 9 as total score and time course over 180 min test session. (c) Left forelimb use determined by the cylinder test in 6–OHDA–lesioned Rhes+/+ and Rhes−/− mice (n = 8 per genotype) before (left) and after (right) L–DOPA administration. All data are expressed as mean ± s.e.m. * p < 0.05, as compared with (a) unlesioned striata and (b) wild type controls. Experiments were conducted in conformity with protocols approved by the veterinary department of the Italian Ministry of Health and in accordance with the ethical and safety rules and guidelines for the use of animals in biomedical research, provided by the relevant Italian laws and European Union directives (n. 86/609/EC).
Previous study noted that therapeutic effects of L–DOPA, monitored by forelimb use of 6–OHDA lesioned mice in the cylinder test, are not mediated by the mTOR pathway as they resist rapamycin treatment1. In our experiments, forelimb use is enhanced by L–DOPA in both wild–type and Rhes mutant mice (Figure 2c). Thus, Rhes mediates the adverse, dyskinetic actions of L–DOPA but not its therapeutic motor effects. Rhes mutants display very little change in motor behavior/coordination7, which thus would not likely contribute notably to the altered L–DOPA responses we observe.
D1 and D2 receptors may play different roles in L–DOPA dyskinesia (See Supplemental Text). L–DOPA can elicits motor effects through striatal dopamine–D1 receptors, signaling via protein kinase A and DARPP–32 to Erk 1/2 and GluR1 glutamate receptors, all upstream of mTOR1, 8. Rhes has previously been shown to modulate dopamine signaling through both D1 and D2 receptors7, 9. If Rhes’ effect on L–DOPA–induced dyskinesia occurred through dopamine signaling upstream of mTOR, then Erk1/2 and GluR1 phosphorylation should be altered in Rhes deleted mice. However, phosphorylation of Erk1/2 and GluR1–S845 increase similarly in wild–type and Rhes deleted mice upon L–DOPA treatment, indicating that Rhes does not affect dopamine signaling and activation of Erk1/2–GluR1 to mediate the dyskinetic effects of L–DOPA (Supplementary Figure 3c). Previous studies have shown that Rhes deleted mice display dopamine–D1 receptor hypersensitivity7, 9. If Rhes acted primarily via the dopamine system, Rhes deleted mice would be predicted to manifest worsened L–DOPA dyskinesia, opposite to our observations. Taken together, these findings buttress the conclusion that Rhes’ mediation of L–DOPA–induced dyskinesia occurs via its activation of mTOR.
Our findings establish that Rhes physiologically binds to and activates mTOR in the striatum, an action previously manifested uniquely by Rheb. A study reported that the Rag–GTPases bind mTOR and facilitate its translocation to lysosomes for activation by Rheb10. Activation of mTOR signaling by Rhes has functional consequences, as it appears to mediate dyskinetic effects of L–DOPA via enhancement of mTOR signaling but not the therapeutic improvement of forelimb movement, which is not blocked by rapamycin nor reduced in Rhes mutants.
The selective mediation by mTOR of dyskinetic but not therapeutic effects of L–DOPA implies that mTORC1 inhibitors such as rapamycin might diminish these adverse effects that markedly interfere with the therapy of PD. These drugs may also be beneficial by preventing neuronal death in PD11. Unfortunately, rapamycin and related drugs are powerful inhibitors of protein synthesis with associated toxicity. Our findings indicate that drugs blocking Rhes binding to mTOR selectively might offer similar therapeutic benefits. However, as Rhes is highly enriched in the striatum with negligible levels in peripheral tissues, drugs blocking Rhes–mTOR interactions may have much less potential for adverse effects, consistent with the lack of major abnormalities in Rhes deleted mice.
Supplementary Material
Acknowledgments
The authors thank valuable technical assistance from R. Romano, D. Vitucci, V. Marsili, P. Scherer and S. Korde, and Dr. R. DiLauro for providing the Rhes deleted mice. Supported by USPHS grant MH18501 (SHS) and a grant of the CHDI Foundation (SHS).
Footnotes
Author Contributions:
S.S initiated the project and conducted the mTOR binding and activation experiments, which were further characterized by R.G.M. A.U initiated the dyskinesia and mTOR experiments in mice. S.S and S.H.S further conceived and designed the experiments. F.N and F.E conducted the L–DOPA and behavioral experiments, under the direction of A.U. R.B generated the Rhes constructs. S.K performed in vitro mTOR activity assay. R.T and N.S contributed to the mTOR activity and binding experiments. S.H.S wrote the manuscript with inputs from R.G.M, S.S and A.U.
References
- 1.Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2:ra36, 1–10. doi: 10.1126/scisignal.2000308. [DOI] [PubMed] [Google Scholar]
- 2.Subramaniam S, Sixt MK, Barrow R, Snyder SH. Rhes, a Striatal Specific Protein, Mediates Mutant-Huntingtin Cytotoxicity. Science. 2009;324:1327–1330. doi: 10.1126/science.1172871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seredenina T, Gokce O, Luthi-Carter R. Decreased striatal RGS2 expression is neuroprotective in Huntington’s disease (HD) and exemplifies a compensatory aspect of HD-induced gene regulation. PLoS One. 6:e22231. doi: 10.1371/journal.pone.0022231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramaniam S, et al. RHES, a physiologic regulator of sumoylation, enhances cross-sumoylation among the basic sumoylation enzymes E1 and UBC9. J Biol Chem. 2010;285:20428–20432. doi: 10.1074/jbc.C110.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Long X, Lin Y, Ortiz-Vega S, Yonezawa K, Avruch J. Rheb binds and regulates the mTOR kinase. Curr Biol. 2005;15:702–713. doi: 10.1016/j.cub.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 6.Vargiu P, et al. The small GTP-binding protein, Rhes, regulates signal transduction from G protein-coupled receptors. Oncogene. 2004;23:559–568. doi: 10.1038/sj.onc.1207161. [DOI] [PubMed] [Google Scholar]
- 7.Errico F, et al. The GTP-binding protein Rhes modulates dopamine signalling in striatal medium spiny neurons. Mol Cell Neurosci. 2008;37:335–345. doi: 10.1016/j.mcn.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Santini E, et al. Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J Neurosci. 2007;27:6995–7005. doi: 10.1523/JNEUROSCI.0852-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quintero GC, Spano D, Lahoste GJ, Harrison LM. The Ras homolog Rhes affects dopamine D1 and D2 receptor-mediated behavior in mice. Neuroreport. 2008;19:1563–1566. doi: 10.1097/WNR.0b013e3283118434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancak Y, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malagelada C, Jin ZH, Jackson-Lewis V, Przedborski S, Greene LA. Rapamycin protects against neuron death in in vitro and in vivo models of Parkinson’s disease. J Neurosci. 2010;30:1166–1175. doi: 10.1523/JNEUROSCI.3944-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.