Abstract
8-Oxo-7,8-dihydroguanine is one the most abundant base lesions in pro- and eukaryotic DNA. In mammalian cells, it is excised by the 8-oxoguanine DNA glycosylases (OGG1) during DNA base excision repair, and the generated free 8-oxoG base (8-oxoG) is one of the DNA-derived biomarkers of oxidative stress in biological samples. The modification of 8-oxoG in the context of nucleoside and DNA has been the subject of many studies; however, the oxidative transformation of the free 8-oxoG base has not been described. By using biochemical and cell biological assays, we showed that in the presence of molecular oxygen, the free 8-oxoG base transformed to a highly reactive hydroperoxide (8-oxoG*). Specifically, 8-oxoG* oxidizes Amplex Red to resorufin, H2DCF to DCF, Fe2+ to Fe3+, and GSH to GSSG. This property of 8-oxoG* was diminished by treatment with catalase, glutathione peroxidase, but not superoxide dismutase. 8-oxoG* formation was prevented by reducing agents or nitrogen atmosphere. Its addition to H2DCF-DA-loaded cells rapidly increased intracellular DCF fluorescence. There were no such properties observed for 8-oxodeoxyguanosine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine, 2’-deoxyguanosine, guanine, adenine, guanosine and 8-hydroxyadenine. These data imply that a free 8-oxoG base is more susceptible to oxidation than is its nucleoside form and, consequently, it stands as unique among intact and oxidatively modified purines.
Keywords: free 8-oxoguanine base, oxidative stress
Introduction
Reactive oxygen and other oxidizing species are the most common chemical entities to modify constituents of DNA, RNA and the nucleotide pool. Among oxygen radicals, the hydroxyl radical (•OH) is one of the most damaging to DNA components, resulting in carbon-centered sugar radicals and OH− or H-adduct radicals of heterocyclic bases via abstractions and addition reactions [1–3]. These reactions yield OH-adduct radicals of bases, while abstraction reactions result in both allyl radicals of thymine and carbon-centered sugar radicals. The most susceptible base among the DNA and RNA bases is guanine (Gua), due to its lowest reduction potential (midpoint potential is −1.29 mV vs. nickel hydrogen electrode: NHE) [4, 5]. In vivo, Gua in DNA and RNA can be modified not only by •OH but also by other reactive species, including reactive oxygen (superoxide anion: O2•– ), non-radical (ozone: O3; singlet oxygen: 1O2; hydrogen peroxide: H2O2), and nitrogen species (nitric oxide: NO•; peroxinitrite: ONOO–), as well as nitrosoperoxycarbonate (ONOOCO2–), carbonate anions (CO3 –) and the UVA component of solar light [2, 6, 7]. For example, the reaction of •OH with Gua results in C4-OH-, C5-OH-, and C8-OH-adduct radicals [8], while one-electron oxidation of Gua-C8-OH results in 7,8-dihydro-8-oxoguanine (8-oxoG). The one-electron reduction of the Gua-C8-OH-adduct radical undergoes a ring opening, resulting in 2,6-diamino-4-hydroxy-5-formamidopyrimidine (FapyG) or its isomer 2,5-diamino-4-hydroxy-6-formamidopyrimidine [3, 9].
Estimates show that under physiological conditions, several hundred 8-oxoG lesions could be formed in DNA per eukaryotic cell daily (rev in [10]). 8-oxoG is one of the most abundant DNA lesions formed in oxidative stress conditions, such as those that exist in diseased and aged cells/tissues [6, 11]. In mammals, the intra-helical 8-oxoG is recognized by its unique electronic properties [12] and excised by the E. coli Fpg homolog 8-oxoguanine DNA glycosylase 1 (OGG1) from nuclear and mitochondrial genomes during base excision repair (BER) processes [13, 14]. Unrepaired 8-oxoG may be paired with adenine during DNA replication, resulting in transversion mutations (rev in [15]). During mRNA synthesis, it may serve as a template to transcriptional mutagenesis [16]. The free 8-oxoG base exists in both neutral (N9-H) and anionic (N9:−) forms at physiologic pH. Its presence as a free base in extracellular fluids is one of the most reliable gauges of the oxidative stress load of an organism [2, 3, 17, 18].
Due to its low redox potential, 8-oxoG is more reactive than guanine and serves as a primary target of reactive oxygen species and considered as a protective element in DNA [5, 19, 20]. These observations were supported by findings showing that oligodeoxynucleotide damage and plasmid cleavage by reactive oxygen species (ROS) were inhibited in the presence of 8-oxodG [21]. We studied whether the free 8-oxoG base could function in a manner similar to that of its intrahelical nucleoside form. Here we show that in an oxygenated environment the 8-oxoG base, but not other nucleotides or nucleosides, is transformed into a hydroperoxide-like specie(s) that oxidized 10-acetyl-3,7-dihydroxyphenoxazine (Amplex red, AR) to resorufin, H2DCF to DCF and GSH to GSSG in the presence of peroxidases. Because of this unexpected behavior, the 8-oxoG base stands uniquely among the intact and oxidatively modified purines by being not only a marker (product) of oxidative stress, but also a substrate for further oxidation.
Materials and Methods
Cells
MRC-5 human lung fibroblast cells were cultured in Minimal Essential Medium. A549, a human lung adenocarcinoma epithelial cell line, and U937, a human monocytic cell line, were grown in Ham’s F12 and RPMI-1640, respectively. The human myelomonocytic KG-1 cells were grown in Iscove's Modified Dulbecco's Medium. All media (Invitrogen, Carlsbad, CA) were supplemented with 10% FBS (Atlanta Biologicals, Lawrenceville, GA), glutamine (292 mg/L) and penicillin (100 units/ml) and streptomycin (100 μg/ml). These cell lines were obtained from the American Type Culture Collection. Balb/c mouse embryonic fibroblast cells were generated locally and maintained in DMEM (low glucose). All cells were cultured under a humidified atmosphere (95% air and 5% CO2) at 37°C and periodically tested for mycoplasma contamination. The monolayer cultures were routinely subcultured by using trypsin-EDTA (Invitrogen).
Preparation of 8-oxoG solution
8-OxoG is provided as a hydroacetate salt and dissolved as recommended by the manufacturer (Cayman Chemical, Ann Arbor, MI). Briefly, a 4-mM stock solution was prepared in 12 mM NaOH (pH 12). Stock solutions were used immediately or stored at room temperature in dark for a maximum of 1 week. Working solutions were diluted in PBS (w/o Ca2+, Mg2+; pH 7.4) or distilled water (pH 7.0). There was no solubility problem observed at 100 μM or lower concentrations. HPLC analysis [250 x 4.6 mm i.d. ACE C18 column (MAC-MOD Analytical, Chadd Ford, PA)] of 8-oxoG showed no detectable contamination (data not shown). All nucleotide bases and nucleosides (2’-deoxyguanosine, guanine, adenine, guanosine, 7,8-dihydro-8-oxo-2'-deoxyguanosine, 8-hydroxyadenine and 2,6-diamino-4-hydroxy-5-formamidopyrimidine) were solubilized in the same manner (12 mM NaOH). HPLC analyses showed no detectable contamination.
Assessment of intracellular ROS levels
Changes in intracellular ROS levels were determined by using the fluorogenic probe 5-(and-6)-chloromethyl-2'7'-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCF-DA; Invitrogen, Eugene, OR) [22]. Briefly, cells were grown to 70% confluence and loaded with 50 μM CM-H2DCF-DA at 37°C for 30 minutes. Cells were then washed with PBS and exposed to nucleobases, nucleosides, and solvents. As positive controls, tert butyl hydroperoxide (tBHP; ACROS Organics, Geel, Belgium) and cumene hydroperoxide (CHP; Spectrum, Gardena, CA) or H2O2 (Fisher, Fair Lawn, NJ) were used. Changes in DCF fluorescence were recorded on an FLx800 (Bio-Tek Instruments Inc., Winooski, VT) microplate reader at 485 nm excitation and 528 nm emission. Results are expressed as fold change or arbitrarily in fluorescence units (FU). The mean V is a calculated value of the mean slope by determining regression on points that are in a linear calculation zone (KC4 software, Bio-Tek Instruments, Inc.). In confirmatory studies, changes in cellular ROS levels were determined by using flow cytometry as we described previously [22, 23].
To visualize an increase in intracellular ROS levels, we loaded cells with 50 μM CM- H2DCF-DA for 15 min at 37°C and then washed them. They were placed in a thermo-controlled microscopic chamber, and 8-oxoG was added. Images were captured with a Photometrix CoolSNAP Fx digital camera mounted on a NIKON Eclipse Ti UV microscope [24]. The microscope was operated via NIS-Elements AR Ver3.22.09 for 64bit edition Software.
Amplex Red (AR) assay
Amplex® UltraRed (10-acetyl-3,7-dihydroxyphenoxazine; Invitrogen, Eugene, OR) reacts with H2O2 (Fisher, Fair Lawn, NJ) in the presence of horseradish peroxidase (HRP; Sigma-Aldrich, St. Louis, MO) to generate a stable product, resorufin [25]. Amplex Red assays were carried out as we previously described [26]. Briefly, test materials (e.g., 8-oxoG and controls; 0.01-100 μM) were diluted in reaction buffer and incubated at 37°C for 30 min with 50 μM Amplex® UltraRed and 0.05 U/ml of HRP (optimal concentrations were determined in preliminary studies). Changes in resorufin fluorescence were determined at 560 nm and 620 nm (excitation and emission, respectively) by using a BioTek FLx800 fluorimeter. To establish the standard curve, increasing concentrations of H2O2 (0 to 10 μM) were used. The addition of catalase (5 U/ml, Sigma-Aldrich, St. Louis, MO) decreased H2O2 levels by ~95%. As additional controls, tBHP or CHP were used.
Assessment of GSH/GSSG ratio
A Bioxytech GSH/GSSG-412 (OxisResearch; Portland, OR) assay kit was used to determine levels of GSH and GSSG by following the basic manufacturer's protocol with a slight modification as described [27]. Briefly, GSSG samples (in triplicate were prepared by gently mixing thiol-scavenging reagent 1-methyl-2-vinylpyridinium trifluoro-methanesulfonate containing H2O2 or 8-oxoG in an assay buffer (50 mM Na3PO4 with EDTA, pH 7.4). Samples were incubated at room temperature for 10 minutes, and the reaction was stopped with cold 5% metaphosphoric acid (MPA, Sigma-Aldrich). Samples were vortexed and combined with the provided GSSG buffer. GSH samples were made by combining 50 μL of 100 μM H2O2 or 8-oxo-Gua in the assay buffer with 350 μL MPA. The samples were combined with chromogen (5, 5’-dithiobis-(2-nitrobenzoic acid)±glutathione peroxidase (GPx, bovine erythrocytes; Sigma-Aldrich). After 5 min incubation at room temperature, NADPH (Sigma-Aldrich) solution (1 μM) was added and changes in absorbance recorded at 412 nm for 3 min on a Beckman DU530 spectrophotometer. Linear regressions were calculated on a five-point curve by using 0, 0.75, 1.50, 2.25 and 3.00 μM for GSH and a four-point curve for GSSG by using 0, 0.10, 0.25 and 0.50 μM.
Glutathione peroxidase assay
Glutathione peroxidase assays were conducted according to the manufacturer’s instructions (Sigma-Aldrich; St. Louis, MO). Briefly, assay buffer (pH = 7.0) contained 48 mM sodium phosphate, 0.12 mM ß-NADPH, 0.95 mM sodium azide, 3.2 units of purified glutathione reductase (GR; Sigma-Aldrich), 1 mM GSH, 0.02 mM dithiothreitol (DTT) and 0.2 units GPx (pH 7.0) [28]. Next, 0.1 to 100 μM of 8-oxoG (or other nucleic acid bases) was added to assay buffer, and changes in NADPH absorbance were monitored at 340 nm in a Beckman DU530 (Beckman Coulter, Inc) spectrophotometer at a constant temperature (25°C) at a light path of 1 cm. H2O2 was used as a positive control. Changes in ∆A340nm/minute were calculated by a formula provided by the manufacturer.
Liquid Chromatography/Mass Spectrometry (LC/MS)
Test samples were placed into liquid nitrogen, then transferred into the freeze-dryer (Dura-Dry MP, Stone Ridge, NY) and lyophilized at −80°C (35 mT). The lyophilized materials were reconstituted and the 8-oxoG level measured by liquid chromatography/isotope dilution mass spectrometry (LC/IDMS). As an internal standard, the stable isotope-labeled analogs of 8- oxoG were used as previously described [11].
PeroXOquant Assay
Assays were performed according to the manufacturer’s recommendations (Pierce, Rockford, IL). Briefly, one volume of reaction buffer A (25 mM ammonium ferrous (II) sulfate and 2.5 M H2SO4) was mixed with 99 volumes of reagent B (100 mM sorbitol and 125 μM xylenol orange). After addition of 8-oxoG (or H2O2 as positive control; 0 μM to 10 μM) mixtures were incubated for 20 min at room temperature. OD was measured at 595 nm (Beckman DU530). Peroxide concentrations in 8-oxoG (and other nucleotides, nucleoside solutions) were calculated from the standard curve of H2O2 [29].
Reagents
The following were purchased from Sigma-Aldrich (St. Louis, MO): L-glutathione reduced (GSH); β-Nicotinamide adenine dinucleotide 2’-phosphate reduced tetrasodium salt (β-NADPH); 2’-deoxyguanosine, guanine, adenine, guanosine, 7,8-dihydro-8-oxo-2'-deoxyguanosine (8-oxodG); N-acetyl-L-cysteine (NAC), and maleic acid diethyl ester (DEM). 8-Aminoguanine was purchased from Carbosynth Inc, Berkshire, UK; 8-hydroxyadenine (8-OHA, Biolog Life Science Institute, Bremen, Germany); and FapyG (2,6-diamino-4-hydroxy-5-formamidopyrimidine) was provided by Dr. Miral Dizdaroglu (National Institute of Standards and Technology, Gaithersburg, MD).
Statistical Analysis
Data are expressed as the mean ±SEM. Results were analyzed for significant differences by using ANOVA procedures and Student’s t-tests (Sigma Plot 11.0). Differences were considered significant at p<0.05 (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Results
8-OxoG base transformed to a hydroperoxide-like molecule
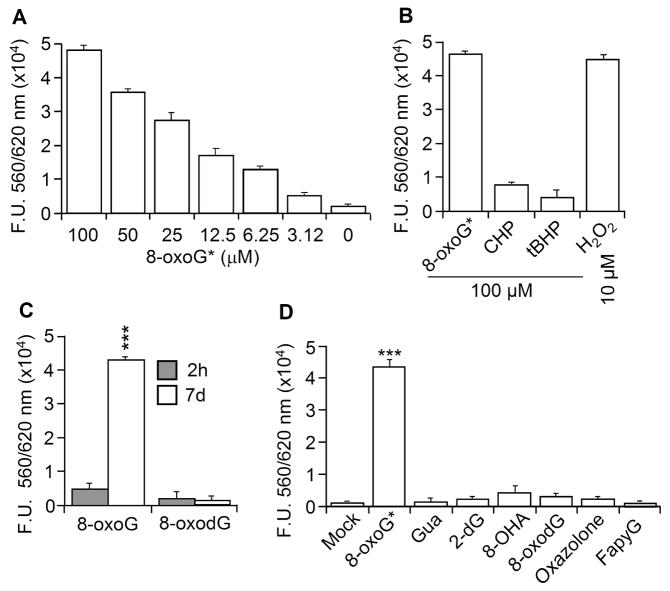
Exposure of cells to a week-old 8-oxoG solution resulted in increased cellular ROS levels, while the freshly solubilized 8-oxoG base did not do so. We speculate that the observed differences were due to changes in the properties of the 8-oxoG base over time. Indeed when the 8-oxoG base solution was stored for a week, it exhibited a concentration-dependent reactivity with Amplex Red (Fig. 1A). Fig. 1C shows that the one-week-old 8-oxoG solution extensively oxidized AR to resorufin, while a 2h old (freshly made) one did so only poorly. 8-OxoG solution also oxidized H2DCF to DCF, in the presence of HRP similar to H2O2 (data not shown). In fact one-week-old 8-oxoG base solution (100 μM) mediated significantly higher resorufin fluorescence than that of organic hydroperoxides, tBHP (100 μM) or CHP (100 μM) (Fig. 1B). On the other hand, the same concentration of H2O2 was ~10-times stronger than 8-oxoG solution. In controls, 8-oxodG, FapyG, guanine, 2-deoxyguanosine (2-dG), 8-hydroxyadenine 8-OHA), oxazolone or adenine prepared similarly and kept for 7 days did not react with AR (Fig. 1D). When taken together, these data imply the presence of a hydroperoxide-like molecule (8-oxoG*) in 8-oxoG solution.
Figure 1.
Hydroperoxide activity in 8-oxoG base solution. (A) Concentration-dependent oxidation of Amplex red by 8-oxoG*. (B) Direct oxidation of AR by 8-oxoG* CHP and tBHP. H2O2 was used as a control. (C) 8-oxoG*, but not 8-oxoG or 8-oxodG oxidizes AR. (D) 8-oxoG*, but not Gua, 2-dG, 8-OHA, 8-oxodG, oxazolone or FapyG solution, has peroxide-like activity. Gua, guanine, 2-dG, 2-desoxyguanine; 8-OHA, 8-hydroxy adenine; 8-oxodG, 7,8- dihydro-8-oxo-2'-deoxyguanosine; FapyG, 2,6-diamino-4-hydroxy-5-formamidopyrimidine; CHP, cumene hydroperoxide; tBHP tert-butyl hydroperoxide. Results are means ±SEM (n=5-11).
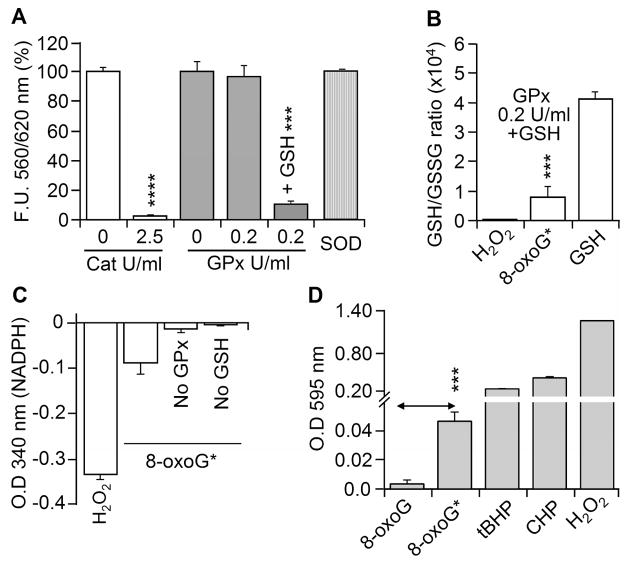
To test further the possibility that 8-oxoG solution contains a hydroperoxide derivative, we mixed it with catalase or glutathione peroxidase (GPx) ± GSH or superoxide dismutase (SOD) in appropriate reaction buffers [30]. Aliquots from parallel mixtures were subjected to AR assays. GPx+GSH and catalase diminished the reactivity of the 8-oxoG* base with AR (Fig. 2A). GPx alone or SOD did not have an effect on 8-oxoG*-mediated AR oxidation (Fig. 2A). In the GPx+GSH-mediated detoxification of hydroperoxides, GSSG was reported to be formed [31]. 8-OxoG* decreased the GSH:GSSG ratio in a manner similar to that found with H2O2 (Fig. 2B). We postulated that if indeed GSSG is formed, then glutathione reductase (GR) should convert it to GSH in the presence of NADPH. As shown in Fig. 2C, there was a significant decrease in NADPH levels in GPx+GSH+8-oxoG*-containing reaction mixtures (Fig. 2C), a finding that suggests the existence of GSSG. Similar results were observed when H2O2 was present. A decrease in NADPH levels could not be measured when GPx or GSH was omitted from these reactions (Fig. 2C). To gain further evidence of 8-oxoG* in 8-oxoG solution, we assessed the conversion of ferrous iron (Fe2+) to ferric iron (Fe3+) in PeroXOquant assays [29]. 8-oxoG*, but not the freshly solubilized 8-oxoG, showed a significant increase in absorbance (Fig. 2D) similar to that seen in the controls CHP, tBHP and H2O2 (Fig. 2D). These results also confirm that a fraction of 8-oxoG is converted to a molecule with the characteristics of hydroperoxide.
Figure 2.
Effect of peroxidases and ferrous iron on 8-oxoG*. (A) Catalase, GPx ,but not SOD, diminishes 8-oxoG*-mediated AR oxidation. (B,C) 8-OxoG* oxidizes GSH to GSSG. 8-oxoG* was incubated with GSH ±GPx, and the GSH:GSSG ratio was determined (Materials and Methods). H2O2 was used as a positive control (B). Utilization of NADPH ± GR was determined at 340 nm (C). H2O2 was used as a positive control. (D) 8-OxoG* oxidizes ferrous iron to ferric iron by a process similar to that seen with CHP, tBHP and H2O2 as shown by the PeroXoquant assay (O.D 595 nm). Cat, catalase, GPx, glutathione peroxidase; GR, glutathione reductase; SOD, superoxide dismutase; GSH, glutathione; GSSG, oxidized GSH; CHP and tBHP, as in legend to Fig 1. Results are means ±SEM (n=3-5).
8-oxoG* formation requires molecular oxygen
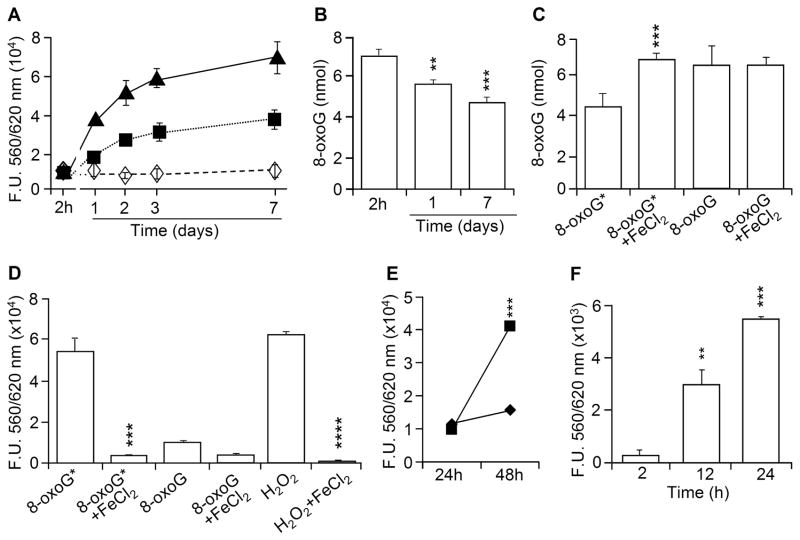
To define conditions that facilitate the formation of 8-oxoG*, freshly made 8-oxoG base (1 mM, pH: 12) solutions were kept under an ambient oxygen or nitrogen (N2) atmosphere (at room temperature in dark). 8-OxoG* formation was assayed immediately and at 1, 2, 3 and 7 days. Under O2 atmosphere, the 8-oxoG* levels were higher by >4.4- (day 1st), 6.1- (day 2nd) and 7.3-fold (day 7th) when compared to freshly prepared 8-oxoG solution, as determined in AR assays. At ambient conditions, there was a ~50% lower 8-oxoG* level at all time points. In contrast, 8-oxoG solution kept under N2 atmosphere did not acquire hydroperoxide properties, between 1 and 7 days (Fig. 3A). The addition of the reducing agent dithiothreitol (DTT; 1 mM), 2-β-mercaptoethanol (100 μM), or FeCl2 (100 μM) prevented 8-oxoG* formation (data not shown). It should be noted that we observed increased fluorescence signals generated by freshly made 8-oxoG solutions or those kept under N2 atmosphere. These fluorescent signals may be due to the rapid formation of 8-oxoG* in aqueous solutions during the solubilization process and mandatory incubations (Fig. 3A). To determine real-time formation of 8-oxoG* is our future challenge.
Figure 3.
Formation of 8-oxoG* requires O2. (A) Formation of 8-oxoG* under ambient (■), O2 (▲) and N2 (◇) atmosphere. Aliquots of 8-oxoG solution were subjected to AR assays at days 1, 2, 3 and 7. (B) Changes in 8-oxoG levels as a function of time and (C) reconstitution of 8-oxoG base level by Fe2+ as determined by LC/MS. (D) AR reactivity of 8-oxoG* and H2O2 is diminished in the presence of ferrous iron. (E) Conversion of 8-oxoG to 8-oxoG* at physiological (■) and alkaline pH (◆). (F) Formation of 8-oxoG* from 8-oxoG when dissolved and stored in PBS. Results are means ±SEM (n=3–6).
Next, if a fraction of 8-oxoG transformed to 8-oxoG*, the 8-oxoG content of the solution should be lower. Indeed, quantification by LC/MS shows a significant decrease in 8-oxoG levels at days 1 and 7 (Fig. 3B). To reinforce the presence of the 8-oxoG*, the solution was incubated for 15 min with Fe2+ (100 μM FeCl2 or solvent), and 8-oxoG levels were assessed. The addition of FeCl2 restored 8-oxoG levels, as assessed by LC/MS (Fig. 3C). In parallel, Fe2+ prevented an increase in 8-oxoG*-mediated resorufin fluorescence (Fig. 3D) in line with the results from the PeroXOquant assay (Fig. 2D).
To evaluate relative quantities of 8-oxoG* generated at alkaline and physiological pH we diluted an alkaline solution of 8-oxoG in PBS (pH, 7.4) and kept it for 24 hours. The capacity of 8-oxoG solutions (PBS) to oxidize AR to resorufin was found to be increased significantly (Fig. 3E) compared to those kept in the alkaline solution. To exclude that the alkaline pH imparted 8-oxoG to form the hydroperoxide-like molecule, we added 8-oxoG directly to PBS (pH 7.4). In PBS ~8 μM (±0.45 μM) of 8-oxoG could be dissolved. When compared to the freshly made one (2 h), the 12-h and 24-h 8-oxoG solutions mediated a significant increase in resorufin fluorescence (Fig. 3F). Taken together, these results provide evidence that a fraction of 8-oxoG was indeed transformed into 8-oxoG*, and its formation occurred both at an alkaline and physiological pH.
8-oxoG* increases cellular oxidative stress levels
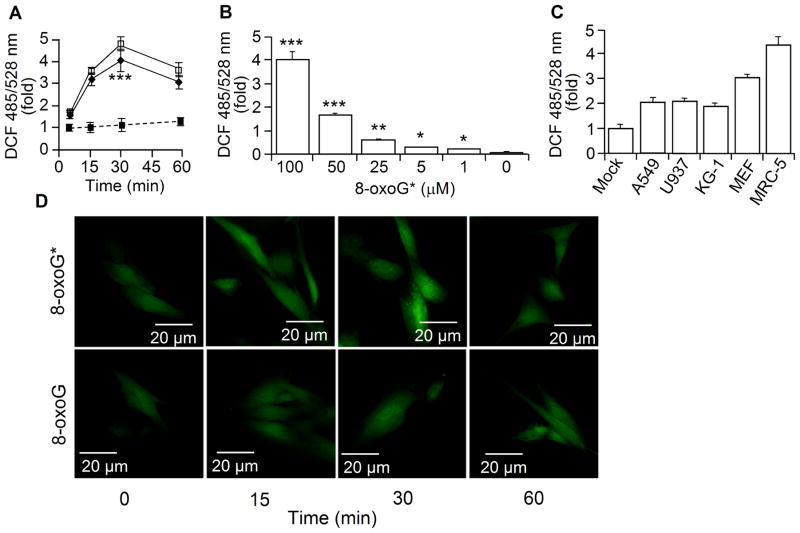
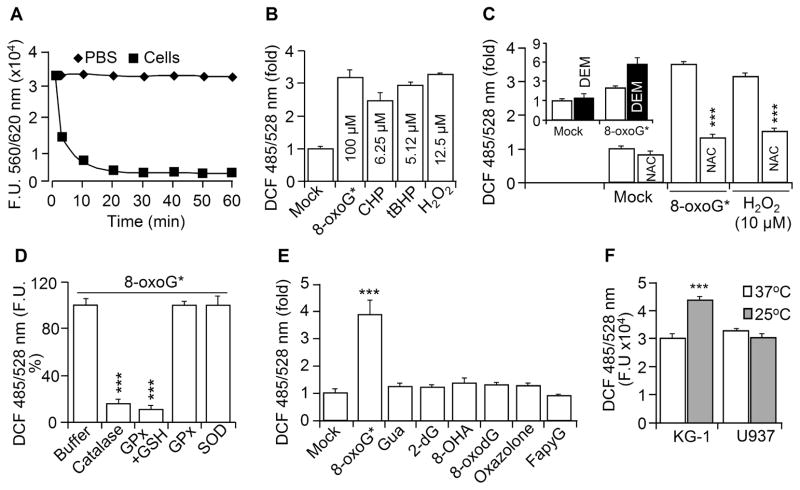
Next we evaluated whether 8-oxoG* increases intracellular ROS levels in the same manner as other hydroperoxides. Fig. 4A shows that 8-oxoG* rapidly increased DCF fluorescence, which peaked at 30 min post-exposure. The changes in fluorescence were similar to those induced by H2O2 (10 μM) and also were cell-type dependent (Fig. 4BC). For example, human (MRC-5; Fig. 4AB and D upper panels) and murine fibroblast cells (MEF, Fig. 4C) were the most sensitive to 8-oxoG,* showing a 3.2- to 4.5-fold increase, while established cell lines, A549, KG1 or U937, exhibited only a ~2-fold increase in DCF fluorescence (Fig. 4C). In parallel with an increase in intracellular DCF fluorescence (Fig. 4A), we observed a rapid decrease (90% at 15 min) in 8-oxoG* levels in cell supernatants (Fig. 5A). In a 100-μM 8-oxoG solution, the level of 8-oxoG* was equivalent to ~12.5 μM H2O2, ~5.1 μM tBHP or ~6.25 μM CHP (MRC-5 cells, Fig. 5B). Freshly prepared 8-oxoG did not increase cellular DCF fluorescence (Fig. 4D, lower panel). Pre-incubation of 8-oxoG solution with catalase (2.5 U/ml) or GPx (0.2 U/ml)+GSH (1 mM) prevented an 8-oxoG*-mediated increase in cellular DCF fluorescence. GPx, GSH alone (controls) or SOD had no effects on redox properties of 8-oxoG* (Fig. 5D). We made similar observation when H2O2 was used in control experiments (data not shown). N-Acetyl-L-cysteine (NAC) pre-treatment of cells (5 mM, for 3 h) prevented a rise in ROS levels, both in 8-oxoG*- and H2O2-treated cells (Fig. 5C). When glutathione was depleted with maleic acid diethyl ester (DEM, 1.25 mM, for 30 min [27]), 8-oxoG* exposure further increased ROS levels (Fig. 5D, inset). In controls, the addition of FapyG, guanine, 8-oxodG, 2-deoxyguanosine (2-dG), 8-hydroxyadenine, oxazolone or adenine did not alter DCF fluorescence (Fig. 5E, MRC-5 cells).
Figure 4.
Exposure of cells to 8-oxoG* increases cellular ROS levels. (A) MRC-5 cells were exposed to 8-oxoG* (◆), 8-oxoG (■) or H2O2 (□) and cellular ROS levels determined at times indicated. (B) Dose- and (C) cell type-dependent changes in ROS level upon 8-oxoG* exposure. (D) 8-OxoG*, but not 8-oxoG, causes changes in cellular ROS levels as shown by microscopic imaging (NIKON Eclipse Ti system). Magnification: 60x. In panels A, B, and C, cells were H2DCF-DA-loaded, and DCF fluorescence was determined at 485/528 nm. Results are means ±SEM (n=3–11).
Figure 5.
Added to cells or generated intracellularly 8-oxoG* increased cellular ROS. (A) 8- oxoG* level decreased in supernatant when added to cells as shown by AR assays. (B) Changes in ROS levels by 8-oxoG* compared to CHP, tBHP, H2O2. The indicated concentrations of peroxides were determined in preliminary studies. (C) NAC pretreatment decreased 8-oxoG*-mediated changes in ROS levels. H2O2 was used as a control. Inset: GSH depletion by DEM increased 8-oxoG*-induced cellular ROS levels. (D) Catalase and GPx(+GSH) pre-treatment of 8-oxoG* prevented an increase in ROS levels. (E) Only 8-oxoG*, but not other nucleotides or nucleosides, changed cellular ROS levels. (F) Endogenously generated 8-oxoG increased DCF fluorescence in KG-1 cells. In B,C,D,E and F, cells were H2DCF-loaded, and DCF fluorescence was assessed at 485/528 nm. NAC, N-acetyl-L-cysteine; DEM, maleic acid diethyl ester; CHP, tBHP, 2-dG, 2-OHA, and FapyG as in legend to Fig 1. GPx, GSH, SOD as in legend to Fig. 2. Results are means ±SEM (n=5–7).
In another study, we used KG-1 cells which express a temperature-sensitive, functionally inactive OGG1 variant (OGG1R229Q) [32]. Moreover, OGG1R229Q regains its 8-oxoG excision activity at 25°C and it is comparable to wild-type OGG1 [32]. Thus we utilized these cells to test whether endogenously generated 8-oxoG forms 8-oxoG*, which oxidizes H2DCF to DCF. Parallel KG-1 cultures were loaded with H2DCF-DA at 37°C and incubated at 25°C for 30 min. We observed an increase in DCF fluorescence of KG1 cells at 25°C, compared to those remaining at 37°C (Fig. 5F). In controls, U937 cells (Fig. 5F) that express wild-type OGG1 showed no change in DCF fluorescence at 25°C. These data imply that when released from DNA by OGG1, the free 8-oxoG base may be converted into 8-oxoG*.
Discussion
Among DNA and RNA bases, guanine is the most susceptible to modification by reactive oxygen and nitrogen species, due to its lowest redox potential [5, 8, 33, 34]. One of its oxidation products is 8-oxoG. Further oxidation of 8-oxoG is well established, and its oxidized derivatives are well-characterized in the context of DNA and nucleosides (rev in [6, 11, 35]. It has also been shown that purine ribonucleotides are scavengers of radicals including •OH [36], and that free 8-oxodeoxyguanosine provides the strongest protection against oxidation of DNA damage [5, 21, 36, 37]. By utilizing biochemical and biological assays, we have documented that the free 8-oxoG base is also a target of oxidation reactions and is transformed to a hydroperoxide (8-oxoG*) form.
In order to be solubilized, free 8-oxoG base was de-protonated in NaOH. When kept in solution, at alkaline or physiological pH (pH: 7.4) it accumulated a derivative showing characteristics of hydroperoxides. These changes were independent from de-protonation by NaOH, as the 8-oxoG base directly dissolved in PBS showed identical characteristics. Formation of 8-oxoG* was prevented by the addition of dithiothreitol, 2-β-mercaptoethanol, FeCl2, in the lack of molecular oxygen (storage under N2 atmosphere) or when it was dissolved in DMSO. The existence of hydroperoxide in 8-oxoG solution was shown by oxidation of AR to resorufin, H2DCF to DCF and GSH to GSSG and Fe2+ to Fe3+. By utilizing the AR assay, the hydroperoxide generated in 8-oxoG solution was a stronger oxidant than organic peroxides, e.g., CHP or tBHP. When added to H2DCF-loaded cells, 8-oxoG* rapidly increased DCF fluorescence. In contrast, 8-oxodG, 8-hydroxyadenine, guanine or other bases (dissolved and stored as 8-oxoG) showed no hydroperoxide-like reactivity. These results strongly support a unique property of the free 8-oxoG base.
The redox potential of the free 8-oxoG base is lower (-0.48 V vs. NHE) than 8-oxodG [20]. Thus, 8-oxoG should be more susceptible to further oxidation and far more reactive than 8-oxodG. Therefore we speculate that the oxidation of the free 8-oxoG base takes place even under mild oxidative conditions such as those in the presence of molecular oxygen (O2). This process could be similar to that reported for guanosine in the presence of 1O2 (rev in [35, 38]). In this scenario, the free 8-oxoG base may undergo a cycloaddition reaction, forming an endoperoxide, which then could rearrange to a reactive hydroperoxy form. We were not able to show a hydroperoxide when 8-oxodezoxyguanosine was dissolved and stored under similar conditions. As an analogy, further oxidation of 8-oxodeoxyguanosine via singlet oxygen (1O2) resulted in an endoperoxide, which rearranges into a 5-hydroperoxy form, at neutral pH [35]. Also, the deoxyguanosine forms an 8-hydroperoxy derivative, an extremely reactive molecule [20, 39] via electron transfer from 1O2 [40, 41].
It is known that activation of dissolved oxygen to the singlet state may occur without sensitizer(s) [42], which may serve as another possible explanation for hydroperoxide formation in 8-oxoG solutions. To our knowledge, there was no known photosensitizer present that was required for 1O2 generation in the solution. We are not aware of pH- and/or heat-initiated 1O2 or other ROS formation, as solvents stored under identical conditions did not show changes in their redox properties and were inactive in AR and DCF assays. The only difference between solvents and 8-oxoG solution is the 8-oxoG base itself. It is extreme to propose that 8-oxoG acts as a photosensitizer-like molecule (e.g., upon light exposure) and initiates formation of 1O2 in an oxygenated environment. If it were correct, that would be a new property of the free 8-oxoG base.
8-OxoG solution has accumulated a ~12 μM H2O2 equivalent 8-oxoG*. This level did not increase significantly after seven days of storage, which implies an equilibrium between 8-oxoG and 8-oxoG* molecules. This observation is supported by LC/MS analysis, as during equilibrium there was a 10-15% decrease in 8-oxoG concentration when compared to a freshly made one. This decrease is in line with an increase in hydroperoxide content in 8-oxoG solution. Ferrous iron added to the solution decreased the level of 8-oxoG* to one which was undetectable (AR assays) and, interestingly, re-established the original 8-oxoG concentration (as shown by LC/MS). Ferrous iron and organic hydroperoxide reactions should follow Fenton’s chemistry, and, indeed, we show generation of ferric iron (PeroXOquant assay). The accurate chemistry surrounding this important observation is beyond the scope of this publication and will be part of future studies.
Despite a significant amount of free 8-oxoG base that converted into 8-oxoG*, we were not able to purify and chemically characterize it. During purification steps, we lost active fractions, most likely a consequence of its high reactivity and/or instability. A highly reactive 8-hydroperoxy derivative of guanosine as strong as peracid was previously identified [20]. 2'-Deoxyguanosine oxidation by 2,2'-azobis(2-amidino-propane) dihydrochloride resulted in a relatively stable product and was characterized [43]. On the other hand, a hydroperoxide form of 8-oxoguanosine was identified only by NMR but was not purified [1, 38].
The addition of 8-oxoG* to cells increased the intracellular DCF fluorescence (a well-established measure of cellular ROS levels) in a time- and dose-dependent manner. The impact on cells was prevented by antioxidants, which leads us to suggest that 8-oxoG* indeed behaved like such other oxidants as H2O2 or organic hydroperoxides. To prove whether these effects were specific, we showed that freshly made 8-oxoG, 8-hydroxydeoxy-guanosine, 2,6-diamino-4-hydroxy-5-formamidopyrimidine, 2’-deoxyguanosine, guanine, adenine, guanosine and 8-hydroxyadenine did not change intracellular DCF fluorescence. Primary human and mouse diploid cells were more sensitive to 8-oxoG* compared to established cell lines (A549, U937). This observation may be explained by the lower antioxidant capacity of primary cells [44]. It is documented that A549 cells contain a higher level of GSH, due to their enhanced γ-glutamylcysteine synthethase expression [45], and U937 cells possess an increased expression of antioxidant enzymes due to an increase in NOX activity [46]. KG-1 cells accumulate excessive amounts of genomic 8-oxoG and behave as OGG1 knockout cells at 37°C due to the expression of a homozygous temperature-sensitive (at 37°C) polymorphic variant of OGG1 (R229Q). At lower temperatures, e.g., at 25°C, R229Q-OGG1 activity is similar to that of wild-type OGG1 [32]. Thus, KG-1 cells allowed us to show that, parallel with the release of a free 8-oxoG base, there was an increase in DCF fluorescence. Similar results were observed after transgenic OGG1 overexpression in Ogg1-/- cells in independent studies [22]. The increased fluorescence rather signifies a rapid reaction between 8-oxoG* and H2DCF than a change in overall cellular redox state. We speculate that in oxidative stress conditions after its liberation by OGG1, the 8-oxoG base rapidly further oxidizes providing protection to DNA. It is also possible that the hydroperoxide form of 8-oxoG serves as a compartment-specific signaling molecule in a manner similar to that of H2O2 (rev in [47]).
8-OxoG is excised by OGG1 during BER processes, and the free 8-oxoG base in the intercellular space is considered as one of the biomarkers of oxidative stress. In the context of DNA, 8-oxoG has been shown to be a primary target of oxidation reactions and has been proposed to play a protective antioxidant role in the genome [37]. Here we show for the first time that the free 8-oxoG base is far more sensitive to oxidation reactions than other intact or oxidized purine nucleotides and nucleosides. According to its biochemical properties, the 8-oxoG* is a hydroperoxide, of which formation requires only molecular oxygen and takes place at physiological pH. When added to cells or generated intracellularly, 8-oxoG* oxidized H2DCF. Accordingly, these results lead us to suggest that 8-oxoG base stands uniquely among the intact and oxidatively modified purines and it may have an impact on cellular environment beyond its being a biomarker of oxidative stress.
Highlights.
Free 8-oxoG base can be transformed to a highly reactive hydroperoxide (8-oxoG*).
8-oxoG* oxidizes Amplex Red, H2DCF, Fe2+, and GSH in vitro.
8-oxoG* act as an organic hydroperoxide and substrate of peroxidases
Increases cellular oxidative stress levels.
8-oxoG* may serve as a compartment-specific signaling molecule.
Acknowledgments
We are grateful to Sankar Mitra for his scientific advice, intellectual input, discussions, and reading/editing of the manuscript. We thank Mardelle Susman, technical editor, for editing our manuscript. We thank Dr. Miral Dizdaroglu (Chemical Science and Technology Laboratory, National Institute of Standards and Technology, Gaithersburg, MD USA) for assessment of 8-oxoG base levels. This work was supported by grants NIEHS RO1 ES018948 (I.B), NIAID/AI062885-01 (I.B), NIA/AG 021830 (I.B), and the, TAMOP 4.2.1/B-09/1/KONV-2010-0007 by European Union and the European Social Fund (A.B).
Abbreviations
- 1O2
singlet oxygen
- 8-oxoG
8-Oxo-7,8-dihydroguanine
- 8-oxoG*
hydroperoxy derivative of 8-oxoG
- OGG1
8-oxoguanine DNA glycosylases
- Gua
guanine
- AR
Amplex Red (10-acetyl-3,7-dihydroxyphenoxazine)
- DCF
dichlorofluorescein
- CHP
cumene hydroperoxide
- CHP
cumene hydroperoxide
- FapyG
2,6-diamino-4-hydroxy-5-formamidopyrimidine
- GPx
glutathione peroxidase
- HRP
horseradish peroxidase
- H2DCF-DA
5-(and-6)-chloromethyl-2'7'-dichlorodihydrofluorescein diacetate acetyl ester
- F.U
fluorescence units
- NAC
N-acetyl-L-cysteine
- •OH
hydroxyl radical
- tBHP
tert butyl hydroperoxide
- ROS
reactive oxygen species
- SOD
superoxide dismutase
Footnotes
COMPETING FINANCIAL INTEREST
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Von Sonntag C. The chemical basis of radiation biology. Taylor and Francis; New York: 1987. [Google Scholar]
- 2.Dizdaroglu M, Jaruga P, Birincioglu M, Rodriguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radic Biol Med. 2002;32:1102–1115. doi: 10.1016/s0891-5849(02)00826-2. [DOI] [PubMed] [Google Scholar]
- 3.Dizdaroglu M, Kirkali G, Jaruga P. Formamidopyrimidines in DNA: mechanisms of formation, repair, and biological effects. Free Radic Biol Med. 2008;45:1610–1621. doi: 10.1016/j.freeradbiomed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Candeias LP, Steenken S. Reaction of HO* with guanine derivatives in aqueous solution: formation of two different redox-active OH-adduct radicals and their unimolecular transformation reactions. Properties of G(-H)*. Chemistry. 2000;6:475–484. doi: 10.1002/(sici)1521-3765(20000204)6:3<475::aid-chem475>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 5.Steenken S, Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 6.Cadet J, Douki T, Ravanat JL. One-electron oxidation of DNA and inflammation processes. Nat Chem Biol. 2006;2:348–349. doi: 10.1038/nchembio0706-348. [DOI] [PubMed] [Google Scholar]
- 7.Dizdaroglu M. Formation of an 8-hydroxyguanine moiety in deoxyribonucleic acid on gamma-irradiation in aqueous solution. Biochemistry. 1985;24:4476–4481. doi: 10.1021/bi00337a032. [DOI] [PubMed] [Google Scholar]
- 8.Steenken S. Purine bases, nucleosides, and nucleotides: aqueous solution redox chemistry and transformation reactions of their radical cations and e- and OH adducts. Chem Rev. 1989;89:503–520. [Google Scholar]
- 9.Jaruga P, Kirkali G, Dizdaroglu M. Measurement of formamidopyrimidines in DNA. Free Radic Biol Med. 2008;45:1601–1609. doi: 10.1016/j.freeradbiomed.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl T, Barnes DE. Repair of endogenous DNA damage. Cold Spring Harb Symp Quant Biol. 2000;65:127–133. doi: 10.1101/sqb.2000.65.127. [DOI] [PubMed] [Google Scholar]
- 11.Dizdaroglu M, Jaruga P, Rodriguez H. Measurement of 8-hydroxy-2'-deoxyguanosine in DNA by high-performance liquid chromatography-mass spectrometry: comparison with measurement by gas chromatography-mass spectrometry. Nucleic Acids Res. 2001;29:E12. doi: 10.1093/nar/29.3.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markus TZ, Daube SS, Naaman R, Fleming AM, Muller JG, Burrows CJ. Electronic structure of DNA--unique properties of 8-oxoguanosine. J Am Chem Soc. 2009;131:89–95. doi: 10.1021/ja804177j. [DOI] [PubMed] [Google Scholar]
- 13.Mitra S, Izumi T, Boldogh I, Bhakat KK, Hill JW, Hazra TK. Choreography of oxidative damage repair in mammalian genomes. Free Radic Biol Med. 2002;33:15–28. doi: 10.1016/s0891-5849(02)00819-5. [DOI] [PubMed] [Google Scholar]
- 14.Dizdaroglu M. Base-excision repair of oxidative DNA damage by DNA glycosylases. Mutat Res. 2005;591:45–59. doi: 10.1016/j.mrfmmm.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura S. Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic Biol Med. 2002;32:813–821. doi: 10.1016/s0891-5849(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 16.Saxowsky TT, Meadows KL, Klungland A, Doetsch PW. 8-Oxoguanine-mediated transcriptional mutagenesis causes Ras activation in mammalian cells. Proc Natl Acad Sci U S A. 2008;105:18877–18882. doi: 10.1073/pnas.0806464105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN. Oxidative damage to DNA during aging: 8-hydroxy-2'-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci U S A. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svoboda P, Maekawa M, Kawai K, Tominaga T, Savela K, Kasai H. Urinary 8-hydroxyguanine may be a better marker of oxidative stress than 8-hydroxydeoxyguanosine in relation to the life spans of various species. Antioxid Redox Signal. 2006;8:985–992. doi: 10.1089/ars.2006.8.985. [DOI] [PubMed] [Google Scholar]
- 19.Kim KH, Rodriguez AM, Carrico PM, Melendez JA. Potential mechanisms for the inhibition of tumor cell growth by manganese superoxide dismutase. Antioxid Redox Signal. 2001;3:361–373. doi: 10.1089/15230860152409013. [DOI] [PubMed] [Google Scholar]
- 20.Sheu CF, Christopher S. Reactivity toward Singlet Oxygen of a 7,8-Dihydro-8-oxoguanosine (“8-Hydroxyguanosine”) Formed by Photooxidation of a Guanosine Derivative. Journal of the American Chemical Society. 1995;117:6439–6442. [Google Scholar]
- 21.Kim JE, Choi S, Yoo JA, Chung MH. 8-Oxoguanine induces intramolecular DNA damage but free 8-oxoguanine protects intermolecular DNA from oxidative stress. FEBS Lett. 2004;556:104–110. doi: 10.1016/s0014-5793(03)01385-1. [DOI] [PubMed] [Google Scholar]
- 22.Bacsi A, Chodaczek G, Hazra TK, Konkel D, Boldogh I. Increased ROS generation in subsets of OGG1 knockout fibroblast cells. Mech Ageing Dev. 2007;128:637–649. doi: 10.1016/j.mad.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das A, Hazra TK, Boldogh I, Mitra S, Bhakat KK. Induction of the human oxidized base-specific DNA glycosylase NEIL1 by reactive oxygen species. J Biol Chem. 2005;280:35272–35280. doi: 10.1074/jbc.M505526200. [DOI] [PubMed] [Google Scholar]
- 24.Boldogh I, Bacsi A, Choudhury BK, Dharajiya N, Alam R, Hazra TK, Mitra S, Goldblum RM, Sur S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J Clin Invest. 2005;115:2169–2179. doi: 10.1172/JCI24422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 26.Bacsi A, Woodberry M, Widger W, Papaconstantinou J, Mitra S, Peterson JW, Boldogh I. Localization of superoxide anion production to mitochondrial electron transport chain in 3-NPA-treated cells. Mitochondrion. 2006;6:235–244. doi: 10.1016/j.mito.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das GC, Bacsi A, Shrivastav M, Hazra TK, Boldogh I. Enhanced gamma-glutamylcysteine synthetase activity decreases drug-induced oxidative stress levels and cytotoxicity. Mol Carcinog. 2006;45:635–647. doi: 10.1002/mc.20184. [DOI] [PubMed] [Google Scholar]
- 28.Wendel A, Cikryt P. The level and half-life of glutathione in human plasma. FEBS Lett. 1980;120:209–211. doi: 10.1016/0014-5793(80)80299-7. [DOI] [PubMed] [Google Scholar]
- 29.Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem. 1994;220:403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 30.Asayama K, Janco RL, Burr IM. Selective induction of manganous superoxide dismutase in human monocytes. Am J Physiol. 1985;249:C393–397. doi: 10.1152/ajpcell.1985.249.5.C393. [DOI] [PubMed] [Google Scholar]
- 31.Giblin FJ, McCready JP, Reddy VN. The role of glutathione metabolism in the detoxification of H2O2 in rabbit lens. Invest Ophthalmol Vis Sci. 1982;22:330–335. [PubMed] [Google Scholar]
- 32.Hill JW, Evans MK. A novel R229Q OGG1 polymorphism results in a thermolabile enzyme that sensitizes KG-1 leukemia cells to DNA damaging agents. Cancer Detect Prev. 2007;31:237–243. doi: 10.1016/j.cdp.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagenknecht HA. Electron transfer processes in DNA: mechanisms, biological relevance and applications in DNA analytics. Nat Prod Rep. 2006;23:973–1006. doi: 10.1039/b504754b. [DOI] [PubMed] [Google Scholar]
- 34.Margolin Y, Cloutier JF, Shafirovich V, Geacintov NE, Dedon PC. Paradoxical hotspots for guanine oxidation by a chemical mediator of inflammation. Nat Chem Biol. 2006;2:365–366. doi: 10.1038/nchembio796. [DOI] [PubMed] [Google Scholar]
- 35.Pratviel G, Meunier B. Guanine oxidation: one- and two-electron reactions. Chemistry. 2006;12:6018–6030. doi: 10.1002/chem.200600539. [DOI] [PubMed] [Google Scholar]
- 36.Gudkov SV, Shtarkman IN, Smirnova VS, Chernikov AV, Bruskov VI. Guanosine and inosine display antioxidant activity, protect DNA in vitro from oxidative damage induced by reactive oxygen species, and serve as radioprotectors in mice. Radiat Res. 2006;165:538–545. doi: 10.1667/RR3552.1. [DOI] [PubMed] [Google Scholar]
- 37.Radak Z, Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravanat JL, Martinez GR, Medeiros MH, Di Mascio P, Cadet J. Mechanistic aspects of the oxidation of DNA constituents mediated by singlet molecular oxygen. Arch Biochem Biophys. 2004;423:23–30. doi: 10.1016/j.abb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 39.McCallum JE, Kuniyoshi CY, Foote CS. Characterization of 5-hydroxy-8-oxo-7,8-dihydroguanosine in the photosensitized oxidation of 8-oxo-7,8-dihydroguanosine and its rearrangement to spiroiminodihydantoin. J Am Chem Soc. 2004;126:16777–16782. doi: 10.1021/ja030678p. [DOI] [PubMed] [Google Scholar]
- 40.Kasai H, Yamaizumi Z, Yamamoto F, Bessho T, Nishimura S, Berger M, Cadet J. Photosensitized formation of 8-hydroxyguanine (7,8-dihydro-8-oxoguanine) in DNA by riboflavin. Nucleic Acids Symp Ser. 1992:181–182. [PubMed] [Google Scholar]
- 41.Hickerson RP, Prat F, Muller JG, Foote CS, Burrows CJ. Sequence stacking dependence of 8-oxoguanine oxidation: Comparison of one-electron vs singlet oxygen mechanisms. Am Chem Soc. 1999;121:9423–9428. [Google Scholar]
- 42.Bruskov VI, Malakhova LV, Masalimov ZK, Chernikov AV. Heat-induced formation of reactive oxygen species and 8-oxoguanine, a biomarker of damage to DNA. Nucleic Acids Res. 2002;30:1354–1363. doi: 10.1093/nar/30.6.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakakibara H, Ashida H, Kanazawa K. A novel method using 8-hydroperoxy-2'-deoxyguanosine formation for evaluating antioxidative potency. Free Radic Res. 2002;36:307–316. doi: 10.1080/10715760290019336. [DOI] [PubMed] [Google Scholar]
- 44.Lorenz M, Saretzki G, Sitte N, Metzkow S, von Zglinicki T. BJ fibroblasts display high antioxidant capacity and slow telomere shortening independent of hTERT transfection. Free Radic Biol Med. 2001;31:824–831. doi: 10.1016/s0891-5849(01)00664-5. [DOI] [PubMed] [Google Scholar]
- 45.Hatcher EL, Chen Y, Kang YJ. Cadmium resistance in A549 cells correlates with elevated glutathione content but not antioxidant enzymatic activities. Free Radic Biol Med. 1995;19:805–812. doi: 10.1016/0891-5849(95)00099-j. [DOI] [PubMed] [Google Scholar]
- 46.Lledias F, Hansberg W. Oxidation of human catalase by singlet oxygen in myeloid leukemia cells. Photochem Photobiol. 1999;70:887–892. [PubMed] [Google Scholar]
- 47.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]