Abstract
Two new xanthone-anthraquinone heterodimers, acremoxanthone C (5) and acremoxanthone D (2), have been isolated from an extract of an unidentified fungus of the Order Hypocreales (MSX 17022) by bioactivity-directed fractionation as part of a search for anticancer leads from filamentous fungi. Two known related compounds, acremonidin A (4) and acremonidin C (3) were also isolated, as was a known benzophenone, moniliphenone (1). The structures of these isolates were determined via extensive use of spectroscopic and spectrometric tools in conjunction with comparisons to the literature. All compounds (1–5) were evaluated against a suite of biological assays, including those for cytotoxicity, inhibition of the 20S proteasome, mitochondria transmembrane potential, and NF-κB.
Keywords: acremonidin, anthraquinone, cytotoxicity, fungus, moniliphenone, 20S proteasome, xanthone
Introduction
Fungi have been a valuable source for drug leads.1,2 As just one contemporary example that originated in the Journal of Antibiotics, the fungal metabolite, myriocin, initially called ISP-1 by Fujita and colleagues when first isolated from the entomopathogenic fungus Isaria sinclairii,3,4 served as a lead-scaffold for the eventual development of fingolimod (FTY720), which received approval by the U.S. FDA in 2010 for the treatment of multiple sclerosis.5 Despite numerous other drug leads from fungi, such as the often cited penicillin, it has been postulated that fewer than 10% of the approximately 1.5M fungi in the world have been investigated taxonomically.6 As part of a larger program to examine diverse natural product source materials, our team has been exploring solid phase cultures of filamentous fungi from the Mycosynthetix library, which has over 55,000 accessions, for new anticancer drug leads.1 In the course of this research, culture MSX 17022, which was isolated in April 1984 from leaf litter collected from a beech tree community near the Mycosynthetix headquarters in Hillsborough, NC, USA, yielded a cytotoxic extract, as evidenced by 93% inhibition of H460 cell growth when tested at 20 μg/mL. Bioactivity-directed fractionation led to the isolation of a series of xanthone-anthraquinone heterodimers, two of which (compounds 2 and 5) were new. All compounds were examined against a suite of biological targets, including those for cytotoxicity, inhibition of the 20S proteasome, NF-κB inhibition assay, and mitochondrial transmembrane potential activity.
RESULTS AND DISCUSSION
Major Isolates
Identification of Acremonidin A (4)
The numbering of the isolated compounds (1–5) originates from their elution order on RP-HPLC (see Experimental). However, the major compounds isolated in the course of this study were compounds 4 and 5, and thus, they will be discussed first. Compound 4 displayed a molecular formula of C33H26O12 from the HRMS data, indicative of an index of hydrogen deficiency (IHD) of 21. From the diode array detector of the HPLC, the UV maxima were 285 and 359 nm. The Dictionary of Natural Products (DNP)7 was utilized for dereplication, using the molecular formula and the UV maxima (with a range of +/− 10 nm from the observed maxima) as search criteria. The NMR data of 4 matched that of the single hit from DNP, which was acremonidin A,8 an antibacterial agent isolated from an Acremonium sp. in 2003.
Structure Elucidation of Acremoxanthone C (5)
The HRMS data of compound 5 yielded a molecular formula of C33H26O12, identical to that of 4. In the NMR spectra of compounds 4 and 5 (both examined in CDCl3), there was a high degree of similarity in those signals attributable to the anthraquinone portion of the molecule (e.g. H-1: δH 5.90 for 4, δH 5.96 for 5; H-3: δH 6.82 for 4, δH 6.88 for 5; H-5: δH 6.75 for 4, δH 6.79 for 5). However, a key difference between the 1H NMR spectra of 4 and 5 was the number of phenolic peaks; 4 had six phenols while 5 only displayed four (Table 1). This could result from the formation of a xanthone in 5 via coupling of two of the phenols from Rings E and F, with a resultant loss of water, as has been reported in the acremoxanthones.9 However, the formula for 5 did not allow for loss of water as compared to 4. The 13C NMR spectrum of 5 showed 33 resonances (Table 1), in agreement with the formula, 19 of which were quaternary, as opposed to 20 quaternary signals with 4. Moreover, the UV spectrum showed maxima at 274 and 369 nm, significantly different from that of 4.
Table 1.
NMR Data for Compounds 2 and 5 (2 in DMSO-d6, 5 in CDCl3, 500 MHz, chemical shifts in δ, coupling constants in Hz).
| Position | Acremoxanthone D (2) | Acremoxanthone C (5) | ||||
|---|---|---|---|---|---|---|
| δC | δH, mult. (J in Hz) | HMBC (H→C) | δC | δH, mult. (J in Hz) | HMBC (H→C) | |
| 2 | 90.6 | --- | --- | 84.9 | --- | --- |
| 3 | 68.4 | 5.15, dd (2.3, 2.3) | C-2,4,5,15 | 74.8 | 5.30, dd (2.8, 2.1) | C-2,4,5,15 |
| 4 | 145.5 | 6.69, dd (10.3, 2.3) | C-2,6 | 143.9 | 6.50, dd (10.3, 2.1) | C-2,6 |
| 5 | 127.9 | 6.02, dd (10.3, 2.3) | C-3 | 123.8 | 6.13, dd (10.3, 2.8) | C-3,7 |
| 6 | 188.2 | --- | --- | 171.2 | --- | --- |
| 7 | 79.1 | --- | --- | 99.2 | --- | --- |
| 8 | 191.5 | --- | --- | 185.3 | --- | --- |
| 9 | 104.6 | --- | --- | 105.6 | --- | --- |
| 10 | 159.7 | --- | --- | 160.0 | --- | --- |
| 11 | 113.5 | 6.21, s | C-9,10,13,15′ | 114.4 | 6.13, s | C-9,10,13,15′ |
| 12 | 148.4 | --- | --- | 147.8 | --- | --- |
| 13 | 114.4 | --- | --- | 115.1 | --- | --- |
| 14 | 154.1 | --- | --- | 154.3 | --- | --- |
| 15 | 168.1 | --- | --- | 168.9 | --- | --- |
| 16 | 52.9 | 3.47, s | C-15 | 53.4 | 3.69, s | C-15 |
| 1′ | 72.2 | 6.02, s | C-2′,3′,7′,9′,13′,14′,15′,17′ | 73.2 | 5.96, s | C-2′,3′,7′,9′,10′,13′,14′,15′,17′ |
| 2′ | 136.6 | --- | --- | 136.5 | --- | --- |
| 3′ | 123.1 | 6.92, s | C-1′,5′,7′,16′ | 123.5 | 6.88, s | C-1′,5′,7′,16′ |
| 4′ | 147.3 | --- | --- | 148.0 | --- | --- |
| 5′ | 118.8 | 6.85, s | C-3′,6′,7′,16′ | 119.6 | 6.79, s | C-3′,6′,7′,16′ |
| 6′ | 160.6 | --- | --- | 161.8 | --- | --- |
| 7′ | 112.1 | --- | --- | 112.7 | --- | --- |
| 8′ | 184.4 | --- | --- | 185.9 | --- | --- |
| 9′ | 105.4 | --- | --- | 105.8 | --- | --- |
| 10′ | 186.2 | --- | --- | 186.1 | --- | --- |
| 11′ | 37.3 | 5.08, d (6.9) | C-12,13,14,9′,10′,13′ | 38.2 | 4.80, d (6.5) | C-12,13,14,9′,10′,13′ |
| 12′ | 131.2 | 6.51, dd (8.1, 6.9) | C-13,10′,11′,13′,14′ | 132.0 | 6.42, dd (8.3, 6.5) | C-10′,11′,14′ |
| 13′ | 133.0 | 6.17, d (8.1) | C-1′,9′,11′,12′,14′ | 132.7 | 6.07, d (8.3) | C-13,1′,9′,11′,14′ |
| 14′ | 40.8 | --- | --- | 41.6 | --- | --- |
| 15′a | 34.1 | 2.63, d (18.4) | C-12,13,1′,9′,13′,14′ | 35.3 | 2.68, d (17.2) | C-12,1′,9′,13′,14′ |
| 15′b | 2.95, d (18.4) | 2.78, d (17.2) | ||||
| 16′ | 21.4 | 2.35, s | C-3′,4′,5′ | 22.3 | 2.37, s | C-3′,4′,5′ |
| 17′ | 169.6 | --- | --- | 170.7 | --- | --- |
| 18′ | 20.7 | 1.96, s | C-17′ | 21.3 | 1.99, s | C-17′ |
| 6-OH | --- | --- | --- | --- | 14.18, s | C-5,6,7 |
| 7-OH | --- | 8.13, s | C-2 | --- | --- | --- |
| 10-OH | --- | 11.21, s | C-9,10,11 | --- | 11.11, s | C-9,10,11,12(weak) |
| 6′-OH | --- | 11.49, s | C-5′,6′,7′ | --- | 11.45, s | C-5′,6′,7′ |
| 8′-OH | --- | 13.92, v br s | not observed | --- | 14.32, br s | not observed |
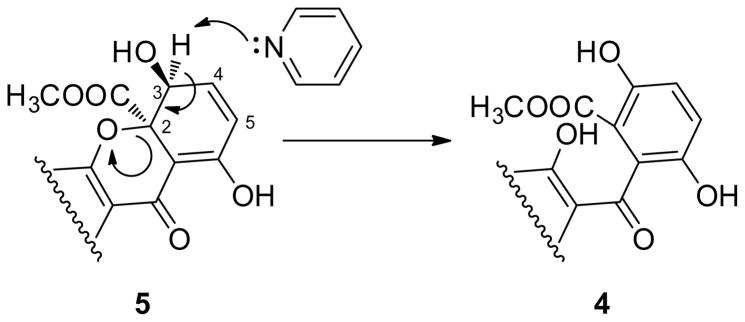
Another ring-closing possibility, which had been reported previously, was via attack of one of the ring E phenols on the aromatic carbon bearing the methyl ester, as in the xanthoquinodins.10 2D-NMR data confirmed this type of ring-closure, and in this case, the linkage was through O-14. One new non-aromatic proton at δH 5.30 (H-3) and two new non-aromatic carbons at δC 74.8 (C-3) and 84.9 (C-2) were present in the NMR spectra of 5 as compared to 4. The signals for δH 5.30 (H-3) and δC 74.8 (C-3) correlated in the multiplicity-edited HSQC experiment, indicative of an aliphatic secondary hydroxyl group; this new H-3 signal correlated to H-4 and H-5 in the COSY spectrum as well. The δH 6.50 signal (H-4) correlated to the new quaternary carbon δC 84.9 (C-2) in the HMBC spectrum, while the H-5 signal (δH 6.13) correlated to the C-3 (δC 74.8) signal. The δH 6.50 resonance (H-4) also correlated to δC 171.2 resonance (C-6) in the HMBC spectrum, which was farther downfield than a typical phenol carbon, consistent with the enhanced keto character of C-6 resulting from tautomerization with the C-8 ketone. These data confirm the proposed E-G-F ring system. The relative stereochemistry of C-2 was confirmed by a 1D nOe Difference spectrum, which showed an enhancement of H-3 upon irradiation of the H3-16 methyl ester signal. The relative stereochemistry of C-1′, C-14′, and C-11′ was established by the ROESY spectrum. The acetate methyl group at C-18′ showed a clear ROESY correlation to H-13′, which established the relative stereochemistry for the anthraquinone portion of the molecule. Compound 5 therefore contained the anthraquinone portion of the acremonidins and acremoxanthones,8,9 and the xanthone portion of the xanthoquinodins.10 This compound was named acremoxanthone C to maintain consistency with the existing acremoxanthones.
Minor Isolates
Identification of Moniliphenone (1)
According to HRMS data, compound 1 had the formula C16H14O6. The 1H NMR showed singlets due to aromatic methyl and methoxy groups. Aromatic region signals at δH 7.12 (doublet), 7.28 (triplet), and 7.47 (doublet), all with a coupling constant of 7.6 Hz, were consistent with a 1,2,3-trisubstituted benzene moiety. Using the AntiMarin11 database for dereplication, the formula was entered into the search query, as was one 1,2,3-trisubstituted benzene and one methoxyl. Only two hits resulted from this query; moniliphenone and nidulalin B12 both of which had 1H-NMR data that were consistent with the spectra of 1. However, the HMBC spectra revealed a key correlation between the δH 7.47 doublet and the δC 166.0 signal (ester carbonyl), establishing moniliphenone as the structure of 1; the 1H- and 13C-NMR data matched the literature.13
Structure Elucidation of Acremoxanthone D (2)
The HRMS data of compound 2 indicated a formula of C33H26O13, establishing an IHD of 21. The NMR data for compounds 2 and 5 were quite similar. The formula indicates the addition of one oxygen, similar to the difference of one oxygen between acremonidin A (4) and acremonidin C (3). Initially, it was expected that the extra oxygen of 2 would present as a hydroxy group at position C-9′, as was noted with acremonidin C (3).8 However, the HMBC spectrum did not confirm this. The oxygen instead was shown to be added as a hydroxy group at position C-7. Indeed, the C-7 resonance in the 13C-NMR spectrum of 2 was at δC 79.1, as opposed to δC 99.2 with 5. Correspondingly, a new singlet appeared in the 1H NMR spectrum of 2 at δH 8.13, with no HSQC correlation, indicating a hydroxy or phenol proton. This -OH proton correlated in the HMBC to the C-2 signal at δC 90.6, identifying the -OH as hydroxy. The carbon resonance for C-6 was farther downfield at δC 188.2 (vs. 171.2 for 5), indicating more keto character due to the lack of tautomerization with C-8. The relative stereochemistry for 2 was established from the ROESY spectrum. The key observation was a strong correlation between the 7-OH proton and H-3. Of the four possible diastereomeric combinations of C-2 and C-7, only one possibility allows close approach of the 7-OH and H-3 according to Chem3D energy minimization, and that is with the C-2 methyl ester in the β position and the C-7 hydroxy in the α position. Due to the similarities between compounds 2 and 5, compound 2 was ascribed the trivial name, acremoxanthone D.
Identification of Acremonidin C (3)
The HRMS of compound 3 indicated a formula of C33H26O13, identical to the formula for 2, however, the NMR spectra showed significant differences. From the diode array detector of the HPLC, the UV maxima were 275 and 341 nm, also differing significantly from 2 (287 and 362 nm). The Dictionary of Natural Products (DNP)7 was again used for dereplication with molecular formula and UV maxima (with a range of +/− 10 nm from the observed maxima) as search criteria. A single hit was identified in DNP, and the NMR data of 3 matched that of acremonidin C.8
Conversion of Compound 5 to 4
To determine the absolute configuration of compound 5, an attempt was made to synthesize the Mosher esters from the MTP chlorides in pyridine.14 However, when the reaction was attempted, compound 4 was by far the major product, with numerous very minor products. When compound 5 was treated with pyridine only, the result was nearly complete conversion to 4 after 4.5 h at room temperature. This would indicate that H-3 of compound 5 is susceptible to basic attack followed by opening of the G-ring to yield 4 (Figure 2). This may not have been an issue with the xanthoquinodins (which also contain the E-G-F ring system), most likely because those compounds have a single bond between C-4 and C-5. The driving force behind the conversion of 5 to 4 may be the creation of aromaticity, which can’t occur with the xanthoquinodins. Therefore, absolute configurations were not determined for either 2 or 5.
Figure 2.
Proposed conversion of compound 5 to 4 by pyridine at room temperature.
Biological Activity
Compounds 1–5 were assayed against three cancer cell lines and showed moderate cytotoxic activity in vitro, with IC50 values summarized in Table 2. Compounds 2, 3, and 4 showed moderate 20S proteasome inhibitory activity as well (Table 2). All compounds were inactive in assays for both NF-κB inhibition and mitochondrial transmembrane potential (i.e. IC50 values > 20 μM; data not shown).
Table 2.
Cytotoxicity Against a Panel of Human Tumor Cell Lines and Inhibition of 20S Proteasome of Compounds Isolated from MSX 17022 (1–5).
| Compound | IC50 values (in μM)a | % Inhibition of 20S Proteasome | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| MCF-7 | H460 | SF268 | HT-29 | MDA-MB-435 | 20 μg/mL | 5 μg/mL | |
|
| |||||||
| moniliphenone (1) | >25 | >25 | >25 | ntb | nt | 1 | 0 |
| acremoxanthone D (2) | 14.0 | 21.4 | >25 | nt | nt | 58 | 39 |
| acremonidin C (3) | >25 | 20.6 | 21.0 | nt | nt | 67 | 32 |
| acremonidin A (4) | 18.1 | 13.6 | 21.4 | >25 | >25 | 55 | 12 |
| acremoxanthone C (5) | 21.0 | 10.9 | 16.1 | >25 | >25 | 13 | 0 |
|
| |||||||
| camptothecinc | 0.06 | 0.01 | 0.05 | nt | nt | nt | |
|
| |||||||
| silvestrolc | nt | nt | nt | 0.004 | 0.006 | nt | |
|
| |||||||
| bortezomibc | nt | nt | nt | nt | nt | 91% inhibition at 25 nM | |
IC50 values are determined as the concentration required to reduce cellular staining with sulforhodamine B by 50% relative to untreated controls following 72 h of continuous exposure.20
Indicates ‘not tested’.
Positive controls.
CONCLUSION
Using bioactivity-directed fractionation, five compounds were isolated from an extract of MSX 17022, an unidentified fungus of the Order Hypocreales. Two of these compounds (2 and 5) were found to be new xanthone-anthraquinone heterodimers. The biological activity of these five compounds was found to be moderate for cytotoxicity and 20S proteasome inhibitory activity (Table 2). Regardless, the biosynthetic potential of this fungus was quite intriguing, particularly the generation of xanthone-anthraquinone heterodimers. Even a non-optimized culture, first isolated nearly three decades ago, produced structurally diverse compounds on the scale of hundreds of mg per 2.8 L culture.
EXPERIMENTAL SECTION
General
Optical rotations, UV spectra, and IR spectra were obtained on a Rudolph Research Autopol III polarimeter, a Varian Cary 100 Bio UV-vis spectrophotometer, and a Perkin-Elmer Spectrum One with Universal ATR attachment, respectively. NMR experiments were conducted in either DMSO-d6 or CDCl3 with TMS as reference using a JEOL ECA-500 (operating at 500 MHz for 1H, 125 MHz for 13C). HRESIMS were performed on a Waters SYNAPT MS system using a capillary voltage of 2000 V (positive mode) and 1000 V (negative mode); sampling cone voltages were 40 V (positive mode) and 10 V (negative mode). Flash chromatography was conducted using a CombiFlash Rf system using a RediSep Rf Si-gel Gold column (both from Teledyne-Isco; Lincoln, NE, USA). HPLC was carried out on Varian Prostar HPLC systems (Walnut Creek, CA, USA) equipped with Prostar 210 pumps and a Prostar 335 photodiode array detector (PDA), with data collected and analyzed using Galaxie Chromatography Workstation software (version 1.9.3.2). For preparative HPLC, a Gemini-NX (5 μm; 250 × 21.2 mm) column was used with a 15 mL/min flow rate, while for analytical HPLC, a Gemini-NX (5 μm; 150 × 4.6 mm) column was used with a 1 mL/min flow rate (both from Phenomenex; Torrance, CA, USA).
Producing Organism and Fermentation
Mycosynthetix fungal strain 17022 was isolated by Dr. Barry Katz of MYCOsearch in April of 1984 from leaf litter from a beech tree community in Hillsborough, NC, USA. DNA analyses were performed by MIDI Labs, Inc. (Newark, DE), and the D2 variable region of the Large Subunit (LSU) rRNA was sequenced and compared to their database. This analysis suggested that this fungus was of the Order Hypocreales; these data were deposited in Genbank (accession No. JN185925). The culture was stored on a malt extract slant and was transferred periodically. A fresh culture was grown on a similar slant, and a piece was transferred to a medium containing 2% soy peptone, 2% dextrose, and 1% yeast extract (YESD media). Following incubation (7 d) at 22 °C with agitation, the culture was used to inoculate 50 mL of a rice medium, prepared using rice to which was added a vitamin solution and twice the volume of rice with H2O, in a 250 mL Erlenmeyer flask. This was incubated at 22°C until the culture showed good growth (approximately 14 d). The scale up culture was grown in a 2.8 L Fernbach flask containing 150 g rice and 300 mL H2O and was inoculated using a seed culture grown in YESD medium. This was incubated at 22°C for 14 d.
Extraction and Isolation
To the scale-up solid fermentation on rice were added 500 mL of 1:1 MeOH-CHCl3, and the mixture was shaken for 16 h on a reciprocating shaker. The solution was filtered and equal volumes of H2O and CHCl3 were added to the filtrate to bring the total volume to 2 L. The solution was stirred vigorously for 1 h, partitioned in a separatory funnel, and the bottom, organic layer was concentrated by rotary evaporation to dryness. This extract was stirred vigorously for 1 h in a mixture of 150 mL MeOH, 150 mL CH3CN, and 200 mL hexane and then partitioned in a separatory funnel. The bottom layer was collected and evaporated to dryness (5.93 g). This defatted extract was adsorbed onto a minimal amount of Celite 545 and dried with mixing via a mortar and pestle. This material was fractionated at 40 mL/min on a RediSep Rf Gold silica gel column (40 g), first with 100% hexanes for 0.7 column volumes (CV) followed by a gradient of 100% hexanes to 100% CHCl3 over 8.9 CV. The elution continued with 100% CHCl3 for 7.4 CV, then with a gradient of MeOH in CHCl3 (0–2% over 9.7 CV, then 2–5% over 5.2 CV, then 5–10% over 5.2 CV, then 10–20% over 3.7 CV, then 20–100% over 2.2 CV. MeOH (100%) was finally held for a further 6.7 CV). Fractions were collected every 24.75 mL. Compounds 1–4 were present in fractions 56–67, which were combined and evaporated (688.9 mg). Compound 5 was present in fractions 22–39 (370.9 mg). The combined fraction 56–67 was then subjected to preparative HPLC in three injections, 40–100% CH3CN in H2O over 30 min. Fractions were collected every 0.5 min. Elution times and amounts isolated were: 1; 15–15.5 min (16.8 mg), 2; 19.5–20 min (8.4 mg), 3; 21–21.5 min (16.1 mg), 4; 21.5–23.5 min (337.7 mg). The combined fraction 22–39 was purified by preparative HPLC using the identical conditions as above (in two injections), and compound 5 was collected between 26–28 min (327.5 mg).
Acremoxanthone C (5)
Compound 5 was isolated as a yellow solid (327.5 mg); [α]D23 +576 (c 0.33, MeOH); UV (MeOH) λmax (log ε), 273 (4.03), 371 (4.43) nm; IR (diamond) νmax 2956, 1736, 1634, 1566, 1469, 1368, 1219, 1097, 871, 711 cm−1; 1H NMR (CDCl3, 500 MHz), and 13C NMR (CDCl3, 125 MHz), see Table 1. HRMS m/z 637.1331 [M + Na]+; 613.1340 [M - H] − (calcd for C33H26O12Na, 637.1322; calcd for C33H25O12, 613.1346).
Acremoxanthone D (2)
Compound 2 was isolated as a yellow solid (8.4 mg); [α]D23 +235 (c 0.33, MeOH); UV (MeOH) λmax (log ε), 287 (4.07), 362 (4.17) nm; IR (diamond) νmax 3055, 1731, 1702, 1609, 1566, 1355, 1278, 1219, 1020, 818 cm−1; 1H NMR (DMSO-d6, 700 MHz), and 13C NMR (DMSO-d6, 175 MHz), see Table 1. HRMS m/z 653.1277 [M + Na]+; 629.1294 [M - H] − (calcd for C33H26O13Na, 653.1271; calcd for C33H25O13, 629.1295).
Conversion of 5 to 4
Compound 5 was stirred in pyridine at room temperature. The progress of the conversion of 5 to 4 was monitored by analytical RP-HPLC at 1 h, 2 h, 3 h, and 4.5 h.
Cytotoxicity Assay
The cytotoxicity measurements against the MCF-715 human breast carcinoma (Barbara A. Karmanos Cancer Center), NCI-H46016 human large cell lung carcinoma (HTB-177, American Type Culture Collection (ATCC), SF-26817 human astrocytoma (NCI Developmental Therapeutics Program), HT-2918 human colorectal adenocarcinoma (HTB-38, ATCC) and the MDA-MB-43519 human melanoma (HTB-129, ATCC) cell lines were performed exactly as described in detail previously.20
20S Proteasome Assay
Human mammary adenocarcinoma cells15 (MCF-7; American Type Culture Collection, Manassas, VA) were propagated at 37°C in 5% CO2 in RPMI 1640 medium supplemented with fetal bovine serum (10%) with penicillin (100 units/mL) and streptomycin (100 μg/mL). Cells in log phase growth were harvested by trypsinization followed by extensive washing to remove all traces of enzyme. A total of 7,500 cells were seeded per well of a 96-well microtiter plate and incubated overnight (37°C in 5% CO2). Samples dissolved in DMSO were then added to achieve the final concentrations as indicated (total volume: 100 μL; DMSO: 0.2%). The cells were incubated in the presence of test substance for 2 h at 37°C and evaluated for proteasome activity using a commercial luminescent assay (Proteasome-Glo™, Promega Corp, Madison, WI) that measures the chymotrypsin-like, trypsin-like or caspaselike protease activity associated with the proteasome complex. Activity was expressed as percent inhibition relative to negative (solvent) control. The positive control was bortezomib tested at 5 nM and 25 nM, which inhibited proteasome activity by 27% and 91% respectively.
NF-κB Assay
An ELISA based NF-κB inhibitory assay was performed exactly as described previously.20 Rocaglamide (Enzo Life Sciences International, Inc.) was used as a positive control (IC50 value of 0.075 μM).
Mitochondrial Transmembrane Potential (ΔΨ) Assay
The mitochondrial transmembrane potential assay kit (Cayman Chemical Company, Ann Arbor, MI) was adapted to detect the ΔΨ using a procedure published previously.21 ΔΨ is used to represent mitochondrial membrane transition events. The specific details were outlined recently,20 and staurosporine (Cayman) was used as a positive control (IC50 value of 2.5 nM).
Supplementary Material
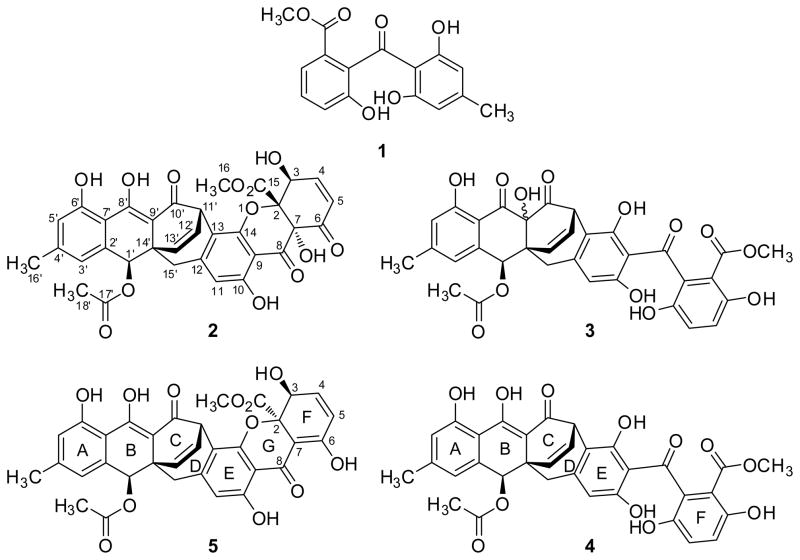
Figure 1.
Structure of Compounds (1–5) isolated from fungus MSX 17022.
Acknowledgments
This research was supported by P01 CA125066 from the National Cancer Institute/National Institutes of Health, Bethesda, MD, USA. The Golden LEAF Foundation (Rocky Mount, NC) provided partial support to D. J. K. Mycology technical support was provided by Maurica Lawrence. The authors thank Mingming Su of the David H. Murdock Research Institute, Kannapolis, NC, for high-resolution mass spectrometry data.
Footnotes
Supporting Information Available
1H and 13C NMR spectra for compounds 2 and 5. This information is available via the internet.
References and Notes
- 1.Orjala J, Oberlies NH, Pearce CJ, Swanson SM, Kinghorn AD. Discovery of potential anticancer agents from aquatic cyanobacteria, filamentous fungi, and tropical plants. In: Tringali C, editor. Bioactive Compounds from Natural Sources. Natural Products as Lead Compounds in Drug Discovery. 2. Taylor & Francis; London, UK: 2011. pp. 37–63. [Google Scholar]
- 2.Pearce C, Eckard P, Gruen-Wollny I, Hanske FG. Microorganisms: Their role in the discovery and development of medicines. In: Buss AD, Butler MS, editors. Natural Product Chemistry for Drug Discovery. The Royal Society of Chemistry; Cambridge, UK: 2010. pp. 215–244. [Google Scholar]
- 3.Fujita T, et al. Fungal metabolites. Part 11 A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot. 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 4.Fujita T, et al. Fungal metabolites. Part 12 Potent immunosuppressant, 14-deoxomyriocin, (2S,3R,4R)-(E)-2-amino-3,4-dihydroxy-2-hydroxymethyleicos-6-enoic acid and structure-activity relationships of myriocin derivatives. J Antibiot. 1994;47:216–224. doi: 10.7164/antibiotics.47.216. [DOI] [PubMed] [Google Scholar]
- 5.Strader CR, Pearce CJ, Oberlies NH. Fingolimod (FTY720): A recently approved multiple sclerosis drug based on a fungal secondary metabolite. J Nat Prod. 2011;74:900–907. doi: 10.1021/np2000528. [DOI] [PubMed] [Google Scholar]
- 6.Hawksworth DL, Rossman AY. Where are all the undescribed fungi. Phytopathology. 1997;87:888–891. doi: 10.1094/PHYTO.1997.87.9.888. [DOI] [PubMed] [Google Scholar]
- 7.Dictionary of Natural Products. Chemical Database. Chapman & Hall/CRC; from www.chemnetbase.com. [Google Scholar]
- 8.He H, Bigelis R, Solum EH, Greenstein M, Carter GT. Acremonidins, new polyketide-derived antibiotics produced by Acremonium sp., LL-Cyan 416. J Antibiot. 2003;56:923–930. doi: 10.7164/antibiotics.56.923. [DOI] [PubMed] [Google Scholar]
- 9.Isaka M, Palasarn S, Auncharoen P, Kornmijit S, Jones EBG. Acremoxanthones A and B, novel antibiotic polyketides from the fungus Acremonium sp BCC 31806. Tetrahedron Lett. 2009;50:284–287. [Google Scholar]
- 10.Tabata N, Tomoda H, Matsuzaki K, Omura S. Structure and biosynthesis of xanthoquinodins, anticoccidial antibiotics. J Am Chem Soc. 1993;115:8558–8564. [Google Scholar]
- 11.Blunt JW, Munro MHG, Laatsch H. AntiMarin Database. University of Canterbury; Christchurch, New Zealand: University of Göttingen; Göttingen, Germany: 2010. [Google Scholar]
- 12.Kawahara N, Sekita S, Satake M, Udagawa S, Kawai K. Structures of a new dihydroxanthone derivate, nidulalin A, and a new benzophenone derivative, nidulalin B, from Emericella nidulans. Chem Pharm Bull. 1994;42:1720–1723. [Google Scholar]
- 13.Kachi H, Sassa T. Isolation of monilphenone, a key intermediate in xanthone biosynthesis from Monilinia fructicola. Agric Biol Chem. 1986;50:1669–1671. [Google Scholar]
- 14.Hoye TR, Jeffrey CS, Shao F. Mosher ester analysis for the determination of absolute configuration of stereogenic (chiral) carbinol carbons. Nat Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 15.Soule HD, Vazguez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 16.Carney DN, Gazdar AF, Bunn PA, Jr, Guccion JG. Demonstration of the stem cell nature of clonogenic tumor cells from lung cancer patients. Stem Cells. 1982;1:149–164. [PubMed] [Google Scholar]
- 17.Rosenblum ML, et al. Stem cell studies of human malignant brain tumors. Part 1: Development of the stem cell assay and its potential. J Neurosurg. 1983;58:170–176. doi: 10.3171/jns.1983.58.2.0170. [DOI] [PubMed] [Google Scholar]
- 18.Fogh J, Trempe G. Human Tumor Cells In Vitro. Plenum; New York, USA: 1975. New human tumor cell lines; pp. 115–159. [Google Scholar]
- 19.Rae JM, Creighton CJ, Meck JM, Haddad BR, Johnson MD. MDA-MB-435 cells are derived from M14 melanoma cells--a loss for breast cancer, but a boon for melanoma research. Breast Cancer Res Treat. 2007;104:13–19. doi: 10.1007/s10549-006-9392-8. [DOI] [PubMed] [Google Scholar]
- 20.Ayers S, et al. Resorcylic acid lactones with cytotoxic and NF-κB inhibitory activities and their structure-activity relationships. J Nat Prod. 2011;74:1126–1131. doi: 10.1021/np200062x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng Y, et al. Bioactive 5,6-dihydro-α-pyrone derivatives from Hyptis brevipes. J Nat Prod. 2009;72:1165–1169. doi: 10.1021/np9001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.