Abstract
Lung cancer is one of the most commonly occurring malignancies. It has been reported that mTOR is phosphorylated in lung cancer and its activation was more frequent in tumors with over-expression of PI3K/Akt. Therefore, dual inhibitors of PI3K/Akt and mTOR signaling could be valuable agents for treating lung cancer. In the present study, we show that fisetin, a dietary tetrahydroxyflavone inhibits cell-growth with the concomitant suppression of PI3K/Akt and mTOR signaling in human non-small cell lung cancer (NSCLC) cells. Using autodock 4, we found that fisetin physically interacts with the mTOR complex at two sites. Fisetin treatment was also found to reduce the formation of A549 cell colonies in a dose-dependent manner. Treatment of cells with fisetin caused decrease in the protein expression of PI3K (p85 and p110), inhibition of phosphorylation of Akt, mTOR, p70S6K1, eIF-4E and 4E-BP1. Fisetin-treated cells also exhibited dose-dependent inhibition of the constituents of mTOR signaling complex like Rictor, Raptor, GβL and PRAS40. There was increase in the phosphorylation of AMPKα and decrease in the phosphorylation of TSC2 on treatment of cells with fisetin. We also found that treatment of cells with mTOR inhibitor rapamycin and mTOR-siRNA caused decrease in phosphorylation of mTOR and its target proteins which were further downregulated on treatment with fisetin, suggesting that these effects are mediated in part, through mTOR signaling. Our results show that fisetin suppressed PI3K/Akt and mTOR signaling in NSCLC cells and thus, could be developed as a chemotherapeutic agent against human lung cancer.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide exceeding the mortality rates of colorectal, breast and prostate cancers combined. In 2010, the American Cancer Society has estimated diagnosis of 222,520 new cases and 157,300 deaths due to lung cancer in the U.S.1 Non-small cell lung cancer (NSCLC) including squamous carcinoma, adenocarcinoma and large cellcarcinoma represents approximately 80–87% of all lung cancer cases in the United States and 65–75% of these cases are detected as locally advanced (Stage III) or metastatic disease (Stage IV), and therefore, palliative treatments are often the only therapeutic option. The majority of lung cancer patients have late-stage disease that is not curable by current therapies and is responsible for low survival.2 The treatment of advanced lung cancer is improving but standard treatments such as chemotherapy and radiotherapy have limited usefulness in improving survival of advanced NSCLC patients. Therefore, there is an urgent need to develop mechanism-based effective non-toxic, preferably dietary origin agents which could be successfully administered to NSCLC patients. Recently, significant efforts have focused on characterizing relevant signaling pathways in developing further strategies for patients with tumors that are insensitive to the targeted agents.
The phosphatidylinositol 3-kinase (PI3K) family is involved in various cellular functions including growth, proliferation, migration and survival. The evolutionarily conserved serine/threonine kinase Akt is one of the most commonly activated protein kinases in human cancer. The PI3K/Akt signaling represents a major cell survival pathway. Its activation has long been associated with malignant transformation and apoptotic resistance.3 It has been well documented that mTOR functions downstream of the PI3K/Akt pathway and is phosphorylated in response to stimuli that activate the PI3K/Akt pathway.4 The PI3K/Akt/mammalian target of rapamycin (mTOR) signaling pathway acts as a key integration point between the extrinsic and intrinsic cellular environments and regulates a broad spectrum of cellular processes.5 The mTOR was first identified as the kinase targeted by rapamycin linked to the cellular protein FKBP12 (FK506-binding protein).6 It is a well-preserved, 289-kDa protein serine/threonine kinase with 95% of its amino acid identity conserved from yeast to human and mouse.7 The mTOR is a serine/threonine-specific protein kinase, downstream of the PI3K/Akt pathway and positively regulates phosphorylation of ribosomal p70S6 kinase (S6K1) and eukaryotic initiation factor 4E (eIF4E) binding protein 1 (4E-BP1). Cumulative evidence supports the hypothesis that mTOR acts as a master switch of cellular catabolism and anabolism, thereby determining whether cells, especially tumor cells grow and proliferate.8 Recently, it has emerged as one of the most significant intracellular signaling enzyme regulating cell growth, survival and motility in lung cancer cells.8 Indeed, PI3K, Akt, and mTOR inhibitors have entered preclinical studies and clinical trials for various human cancers.9 The PI3K/Akt/mTOR pathway therefore represents an attractive and promising target for therapeutic intervention.
Fisetin (3,3',4',7-tetrahydroxyflavone), a naturally occurring flavonoid is found in several fruits and vegetables such as strawberry, apple, persimmon, grape, onionand cucumber.10 It possesses antiproliferative11–17, apoptotic15, 17–19, neuroprotective20 and antioxidative21 activities. In this study, we provide information that fisetin at physiologically attainable concentrations exerts dual inhibition of PI3K/Akt and mTOR signaling in human NSCLC cells without affecting Normal Human Bronchial epithelial (NHBE) cells.
Materials and Methods
Materials
Akt, p-Akt (Ser473 and Thr308), PI3K (p85 and p110), p-mTORSer2448, p-4EBP1Ser65, p-eIF4ESer209, p-p70S6K, p-AMPKαThr172, p-PRAS40, TSC2, p-TSC2Thr1462, LKB1, PTEN, Rictor, Raptor and GβL antibodies were obtained from Cell Signaling Technology (Danvers, MA). Anti-mouse and anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Amersham Life Science Inc. (Arlington Height, IL). Rapamycin was purchased from Calbiochem (Darmstadt, Germany). mTOR-siRNA and scrambled-siRNA were purchased from Dharmacon (Lafayette, CO). Fisetin was purchased from Sigma Chemical Co. (St. Louis, MO). BCA Protein assay kit was obtained from Pierce (Rockford, IL). Novex precast Tris-glycine gels were obtained from Invitrogen (Carlsbad, CA). PathScan p-Akt (Ser473) ELISA kit was purchased from Cell Signaling Technology (Danvers, MA).
Cell culture and treatment
The human lung carcinoma A549 and H1792 cells were obtained from American Type Culture Collection (Manassas, VA, USA) and cultured in F12K medium (ATCC, Manassas, VA, USA), supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin (P-S). H1792 cells were grown in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and 1% P-S. NHBE cells were obtained from Clonetics Airway Epithelial Cell Systems (Cambrex Bio Science, Walkersville, Inc, USA) and cultured in Bronchial Epithelial Growth Media supplemented with growth factors (Cambrex Bio Science, Walkersville, Inc, USA). A549 and H1792 cells were tested by ATCC for post-freeze viability, growth properties, morphology, mycoplasma contamination and species determination (COI assay and STR analysis). The cells were maintained under standard cell culture conditions at 37°C and 5% CO2 in a humid environment. Fisetin dissolved in dimethyl sulfoxide (final concentration 0.1% v/v) was used for the treatment of cells. The cells (60–70% confluent) were treated with fisetin (5–20 μM) for 24 and 48 h in complete growth medium.
Cell viability
The effect of fisetin on the viability of cells was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (MTT) assay. NHBE, A549 and H1792 cells were plated at 1 × 104 cellsper well in 200 μl of complete culture medium containing5–20 μM concentrations of fisetin in 96-well microtiter plates for 24 and 48 h. After incubation for specified times at 37°C in a humidified incubator, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide (5 mg/ml in PBS) was added to each well andincubated for 2 h, after which the plate was centrifuged at1,800 × g for 5 min at 4 °C. The supernatant was discarded and the pellet dissolved in 200 μl of DMSO and absorbance at the wavelength of 540 nm was recordedon a microplate reader. The effect of fisetin on growth inhibition was assessed as percent cell viability where DMSO-treated cells were taken as 100% viable. DMSO at the concentrations used was without any effect on cell viability.
Colony Formation Assay
Cells were seeded in top agar containing 0.3% agar with F-12K media and 10% FBS. Bottom agar consisted of 0.5% agar, F-12K media and 10% FBS. Media with DMSO or indicated doses of fisetin (5–20 μM) was added and replaced every 3 days. The cells were treated with fisetin and maintained at 37°C in a humidified 10% CO2 atmosphere. The media with DMSO or indicated doses of fisetin was replaced every 3 days and the number of colonies was counted after three weeks. Clonogenic survival was expressed as a percentage relative to the untreated controls.
Docking study
Blind docking of fisetin to the mTOR target was performed with Autodock4 by setting grid sizes that included the entire mTOR molecule. The receptor site was prepared with Sybyl (Tripos, St. Louis) using the NMR structure 2NPU model 1 from the Protein Data Bank (www.pdb.org).22 The structure consists of 4 stacked alpha helices. The grid size for the docking site was expanded to include the entire mTOR molecule and fisetin was docked. The results placed the ligand in two clustered sites located between the helices and on either side of the 4 helices. The binding energies were in the −7 to −8 Kcal/mol range for the binding constant. The binding in the best site included hydrogen bonding to a glutamate by two hydroxyl groups. The second site is mostly hydrophobic, with the ring of fisetin stacking on rings from the peptide.
Protein extraction and western blotting
Following the treatment of A549 cells with fisetin (5–20 μM; 48 h), the media was aspirated, the cells were washed with cold PBS (pH 7.4), and ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM PMSF (pH 7.4) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Set III, Calbiochem, La Jolla, CA) over ice for 30 min. The cells were scraped and the lysate was collected in a microfuge tube and passed through needle to break up the cell aggregates. The lysate was cleared by centrifugation at 14,000 g for 15 min at 4°C and the supernatant (whole cell lysate) was used or immediately stored at −80 °C.
For western blotting, 30–50 μg protein was resolved over 8–12% polyacrylamide gels and transferred to a nitrocellulose membrane. The blot was blocked in blocking buffer (5% non-fat dry milk/1% Tween 20; in 20 mM TBS, pH 7.6) for 1 h at room temperature, incubated with appropriate monoclonal or polyclonal primary antibody in blocking buffer for one and half h to overnight at 4°C, followed by incubation with anti-mouse or anti-rabbit secondary antibody horseradish peroxidase conjugate obtained from Amersham Life Science Inc. (Arlington Height, IL, USA) and detected by chemiluminescence and autoradiography using XAR-5 film obtained from Eastman Kodak Co. (Rochester, NY, USA). Densitometric measurements of the band in Western blot analysis were performed using digitalized scientific software program UN-SCAN-IT (Silk Scientific Corporation, Orem, UT).
Phospho-Akt ELISA
To quantify the endogenous levels of p-Akt (Ser473) in cells, PathScan p-Akt (Ser473) ELISA assay (Cell Signaling Technology, Danvers, MA) was performed as per manufacturer’s manual. Briefly, p-Akt (Ser473) proteins in cell lysate were captured by the corresponding antibody that was coated in the microplate. After adding the HRP-linked secondary antibody and chemiluminescent substrate, the magnitude light emission, which is proportional to the quantity of p-Akt (Ser473) protein was measured.
Silencing of mTOR by Small Interfering RNA
A549 cells were transfected with mTOR-siRNA (75 nmol) and scrambled siRNA (75 nmol) procured from Dharmacon (Lafayette, CO) using the nucleofection kit from Amaxa Biosystems (Gaithersburg, MD). Cells were resuspended in a solution from nucleofector kit following the manufacturer's guidelines. 100 μl of nucleofector solution was mixed with 2×106 cells and siRNA. They were then transferred to the cuvette provided with the kit and were nucleofected withan Amaxa Nucleofector apparatus. Cells were transfected usingthe T-001 pulsing parameter and were transferred into 100-mm plates containing 37 °C prewarmed culture medium. After transfection, cells were cultured and the medium was replaced with fresh medium. Cells were treated with15 μM fisetin for 24 h, and protein lysates were prepared. For assessing transfection efficiency cells were co-transfected with 2 μg of GFP and 70–80% transfection efficiency was observed with this protocol.
Statistical Analysis
Results were analyzed using a two-tailed Student’s t-test to assess statistical significance and p values <0.05were considered significant.
RESULTS
Inhibition of cell growth and colony formation by fisetin in human non-small cell lung cancer cells
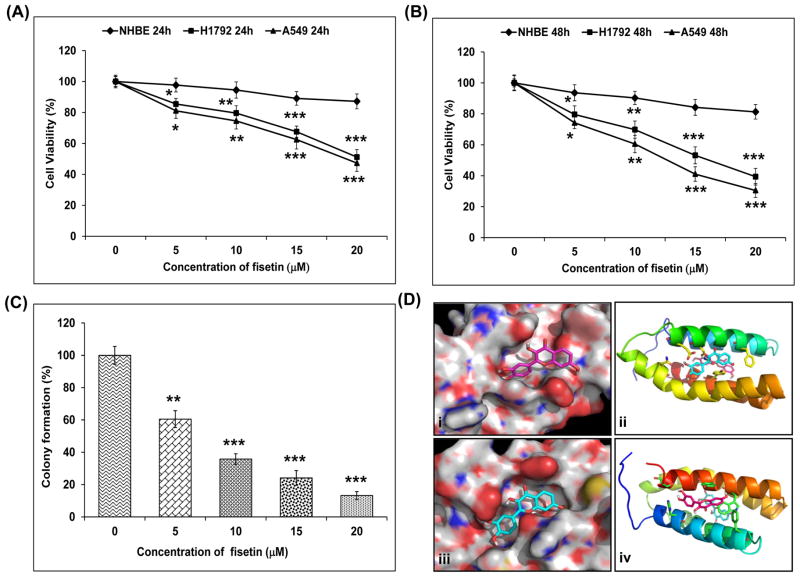
First, we investigated the dose- and time-dependent effect of fisetin treatment at dose levels of 5–20 μM on the growth of NHBE and human NSCLC A549 and H1792 cells. These doses of fisetin are physiologically attainable concentrations as pharmacokinetic analysis demonstrated a Cmax for total fisetin to be 22.18 μM/ml, the AUC was 19.12 μM hr/ml and the Tmax was 60 minutes in athymic nude mice. For these studies, 5 athymic nude mice (25 + 5 gm body weight) were administered 1mg of fisetin by single intraperitoneal injection and serum collected over time (data not shown).
We used MTT assay to evaluate the effect of fisetin on the growth of these cells. Treatment with fisetin (5–20 μM) for 24 h decreased cell viability in A549 cells by 19, 25, 37 and 52% and in H1792 cells by 12, 20, 32 and 49% but had minimal effect on NHBE cells at these doses (Fig. 1A). There was more prominent decrease in cell viability on treatment with fisetin (5–20 μM) for 48 h in A549 cells by 26, 39, 58 and 70% and in H1792 cells by 20, 30, 47 and 61% but very nominal effect on NHBE cells (Fig. 1B). Based on this data, we selected A549 cells for our study, since fisetin treatment caused maximum decrease in cell-viability in A549 cells as compared to H1792 cells.
Figure 1.
Effect of fisetin on cell growth and blind docking of fisetin to the mTOR target. A, Effect of fisetin on cell growth. As detailed in “Materials and Methods”, NHBE, A549 and H1792 cellss were treated with fisetin (5–20 μM) for 24 and B, 48h and the viability of cells was determined by the MTT assay. The data is expressed as the percentage of cell viability and represent the mean ± SEM of three experiments in which each treatment was performed in multiple wells. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group. C, Effect of fisetin on colony formation. The assay was performed in 6-well plates in triplicate and A549 cells were treated with 5–20 μM of fisetin. Primarily, 5,000 cells were seeded in top agar and grown for three weeks. Colonies were counted from 3 different fields and compared to that of control. The data is presented as mean ± SEM. **P < 0.01, ***P < 0.001 versus control group. D, Hypothetical model of the mTOR molecule in complex with fisetin. (i and iii), Blind docking of fisetin to the mTOR target was performed with Autodock4 by setting grid sizes that included the entire mTOR molecule. The structure consists of 4 stacked alpha helices. The grid size for the docking site was expanded to include the entire mTOR molecule and fisetin was docked. Using autodock 4, fisetin bound to two sites on the mTOR target. The binding energies were in the −7 to −8 Kcal/mol range for the binding constant. (ii and iv), The binding in the best site included hydrogen bonding to a glutamate by two hydroxyl groups.
Next, we investigated the effect of fisetin on clonogenic survival of A549 cells. Fisetin treatment (5–20 μM) caused inhibition in the ability of A549 cells to form colonies by 39–87% (Fig. 1C).
Fisetin physically interacts with the mTOR complex at two sites
Using autodock 4, fisetin bound to two sites on the mTOR target (Fig.1D-i and iii). The binding energies were in the −7 to −8 Kcal/mol range for the binding constant. The binding in the best site included hydrogen bonding to a glutamate by two hydroxyl groups. The second site is mostly hydrophobic, with ring of fisetin stacking on rings from the peptide (Fig. 1D-ii and iv).
Activation of PTEN and p-AMPKα in human non-small cell lung cancer cells
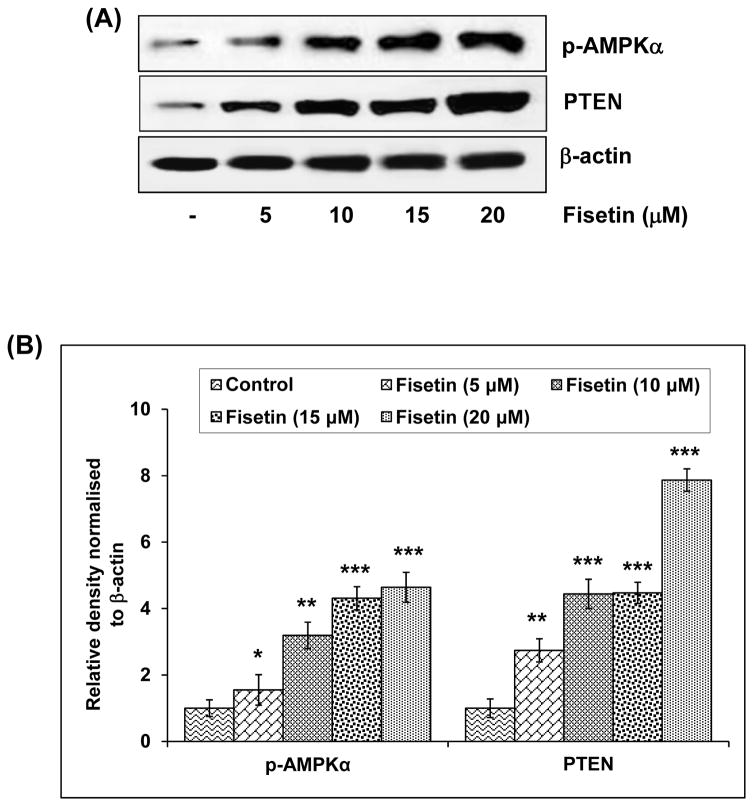
The phosphatase and tensin homologue (PTEN) gene is a multifunctional phosphatase, and its lipid phosphatase activity is associated with tumor suppression.23 It is the second most frequently mutated tumor suppressor gene in human sporadic cancers, and reduced PTEN protein expression occurs in approximately half of all tumors.24 Immunoblot analysis (Fig. 2A) and relative density of the bands (Fig. 2B) revealed that treatment with fisetin resulted in 1.7 fold activation of PTEN even at the lowest concentration of 5μM with a significant increase of 6.8 fold at the highest concentration of 20 μM.
Figure 2.
Effect of fisetin on protein expression of PTEN and phosphorylation of p-AMPKα in A549 cells. A, As detailed in “Materials and Methods”, the cells were treated with fisetin (5–20 μM; 48h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. B, Histogram represents relative density data of the immunoblots, from all experiments, shown in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group.
AMP-activated protein kinase (AMPK) is the central component of a protein kinase cascade that plays a major role in the regulation of energy control. It has been reported that there is a link between AMPK and the growth and survival of cancer cells.25 The phosphorylation of AMPK negatively regulates protein synthesis by directly phosphorylating and inhibiting mTOR.26 We found that there was a significant increase (0.5–3.6 fold) in the phosphorylation of AMPKα at 5–20 μM concentration of fisetin (Fig. 2A and B).
Inhibition of PI3K and phosphorylation of Akt by fisetin in human non-small cell lung cancer cells
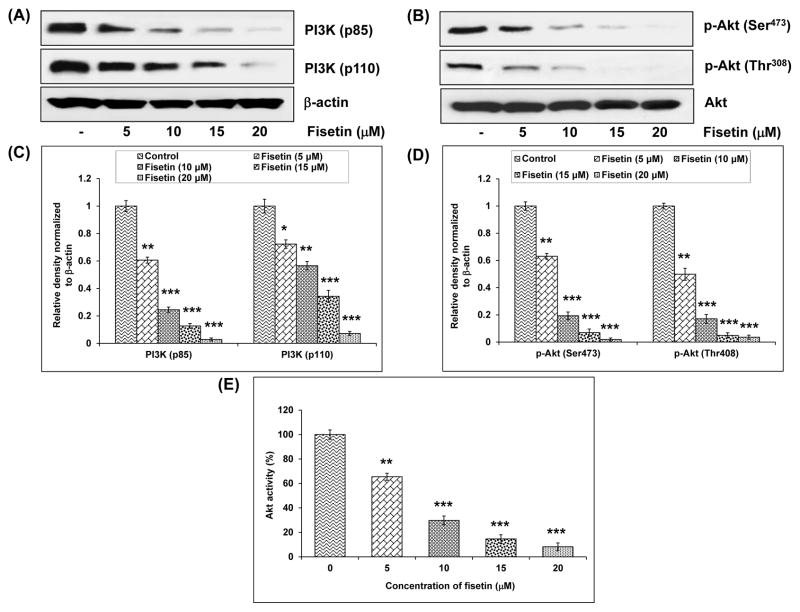
Deregulation of PI3K has been implicated in the induction and progression of several diseases including cancer.27 Increased cell growth, cell proliferation, resistance to apoptosis and cellular energy metabolism are connected with hyperactivation of Akt.8 Treatment with fisetin (5–20 μM) caused 39–94% and 28–92% inhibition in the expression of regulatory (p85) and catalytic (p110) subunits of PI3K, respectively (Fig. 3A and C). Fisetin also caused inhibition in the phosphorylation of Akt at both Ser473 (37–98%) and Thr308 (51–96%) in A549 cells (Fig. 3B and D). Further, enzyme-linked immunosorbent assay (ELISA) was conducted to evaluate the effect of fisetin on the phosphorylation of Akt. Fisetin treatment at 5, 10, 15 and 20 μM resulted in 34, 70, 85 and 92% decrease, respectively, in the levels of p-Akt as compared to control group in a dose-dependent manner (Fig. 3E).
Figure 3.
Effect of fisetin on protein expression of PI3K and Akt in A549 cells. A, Effect of fisetin on protein expression of PI3K (p85 and p110. B, Effect of fisetin on phosphorylation of Akt. As detailed in “Materials and Methods”, the cells were treated with fisetin (5–20 μM; 48h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. C and D, Histogram represents relative density data of the immunoblots, from all experiments, shown in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, *P < 0.01 and *P < 0.001 versus control group. E, The PathScan p-Akt (Ser473) sandwich ELISA kit was used to measure Akt activity. The data shown are representative results of three independent experiments and are presented as mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group.
Activation of TSC2 and inhibition of the phosphorylation of mTOR and its constituents by fisetin in human non-small cell lung cancer cells
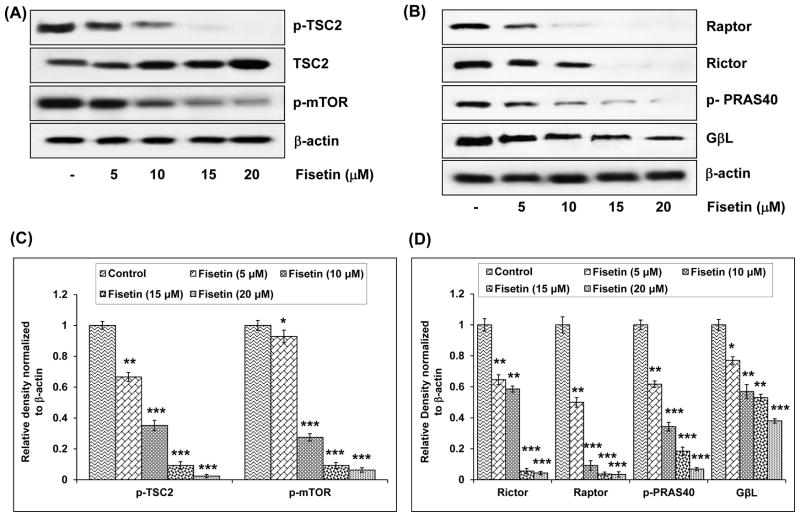
The TSC1/TSC2 complex is the only known GTPase for Rheb, serving to reduce Rheb-GTP levels, and thereby inhibit the activation of mTOR.28 TSC1 and TSC2 function as critical integrators of growth signals within the cell and are targets of multiple kinases, which regulate the GTPase activity of the complex. We found that treatment with fisetin (5–20 μM) caused 34–98% inhibition in the phosphorylation of TSC2 (Figure 4A and C), which is mediated by Akt. Fisetin also caused dose-dependent increase in the protein expression of TSC2 (Fig. 4A).
Figure 4.
Effect of fisetin on phosphorylation of TSC2, mTOR and its constituents in A549 cells. A, Effect of fisetin on phosphorylation of TSC2 and mTOR. B, Effect of fisetin on protein expression of Rictor, Raptor, GβL and phosphorylation of PRAS40. As detailed in “Materials and Methods”, the cells were treated with fisetin (5–20 μM; 48h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. C & D, Histogram represents relative density data of the immunoblots, from all experiments, shown in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group.
Phosphorylation of mTOR at Ser2448 has been shown to be associated with the activity of mTOR and Ser2448 of mTOR is phosphorylated by Akt.29 We investigated the effect of fisetin on the phosphorylation of mTOR at Ser2448. Treatment with fisetin (5–20 μM) caused dose-dependent (8–94%) inhibition in the phosphorylation of mTOR at Ser2448 as detected by immunoblot analysis (Fig. 4A) and relative density of the bands (Fig. 4C). We next examined whether fisetin affects mTOR complexes. Both raptor (forming mTOR complex 1, mTORC1) and rictor (forming mTOR complex 2, mTORC2) levels were decreased 50–97% and 36–96% respectively on treatment of cells with fisetin (Fig. 4B and D). The main pathway that proline-rich Akt-substrate PRAS40 is involved in is the PI3K-Akt pathway, and Akt is the upstream kinase of PRAS40.30 Since treatment with fisetin caused downregulation of PI3K/Akt pathway, we investigated the effect of fisetin on the protein expression of PRAS40. We found that there was 39–93% inhibition in the level of PRAS40 on treatment of A549 cells with fisetin (Fig. 4B and D). The protein expression of G protein β-like protein (GβL), which constitute part of mTORC1 and mTORC2, was also 23–62% downregulated dose-dependently on fisetin treatment (Fig. 4B and D). These results clearly indicate that fisetin inhibits both mTOR/raptor and mTOR/rictor complexes.
Inhibition of the phosphorylation of mTOR target proteins by fisetin in human non-small cell lung cancer cells
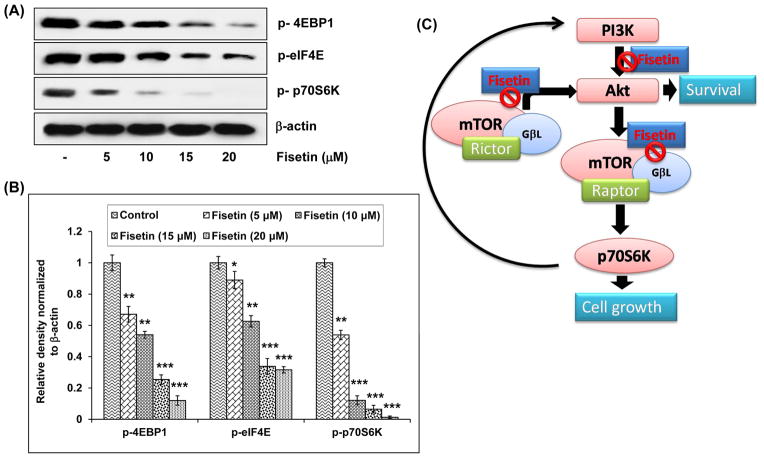
The activity of mTOR leads to S6K1/2 phosphorylation and activation, phosphorylation of 4E-BP1 and release from the cap-dependent translation initiation factor eIF4E. These two events, likely combined with other mTOR targets, lead to an increase in ribosomal biogenesis and the selective translation of specific mRNA populations.31, 32 We examined the effect of fisetin on the expression of 4E-BP1, eIF4E and p70S6K. Treatment of cells with fisetin caused 33–88%, 11–69% and 46–98% dose-dependent decrease respectively, in the phosphorylation of 4E-BP1, eIF4E and p70S6K proteins which are downstream targets of mTOR (Fig. 5A and B).
Figure 5.
Effect of fisetin on phosphorylation of its downstream targets in A549 cells. A, Effect of fisetin on phosphorylation of 4EBP1, eIF4E and p70S6K in A549 cells. As detailed in “Materials and Methods”, the cells were treated with fisetin (5–20 μM; 48h) and then harvested. Total cell lysates were prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of three independent experiments with similar results. B, Histogram represents relative density data of the immunoblots, from all experiments, shown in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group. C, Schematic representation of the effect of fisetin on PI3K/Akt and mTOR signaling. mTOR/Raptor drives a feedback loop that normally keeps PI3K activity in check. One consequence of mTOR/raptor inhibition is alleviation of this negative feedback loop resulting in activation of PI3K and subsequent activation of AKT. Therefore, simultaneously targeting both PI3K and mTOR has the potential to simultaneously inhibit both upstream and downstream signaling in the pathway. Fisetin inhibits the mTOR pathway and keeps the feedback loop in check by also inhibiting the PI3K/Akt pathway and inhibits cell survival and growth.
Inhibition of mTOR and its downstream targets by Rapamycin in human non-small cell lung cancer cells
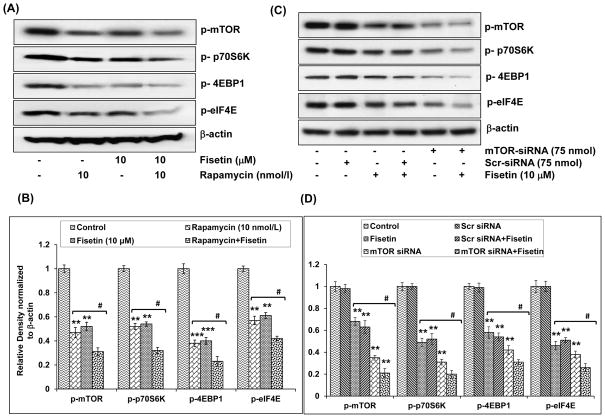
To assess whether fisetin-induced decrease in mTOR and its target proteins was due to inhibition of mTOR signaling, we treated cells with rapamycin, an inhibitor of mTOR. As shown in Fig. 6A and B, treatment of cells with rapamycin (10 nmol/l) caused decrease in the phosphorylation of mTOR (53%), 4E-BP1 (62%), eIF4E (43%) and 4E-BP1 (48%). When fisetin (10 μM) was added to rapamycin-treated cells, there was further downregulation in the phosphorylation of mTOR (69%), 4E-BP1 (77%), eIF4E (58%) and 4E-BP1 (68%), suggesting that these effects are mediated in part through mTOR signaling and fisetin is likely to have other modes of action (Fig. 6A and B).
Figure 6.
Effect of rapamycin and mTOR-siRNA on fisetin-induced inhibition of the phosphorylation of mTOR and its downstream targets in A549 cells. A, Effect of rapamycin on fisetin-induced inhibition of the phosphorylation of mTOR, p70S6K, 4EBP1 and eIF4E in A549 cells. Cells were treated with rapamycin (10 nmol/L) for 2 h, followed by addition of fisetin (10 μM; 48h). Whole cell lysate was prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of 3 independent experiments with similar results. B, Histogram represents relative density data of the immunoblots, from all experiments, shown in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group. #P < 0.05 versus fisetin-treated and rapamycin-treated groups. C, Effect of mTOR-siRNA on the inhibition of the downstream targets of mTOR in A549 cells. The A549 cells were transfected with mTOR-siRNA (75 nM) or scrambled siRNA (75 nM) and were then treated with 10 μM fisetin for 24 h. Whole cell lysate was prepared and 40 μg protein was subjected to SDS-PAGE followed by immunoblot analysis and chemiluminescence detection. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. The immunoblots shown here are representative of 3 independent experiments with similar results. D, Histogram represents relative density data of the immunoblots shown in 'C' in relative units ± SEM. Values represent mean ± SEM. *P < 0.05, **P < 0.01 and ***P < 0.001 versus control group. #P < 0.05 versus fisetin-treated and mTOR siRNA-treated groups.
Inhibition of the downstream targets of mTOR by knockdown of mTOR in human non-small cell lung cancer cells
To further investigate whether fisetin-induced downregulation of mTOR and its downstream targets was regulated by mTOR signaling, we knocked down mTOR by siRNA in cells. Silencing of mTOR by siRNA led to a decline in the phosphorylation of p70S6K (69%), eIF4E (62%) and 4EBP1 (58%), suggesting that the phosphorylation of these proteins is mediated by mTOR or one of its downstream targets (Fig. 6C and D). Treatment of cells with fisetin (10 μM) to mTOR-siRNA treated cells caused further decrease in the phosphorylation of p70S6K (80%), eIF4E (74%) and 4E-BP1 (69%). These results, with the data presented in Fig. 6, demonstrate that these effects are mediated partly through mTOR and other modes of actions are also involved (Fig. 6C and D).
DISCUSSION
The most important finding of our study is that treatment with fisetin caused dual inhibition of PI3K/Akt and mTOR signaling in human NSCLC cells. To our knowledge, no other dietary agent at physiologically attainable concentrations has been shown to exert this dual inhibitory effect. Finally, fisetin did not inhibit cell-growth, PI3K/Akt and mTOR signaling in NHBE cells (data not shown). While it remains unclear as to why fisetin behaves differently in cancer cells compared to normal cells, it could be speculated that uptake mechanisms could partially explain this paradox. It is speculated that fisetin is rapidly taken up by cancer cells, while its uptake is slow and regulated in normal cells.
The mTOR pathway has emerged as an important cancer therapeutic target. The discovery of the highly specific and potent mTOR inhibitor rapamycin and its derivatives that specifically inhibit mTOR are now being actively evaluated inclinical trials.33 A potential mechanism of resistance to mTOR inhibitors is caused by a negative feedback loop in which mTOR inhibition leads to AKT activation through upregulation of receptor tyrosine kinases such as platelet derived growth factor receptors34 and insulin receptor substrate-1.35 The relevance of this feedback is underscored by its existence in cancer patients.36 We found that fisetin inhibits the mTOR pathway and keeps the feedback loop in check by also inhibiting the PI3K/Akt pathway and inhibits cell survival and growth (Fig. 5C).
In the present study, we have shown for the first time that fisetin inhibited PI3K/Akt and mTOR signaling in human NSCLC cells. Treatment of A549 and H1792 human lung cancer cells with fisetin caused decrease in cell-viability but had minimal effects on NHBE cells. There was also inhibition in the ability of A549 cells to form colonies on treatment with fisetin. Using autodock4, we also found that fisetin bound to two sites on the mTOR target. The binding energies were in the −7 to −8 Kcal/mol range for the binding constant.
Since the discovery of PTEN as a putative tumor suppressor in 1997, its importance as a tumor suppressor has been validated by its mutation and/or loss of expression in a variety of sporadic cancers and its association with Cowden disease, an autosomal dominant cancer syndrome. PTEN plays an important role in multiple cellular functions such as cell metabolism, proliferation and survival. Loss of the tumor suppressor PTEN is common in various kinds of human solid tumors. Therefore, development of genes and materials that regulate PTEN in tumors is one of the important fields in overcoming resistance against anticancer agents.37 The major substrate of the lipid phosphatase activity of PTEN is PIP3 (phosphatidylinositol 3,4,5-triphosphate), an important intracellular second messenger. By dephosphorylating the D3-position of PIP3, PTEN negatively regulates the PI3K pathway and Akt activation and thus suppresses tumorigenesis. We also found that fisetin increased the protein levels of PTEN dose-dependently. AMPK is a member of a metabolite-sensing protein kinase family which plays an essential role as an energy-sensor mainly in ATP-deprived conditions.38 Therefore, AMPK is known to play a major protective role under metabolic stressed conditions. In the activated states, AMPK down-regulates several anabolic enzymes and thus shuts down the ATP-consuming metabolic pathways. Activation of AMPK inhibits mTOR signaling and is associated with inhibition of cancer cell growth.39 Consistent with these studies, we found that fisetin caused inhibition of the phosphorylation of mTOR, upregulation of AMPKα and decrease in the expression of Raptor, Rictor, PRAS40 and GβL causing less formation of both mTORC1 and mTORC2 in lung cancer cells.
Since we observed a decrease in the phosphorylation of mTOR on treatment with fisetin, we investigated the effect of fisetin on PI3K/Akt pathway. Fisetin treatment resulted in the inhibition of the expression of regulatory and catalytic subunits of PI3K and inhibition of the phosphorylation of Akt, suggesting that fisetin-induced decrease in mTOR phosphorylation is dependent on PI3K/Akt pathway as well.
Tuberous sclerosis, an autosomal dominant disorder is caused by mutations of TSC1 and TSC2, which in humans is associated with hamartomatous polyps in multiple tissues and an increased risk of cancers. TSC2 is a tumor suppressor that has been linked to AMPK and it forms an inhibitory complex with TSC1 that binds to and inhibits mTOR, leading to negative regulation of cell size and growth.40 TSC1/TSC2 complex inhibits mTOR activity by activating the GTPase activity of Ras homologue enriched in brain, and both Akt and AMPK converged at TSC1/TSC2 to regulate mTOR activity.41 Fisetin caused inhibition of the phosphorylation of TSC2 and increase in the protein expression of TSC2 consistent with the fact that Akt phosphorylates TSC2 and disrupts the TSC1/TSC2complex, leading to activation of mTOR.42
The ribosomal S6 kinase and the 4E-BP1 are the two major downstream signaling pathways of mTOR and have a role in control of protein translation.43 The phosphorylation of 4EBP-1 by mTOR results in the release of a cap-binding protein eIF4E, which is held inactive when bound to the hypophosphorylated 4EBP-1 complex.4, 44 Studies have shown that higher levels of eIF4E are found in many transformed cell-lines and various cancers overexpress eIF4E.45 Seki et al. have shown that eIF4E seems increased in peripheral lung adenocarcinomas and suggests a correlation between the magnitude of the eIF4E increase and the invasiveness of the tumors.46 Fisetin-treated cells showed decreased phosphorylation of mTOR protein expression and its downstream targets (4EBP1, eIF-4E, p70S6K), suggesting the effect of fisetin on mTOR signaling. To confirm that these effects are mediated in part through mTOR signaling, we have shown that when fisetin was added to rapamycin-treated cells, there was further downregulation in the phosphorylation of these proteins. To further validate this, we silenced mTOR and found that it caused decrease in the phosphorylation of the downstream targets of mTOR which was further augmented by the addition of fisetin, suggesting that these effects are in part, due to mTOR signaling and fisetin is likely to have other modes of action, as is the case for other dietary agents. Taken together, these findings show that fisetin, a natural dietary flavonoid inhibits PI3K/Akt and mTOR signaling in human non-small cell lung cancer cells and could be developed as a potential lung cancer chemopreventive/chemotherapeutic agent.
Novelty and Impact Statement.
This study identifies fisetin, a dietary flavonoid, as a dual inhibitor of PI3K/Akt and mTOR signaling in human non-small cell lung cancer cells and provides a basis for its development as a chemotherapeutic agent, alone or as an adjuvant. The mTOR is phosphorylated in lung cancer and its activation is more frequent in tumors with over-expression of PI3K/Akt. The fact that lung cancer is the second most common cancer, the most deadly and a common cause of cancer-related death in both men and women underscores the importance of this discovery.
Acknowledgments
The authors thank Dr. Kenneth Satyshur from the Small Molecule Screening Facility, University of Wisconsin-Madison for help with in-silico studies. This study was supported by National Institutes of Health, National Cancer Institute Grants (R01CA120451 and R03CA153961).
List of abbreviations
- AMPK
AMP-activated protein kinase
- ATCC
American Type Culture Collection
- AUC
Area Under Curve
- Cmax
Maximum Concentration
- DMSO
Dimethyl Sulfoxide
- ELISA
Enzyme linked immunosorbent assay
- mTOR
Mammalian target of rapamycin
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide
- NHBE
Normal Human Bronchial Epithelial
- NSCLC
Non-small cell lung cancer
- PI3K
Phosphatidylinositol 3-kinase
- PTEN
Phosphatase and tensin homologue
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Lynch TJ, Adjei AA, Bunn PA, Jr, Eisen TG, Engelman J, Goss GD, Haber DA, Heymach JV, Janne PA, Johnson BE, Johnson DH, Lilenbaum RC, et al. Summary statement: novel agents in the treatment of lung cancer: advances in epidermal growth factor receptor-targeted agents. Clin Cancer Res. 2006;12:4365s–71s. doi: 10.1158/1078-0432.CCR-06-1005. [DOI] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 5.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008;11:32–50. doi: 10.1016/j.drup.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, Abraham RT. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–22. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 7.Gridelli C, Maione P, Rossi A. The potential role of mTOR inhibitors in non-small cell lung cancer. Oncologist. 2008;13:139–47. doi: 10.1634/theoncologist.2007-0171. [DOI] [PubMed] [Google Scholar]
- 8.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–83. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 2010;5:29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 10.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000;130:2243–50. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 11.Haddad AQ, Venkateswaran V, Viswanathan L, Teahan SJ, Fleshner NE, Klotz LH. Novel antiproliferative flavonoids induce cell cycle arrest in human prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2006;9:68–76. doi: 10.1038/sj.pcan.4500845. [DOI] [PubMed] [Google Scholar]
- 12.Syed DN, Afaq F, Maddodi N, Johnson JJ, Sarfaraz S, Ahmad A, Setaluri V, Mukhtar H. Inhibition of Human Melanoma Cell Growth by the Dietary Flavonoid Fisetin Is Associated with Disruption of Wnt/beta-Catenin Signaling and Decreased Mitf Levels. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh Y, Afaq F, Khan N, Johnson JJ, Khusro FH, Mukhtar H. Fisetin induces autophagic cell death through suppression of mTOR signaling pathway in prostate cancer cells. Carcinogenesis. 2010;31:1424–33. doi: 10.1093/carcin/bgq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murtaza I, Adhami VM, Hafeez BB, Saleem M, Mukhtar H. Fisetin, a natural flavonoid, targets chemoresistant human pancreatic cancer AsPC-1 cells through DR3-mediated inhibition of NF-kappaB. Int J Cancer. 2009;125:2465–73. doi: 10.1002/ijc.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30:300–7. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan N, Asim M, Afaq F, Abu Zaid M, Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–63. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan N, Afaq F, Syed DN, Mukhtar H. Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle arrest in human prostate cancer LNCaP cells. Carcinogenesis. 2008;29:1049–56. doi: 10.1093/carcin/bgn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YC, Shen SC, Lee WR, Lin HY, Ko CH, Shih CM, Yang LL. Wogonin and fisetin induction of apoptosis through activation of caspase 3 cascade and alternative expression of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch Toxicol. 2002;76:351–9. doi: 10.1007/s00204-002-0346-6. [DOI] [PubMed] [Google Scholar]
- 19.Sung B, Pandey MK, Aggarwal BB. Fisetin, an inhibitor of cyclin-dependent kinase 6, down-regulates nuclear factor-kappaB-regulated cell proliferation, antiapoptotic and metastatic gene products through the suppression of TAK-1 and receptor-interacting protein-regulated IkappaBalpha kinase activation. Mol Pharmacol. 2007;71:1703–14. doi: 10.1124/mol.107.034512. [DOI] [PubMed] [Google Scholar]
- 20.Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci U S A. 2006;103:16568–73. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou DX, Fukuda M, Johnson JA, Miyamori K, Ushikai M, Fujii M. Fisetin induces transcription of NADPH:quinone oxidoreductase gene through an antioxidant responsive element-involved activation. Int J Oncol. 2001;18:1175–9. doi: 10.3892/ijo.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 22.Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene. 2008;27:585–95. doi: 10.1038/sj.onc.1210693. [DOI] [PubMed] [Google Scholar]
- 23.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–7. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 24.Stokoe D. Pten. Curr Biol. 2001;11:R502. doi: 10.1016/s0960-9822(01)00303-7. [DOI] [PubMed] [Google Scholar]
- 25.Xiang X, Saha AK, Wen R, Ruderman NB, Luo Z. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–7. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 26.Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226–32. doi: 10.1074/jbc.M202489200. [DOI] [PubMed] [Google Scholar]
- 27.Thomas M, Owen C. Inhibition of PI-3 kinase for treating respiratory disease: good idea or bad idea? Curr Opin Pharmacol. 2008;8:267–74. doi: 10.1016/j.coph.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Sekulic A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, Abraham RT. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60:3504–13. [PubMed] [Google Scholar]
- 30.Huang B, Porter G. Expression of proline-rich Akt-substrate PRAS40 in cell survival pathway and carcinogenesis. Acta Pharmacol Sin. 2005;26:1253–8. doi: 10.1111/j.1745-7254.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 31.Rowinsky EK. Targeting the molecular target of rapamycin (mTOR) Curr Opin Oncol. 2004;16:564–75. doi: 10.1097/01.cco.0000143964.74936.d1. [DOI] [PubMed] [Google Scholar]
- 32.Tee AR, Blenis J. mTOR, translational control and human disease. Semin Cell Dev Biol. 2005;16:29–37. doi: 10.1016/j.semcdb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–88. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 34.Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–8. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, et al. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–23. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simpson L, Parsons R. PTEN: life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 38.Hardie DG, Carling D, Carlson M. The AMP-activated/SNF1 protein kinase subfamily: metabolic sensors of the eukaryotic cell? Annu Rev Biochem. 1998;67:821–55. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 39.Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Corradetti MN, Inoki K, Guan KL. TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem Sci. 2004;29:32–8. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14(Spec No 2):R251–8. doi: 10.1093/hmg/ddi260. [DOI] [PubMed] [Google Scholar]
- 42.Dan HC, Sun M, Yang L, Feldman RI, Sui XM, Ou CC, Nellist M, Yeung RS, Halley DJ, Nicosia SV, Pledger WJ, Cheng JQ. Phosphatidylinositol 3-kinase/Akt pathway regulates tuberous sclerosis tumor suppressor complex by phosphorylation of tuberin. J Biol Chem. 2002;277:35364–70. doi: 10.1074/jbc.M205838200. [DOI] [PubMed] [Google Scholar]
- 43.Ruggero D, Montanaro L, Ma L, Xu W, Londei P, Cordon-Cardo C, Pandolfi PP. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–6. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 44.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–35. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 45.De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene. 2004;23:3189–99. doi: 10.1038/sj.onc.1207545. [DOI] [PubMed] [Google Scholar]
- 46.Seki N, Takasu T, Mandai K, Nakata M, Saeki H, Heike Y, Takata I, Segawa Y, Hanafusa T, Eguchi K. Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin Cancer Res. 2002;8:3046–53. [PubMed] [Google Scholar]