Abstract
Curcuma longa is a perennial member of the Zingiberaceae family, and cultivated mainly in India, and Southeast Asia. The hypothesis for this study is that turmeric will have distinctive effects from curcumin due to the presence of other bioactive compounds. Thirty Eight-week old Sprague-Dawley rats were separated into 3 oral feeding groups. Group 1, standard rat chow, Control diet - AIN 93M, group 2 Curcumin- 700 ppm or 0.7 g/kg diet, and group 3 - Turmeric -14,000 ppm or 14 g/kg diet for a total of 3 weeks. One group of rats were feed all three diets only and another group underwent esophagoduodenal anastomosis to evaluate the effects of bioavailability. Curcumin diet did not increase the transcription of mRNA of TNF-alpha, IL-6, iNOS and COX-2. The average fold change in the mRNAs level was not significant. Whereas turmeric diet increases the levels of IL-6 (1.9 fold, p=0.05) iNOS (4.39 fold, p=0.02), IL-8 (3.11 fold, p=0.04) and COX-2 (2.02 fold, p=0.05), suggesting that turmeric either was more bioavailabile or had more affect on pro-inflammatory genes compare to curcumin diet. We have demonstrated the molecular effects of curcumin and turmeric in the role as an anti-inflammatory therapy. However, significant bioavailable differences do occur and must be considered in further chemopreventative investigative trials the setting of reflux esophagitis, Barrett’s esophagus, and other upper gastrointestinal cancers.
Keywords: Curcumin, Turmeric, Bioavailability, Antioxidant
Introduction
Turmeric (common name for Curcuma longa) has been used for centuries in Ayurvedic medicine for its anti-inflammatory properties. Curcuma longa is a perennial member of the Zingiberaceae family, and cultivated mainly in India, and Southeast Asia1. Curcuma is commonly used as a spice, flavoring agent, food preservative, and color agent. The primary active ingredient of turmeric is in a group of three cuminoids: curcumin (Difeurloylmethane), the yellow pigment of turmeric, demethoxycurcumin, and bisdemethoxycurcumin2. While Curcumin has other properties such as anti-oxidative, anti-microbial, anti-fungal, and anti-viral properties, recent laboratory research has focused on its anti-inflammatory properties3. In addition further research involving turmeric and its bioactive components has lead many scientists to believe curcumin has vast potential to prevention, and therapy of cancer, and other ailments2. Recent studies, and extensive review literature has also proved curcumin role in enhancement of wound healing4-7, reducing blood cholesterol8,9, inhibition with anti-viral activity against HIV replication10,11, increasing bile secretion4, and other medical use for relevant studies both in basic science, and clinical trials have been the imposable research of the past three decades. There is extensive research, and literature to the effects of curcumin in biomedical use, but its derivative turmeric has not been extensively questioned until now.
The questions raised for turmeric use in biomedical research is related to the correlation and bioavailability of curcumin versus turmeric. Curcumin has had limited clinical efficacy because of its inefficient bioavailability, remaining highly stable in acidic solutions such as gastric acid and poor solute in water, thus limiting its availability. Curcumin’s major degradative ingredient in acidic solvents relies heavy on the components structure that results in its quick metabolic activity within the digestive tract that results to such poor bioavailability5. The degradation process occurs with the help of enzymes such as UDP-glucuronosyltransferase, sulfotransferase, alcohol dehydrogenase, and p4502. Limiting factors of curcumin has lead to recent studies that have developed insights to enhancing curcumin bioavailability by the use of heat and mild alkali treatment6, natural compounds, such as piperin, quercetin, and genistein2, and liposome encapsulation15.
There has been limited research on turmeric’s bioavailability or other constituents and only until recent awareness on the lack of curcumin bioavailability in most bio-systems are these derivatives becoming a greater interest. Despite turmeric’s active ingredient cuminoids; turmeric oleorensin has phytoconstituent of essential oil, which has sesquiterpenoids of aromatic-turmerone, alpha-turmerone, beta-turmerone, and curlone7 which has properties of anti-oxidation8, suppression of blood glucose levels18,19, inducing apoptosis in cancer cells9, also including anti-inflammatory, anti-bacterial, and others properties that coincide with cuminoid for biomedical advantage.
Thus the aim of this study was to compare and contrast the bioavailable distribution of Curcumin and Turmeric as well as to look at the molecular effects these compounds have on pro- and anti-inflamatory markers
Materials and Methods
All animal studies were approved per the University of Louisville Institutional of Animal Care and Use Committee.
Non-Surgical Animal Model
Thirty Eight-week old Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed three per cage, given commercial rat chow and tap water, and maintained on a 12-hour light/dark cycle. They were allowed to acclimate for 2 weeks feed initiation. Animals were then separated into 3 oral feeding groups. Group 1 (n=15), standard rat chow, Control diet - AIN 93M, group 2 (N=15) Curcumin- 700 ppm or 0.7 g/kg diet, and group 3 (N=15)- Turmeric -14,000 ppm or 14 g/kg diet for a total of 3 weeks.
Surgical Esophagoduodenal Anastomosis Animal Model21,22
Additional Eight-week old Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed three per cage, given commercial rat chow and tap water, and maintained on a 12-hour light/dark cycle. They were allowed to acclimate for 2 weeks prior to surgery. Solid food was withdrawn 1 day before and 1 day after surgery. The animals were anesthetized with 60 mg/kg sodium pentobarbital, and the pedal reflex was used to monitor the depth of anesthesia. The gastroesophageal junction was ligated flush with stomach and the distal esophagus was transected proximal to the ligature. A 4 mm enterotomy was made 1 cm distal to the pylorus on the anti-mesenteric border. The distal esophagus was then anastomosed to the duodenal enterotomy with mucosal to mucosal opposition10. The animals were weighed weekly and feed the same 3 types of diet as above (Group 1 (n=5), standard rat chow, Control diet - AIN 93M, group 2 (N=15) Curcumin- 700 ppm or 0.7 g/kg diet, and group 3 (N=15)- Turmeric -14,000 ppm or 14 g/kg diet for a total of 3 months), in order to evaluate the effects that bypassing the stomach would have on the bioavailability of both turmeric and curcumin respectively.
Curcumin and Whole Turmeric Dosing
Curcumin was purchases from LKT Laboratories, Inc. 545 Phalen Blvd. St. Paul, MN 55130 and Whole Turmeric from Frontier Natural Products Co-op PO Box 299 3021 78th St. Norway, IA 52318. Utilizing our past publication of curcuma dosing used which was 100 mg/kg body weight11. Considering the average body weight of the rats over 4 weeks after EDA surgery to be 350 g12. We have chosen a curcumin dose received by each rat is approximately 35 mg/rat. This was delivered twice a week for a total dose received by each rat = 70 mg/rat/week. To translate this dose in to dietary dosage, we did not take the systemic bioavailability into account because we are primarily dealing with a GI condition and we assumed the agent to be 100% bioavailable to the GI tract. Thus the dose of curcumin in the diet = 70 mg/ 100 g diet = 0.7 mg/g diet = 0.07% = 0.7g/kg diet = 700 mg/kg diet = 700 ppm. Past reports have reported dietary curcumin dosage as 2% is the highest dose tested. This is equal to 20,000 ppm. (2% is 20g/kg diet= 20,000 mg/kg diet. 1mg/kg diet is 1 ppm). We did not feel we needed to use such a high dose as our prior study did not use such a dose. So we will stay with 700 ppm for oral curcumin. For turmeric with known content of curcumin (5%) is available ((http://www.ediblenature.com/index.asp?PageAction=VIEWPROD&ProdID=6309) If we are using 700 ppm curcumin in the diet, we want to use the amount of turmeric that has a equivalent of curcumin. Since this turmeric has 5% curcumin, we will use a turmeric dose of 14,000 ppm (5% of this is 700 ppm) or 14 g/kg diet. Turmeric was also administered twice a week. Fresh food was administered every day to record and ensure amounts consumed by each rat each day.
RNA Isolation and quantitative real-time PCR
Intestinal tissue was collected by excising 5 cm of small intestine in the ileal section and thoroughly washing it in 3 changes of sterile PBS to remove both residual intestinal contents and flora. The intestinal epithelium was then collected by scraping the epithelial layer from the submucosal layer using a sterile glass slide. The epithelial cells were immediately suspended in RNAlater (Ambion, CA) and stored at -80°C until isolation. The cells were sedimented from RNALater by centrifuging at 2000 Xg for 15m. The cell pellet was then washed once with PBS and resuspended in TRizol. The RNA was subsequently isolated using the TRizol method. THe quality of the RNA was assessed using an Agarose gel and it was quantitated using NanoDrop () before being used for qRTPCR. The Taqman® primer/probes for each gene (TNF alpha, IL6, IL8, COX-2 and B-actin) was ordered from Applied Biosystems (Foster City, CA) as readymade gene-expression assays. One hundred ng of RNA was reverse transcribed into cDNA using the High Capacity Reverse Transcription KIt (Applied Biosystems,Foster City, CA). Three ng of cDNA was subsequently used for PCR along with 1X Taqman primer according to anufacturer’s instructions in a final volume of 10μL. The fold change was calculated using the ddCT mathod as previously described13.
HPLC Methods
We utilized a waters system: Isocratic of W2487 Dual λ detector, λ=430, 717 plus Auto sampler, 515 plus Pump, and Flow rate 1 ml/min14. The Column was Waters Symmetry Shield RP C18, 5μm 150×3.9mm column and RP C18 20 × 3.9 mm guard cartridge. The chemical utilized were Curcumin (C21H20O6 FW 368.35), Chloroform, tetrahydrofuran (C4H8O FW 72.11),Methanol, Citric acid (C6H8O7 FW 192.1), and Sodium Phosphate (dibasic) (Na2HPO4*7H2O FW 268.07). The Mobile phase: (isocratic) used 50% 1% Citric Acid at a pH 3.0 and 50% tetrahydrofuran. The sample involved Tissue homogenization with 1x PBS (w/v) at Liver I:2 Intestine (1:4), then 1:6(v/v) extracted with chloroform, followed by Centrifuge 1500g for 5 minutes, with Transferring the organic layer to a clean glass tube and evaporated to dryness ~ 30minutes and then Reconstituted in 100 micro liter methanol, and injected 50 micro liter onto HPLC
Statistics
Biweekly weight measurements were plotted and a linear regression analysis was carried out to see if there were significant differences in the weight gains among the groups. To assess the differences in diet intake a one-way analysis of variance (ANOVA) was used followed by a Newman Keul’s posttest to compare means. All data represented are mean ± SE.
Results
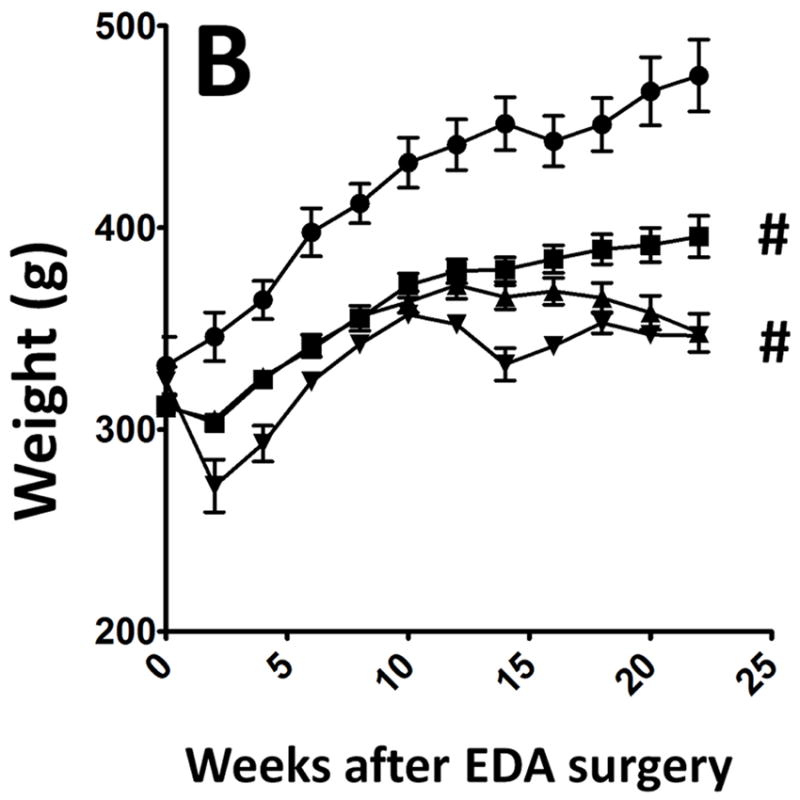
All surgical animals showed stable weight throughout the 3 week diet (Figure 1a). All had normal consumption and equivalent diet intake regardless of the feed. Similar weight loss was seen in the EDA animal as has been previously presented by our group (Figure 1b)
Figure 1.
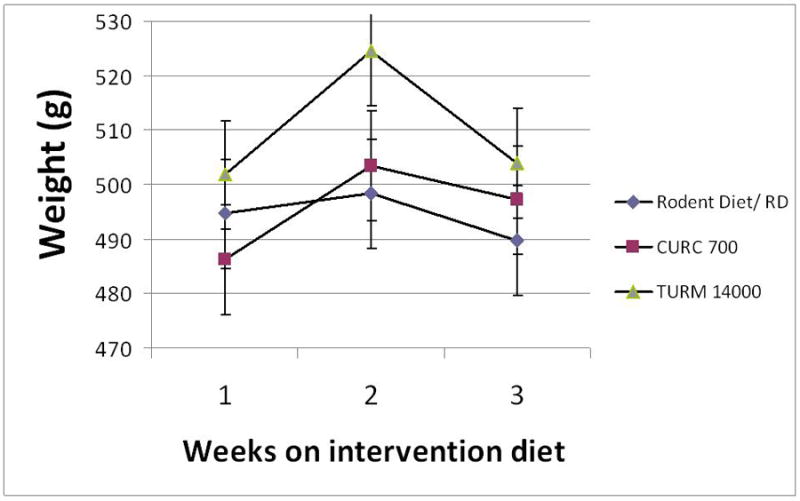
a: Distribution of weight gain among the non-surgical animals feed three different types of diet: Control Rodent diet - AIN 93M, Curcumin- 700 ppm or 0.7 g/kg diet, and Turmeric -14,000 ppm or 14 g/kg diet for a total of 3 weeks.
b: Distribution of weight gain among the surgical animals feed four different types of diet- Sham-CD (Open bars; ●; n=5); EDA-CD (Solid bar; ▀; n=15); EDA Curcumin- 700 ppm or 0.7 g/kg diet (gray bar; ▲; n=15) and EDA Turmeric -14,000 ppm or 14 g/kg diet (Striped bars; ▼; n=15).
In an evaluation of animal intestine in the non-surgical animals we based results on the average fold calculations, the data provides us with an overall insight that turmeric has an increased bioavailability, thus leading to distinctive effects from curcumin under basal conditions (Figure 2). Our data suggests that curcumin diet did not increase the transcription of mRNA of TNF-alpha, IL-6, iNOS and COX-2. The average fold change in the mRNAs level was not significant. Whereas turmeric diet increases the levels of IL-6 (1.9 fold, p=0.05) iNOS (4.39 fold, p=0.02), IL-8 (3.11 fold, p=0.04) and COX-2 (2.02 fold, p=0.05), suggesting that turmeric either was more bioavailable or had more affect on pro-inflammatory genes compare to curcumin diet. In contrast curcumin diet marginally elicits a pro-inflammatory response in these animals looking at the COX-2 and iNOS mRNA levels. TNF-alpha which can signal both pro-apoptosis and anti-apoptosis response did not change with either diet.
Figure 2.
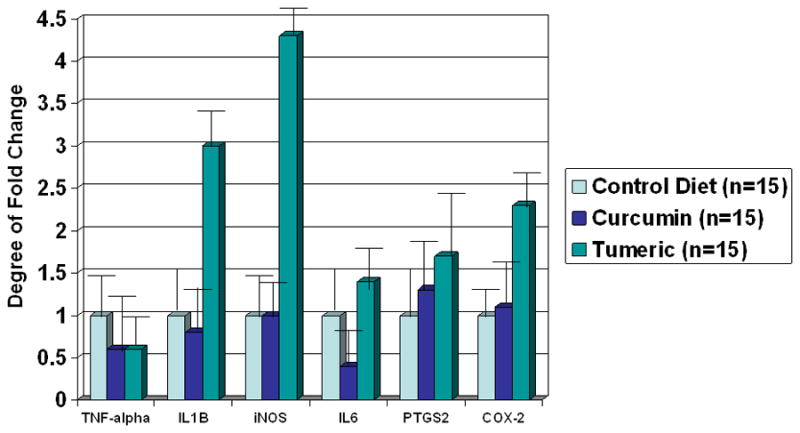
The fold changes of molecular markers in the non-surgical animals feed three different types of diet: Control Rodent diet - AIN 93M, Curcumin- 700 ppm or 0.7 g/kg diet, and Turmeric -14,000 ppm or 14 g/kg diet for a total of 3 weeks.
In an evaluation of only curcumin bioavailability by HPLC in both non-surgical and surgical (EDA) animals, significant differences were seen in curcumin distribution (Table 1). Both non-operative normal rats as well as rats undergoing EDA anastamosis who are evaluated by available distribution of curcumin after receiving curcumin or whole turmeric. In the non-operative rats the distribution of curcumin in the rat intestine were significantly greater than in the turmeric fed animals, with a near 10-fold increase in mean curcumin levels as demonstrated (median: turmeric fed 228 vs curcumin fed 24.6, p<0.01). Serum distribution of curcumin was similar for both the curcumin fed and turmeric fed animals, with a non significant increase in turmeric, but significant increase in the curcumin and turmeric when compared to control diet. Similar differences were seen in liver in the non-surgical treated animals with a significantly greater curcumin level in the turmeric feed animals (median, turmeric fed 12.1 vs curcumin fed 1, p<0.01).
Table 1.
Bioavailable distribution of Curcumin in Non-operated rats and EDA rats following Curcumin or Whole Turmeric Diet.
| Type of Oral Diet | Mean ng/g or ml of tissue | SD | Median | Range | |
|---|---|---|---|---|---|
| Non Surgical Rat | Rat Intestine* | ||||
| Controls | 3.8 | 2.3 | 1.7 | 0.2-5.8 | |
| Tumeric | 482 | 89 | 228 | 46.4-3726 | |
| Curcumin | 21.2 | 8.3 | 24.6 | 13.1-39.3 | |
| Rat Serum* | |||||
| Controls | 8.9 | 3.8 | 5.4 | 3.8-12.3 | |
| Tumeric | 27.9 | 8.9 | 15.4 | 12.5-73.0 | |
| Curcumin | 17.1 | 8.4 | 17.3 | 5.0-31.7 | |
| Rat Liver | |||||
| Controls | 5.3 | 8.48 | 3.4 | 0.1-7.34 | |
| Tumeric | 12.2 | 16,1 | 12.1 | 2.1-20.4 | |
| Curcumin | 1.2 | 3.3 | 1 | Nd-4.5 | |
| Rat Fat | |||||
| Controls | nd | nd | nd | nd | |
| Tumeric | 19.39 | 8.9 | 10.8 | 5.35-43.9 | |
| Curcumin | 10.1 | 8.8 | 6.6 | 3.3-22.1 | |
| Surgical Rat EDA | |||||
| Rat Serum | |||||
| Control Diet | 6.9 | 3.1 | 4.4 | 2.8-12.3 | |
| Tumeric | 20.5 | 3.6 | 19.8 | 17.3-24.4 | |
| Curcumin | 16.2 | 7 | 18.1 | 12.8-28.6 | |
| Rat Liver | |||||
| Control | 1.6 | 1.1 | 1.4 | 0.9-1.8 | |
| Tumeric | 4.1 | 1.95 | 4.38 | 1.2-7.3 | |
| Curcumin | nd | nd | nd | nd | |
| Rat Intestine* | |||||
| Control | 3.8 | 2.1 | 1.6 | 0.2-5.8 | |
| Tumeric | 104 | 26 | 45 | 37.1-125 | |
| Curcumin | 21.2 | 8.3 | 24.6 | 13.1-39.3 | |
| Rat Colon* | |||||
| Control | 3.7 | 2.2 | 1.8 | 0.2-5.8 | |
| Tumeric | 37.8 | 2.3 | 39 | 36.3-43.7 | |
| Curcumin | 18.4 | 3.4 | 19 | 15.5-21.4 | |
| Rat Fat | |||||
| Control | nd | nd | nd | nd | |
| Tumeric | 8.5 | 1.39 | 8.08 | 7.05-10.8 | |
| Curcumin | 8.2 | 1.42 | 8.2 | 7.05-10.8 |
Nd = none detected,
= P<0.05
In contrast, in rats undergoing EDA anastamosis there were similar levels in the rat serum (median 19.8 vs 18.1), liver (4.4 vs none detected), and fat (8.08 vs 8.2). There were significant greater levels in the rat intestine (median 45 vs 24.6, p<0.03) and colon (39 vs 19, p<0.02). These results demonstrate the potential decrease by availability and distribution when oral agents bypass the gastric mucosa and gastric acidity. These results demonstrated the potential benefits of lower availability if indeed the gastric mucosa and gastric acids could essentially be bypassed, leading to a change in distribution of curcumin in the rat intestine as well as colon.
Discussion
Curcumin is a naturally occurring phytochemical derived from turmeric, a rhizome of the curcuma longa plant. It is widely used in India as both a curry spice and as an herbal medicine. Numerous in vitro and in vivo studies have shown curcumin to have several potential health benefits including prevention of atherosclerosis, enhancing wound healing, antiviral activity, antithrombotic activity, antimutagenic activity and antiamylodiogenic activity. In particular curcumin, has been shown to have anticarcinogenic properties in several cancer types 15. Curcumin has previously been investigated as a chemopreventive agent in a small number of phase I and phase II trials and found to be safe and well tolerated25-27. However, a primary impediment to the use of curcumin has been its poor bioavailability as a result of extensive metabolization in the human gut28. We here in present the potential concept of the use of turmeric as a way to increase bioavailability. However, true bioavailability in an end organ may not be the optimal evaluation of oral agents, and that the molecular effects of that end organ maybe a better evaluation of true effects.
Curcumin seems to exert its anticarcinogenic effect through a variety of different mechanisms. Curcumin has been shown to inhibit a number of cell signaling pathways including suppression of NF-KB, suppression of activation of Ap-1, and inhibition of EGF receptor kinase activity16. Curcumin also inhibits the proliferation of tumor cells by affecting the cell cycle. In particular, it has been shown to down regulate the expression of cyclin D1 protein 17. Furthermore, curcumin has been shown to induce apoptosis in malignanat cells 18. Curcumin also acts as a potent antioxidant and has been shown to protect against damage by free radicals 19.
Our own experience has shown that intraperitoneal injections of curcumin can inhibit esophageal carcinogenesis in rats following esophagodoudenal anastamosis 20. Direct tumor injection and topical curcumin have also been shown to inhibit growth of some tumor types 21, 22. Further work needs to be done to assess if oral curcumin has a similar effect. Studies looking at the oral absorption of curcumin in rats and other species have found that over all it displays poor absorption and rapid excretion. Experiments involving radioactively labeled curcumin revealed that it is predominantly excreted in the feces 23. Other work has suggested that curcumin is extensively metabolized to other compounds during absorption from the rat intestine 24,25.
This may be less of a drawback for curcumin use in the esophagus, as initial exposure to curcumin would occur prior to intestinal metabolism. Also, current work on developing lipolized or heat-solubilized forms of curcumin may help overcome this issue26,28. The current study provides a basis for possible use of curcumin and/or the potentially more active turmeric for chemoprevention of esophageal adenocarcinoma through its effects on bile acid-induced alterations of COX-2 and SOD gene expression. In the non-surgical animals it is demonstrated that curcumin diet better serve the purpose of inhibition of pro-inflammatory genes as compared to turmeric diet in these animals.
Recent studies have confirmed the effects on pro-inflammatory cytokines and attenutated oxidative stress. Seehofer et al demonstrated curcumin revealed significant hepatoprotective effects with stabilization of redox state, reduced liberation of liver enzymes, and attenuated expression of pro-inflammatory cytokines. However, when the digestive pattern is altered through bypassing the gastric secretions through our EDA model then the bio-distribution is significantly changed. The effects of minimizing the gastric secretions or removing them from the digestive process have significant effects on the distribution of curcumin throughout the major organs. These results, could potentially provide information on the effects of gastric bypass my play in the next 15-20 years of changes in the epidemiology of upper gastrointestinal cancers. However, The low bioavilabilty of curcumin through the oral route represents a possible hurdle to therapeutic benefit, but oral trials have demonstrated possible therapeutic levels in the gastrointestinal tract of rats and humans.
Turmeric (curcuma longa) is the perennial plant of the ginger family, to which one of its active ingredients is curcumin. Our data presented in this manuscript is the first to demonstrate the possible answer for oral curcumin delivery – i.e. to increase the bioavailability of curcumin. It has been well established that the reason for the lack of efficacy of oral curcumin alone is because of the rapid metabolism of curcumin into its less effective conjugated forms of curcumin glucuronides and curcumin sulfates or, alternately reduced to hexahydrocurcumin26. Our data presented here, begins to suggest that the precursor form of curcumin may provide resistance to the rapid metabolism that occurs in the upper gastrointestinal tract and provide better organ penetrance than has been previously reported with curcumin alone. Obviously, further larger studies with longer follow up will need to be confirmed in order to confirm these preliminary results.
Thus in conclusion we have demonstrated the molecular effects of curcumin and turmeric in the role as an anti-inflammatory therapy as well as its effected on certain pro-inflammatory genes. However, significant bioavailable differences do occur and must be considered in further chemopreventative investigative trials the setting of reflux esophagitis, Barrett’s esophagus, and other upper gastrointestinal cancers.
Highlights.
Demonstrated the molecular effects of curcumin and turmeric.
The distribution of curcumin in the rat intestine versus tumeric.
Bioavailable differences of curcumin and turmeric.
Acknowledgments
Supported by: National Institutes of Health Grant (1R03CA137801).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- 2.Patil BS, Jayaprakasha GK, Chidambara Murthy KN, Vikram A. Bioactive compounds: historical perspectives, opportunities, and challenges. J Agric Food Chem. 2009;57:8142–8160. doi: 10.1021/jf9000132. [DOI] [PubMed] [Google Scholar]
- 3.Bower MR, Aiyer HS, Li Y, Martin RC. Chemoprotective effects of curcumin in esophageal epithelial cells exposed to bile acids. World J Gastroenterol. 2010;16:4152–4158. doi: 10.3748/wjg.v16.i33.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deters M, Siegers C, Hansel W, Schneider KP, Hennighausen G. Influence of curcumin on cyclosporin-induced reduction of biliary bilirubin and cholesterol excretion and on biliary excretion of cyclosporin and its metabolites. Planta Med. 2000;66:429–434. doi: 10.1055/s-2000-8584. [DOI] [PubMed] [Google Scholar]
- 5.Wang YJ, Pan MH, Cheng AL, Lin LI, Ho YS, Hsieh CY, Lin JK. Stability of curcumin in buffer solutions and characterization of its degradation products. J Pharm Biomed Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- 6.Kurien BT, Scofield RH. Increasing aqueous solubility of curcumin for improving bioavailability. Trends Pharmacol Sci. 2009;30:334–335. doi: 10.1016/j.tips.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh G, Kapoor IP, Singh P, de Heluani CS, de Lampasona MP, Catalan CA. Comparative study of chemical composition and antioxidant activity of fresh and dry rhizomes of turmeric (Curcuma longa Linn.) Food Chem Toxicol. 2010;48:1026–1031. doi: 10.1016/j.fct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z Naturforsch C. 2002;57:828–835. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 9.Xiao Y, Yang FQ, Li SP, Hu G, Lee SM, Wang YT. Essential oil of Curcuma wenyujin induces apoptosis in human hepatoma cells. World J Gastroenterol. 2008;14:4309–4318. doi: 10.3748/wjg.14.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Martin RC. Reflux injury of esophageal mucosa: experimental studies in animal models of esophagitis, Barrett’s esophagus and esophageal adenocarcinoma. 2007;20:372–378. doi: 10.1111/j.1442-2050.2007.00713.x. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wo JM, Liu Q, Li X, Martin RC. Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann Surg Oncol. 2009;16:515–523. doi: 10.1245/s10434-008-0228-0. [DOI] [PubMed] [Google Scholar]
- 12.Aiyer HS, Li Y, Martin RC. Diet Composition Affects Surgery-Associated Weight Loss in Rats with a Compromised Alimentary Tract. J Surg Res. 2009 doi: 10.1016/j.jss.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Schiborr C, Eckert GP, Rimbach G, Frank J. A validated method for the quantification of curcumin in plasma and brain tissue by fast narrow-bore high-performance liquid chromatography with fluorescence detection. Anal Bioanal Chem. 2010;397:1917–1925. doi: 10.1007/s00216-010-3719-3. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 16.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 17.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 18.Wolanin K, Magalska A, Mosieniak G, Klinger R, McKenna S, Vejda S, Sikora E, Piwocka K. Curcumin affects components of the chromosomal passenger complex and induces mitotic catastrophe in apoptosis-resistant Bcr-Abl-expressing cells. Mol Cancer Res. 2006;4:457–469. doi: 10.1158/1541-7786.MCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 19.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21:8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Wo J, Liu Q, Li X, Martin R. Chemoprotective Effects of Curcuma Aromaica on Esophageal Carcinogenesis. 2008 doi: 10.1245/s10434-008-0228-0. [DOI] [PubMed] [Google Scholar]
- 21.LoTempio MM, Veena MS, Steele HL, Ramamurthy B, Ramalingam TS, Cohen AN, Chakrabarti R, Srivatsan ES, Wang MB. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11:6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, Han C, Chen J, Huang MT, Yang CS. Inhibition of 7,12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis. 2002;23:1307–1313. doi: 10.1093/carcin/23.8.1307. [DOI] [PubMed] [Google Scholar]
- 23.Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980;16:259–265. doi: 10.1016/0300-483x(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 24.Ravindranath V, Chandrasekhara N. In vitro studies on the intestinal absorption of curcumin in rats. Toxicology. 1981;20:251–257. doi: 10.1016/0300-483x(81)90056-1. [DOI] [PubMed] [Google Scholar]
- 25.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]
- 26.Ireson CR, Jones DJ, Orr S, Coughtrie MW, Boocock DJ, Williams ML, Farmer PB, Steward WP, Gescher AJ. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol Biomarkers Prev. 2002;11:105–111. [PubMed] [Google Scholar]


