Summary
Concrete examples of computation and implementation of cost-benefit decisions at the level of neuronal circuits are largely lacking. Such decisions are based on appetitive state, which is the integration of sensation, internal state and memory. Value-based decisions are accessible in neuronal circuitry of simple systems [1]. In one such, the predatory sea-slug Pleurobranchaea, appetite is readily quantified in behavior [2] and related to approach-avoidance decision [3]. Moreover, motor aspects of feeding and turning can be observed as fictive motor output in the isolated CNS [4,5]. Here we found that the excitation state of the feeding motor network both manifested appetitive state and controlled expression of orienting vs. avoidance. In isolated CNS’s spontaneous feeding network activity varied proportionally to donor feeding thresholds. CNS’s from low and high feeding threshold donors expressed fictive orienting or avoidance, respectively, in response to brief stimulation of sensory nerves. Artificially exciting the feeding network converted fictive avoidance to orienting. Thus, the feeding network embodied appetitive state and toggled approach-avoidance decision by configuring response symmetry of the premotor turn network. A resulting model suggests a basic cost-benefit decision module from which to consider evolutionary elaboration of the circuitry to serve more intricate valuation processes in complex animals.
Results
Appetitive State Conserved in Isolated CNS
The feeding thresholds for proboscis extension and biting of 25 CNS donors, measured by applying dilutions of the appetitive stimulus betaine to the oral veil, were directly related to the level of spontaneous bursting activity recorded from the feeding network of the isolated CNS (Fig. 1A, B). Recordings of the R3 buccal motor nerve (Fig. 1A), which innervates musculature of the buccal feeding apparatus in the intact animal, showed spike bursts whose frequencies varied directly with donors’ readiness-to-feed (Fig. 1B). R3 burst activity was an approximately linear function of donor feeding thresholds on a log-log plot (Fig. 1C). In the absence of sensory input, such spontaneous activity is likely to reflect the intrinsic excitatory state of the feeding network. These results showed the conservation of appetitive state in the excitation state of the feeding motor network in the isolated CNS.
Figure 1.
Conservation of donor appetitive state in the isolated CNS. A: The isolated CNS, showing nerves stimulated and recorded in these experiments. Excitation state of the feeding network was recorded from feeding motor nerves R3. In some experiments the feeding network was excited by stimulating the stomatogastric Nerve (SGN) innervating the gut and buccal cavity, or by driving a PCp feeding command neuron [6]. Unilateral stimulation of large oral veil nerves (LOVNs) triggered the fictive turn response recorded from ipsi- and contralateral Lateral Body Wall Nerves (LBWNs; [4]). B: Spontaneous burst frequency recorded from nerve R3 of isolated CNS’s was less from high-threshold donors than for low threshold animals. C: R3 burst frequency was an approximately linear function of donor feeding thresholds on a log-log plot (N=25; R2=0.54 and 0.59 for proboscis extension and biting, respectively). Line fits were by Least Squares. Three high threshold donor CNS’s did not show burst patterns in R3 and were excluded here.
It was especially interesting to find that induced fictive turns in isolated CNS’s also reflected donor appetitive state. Isolated CNS’s effectively sorted themselves into distinct groups based on donor feeding thresholds and their fictive turn. Previously, it was shown that unilateral stimulation of nerves innervating the oral veil induced fictive turn motor output, recorded in the nerves of the lateral body wall muscles, shown in higher spike activity in contralateral or ipsilateral body wall nerves LBWNs for avoidance and orienting turns, respectively [4]. Here we observed fictive orienting turning in isolated CNS’s specifically from relatively low feeding threshold donors, and either fictive avoidance or null responses in those from high threshold donors. We compared behavioral thresholds and fictive turn responses of 40 CNS donors. Of them, 11 CNS’s showed fictive orienting to large oral veil nerve (LOVN) stimulation, 12 showed no clear turn response and 17 expressed fictive avoidance turns (Fig. 2A). Donor feeding thresholds of the fictive orienting CNS’s were significantly lower than donor thresholds of the non-turning and fictively avoiding CNS’s. A clear transition existed in donor feeding thresholds and avoidance/null responding vs. orienting preparations. In intact animals high feeding thresholds are characteristically associated with either active avoidance or no reactions to food stimuli [2].
Figure 2.
Fictive turn direction was a function of donor feeding thresholds. A: CNS’s of high threshold donors responded to sensory LOVN stimulation with fictive avoidance turns (N=17) or null responses (N=12), while orienting turns (N=11) characterized CNS’s from animals with lower feeding thresholds (p<0.001 for both biting and proboscis extension thresholds, two-tailed Mann-Whitney U Test). Transition from avoidance/null to orienting responses occurred at proboscis extension thresholds of 10−3 and between 10−1 and 100 for biting. B: Fictive turn direction was represented in differing relative spike rates of the LBWNs [4] following LOVN stimulation (bar). In the two representative experiments shown, an avoidance turn (left) was seen in higher spike rates in LBWN contralateral to the stimulated nerve, while an orienting turn (right) was shown in higher relative activity in the ipsilateral LBWN (p<0.0001 in both cases, two-tailed Mann-Whitney U Tests). The initial post-stimulation peaks corresponded to fictive withdrawal preceding the fictive turn response (solid arrow) [4], as indicated.
Figure 2B illustrates fictive avoidance and orienting in enhanced motor activity of the lateral body wall nerve (LBWN) of the side contralateral or ipsilateral to the stimulus, respectively. In the intact animal this shortens that side of the body relative to the other, resulting in a turn away or toward the stimulus to the oral veil [4]. LBWN activity differences tended to be less marked in orienting than avoidance, as in normal behavior where aversive turns are typically greater in amplitude and more stereotypic in postural involvement, as might be expected of an escape-like behavior [5].
Feeding Network Excitation Governs Turn Direction
These results suggested that the excitation state of the feeding network might control the response symmetry of the turn network, where higher spontaneous feeding activity would promote orienting activity over avoidance. Accordingly, we manipulated excitation state of the feeding network in two ways: First, through intracellular stimulation of identified feeding command neurons, and second, by stimulation of a buccal ganglion nerve innervating the buccal cavity and esophagus. .
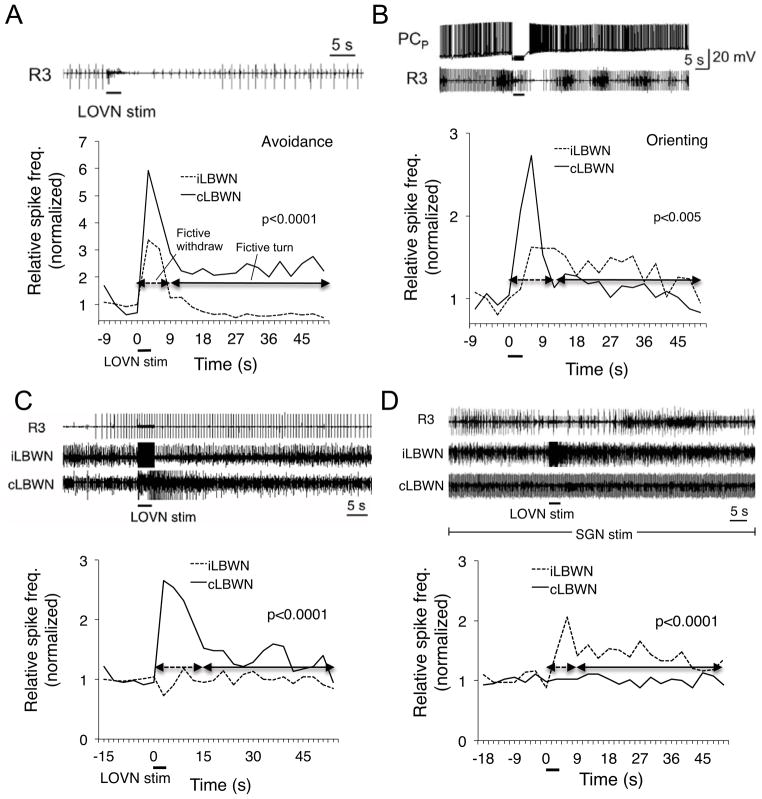
Increasing excitation of the feeding motor network reversibly switched the fictive turn response from avoidance to orienting. Activity in identified feeding command neurons, the PCP’s, is necessary and sufficient to activate and sustain feeding [6]. Single PCP’s were penetrated in four CNS’s of donors with high feeding thresholds, whose initial responses to LOVN stimulation were fictive avoidance (Fig. 3A). Depolarization with injected current caused rapid spiking followed by onset of fictive feeding motor output, recorded as rhythmic bursting in the feeding motor nerve R3. When the LOVN was then stimulated, turning motor output recorded was switched to orienting (Fig. 3B). Hyperpolarization of the PCP then abolished bursting activity in the feeding nerve and concomitantly caused the induced turn response to revert to avoidance, and releasing the PCP to increase feeding network excitation restored the orienting turn (Fig. S1).
Figure 3.
Increasing feeding network excitation state switched the fictive turn from avoidance to orienting. In CNS's from high feeding threshold donors avoidance turns (A) were converted to orienting (B) when a PCP feeding command neuron was penetrated with a microelectrode and driven to induce rhythmic bursting in the feeding motor nerve R3 (N=4). Increasing feeding network excitation by stomatogastric nerve stimulation (4 hz, 2 msec duration pulses) to drive slow rhythmic bursting in the buccal nerve R3 switched the avoidance turn (C) to orienting (D; N=5). Fictive avoidance (A, C) corresponded to higher spike frequency in contralateral LBWN (cLBWN) (p<0.0001 in both cases, Mann-Whitney U Test). Fictive orienting (B, D) matched higher activity in ipsilateral LBWN (iLBWN) (B, p<0.005; D, p<0.0001). Such significant differences were observed in all experiments. See also Figure S1.
Similar results were found for stimulation of the stomatogastric nerve (SGN) of the buccal ganglion, which drives rhythmic protraction/retraction feeding cycles in Pleurobranchaea and other opisthobranch gastropods [7]. When SGN stimulation excited the feeding network in five fictively avoiding CNS’s, the responses to LOVN stimulation also converted from avoidance (Fig. 3C) to orienting (Fig. 3D). In the example, SGN stimulation caused weak rhythmic bursts in the feeding motor nerve R3. The increase in feeding network excitation was sufficient to switch the fictive turn response.
Discussion
Neural Nature of Appetite
The appetites were described by Plato, ~380 BCE [8], as the set of baser cravings, and appetitive state remained until the 19th century as an inferred multidimensional construct based on observations of cravings for food, hydration, sex and other goal-directed motives generally allayed by consummatory acts. Physiological representations emerged from inquiries into hunger and thirst related to diabetic conditions (c.f. [9]), which then led to present appreciation of endocrine, neurotransmitter and electrophysiological activities as sequelae of motivation in nutrition and thirst [10–13].
The present work goes beyond unitary physiological elements of appetitive state to document its manifestation in a well described, homeostatic neuronal network. Further, its control of approach/avoidance decision is demonstrated in a similarly well described premotor circuit. The results suggest a testable general model of neuronal circuitry context in which physiological parameters of motivation and choice operate.
Appetitive state, measured behaviorally in readiness-to-feed, was found here to be a direct function of the excitation of the homeostatic feeding network. The appetitive states of intact animal donors were conserved in their isolated CNS’s and reflected as rates of spontaneous rhythmic output of the feeding network. The marked linearity of the double logarithmic plot for CNS donor feeding thresholds vs. spontaneous burst frequency (Fig. 1) directly related responsiveness for appetitive stimuli to feeding network excitation state.
Appetitive Control of Decision
A manifestation of appetitive state in the excitation of the feeding motor network was further indicated by the relationship of CNS donors’ readiness-to-feed to the fictive approach-avoidance decision of the isolated CNS (Fig. 2). CNS’s from donor animals with low feeding thresholds showed orienting turns, while those with high feeding thresholds exhibited avoidance or null turn responses. These results matched previous behavioral studies showing that satiation exchanges orienting turn responses for avoidance or null responses [2]. The identity of appetitive state was further confirmed by inducing the switch from avoidance to orienting by artificially adding and subtracting excitation in the feeding network via feeding command neuron activity and SGN stimulation (Fig. 3).
The control of the turn response by excitation state in the feeding motor network predicts two aspects of the choice mechanism at the neuronal level: 1) Corollary outputs from feeding to turn network must exist that promote the orienting turn, and 2) The default organization of the turn network circuitry is likely to be that for avoidance turns. In default mode, turn interneurons ipsilateral to sensory stimuli are activated and the consequent asymmetric network response drives contralateral, avoidance turn output [4]. However, in low feeding threshold animals, corollary output from the feeding network would reconfigure the premotor turn network into its alternative activity mode of orienting, for ipsilateral output. The regulation of the turn network by the feeding network resembles control of vertebrate spinal reflexes, whose default circuits are redirected to other, even oppositely directed, behaviors by descending voluntary control [14,15].
Modeling Basic Cost-Benefit Decision
These and previous observations point to the feeding network as a final integration site for sensation, internal state and memory in cost-benefit decision. The effects of learning and satiation were found earlier to be expressed at strategic sites in the feeding network, where they controlled responses to aversively learned odors and appetitive stimuli [16,17]. In naïve hungry animals food stimuli induce network excitation, culminating in rhythmic feeding motor output [6]. However, in odor-avoidance trained and partially satiated animals the feeding network is inhibited, raising feeding threshold to appetitive stimuli by 100–1000 fold [16]. In these cases, active feeding can be released by increasing the stimulus strength or by providing excitation directly to the feeding network via PCp feeding command neurons. Excitation state of the feeding network thus integrates the contributions of learning and motivation into its responsiveness to sensory input, and so embodies appetitive state in the animal’s moment-to-moment regulation of readiness to feed.
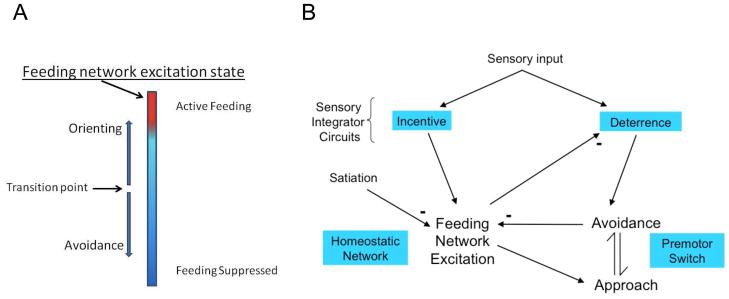
Collectively, these results can be summarized in a simple model (Fig. 4). In it, the excitation state of the homeostatic feeding network controls a switch between avoidance and approach responses (Fig. 4A). Sensory signals are processed through integrative sensory networks for Incentive and Deterrence to promote orienting or avoidance, dependent on homeostatic feeding network activity (Fig. 4B). Active avoidance and the feeding network are reciprocally inhibitory. The simple design elaborates an earlier model proposed to explain effects of satiation on the approach/avoidance decision [2]. The excited homeostatic network is shown simply as existing in a continuum of excitatory state (although several states are actually possible in Pleurobranchaea) culminating in active feeding (cf. [18]). The model can be further elaborated on the basis of new observations and is markedly accessible to computational simulation.
Figure 4.
A summary model for cost-benefit decision in a simple forager, summarizing appetitive regulation of approach-avoidance. A. The excitation state of the homeostatic (feeding) network controls expression of orienting vs. avoidance via corollary outputs to the directional turn motor network. The transition is sub-threshold to active feeding. B. Sensory inputs for resource quality, sensory signatures and nociception access integrating sensory networks for Incentive and Deterrence, which each promotes excitation of feeding and avoidance, respectively. Excitation in the homeostatic feeding network suppresses stimulus avoidance behavior and promotes transition to orienting approach from avoidance. Active avoidance and satiation are inhibitory to appetitive state in the homeostatic network, while homeostatic network activity also suppresses Deterrence input [cf. ref. 21]. Except where noted with minus signs, all arrows represent excitatory effect.
Not explicitly shown in the model is the sea-slug’s ability for associative learning of odor value [19; in preparation]. Moreover, the feeding network also exchanges outputs with conflicting neuronal networks, whose interactions mediate decision in escape swimming [20] and local withdrawal vs. feeding [21,22], and which also manifest effects of behavioral conflict [17,23–25]. Incorporating these interactions may eventually result in a more complete model of animal behavior.
Neuroeconomics of a Simpler Generalist Forager
In the predator’s natural environment these neural relations are likely to sustain the essential behavioral economics of foraging [2]. Thus, avoidance of appetitive stimuli below orienting threshold may represent a negotiation of expected nutritive benefit against costs of meal acquisition and risk of becoming prey to another predator attracted by the scent (e.g., a cannibal conspecific). Otherwise, orienting and attacking a stimulus reflects a decision in which estimated benefit offsets costs and risks. Here, appetitive state itself captures the cost-benefit computation as it is embodied in the excitatory configuration of the goal-directed feeding network.
Finally, the interactions of the goal-directed feeding network of the mollusk with its turn network form a simplest decision module for approach/avoidance, acting at a precognitive level in this solitary, cannibal predator. The simple module forms a potentially fundamental type of core circuitry around which the more complex neuronal circuit functions of valuation and comparison are elaborated in the social vertebrates. As such, it can provide a useful starting point for considering the evolution of more complex systems, and it invites future modeling for adding neural and behavioral complexity.
Experimental Procedures
Animals
Specimens of Pleurobranchaea californica (50–800 ml volume) were obtained from Monterey Abalone, Inc. (Monterey, CA) and maintained in artificial seawater at 12–13 °C until use.
Feeding Thresholds
Appetitive state, or behavioral readiness-to-feed, in Pleurobranchaea is controlled by sensation, nutritional state, learning, reproductive condition and health. Readiness-to-feed is quantitated in terms of feeding thresholds measured as the minimal concentrations of appetitive stimuli that elicit proboscis extension and active biting [2,7]. Feeding thresholds were measured as previously described [2,7] in responses to betaine (trimethylglycine; Sigma-Aldrich) solutions in seawater + 10 mM MOPS at pH 8.0 applied in 1.5 ml volumes to the oral veil with a hand-held Pasteur pipette over 10 seconds in a series of ascending concentrations from 10−6 to 10−1 M. Feeding thresholds measured were those concentrations at which animals showed proboscis extension and biting. When specimens failed to respond to the highest concentration (10−1 M) the next highest value, 100, was assigned for proboscis extension and 101 for biting threshold, since biting threshold is normally higher than proboscis extension threshold. Tests began with a control sea-water application assigned a value of 10−7. These conventions assign conservative finite values to essentially infinitely high or low thresholds. Data were analyzed and presented as the logarithms of the dilutions; thus, 10−1 is −1.0 and so on [26]. Data were analyzed with non-parametric tests that best accommodate the threshold conventions described above. In the laboratory population, thresholds are somewhat skewed to the high end as result of the sample population being largely larger reproductive animals, an artifact of both trapping methods and seasonality.
Isolated CNS Preparation
Shortly after feeding threshold measures, animals were anesthetized by cooling to 4°C. CNS’s, consisting of cerebropleural, pedal, and buccal ganglia, were dissected out and pinned in a Sylgard dish under saline (in mM) 460 NaCl, 10 KCl, 25 MgCl2, 25 MgSO4, 10 CaCl2, and 10 MOPS buffer at pH 7.5 and 12–13 °C.
Electrophysiology
For intracellular recordings, connective tissues were removed over neuron cell bodies. Intracellular and extracellular recordings and nerve stimulation were made with 3 M KCl-filled glass micropipettes and polyethylene suction electrodes, respectively. Data were acquired with Power Lab software (AD Instruments). Fictive turns were induced by brief, unilateral stimulation of one of the bilateral pair of Large Oral Veil Nerves (LOVNs; 15 hz, 2 msec pulse duration; [4]). Fictive feeding was induced by driving PCP feeding command neurons [6] or by stimulating stomatogastric nerve (SGN) at 2–4 hz, 2 msec duration pulses; [7]). Fictive feeding was recorded from buccal ganglion nerve root 3 as bursts of fictive radular retraction activity. Data were captured and analyzed with Chart 5 Pro (AD Instruments). Fictive turn events were characterized by comparing mean spike frequencies in bilateral lateral body wall nerves (LBWNs), which are motor outputs for the turn network [4]. Spikes were counted after selecting a threshold level above spontaneous noise. Spike frequencies were normalized to spike counts for 20 seconds prior to the stimulus event and plotted in 2–3 second bins. The non-parametric Mann-Whitney U-test was used for statistical analyses. The p values were calculated by comparing the spike counts in ipsilateral and contralateral LBWNs for 30 seconds from the first steep inflection following the initial peak. The initial peak corresponds to a fictive withdrawal [4] preceding the turn, as in intact animals. Criterion for assigning “fictive avoidance” vs. “fictive orienting” to LBWN activity was a significant difference with at least p<0.05 for bilateral spike counts; in fact, for all avoidance results, p values were <0.0001; and for orienting, p values ranged p<0.01 to 0.0001.
Supplementary Material
Highlights.
Appetitive state determined by excitation state in a homeostatic network
Homeostatic network controls a switch for motor outputs of approach-avoidance
Acknowledgments
Supported by NSF IOB 04-47358 and NIH R21 DA023445. Thanks are due Jeffrey Brown and anonymous reviewers for editorial comments, and to Trevor Fay and Art Seavey of Monterey Abalone Co. for animal supply.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kristan R, Gillette R. Behavioral choice. In: North G, Greenspan RJ, editors. Invertebrate Neurobiology. New York: Cold Spring Harbor Laboratory Press; 2007. pp. 533–553. [Google Scholar]
- 2.Gillette R, Huang RC, Hatcher N, Moroz LL. Cost-benefit analysis potential in feeding behavior of a predatory snail by integration of hunger, taste, and pain. Proc Natl Acad Sci U S A. 2000;97:3585–3590. doi: 10.1073/pnas.97.7.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yafremava LS, Anthony CW, Lane L, Campbell JK, Gillette R. Orienting and avoidance turning are precisely computed by the predatory sea-slug Pleurobranchaea californica Mcfarland. J Exp Biol. 2007;210:561–569. doi: 10.1242/jeb.02697. [DOI] [PubMed] [Google Scholar]
- 4.Jing J, Gillette R. Directional avoidance turns encoded by single interneurons and sustained by multifunctional serotonergic cells. J Neurosci. 2003;23:3039–3051. doi: 10.1523/JNEUROSCI.23-07-03039.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gillette R, Saeki M, Huang RC. Defense mechanisms in notaspidean snails: Acid humor and evasiveness. J Exp Biol. 1991;156:333–347. [Google Scholar]
- 6.Gillette R, Kovac MP, Davis WJ. Control of feeding motor output by paracerebral neurons in the brain of Pleurobranchaea californica. J Neurophysiol. 1982;47:885–908. doi: 10.1152/jn.1982.47.5.885. [DOI] [PubMed] [Google Scholar]
- 7.Davis WJ, Mpitsos GJ. Behavioral choice and habituation in the marine mollusk Pleurobranchaea californica. Z Vergl Physiol. 1971;75:207–232. [Google Scholar]
- 8.Plato. (~380 BCE). The Republic, Books IV, VIII and IX.
- 9.Bernard C. Introduction to the study of experimental medicine. New York: Dover pub; 1865. [Google Scholar]
- 10.Egan G, Silk T, Zamarripa F, Williams J, Federico P, Cunnington R, Carabott L, Blair-West J, Shade R, McKinley M, Farrell M, Lancaster J, Jackson G, Fox P, Denton D. Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci U S A. 2003;100:15241–15246. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami KI, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 12.Hatcher NG, Zhang X, Stuart JN, Moroz LL, Sweedler JV, Gillette R. 5-HT and 5-HT-SO4, but not tryptophan or 5-HIAA levels in single feeding neurons track animal hunger state. J Neurochem. 2008;104:1358–1363. doi: 10.1111/j.1471-4159.2007.05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powley TL. Hunger. In: Berntson GG, Cacioppo JT, editors. Handbook of Neuroscience for the Behavioral Sciences. Vol. 2. New York: Wiley & Sons Pub; 2009. pp. 659–679. [Google Scholar]
- 14.Sherrington C. The Integrative Action of the Nervous System. 1947. Yale University Press; 1906. p. 7. [Google Scholar]
- 15.Stuart DG. Reflections on spinal reflexes. Adv Exp Med Biol. 2002;508:249–257. doi: 10.1007/978-1-4615-0713-0_30. [DOI] [PubMed] [Google Scholar]
- 16.Davis WJ, Gillette R, Kovac MP, Croll RP, Matera E. Organization of synaptic inputs to paracerebral feeding command interneurons of Pleurobranchaea californica III. Modifications induced by experience. J Neurophysiol. 1983;49:1557–1572. doi: 10.1152/jn.1983.49.6.1557. [DOI] [PubMed] [Google Scholar]
- 17.London JA, Gillette R. Mechanism for food avoidance learning in the central pattern generator of feeding behavior of Pleurobranchaea californica. Proc Natl Acad Sci U S A. 1986;83:4058–4062. doi: 10.1073/pnas.83.11.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillette R. Behavioral hierarchies. In: Squire LR, editor. The New Encyclopedia of Neuroscience. Amsterdam: Elsevier Pub; 2008. pp. 145–154. [Google Scholar]
- 19.Davis WJ, Villet J, Lee D, Rigler M, Gillette R, Prince E. Selective and differential avoidance earning in the feeding and withdrawal behavior of Pleurobranchaea californica. J Comp Physiol. 1980;138:157–165. [Google Scholar]
- 20.Jing J, Gillette R. Neuronal elements that mediate escape swimming and suppress feeding behavior in the predatory sea slug Pleurobranchaea. J Neurophysiol. 1995;74:1900–191. doi: 10.1152/jn.1995.74.5.1900. [DOI] [PubMed] [Google Scholar]
- 21.Kovac MP, Davis WJ. Behavioral choice: neural mechanisms in Pleurobranchaea. Science. 1977;198:632–634. doi: 10.1126/science.918659. [DOI] [PubMed] [Google Scholar]
- 22.Kovac MP, Davis WJ. Neural mechanism underlying behavioral choice in Pleurobranchaea. J Neurophysiol. 1980;43:469–487. doi: 10.1152/jn.1980.43.2.469. [DOI] [PubMed] [Google Scholar]
- 23.Davis WJ, Gillette R. Neural correlate of behavioral plasticity in command neurons of Pleurobranchaea. Science. 1978;199:801–804. doi: 10.1126/science.622572. [DOI] [PubMed] [Google Scholar]
- 24.London JA, Gillette R. Functional roles and circuitry in an inhibitory pathway to feeding command neurons in Pleurobranchaea. J Exp Biol. 1984;113:423–446. doi: 10.1242/jeb.113.1.423. [DOI] [PubMed] [Google Scholar]
- 25.Kovac MP, Matera EM, Volk PJ, Davis WJ. Food avoidance learning is accompanied by synaptic attenuation in identified interneurons controlling feeding behavior in Pleurobranchaea. J Neurophysiol. 1986;56:891–905. doi: 10.1152/jn.1986.56.3.891. [DOI] [PubMed] [Google Scholar]
- 26.Davis WJ, Mpitsos GJ, Pinneo JM. The behavioral hierarchy of the mollusk Pleurobranchaea I. The dominant position of the feeding behavior. J Comp Physiol. 1974;90:207–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.