Abstract
This is a case report of a patient presenting with a destructive lesion with histologic features of a low grade malignancy in a predominantly myxoid matrix. Various low grade myxoid malignancies were considered in the differential diagnosis of which an overview is presented. A literature review of the ultrastructural findings and possible histogenesis is discussed along with the diagnostic criteria and recent change in the terminology regarding the malignancies previously diagnosed as myxoid malignant fibrous histiocytomas. A final diagnosis of a myxofibrosarcoma was only possible after assessing the immuno-histochemical profile, results of histochemical stains and ultrastructural features of this lesion.
Keywords: Myxoid sarcoma, Myxoid malignant fibrous histiocytoma, Maxillary sinus, Undifferentiated pleomorphic sarcoma, Myxofibrosarcoma
Introduction
The terms “myxofibrosarcoma” and “myxosarcoma” were previously reserved for a low grade malignant myxoid tumor that presents with a deceptively benign microscopic appearance [1]. In 1977, Angervall et al. [2] redefined this concept and excluded all myxoid lesions with evidence of lipoblastic, myoblastic or chondroblastic differentiation. Angervall et al. [1] and Merck et al. [2] divided the lesion into four grades according to cellularity, atypical features, mitotic activity and myxoid content. Grade I myxofibrosarcomas, representing one end of the spectrum, were predominantly myxoid and hypocellular, while grade IV tumors at the other end were cellular lesions with atypical features, a high mitotic index, and admixed giant cells. O’Brien and Stout [3] introduced the term “malignant fibrous histiocytoma” (MFH) for malignancies of supposed fibrohistiocytic lineage and several subtypes were reported [4, 5]. The myxoid MFH described by Weiss and Enzinger [6] shared many similarities with myxofibrosarcomas as redefined by Angervall et al. [2]. Weiss and Enzinger [6] and others [6, 7] separated myxoid MFHs into predominantly myxoid (low grade), mixed myxoid and cellular (intermediate grade), and predominantly cellular (high grade) types. Predominantly myxoid MFH’s were regarded by some authors to be the same as the low-grade myxofibrosarcomas [1, 8–11]. Siverman and Caolson [12] observed histiocyte-like cells in a myxofibrosarcoma. Weiss and Enzinger [6] and others [4, 7, 9, 13, 14] applied the term myxoid MFH or myxofibrosarcoma to those tumors in which 50% or more of the stroma was myxoid. The diagnostic criteria with regards to the amount of myxoid material and the presence of histiocyte-like cells, account for the variable terminology used in the literature, whether myxofibrosarcoma or myxoid MFH.
In 2002, the WHO declassified the MFH as a diagnostic entity and determined that the myxoid MFH without myogenic-, lipoblastic- and chondrogenic features be diagnosed as myxofibrosarcoma [15]. There seems to be a spectrum of microscopic views ranging from hypocellular myxoid tumors to cellular tumors with little or no myxoid substance, increased pleomorphism and mitoses, such as seen in malignancies previously known as storiform-pleomorhic MFH’s [1, 11, 16, 17]. Evidence suggest that the latter tumor (UPS with giant cells) represents a final common pathway in malignancies that undergo dedifferentiation [18] or, that it represents a second component in another sarcoma undergoing morphologic modulation resulting from tumor progression [19]. This reasoning stems from the fact that similar recurrent chromosome deletions were noted in tumors such as leimyosarcomas [19] and many cases previously diagnosed as MFH were then later rediagnosed as pleomorphic variants of other sarcomas [20, 21]. Similar to a myxofibrosarcoma, a pleomorphic variants with giant cells should only be diagnosed after ultrastructural and immuno-histochemical examination excluded all other sarcoma types [20, 22]. It is the most common soft tissue sarcoma that occurs in late adult life, peaking in the seventh decade [4, 12, 19, 23–25] and is mainly encountered in the lower extremities [1, 2, 6, 7, 9, 11, 25–28]. They have also been reported in the paranasal sinuses [23, 29–32], nasal cavity [1], maxillary bone and maxillary sinus [33]. The myxofibrosarcoma has only rarely been reported in the sinuses and orofacial region [30, 34–37]. The myxofibrosarcoma reportedly pursue a less aggressive course [8], as compared to the pleomorphic sarcomas with giant cells, which has a much higher risk for early metastasis and recurrence [38].
The purpose of this report is to present the clinical, histological, immunohistochemical and ultrastructural features of a myxofribrosarcoma that occurred in the maxillary sinus.
Case Report
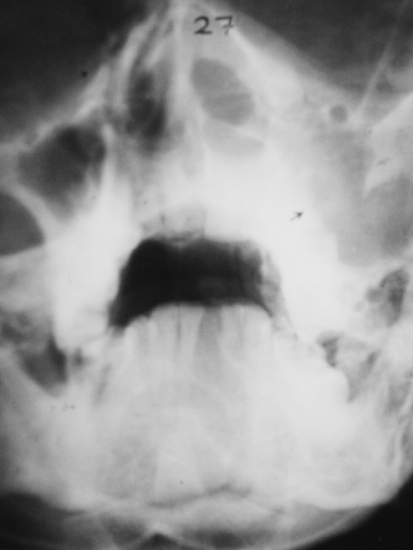
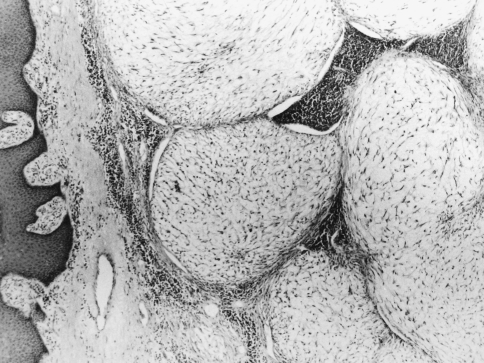
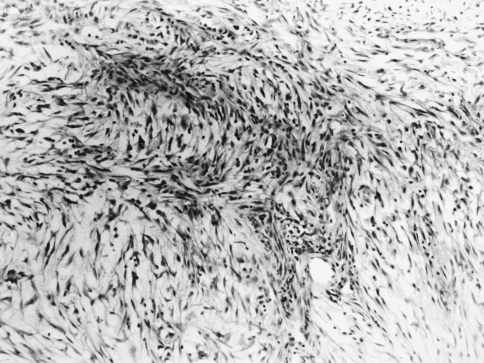
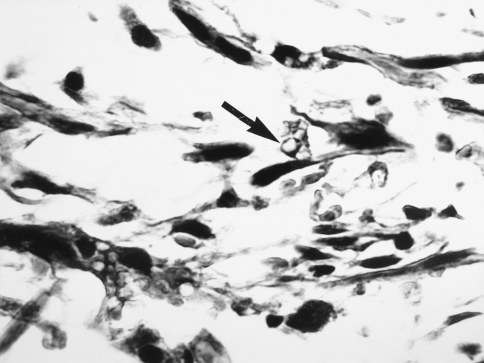
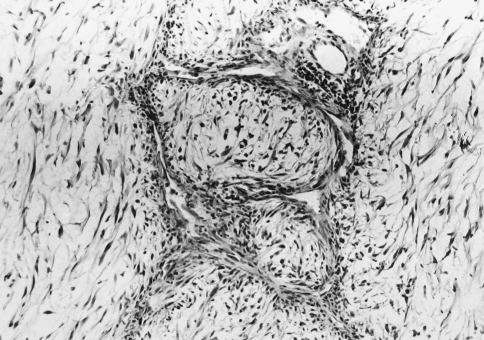
A 69 year old male complained of pain refractile to analgesics in the left maxilla that radiated to the left zygoma. Intraoral examination revealed a 3 × 5 cm exophytic mass on the left maxillary alveolus that crossed the midline. No lymphadenopathy was apparent and the patient had smoked 40 cigarettes per day, but had stopped smoking 17 years previously. An occipitomental radiograph revealed opacification of the left maxillary sinus and destruction of its lateral wall (Fig. 1). The mass extended to the floor of the orbit, as far back as the pterygoid plates, and included the lateral nasal wall. The clinical features were those of a destructive lesion originating in the maxillary sinus and a carcinoma or an infection such as mucormycosis were considered. There was no radiographic evidence of lung involvement. Histologic examination revealed a poorly circumscribed tumor that was divided into nodules by delicate fibrous septa (Fig. 2). The nodules consisted of a myxoid matrix containing atypical spindle-shaped and stellate cells. In the more cellular areas, the cells were arranged in short fasicles with focal storiform patterns (Fig. 3). Lipoblast-like cells with small cytoplasmic vacuoles were also observed (Fig. 4). The cells at the periphery of the nodules had anastomosing processes and were more compactly arranged than those in the centre. In the more cellular areas pleomorphic cells with hyperchromatic nuclei and a mean of two mitoses per 10 HPF were observed. Curvilinear capillaries were present in the nodules as well as in the fibrous septa (Fig. 5). Angiocentrically distributed aggregates of lymphocytes and plasma cells were seen and no areas of necrosis were noted. The vacuoles of the lipoblast-like cells and the myxoid areas stained with Alcian-blue at pH 2.5 but not at pH 0.5 and the staining was abolished after predigestion with hyaluronidase. Except for vimentin, no reactivity with a battery of other markers for mesenchymal differentiation was noted.
Fig. 1.
Occipitomental radiograph showing left maxillary clouding and sinus wall destruction
Fig. 2.
Photomicrograph showing myxoid nodules and fibrous septa. (Hematoxylin and eosin, original magnification ×40)
Fig. 3.
Photomicrograph showing spindle-shaped cells in a fasicular arrangement. (Hematoxylin and eosin, original magnification ×100)
Fig. 4.
Photomicrograph showing lipoblast-like cells with vacuolated cytoplasm (arrow). (Hematoxylin and eosin, original magnification ×400)
Fig. 5.
Photomicrograph of curvilinear capillaries and angiocentrically distributed lymphocytes and plasma cells. (Hematoxylin and eosin, original magnification ×100)
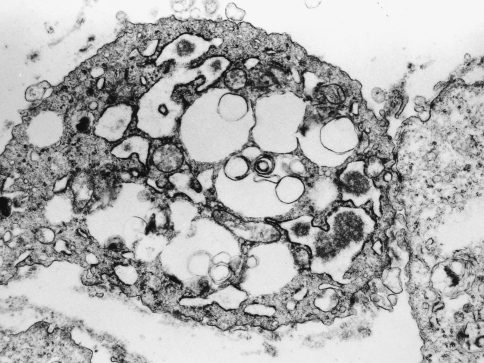
Electron-microscopical examination revealed the presence of five cell types of which the fibroblast-like cells predominated. They were identified by their fusiform shape, oval or elongated nuclei, abundant parallel ribosome-studded membranes, and close association with collagen bundles. Polygonal cells with indented nuclei, sparse ribosomal endoplasmic reticulum, pseudopods, and lysomomes of varying shape and density were regarded as histiocyte-like cells. Smaller cells with features of both fibroblasts and histiocytes, and primitive appearing round cells with euchromatic nuclei, were also noted. The fifth cell type had prominent filopodic extensions (Fig. 6). Many of these vacuoles contained granular material similar to the extracellular matrix.
Fig. 6.
Electronmicrograph demonstrating a xanthomatous cell with anastomosing processes. (original magnification ×16,000)
An initial diagnosis of a myxoid MFH was made due to the immuno-histochemical and electron-microscopical findings. The tumor was considered not resectable and the patient was treated with a combination of radiotherapy (60 Gy given over 4 week period) and chemotherapy. However, the tumor did not respond and the patient was subject to chemotherapy but this was stopped after his condition deteriorated. He died 1 year later.
Discussion
The diagnosis of the case was challenging. Light microscopic examination showed well demarcated nodules and a low mitotic count, indicative of a benign soft tissue lesion such as a nodular or pseudosarcomatous fasciitis [18, 39], a myxoma [2, 26, 27, 40], a myxoid neurofibroma [27] or chondromyxoid fibroma [41]. However, the clinical and radiologic features were indicative of a malignant growth. The lack of epithelial differentiation excluded a carcinoma and infective conditions such as mucormycosis were not considered due to the absence of necrosis and the cellular nature of the growth. The presence of curvilinear vessels and atypia ruled out benign fibrous lesions, which present with radiating vascular patterns and absence of atypia. Malignancies that were considered included myxoid liposarcoma [42], myxoid chondrosarcoma [26, 42], myxoid leimyosarcoma [43], myxofibrosarcoma [44, 45], myxoid neurogenic sarcoma [42], low-grade fibromyxoid sarcoma [46] and myxoid squamous carcinoma [47]. The lack of positive staining with a panel of monoclonal antibodies aided in excluding the myxoid neurogenic sarcoma, leimyosarcoma and myxoid squamous carcinoma. Distinction is important as the myogenic sarcomas carry a poorer prognosis that tumors diagnosed as myxofibrosarcomas [48].
A myxoid liposarcoma was excluded because it lacks nodularity, has indented nuclei, cytoplasmic vacuoli and a plexiform or so-called “crow’s feet” vascular pattern, rather than a curvilinear capillary pattern [2, 6, 24]. Additionally, the lipoblast-like cells stained with Alcian blue and lacked the osmophilic properties of a lipoblast. Silverman and Coalson [12] and Vuzevski [42] studied a myxoid MFH electron microscopically and described five cell types, similar to those seen in this case. No myfibroblast-like cells were seen in this case, as were described by Kindblom and coworkers [26]. The vesiculated cells containing extracellular matrix-like material and anastomosing filopodia resembled those described by these authors as xanthomatous cells. This case presented with primitive cells seemingly giving rise to, or differentiating along fibroblastic and histiocytic lines and differs from many low-grade myxofibrosarcomas that are made up of fibroblast-like cells only [11, 16]. Myxoid chondrosarcoma presents with myxoid nodules in which chondroblasts with hyperchromatic nuclei occur. However, it has scant vascularity and the interstitial matrix retains the Alcian blue stainability when pretreated with hyaluronidase [1]. This feature substantiates the presence of sulphated glucosaminoglycans such as chondroitin sulphate that is present in chondroid neoplasms.
Electronmicroscopic examination failed to demonstrate Luse bodies and the whorled cellular processes seen in neurogenic tumors or tonofilaments associated with squamous carcinoma. Myofibroblastic sarcoma, characterized by spindle cells in a variable amount of myxoid matrix requires positive identification of smooth muscle markers and ultrastructural demonstration of myofilaments extending peripherally to the cell surface [45, 49]. The myxoid leiomyosarcoma is identified by SMA- and MSA-positivity in most cases and the ultrastructural presence of cytoplasmic dense bodies, marginal dense plaques and myofilaments [50]. The low-grade fibromyxoid sarcoma is made up of bland uniform spindle- or stellate-shaped fibroblasts, without the characteristic curvilinear vascular pattern [8, 46, 51] and pseudolipoblasts as seen in this case.
The final diagnosis of a myxofibrosarcoma was reached after evaluating all the clinical, radiologic, light microscopic, immunohistochemical and ultrastructural features of this lesion. The electron microscopic demonstration of fibroblast-like cells, histiocyte-like cells, intermediate cells, primitive appearing cells, and vacuolated cells aided in the diagnosis of this tumor, previously described as a low-grade myxoid MFH. This combination of cells were at one time regarded as diagniostic for the diagnosis of a MFH [26, 39, 42, 50–54]. Suh et al. [49] examined 32 cases of myxoid MFH’s and found no evidence of true histiocytic differentiation in any, which led some authors to conclude that these are tumors of the fibroblast lineage [20].
The histogenesis remains unresolved and diagnosis is usually reached by a process of exclusion [55]. Nevertheless, grading is mandatory because this has a direct bearing on recurrence rate and metastatic potential. The myxoid content must be determined by adequate sampling and microscopic examination. Predominantly myxoid tumors are regarded as low grade and they may have a more favorable prognosis than high grade tumors [15]. After treatment they seldom recur [27] and are less likely to metastasize [6].
Low-grade myxoid malignancies of bone, soft tissue and neural tissue may all present with similar clinico-pathological and radiological features. They may all develop from undifferentiated primitive cells along different phenotypic pathways and only differ ultrastructurally with regards to their myogenic, neurogenic, chondrogenic, lipoblastic, myofibroblastic or fibroblast-like and fibrohistiocyte-like lineage. The diagnosis of a myxobibrosarcoma based solely on morphologic features is unacceptable and only those with immuno-histochemical and ultrastrucural evidence of a fibrohistiocyte-like phenotype should at present be regarded as myxofibrosarcomas [15].
References
- 1.Merck C, Angerval L, Kindblom L, Oden A. Myxofibrosarcoma: a malignant soft tissue tumor of fibroblastic-histiocytic origin. Acta Path Microbiol Scand. 1983;91(282):1–40. [PubMed] [Google Scholar]
- 2.Angervall I, Kindblom L, Merck C. Myxofibrosarcoma. Acta Path Microbiol Scand. 1977;85:127–140. [PubMed] [Google Scholar]
- 3.O’Brien JE, Stout AP. Malignant fibrous xanthomas. Cancer. 1964;17:1445–1455. doi: 10.1002/1097-0142(196411)17:11<1445::AID-CNCR2820171112>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Anavi Y, Herman GE, Graybrill S, MacIntosh RB, Tiqva P. Malignant fibrous histiocytoma of the mandible. Oral Surg Oral Med Oral Pathol. 1987;68:436–443. doi: 10.1016/0030-4220(89)90142-4. [DOI] [PubMed] [Google Scholar]
- 5.Bras J, Batsakis JG, Luna MA. Malignant fibrous histiocytoma of the oral soft tissues. Oral Surg Oral Med Oral Pathol. 1987;64:57–67. doi: 10.1016/0030-4220(87)90117-4. [DOI] [PubMed] [Google Scholar]
- 6.Weiss SW, Enzinger FM. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1977;39:1672–1685. doi: 10.1002/1097-0142(197704)39:4<1672::AID-CNCR2820390442>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Hollowood K, Fletcher CDM. Malignant fibrous histiocytoma: morhologic pattern or pathologic entity. Semin Diagn Pathol. 1995;12(3):210–220. [PubMed] [Google Scholar]
- 8.Wada T, Hasegawa T, Nagoya S, Kwaguchi S, Kaya M, Ishii S. Myxofibrosarcoma with infitrative growth pattern: a case report. Jap Jnl Clin Oncol. 2000;30:458–462. doi: 10.1093/jjco/hyd115. [DOI] [PubMed] [Google Scholar]
- 9.Waters B, Panice KDM, Lefkowitz RA, Antonescu CR, Healy JH, Athanason EA, Brennan MF. Low-grade myxofibrosarcoma: CT and MRI patterns in recurrent disease. Am J Roentgenol. 2007;188(2):W193–W198. doi: 10.2214/AJR.05.1130. [DOI] [PubMed] [Google Scholar]
- 10.Pezzi CM, Rawlings MS, Esqro JJ, Pollock RE, Romsdhal MM. Prognostic factors in 227 patients with malignant fibrous histiocytoma. Cancer. 1992;69(8):2013–2098. doi: 10.1002/1097-0142(19920415)69:8<2098::AID-CNCR2820690815>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Huang HY, Quin J, Brennan MF, Antonescu CR. Low-grade myxofibrosarcoma: a clinicopathologic analysis of 49 cases treated at a single institution with simultaneous assessment of the efficacy of 3-tier and 4-tier grading systems. Human Pathol. 2004;35(5):612–621. doi: 10.1016/j.humpath.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Silverman JF, Coalson JJ. Primary myxoid malignant fibrous histiocytoma of the lung. Arch Pathol Lab Med. 1984;108:49–54. [PubMed] [Google Scholar]
- 13.Rooser B, Willen H, Gustafson P, Alvegard TA, Rydbholm A. Malignant fibrous histiocytoma of soft tissue. Cancer. 1991;67:499–505. doi: 10.1002/1097-0142(19910115)67:2<499::AID-CNCR2820670230>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento AF, Bertoni F, Fletcher CD. Epithelioid variant of myxofibrosarcoma: expanding the clinicomorphologic spectrum of myxofibrosarcoma in a series of 17 cases. Am J Surg Pathol. 2007;115(7):1237–1240. doi: 10.1097/01.pas.0000213379.94547.e7. [DOI] [PubMed] [Google Scholar]
- 15.Fletcher CDM, Mehrtens F. World heath organization classification of tumors: pathology and genetics of tumors of soft tissue and bone. France: IARC Press; 2002. [Google Scholar]
- 16.Mentzel T, Calonje E, Wadden C, Camplejohn RS, Beham A, Smith MA, Fletcher C. Myxofibrosarcoma: clinicopathological analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol. 1996;20(4):391–405. doi: 10.1097/00000478-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Mansoor A, White CR. Myxofibrosarcoma presenting in the skin: clinicopathological features and differential diagnosis with cutaneous myxoid neoplasms. Am J Dermatopathol. 2003;25(4):281–286. doi: 10.1097/00000372-200308000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Ackerman M. Malignant fibrous histiocytoma—the commonest soft tissue sarcoma or a nonexistent entity? Acta Orthop Scand Suppl. 1997;273:41–46. [PubMed] [Google Scholar]
- 19.Lagare R, Aurias A. Does malignant fibrous histiocytoma exist? Ann Pathol. 2002;22(1):29–34. [PubMed] [Google Scholar]
- 20.Erlandsen RA, Antonescu CR. The rise and fall of malignant fibrous histiocytoma. Ultrastruct Pathol. 2004;28:283–289. doi: 10.1080/019131290882150. [DOI] [PubMed] [Google Scholar]
- 21.Oda Y, Tamiya S, Oshiro Y, Hachitanda Y, Kinukawa N, Iwamoto Y, Tsuneyoshi M. Reassessment and clinicopathological prognostic factors of malignant fibrous histiocytoma of soft parts. Pathol Int. 2002;52(9):595–606. doi: 10.1046/j.1440-1827.2002.01399.x. [DOI] [PubMed] [Google Scholar]
- 22.Al-Agha OM, Igbokwe AA. Malignant fibrous histiocytoma: between the past and the present. Arch Path Lab Med. 2008;132(6):1030–1035. doi: 10.5858/2008-132-1030-MFHBTP. [DOI] [PubMed] [Google Scholar]
- 23.Blitzer A, Lawson W, Zak FG, Biller HF, Som ML. Clinicopathological determinants in prognosis of fibrous histiocytomas of head and neck. Laryngoscope. 1981;87(9):2053–2070. doi: 10.1288/00005537-198112000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Allen PW. Myxoid tumors of soft tissues. Path Ann. 1980;15(1):133–192. [PubMed] [Google Scholar]
- 25.Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41(6):2250–2260. doi: 10.1002/1097-0142(197806)41:6<2250::AID-CNCR2820410626>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 26.Kindblom L, Merck C, Angercvall L. The ultrastructre of myxofibrosarcoma: a study of 11 cases. Virchows Arch Anat Pathol. 1979;381:121–139. doi: 10.1007/BF01257879. [DOI] [PubMed] [Google Scholar]
- 27.Hollowood K, Fletcher CDM. Soft tissue sarcomas that mimick benign lesions. Semin Diagn Pathol. 1995;12(1):87–97. [PubMed] [Google Scholar]
- 28.Nuamah IK, Browne RM. Malignant fibrous histiocytoma presenting as a perioral abscess. Int J Oral Maxillofac Surg. 1995;24:158–159. doi: 10.1016/S0901-5027(06)80092-5. [DOI] [PubMed] [Google Scholar]
- 29.Parka SW, Kimb H-J, Leed JH, Koc YH. Malignant fibrous histiocytoma of the head and neck: CT and MRI findings. Am J Neuroradiol. 2009;30:71–76. doi: 10.3174/ajnr.A1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogura JH, Toomey JM, Setzen M, Sobol S. Malignant fibrous histiocytoma of the head and neck. Laryngoscope. 1980;90(9):1429–1440. [PubMed] [Google Scholar]
- 31.Crissman JD, Henson SL. Malignant fibrous histiocytoma of the maxillary sinus. Arch Otolaryngol. 1987;104:228–230. doi: 10.1001/archotol.1978.00790040050010. [DOI] [PubMed] [Google Scholar]
- 32.Schaefer SD, Denton RA, Blend BL, Carder HM (1983) Malignant fibrous histiocytoma of the frontal sinus. Laryngoscope 93(2):2021–2026 [DOI] [PubMed]
- 33.Al-Salihi KA, Al-Jashamy KA, Ab Rahman S, Samsudin AR. Maxilla tuberosity malignant fibrous histiocytoma with giant fibroblastic cells: case report and review of the literature. Oral Oncol Extra. 2006;42(3):112–115. doi: 10.1016/j.ooe.2005.09.013. [DOI] [Google Scholar]
- 34.Barnes L, Kanbour A. Malignant fibrous hiostiocytoma of the head and neck. A report of 12 cases. Arch Otolaryngol Head Neck Surg. 1988;114:1149–1156. doi: 10.1001/archotol.1988.01860220083030. [DOI] [PubMed] [Google Scholar]
- 35.Hale HM, Handlers JP, Abrams AM, Strahs G. Malignant fibrous histiocytoma, myxoid variant metastatic to the oral cavity. Oral Surg Oral Med Oral Pathol. 1981;51(2):156–163. doi: 10.1016/0030-4220(81)90034-7. [DOI] [PubMed] [Google Scholar]
- 36.Lam PK, Trendell-Smith N, Li JHC, Fan YW, Yuen PW. Myxofibrosarcoma of the sphenoid sinus. J Laryngol Otol. 2002;116:464–466. doi: 10.1258/0022215021911086. [DOI] [PubMed] [Google Scholar]
- 37.Enoz M, Suoglu Y (2007) Myxofibrosarcoma of the maxillary sinus. Int J Head and Neck Surg 1(1):1–5
- 38.Shinozaki T, Kato F, Watanabe H, Yanagawa T, Ahmed AR, Takagishi K. Discriminant analysis of prognostic factors for malignant fibrous histiocytoma in soft tissue. J Orthop Sci. 2001;6(4):339–342. doi: 10.1007/s007760100029. [DOI] [PubMed] [Google Scholar]
- 39.Blumber AK, Kay S, Adelaar RS. Nerve sheath myxoma of digital nerve. Cancer. 1989;63:1215–1218. doi: 10.1002/1097-0142(19890315)63:6<1215::AID-CNCR2820630629>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 40.Lagace R, Delage C, Seemayer TA. Myxoid variant of malignant fibrous histiocytoma. Cancer. 1979;43:526–534. doi: 10.1002/1097-0142(197902)43:2<526::AID-CNCR2820430218>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 41.Kreicbergs A, Lonnquist P, Willems J. Chondromyxoid fibroma. A review of the literature and a report on our own experience. Acta Pathol Microbiol Scand A. 1985;93(4):189–197. [PubMed] [Google Scholar]
- 42.Vuzevski VD, Heul RO. Comparative ultrastructure of soft-tissue myxoid tumors. Ultrastr Pathol. 1988;12(1):87–105. doi: 10.3109/01913128809048478. [DOI] [PubMed] [Google Scholar]
- 43.Fujimura T, Okuyama R, Terui T, Okuno K, Masu A, Masu T, Chiba S, et al. Myxofibrosarcoma (MFH) showing cutaneous presentation: report of two cases. J Cut Pathol. 2005;32(7):512–515. doi: 10.1111/j.0303-6987.2005.00368.x. [DOI] [PubMed] [Google Scholar]
- 44.Vasuder S, Harris M. Sarcoma of myofibroblasts. Arch Pathol Lab Med. 1978;102:185–188. [PubMed] [Google Scholar]
- 45.Mentzel T, Dry S, Katenkamp D, Fletcher CD. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22(10):1228–1238. doi: 10.1097/00000478-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Evans HL. Low-grade fibromyxoid sarcoma: a report of the metastatizing neoplasm having a deceptively benign appearance. Am J Clin Pathol. 1987;88(5):615–619. doi: 10.1093/ajcp/88.5.615. [DOI] [PubMed] [Google Scholar]
- 47.Foshini MP, Fulcheri, Boracchini C, Betts CM, Eusebi V. Squamous cell carcinoma with prominent myxoid stroma. Human Pathol. 1990;21:859–865. doi: 10.1016/0046-8177(90)90057-C. [DOI] [PubMed] [Google Scholar]
- 48.Fletcher CD, Gustafson P, Rydholm A, Willen H, Ackerman M. Clinicopathologic re-evaluation of 100 malignant fibrous histiocytomas: prognostic relevance of subclassification. J Clin Oncol. 2001;19(2):3045–3050. doi: 10.1200/JCO.2001.19.12.3045. [DOI] [PubMed] [Google Scholar]
- 49.Suh CH, Ordonez NG, Mackay B. Malignant fibrous histiocytoma: an ultrastructural perspective. Ultrastruct Pathol. 2000;24:243–250. doi: 10.1080/01913120050176699. [DOI] [PubMed] [Google Scholar]
- 50.Bloustein PA. Hepatic leiomyosarcoma; ultrastructural study and review of the differential diagnoses. Human Pathol. 1978;9(6):713–715. doi: 10.1016/S0046-8177(78)80054-9. [DOI] [PubMed] [Google Scholar]
- 51.Evans HL. Low-grade fibromyxoid sarcoma: a report of 12 cases. Am J Surg Pathol. 1993;17(6):595–600. doi: 10.1097/00000478-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Block MS, Cade JE, Rodriquez FH. Malignant fibrous histiocytoma of the maxilla. J Oral Maxillofac Surg. 1986;44:404–412. doi: 10.1016/S0278-2391(86)80039-8. [DOI] [PubMed] [Google Scholar]
- 53.Hirose T, Toshiaki S, Hizawa K. Ultrastructural study of the myxoid area of malignant fibrous histiocytomas. Ultrastruct Pathol. 1988;12:621–630. doi: 10.3109/01913128809056487. [DOI] [PubMed] [Google Scholar]
- 54.Tsuneyoshi M, Hashimoto H, Enjoji M. Myxoid malignant fibrous histiocytoma versus myxoid liposarcoma. Virchows Arch (Anat Pathol) 1983;400:187–199. doi: 10.1007/BF00585500. [DOI] [PubMed] [Google Scholar]
- 55.Matushansky I, Charytonowicz E, Mills J, Siddiqqi S, Hricik T, Cordon-Cardo C. MFH classification: differentiating undifferentiated pleomorphic sarcoma in the 21st century. Expert Rev Anticancer Ther. 2009;9(8):1113–1144. doi: 10.1586/era.09.76. [DOI] [PMC free article] [PubMed] [Google Scholar]