Abstract
A method is described to generate and validate antibodies based on mapping the linear epitopes of a polyclonal antibody followed by sequential epitope-specific capture using synthetic peptides. Polyclonal antibodies directed towards four proteins RBM3, SATB2, ANLN, and CNDP1, potentially involved in human cancers, were selected and antibodies to several non-overlapping epitopes were generated and subsequently validated by Western blot, immunohistochemistry, and immunofluorescence. For all four proteins, a dramatic difference in functionality could be observed for these monospecific antibodies directed to the different epitopes. In each case, at least one antibody was obtained with full functionality across all applications, while other epitope-specific fractions showed no or little functionality. These results present a path forward to use the mapped binding sites of polyclonal antibodies to generate epitope-specific antibodies, providing an attractive approach for large-scale efforts to characterize the human proteome by antibodies.
Keywords: epitope mapping, monospecific antibody, affinity chromatography
Introduction
One of the challenges in the study of human biology and disease is the lack of well-validated antibodies to many of the human proteins.1 This has led to several initiatives to systematically generate antibodies, such as the Human Protein Atlas,2 the SH2-consortium,3 protein binder consortiums,4 and efforts to generate antibodies to cancer-related targets.5 Together with complementary efforts from numerous commercial providers, this has greatly increased the availability of antibodies to human targets during the last few years. Recently, a milestone was achieved by the Human Protein Atlas effort with the inclusion of antibody-based data for more than 50% of the human protein-encoding genes.6 The Antibodypedia portal7 listing publicly available antibodies to human proteins currently includes approximately 150,000 antibodies corresponding to more than 70% (n = 15,000) of the human protein-encoding genes. The availability of well-validated antibodies provides a valuable resource for functional studies of the corresponding proteins and facilitates the systematic identification of protein profiles, including subcellular locations and tissue-specificity.
Today, more than 70% of the antibodies in Antibodypedia and 80% of the antibodies in the Human Protein Atlas are polyclonal antibodies. These antibodies have the advantageous characteristic of being directed to several binding sites (epitopes) of the target protein, but this also implies that binding to multiple epitopes can increase the risk of cross-specificity towards other proteins. Furthermore, polyclonal antibodies exhibit limitations with regards to renewability, due to the limited amounts obtained from single immunizations and the batch-to-batch variations obtained when several immunizations are performed to generate larger quantities of antibodies.8 This emphasizes the need for the development of single epitope-specific antibodies with defined binding sites of the target proteins. This could be achieved with the generation of monoclonal antibodies or recombinant affinity reagents, but an alternative might be to use the multiple binding sites of polyclonal antibodies to generate one or several epitope-specific antibodies as an alternative to monoclonal antibodies. In this manner, the already existing tens of thousands of polyclonal antibodies could be used to create a valuable resource of epitope-specific antibodies.
Here, we describe such a strategy based on epitope mapping using peptide bead arrays and affinity purification using synthetic peptides. Four proteins implicated as potential biomarkers for various human cancers, including breast, colorectal, lung, and prostate cancer, were chosen as targets for the approach. In all cases, monospecific antibodies were generated and subsequently used for the analysis across several immunological platforms, including Western blot, immunohistochemistry, immunofluorescence, and sandwich immunoassays.
Results
The principle for generation of monospecific antibodies
A method to generate epitope-specific antibodies based on sequential affinity purification of polyclonal sera has been developed as outlined schematically in Figure 1. The linear epitopes of the polyclonal antibody is determined by overlapping synthetic peptides as pioneered already in 1987 by Geysen et al.,9 but here using a suspension bead array approach to allow high-throughput, multiplex assays. The synthetic peptides spanning the identified epitopes are immobilized to a solid support and used for specific affinity capture. Since the polyclonal antibodies have been generated by a fusion protein containing a solubility-enhancing tag, a matrix with the fusion tag is used prior to the peptide-specific affinity capture to deplete all antibodies towards the tag. The remaining polyclonal antibodies are passed through a series of peptide columns followed by a final column comprising the fusion protein, which was used for immunization. The last column thus captures all antigen-specific antibodies that did not bind to any of the linear epitopes in the previous columns. An automated chromatography system was used to allow affinity capture, washing, and elution in a standardized manner. The various columns were eluted separately with low pH buffer to recover the various monospecific fractions and one last fraction with antibodies specific for the remaining epitopes. The different fractions are subsequently mapped using the peptide bead arrays to confirm the epitopes of the antibodies.
Figure 1.
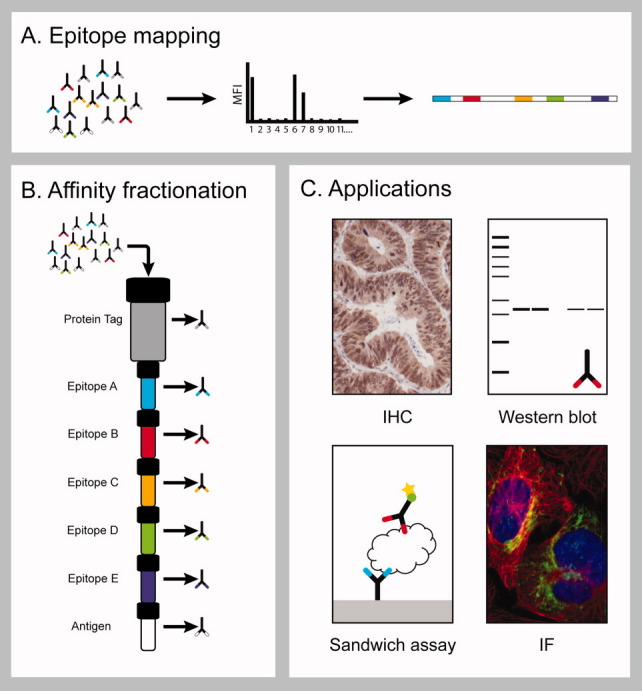
The principle for generation of monospecific antibodies. The polyclonal serum is assayed with a peptide array spanning the antigen sequence to identify the epitopes of the antibodies (A). The epitope corresponding peptides as well as the recombinant antigen are coupled to columns which are mounted in a serial manner after a protein tag specific column onto which the polyclonal serum is applied (B). Bound antibodies are eluted in parallel to create monospecific antibodies which are evaluated in different paired antibody applications (C).
Monospecific antibodies towards the human RNA binding protein RBM3
The presence of human RNA binding protein RBM3 is associated with good prognosis in both breast cancer10 and epithelial ovarian cancer.11 Thus, antibodies towards this interesting target were generated via a 134 amino acids antigen fragment of RBM3 and epitope mapped. A suspension bead array was set-up using 15-mer peptides with a lateral shift of five amino acids. The analysis revealed five distinct linear epitopes as outlined in Figure 2 with predominant binding to peptides 6 and 24. The peptides corresponding to the epitopes (peptide 1, 6, 15, 19, and 24) were coupled to columns and used for the affinity purification. All fractions revealed monospecific binding characteristics with high selectivity for the corresponding peptide (Fig. 2), except antibodies purified against peptide 6, which showed some cross-reactivity towards peptide 15 possibly due to a homologous stretch in the center of the two peptides (RGFGF and RGGGF). In Western blotting, three of the monospecific antibodies (peptide 6, 19, and 24) revealed a band of predicted mass, while the monospecific antibodies towards peptides 1 and 15 were found to be nonfunctional using the applied Western blot protocol (Supporting Information Fig. 1). The results for the immunohistochemistry analysis showed that only two of the epitope-specific fractions stained breast cancer cells with expected nuclear positivity, while all other fractions yielded negative staining (Fig. 2). Similarly, the immunofluorescence analysis showed nuclear staining by the original polyclonal antibody and the epitope-specific fractions from peptide 6, 19, and 24, while the fractions towards peptide 1 and 15 gave no specific staining (Supporting Information Fig. 2).
Figure 2.
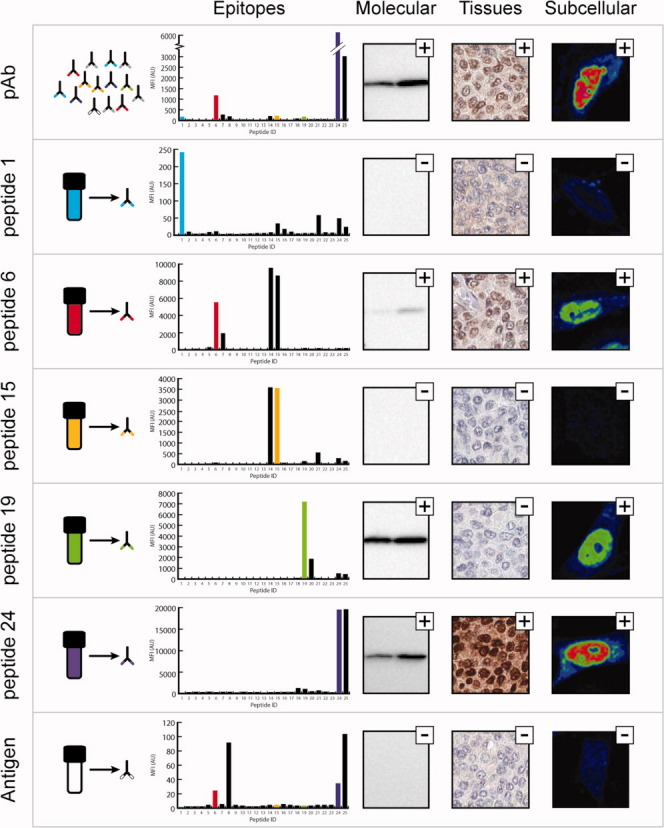
Generation of monospecific antibodies towards the human RNA-binding protein RBM3. Each panel shows the epitopes, the molecular identification in Western blot (Lane 1: RT-4 cell lysate Lane 2: U-251 MG cell lysate), staining of breast cancer tissue, and subcellular localization in U-251 MG cells (images are shown in a rainbow RGB look up table (LUT) where red indicates strong signal and blue low signal) for the polyclonal serum purified from tag specific antibodies (pAb) targeting RBM3, five monospecific and one conformational antibody purified from the polyclonal sera, respectively. The peptide selected for purification are indicated in the epitope mapping barplots in different colors, where the x-axis represents the peptide ID and the y-axis indicate the mean fluorescence intensity. A small plus indicate the detection of a band of correct molecular mass, the right staining or localization according to literature while a minus indicate aberrant or negative result.
In summary, the results suggest that out of the five epitopes recognized by the polyclonal antibody, two antibodies (towards peptides 1 and 15) are not functional in any of the applied assays, while two of the epitope-specific fractions (towards peptides 6 and 24) show specific staining in all three applications tested here. The antibody toward peptide 19 yields good results in Western blots and immunofluorescence, but fails to generate specific staining in immunohistochemistry. Thus, the affinity purification has revealed two independent monospecific antibodies with specific staining across the three application platforms. Since these antibodies have non-overlapping epitopes they can be used as paired antibodies to generate a knowledge-based interpretation, in which the staining of the two antibodies in all the different tissues is compared to generate annotated protein expression.6 In Supporting Information Figure 3, the protein annotations based on the staining of the polyclonal antibodies and the two monospecific antibodies towards peptide 6 and 24 are shown. The data suggest that human RBM3 is expressed in a tissue-specific manner in the epithelial cells of the respiratory system and oral mucosa, as well as the glandular cells of the breast and fallopian tube. The protein is also expressed in the heart and in a subset of cells in the hematopoietic system.
Monospecific antibodies towards the human transcription factor SATB2
The human transcription factor SATB2 is suggested to be a diagnostic biomarker in colorectal cancer that can be used in differential diagnostics to indicate whether a metastasis is of colorectal origin.12 The protein is highly homologous to the transcription factor SATB1, and therefore an affinity reagent specific for SATB2 is difficult to achieve. A 123 amino acid fragment of SATB2 was used to generate a polyclonal antibody and epitope mapping using a suspension bead array revealed several major epitopes (Fig. 3). Five peptides (peptides 3, 6, 10, 13, and 18) were chosen for affinity purification to generate monospecific antibodies and the re-mapping of the purified fractions demonstrates high specific binding to the expected peptide epitopes (Fig. 3). The application specific validation demonstrated that only one of the five monospecific antibodies (peptide 18) yielded desired staining, while all the others, including the recombinant antigen fraction obtained after the capture of the five epitope-specific fractions, showed negative staining both in the Western blot analysis and the immunofluorescence application (Fig. 3 and Supporting Information Figs. 4 and 5). Interestingly, peptide 18 has very low sequence identity to the related transcription factor SATB1, while some of the other epitopes show very high sequence identity (Supporting Information Fig. 6). This suggests that the cross-reactivity to the homologous transcription factor SATB1 can be avoided using the monospecific antibody towards peptide 18.
Figure 3.
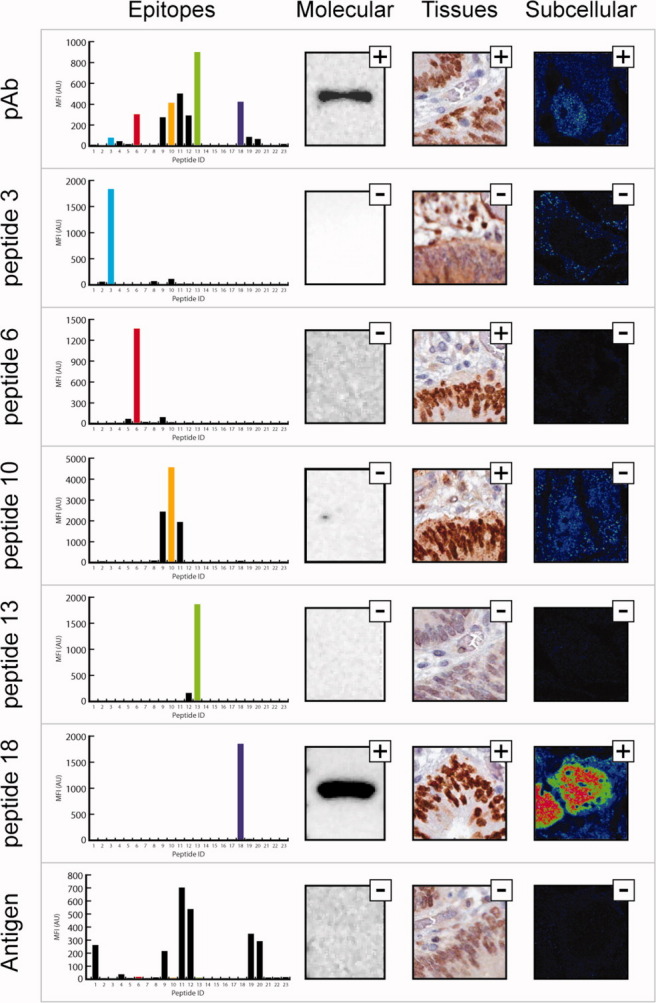
Generation of monospecific antibodies towards the human transcription factor SATB2. Each panel shows the epitopes, the molecular identification in Western blot (Lane 1: Caco-2 cell lysate), staining of colon cancer tissue, and subcellular localization in CACO-2 cells for the polyclonal sera purified from tag specific antibodies (pAb) targeting SATB2 and five monospecific and one conformational antibody purified from polyclonal sera, respectively.
A tissue microarray with 46 different tissues from three independent individuals was stained with monospecific antibodies generated by affinity chromatography using peptide 18, as well as the original polyclonal antibody. The monospecific fraction towards peptide 6 was also included in the analysis to generate IHC staining with an antibody with a non-overlapping epitope as compared to peptide 18. The staining in the different tissues with the three antibody fractions were annotated by certified pathologists and the staining patterns were compared to determine an annotated protein expression score. The results suggest a tissue-specific expression of the human transcription factor SATB2 restricted to the lower GI-tract, more specifically to the glandular cells in the appendix, colon, and rectum, but not to the stomach, duodenum and small intestine (Supporting Information Fig. 7). SATB2 is also expressed in a subset of neuronal cells in the cerebral cortex and lateral ventricle of the brain.
Monospecific antibodies towards the human actin binding protein ANLN
The human actin binding protein ANLN is a protein involved in cytokinesis13 and has been implicated as a biomarker for lung carcinogenesis.14 A polyclonal antibody has been generated towards a 136 amino acids fragment of ANLN and this polyclonal antibody showed adequate specificity in three applications (Fig. 4). However, the staining with the polyclonal antibody reveals a weak cytoplasmic staining in addition to the expected strong staining of the nucleus (Fig. 4). The epitope mapping of the polyclonal antibody showed three dominating epitopes and some additional subordinate epitopes (Fig. 4). Four peptides corresponding to one minor (peptide 5) and three major (peptides 7, 14, and 25) epitopes were synthesized and used for affinity purification. Re-mapping of the epitope-specific antibodies showed the expected specificity of all the antibodies to the respective peptides (Fig 4). Again, only one monospecific antibody (peptide 14) showed specific staining across all three applications (Fig. 4 and Supporting Information Figs. 8 and 9). This antibody towards peptide 14 showed exclusive nuclear staining in the immunofluorescence, while the antibodies towards peptide 7 and 25 yielded mainly cytoplasmic staining with a vesicle-like pattern.
Figure 4.
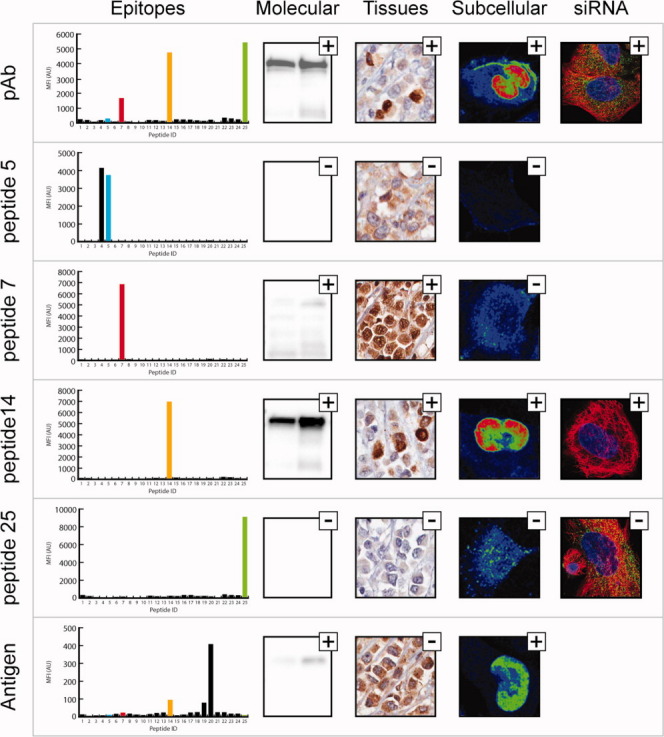
Generation of monospecific antibodies towards the human enzyme ANLN. Each panel shows the epitopes, the molecular identification in Western blot (Lane 1: RT-4 cell lysate Lane 2: U-251 MG cell lysate), staining of breast cancer tissue, and subcellular localization in U-251 MG cells for the polyclonal purified from tag specific antibodies (pAb), four monospecific and one conformational antibody targeting ANLN respectively. To the right, staining of ANLN siRNA treated U-251 MG cells, microtubuli (red), nucleus (blue), and respective antibody in green.
The separation of vesicle staining (with no support from literature) from nucleus staining in the different monospecific antibodies is an interesting observation. To further investigate this phenomenon, we performed protein knockdown experiments in human U-2 OS cells treated with siRNA corresponding to the human ANLN gene. The cells, with or without ANLN targeted siRNA treatment, were stained after 3 days using the original polyclonal antibody and the monospecific antibodies against peptides 14 and 25, respectively. The result demonstrated that the nuclear staining of the polyclonal antibody and the monospecific antibody towards peptide 14 were significantly down-regulated upon ANLN targeted siRNA-treatment (Fig. 4 and Supporting Information Fig. 10), while the vesicle staining by the polyclonal antibody and monospecific antibody to peptide 25 were not affected by the siRNA treatment. These results support the conclusion that the nuclear staining is specific for the ANLN protein target, while the weak vesicle staining is due to cross-reactivity to another unknown human protein. Thus, the monospecific antibody towards peptide 14 has improved specificity as compared to the original polyclonal antibody.
Since only one of the four epitopes (peptide 14) was found to give desired specificity in the applications tested here, no annotated expression profile using two independent monospecific antibodies with non-overlapping epitopes was achieved. However, tissue microarrays containing 46 different tissues were stained with the original antibody or the monospecific antibody towards peptide 14 and the staining of the 65 cell types were scored by a certified pathologist. The results are shown in Supporting Information Figure 11 suggesting a relatively ubiquitous nuclear expression across human tissues. Interestingly, the comparison suggests that the protein is not expressed in the brain (cerebellum and cortex) as was suggested using the original polyclonal antibody. This cytoplasmic annotation in the brain could hence be assumed to originate from the cross-specificity of antibodies binding to peptide 25.
Monospecific antibodies towards the human enzyme CNDP1
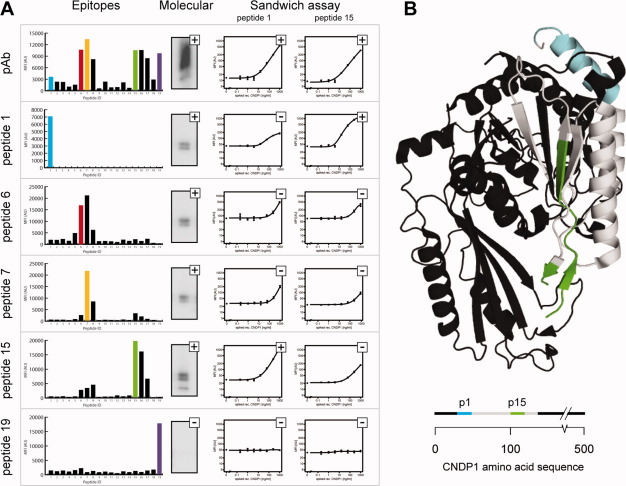
Recently, the human dipeptidase CNDP1 was described as a potential biomarker in plasma for prostate cancer as a complement to the biomarker prostate-specific antigen (PSA).15 To investigate the role of this potential biomarker, sandwich immunoassays based on monospecific antibodies were developed and evaluated. An N-terminally located 101 amino acid fragment of the protein was used as immunogen to produce a polyclonal antibody, which was epitope mapped to generate a binding profile [Fig. 5(A)]. Five peptides corresponding to the main epitopes were employed for affinity purification and epitope re-mapping. All epitope-specific antibodies except one corresponding to peptide 19 detected a band of predicted molecular mass (57 kDa) in Western blot (Supporting Information Fig. 12). The functionality of these epitope-specific antibodies was examined in a multiplexed fashion using a 6-plex bead-based sandwich immunoassay to detect CNDP1 spiked into in human plasma. Dilution series of recombinant CNDP1 were analyzed with 36 sandwich combinations built on monospecific and polyclonal antibody pairs, where all antibodies served as capture or/and detection reagents. From a first screening, relative detection limit levels were determined to rank antibody pairs and to judge their compatibility (Supporting Information Table 1). Identical antibodies were found to be functional sandwich pairs, suggesting that CNDP1 is detected in a homodimeric form.16 Here the monospecific antibodies for peptide 1 and 15 showed the most promising results and titration experiments were repeated using these detection candidates in combination with all capture antibodies. This revealed concordant results to the screen, low coefficients of variation, and limits of detection around 10 ng/mL (Supporting Information Table 1). As shown from the 3-dimensional structure of the native protein [Fig 5(B)], the binding sites for the antibodies towards peptide 1 and 15 are exposed on the surface of the protein. The results confirm that paired antibodies selected by affinity purification from a single polyclonal antibody serum are functional as capture and detection reagents in a sandwich-based immunoassay.
Figure 5.
Generation of monospecific antibodies towards the human enzyme CNDP1. A: In the left panel, epitopes and molecular identification in Western blot (Lane 1: Purified recombinant protein CNDP1) is shown for the polyclonal sera purified from tag specific antibodies (pAb) and five monospecific antibodies purified from the sera. To the right, titration curves resulting from the detection of CNDP1 spiked into plasma are shown. Sandwich pairs built on monospecific antibodies were compared using a 6-plex bead array, and two detection antibody candidates are presented in combination with all six antibodies as capture reagents. Rating a pair as functional was based on a relative detection limit for CNDP1 <10 ng/mL and is indicated with a plus. B: Crystal structure of CNDP1 (http://www.pdb.org/pdb/explore/explore.do?pdbId=3DLJ) with peptide 1 (cyan) and peptide 15 (green) indicated. Below a linear view of the amino acid sequence of CNDP1 with the antigen used for generation of polyclonal antibody marked in grey.
Generation of monoclonal antibodies against the RBM3 epitope
The method described above shows a path forward to design epitopes for generation of monoclonal antibodies. To test this hypothesis, synthetic peptides corresponding to peptides 6 and 24 of different length of the RBM3 antigen (Fig. 2 and Supporting Information Fig. 13) was used for immunization and subsequent generation of mouse hybridoma cells. The supernatants of the hybridomas generated in this manner were screened for antigen binding and then tested for functionality in Western blots using RT-4 cell lysates and immunohistochemistry using tissue microarrays.17 The Western blot analysis (Supporting Information Fig. 13) shows that monoclonal antibodies were obtained with the same staining as the corresponding monospecific antibodies.
Discussion
We here describe a concept to generate epitope-specific antibodies for use in different assays relevant in research and clinical diagnostics. In all cases, four to five linear epitopes were observed for these antigens that were between 100 and 130 amino acids in length, selected on their low sequence identity to other human proteins. Dramatic differences in the functionality of the various monospecific antibodies generated towards these epitopes were observed for all four targets. Interestingly, some epitopes gave antibodies functional across all applications tested, while epitope-specific antibodies towards other binding regions were not functional in any of the assays. For the human protein ANLN, the analysis, supported by an siRNA knock-down assay, shows that the polyclonal antibody has two staining patterns, nuclear and cytoplasmic, respectively, and the evidence suggests that only the nuclear staining represents binding to the expected target and that one of the monospecific antibodies show this specificity.
It is noteworthy that some of the epitope-specific antibodies do not show any binding to the target protein in the subsequent Western blot analysis, although the proteins normally are considered as denatured in such an analysis. This suggests that the epitope of the target protein is hidden from active binding to the antibody protein target during this analysis, as a result of binding the target protein to the blotting membrane and removing the detergent sodium dodecyl sulfate (SDS). One could speculate that this could be due to either partial renaturation of the target protein or, on the other hand, proteins collapsing into an insoluble complex.
This method of generating monospecific antibodies allows for an efficient way to validate different epitopes across several application platforms, such as Western blots, immunhistochemstry, and immunofluorescence, as well as creating a path for developing sandwich immunoassays for clinical diagnostics. For some of the targets, it was possible to generate paired antibodies towards separate and non-overlapping epitopes of the proteins to allow for annotated protein expression6 in which the staining of one antibody is validated by the staining of a second independent antibody. We also show that sandwich assays with separate capture and detection antibodies can be developed using this approach starting from a single polyclonal antibody.
Several reports18,19 have suggested that the majority of antibodies recognize conformational epitopes consisting of amino acids that are brought together in space by the folding of several chains of the protein target. In contrast, the method described here relies on antibodies binding to linear epitopes, while the conformational epitopes are only recovered on the last antigen matrix. For all the four targets, it was possible to recover a majority of the polyclonal antibodies using the synthetic peptides suggesting that the strategy to generate polyclonal antibodies using recombinant protein fragments yield a large fraction of linear epitopes. The fraction linear epitopes ranged from 49% in the case of the protein target RBM3 to 94% for the protein ANLN. It is noteworthy that the polyclonal antibodies used in this study are directed to protein fragments of approximately 100 amino acids. Obviously, this might cause large part of the antigen to not have a native fold. The conformational antibodies showed in all cases weak or no staining indicating that linear epitopes perform better in the applications used here, which is not unexpected, since the applications Western blot, immunohistochemistry, and immunofluorescence all involve sample treatment where the analyzed sample is challenged with agents known to denature proteins, such as SDS, heat or alcohol.
The method described here relies on a precise mapping of the polyclonal antibody to establish the epitopes of the original antigen used for immunization. We have used suspension bead arrays with overlapping synthetic peptides, which requires relatively large numbers of peptides synthesized individually. In the future, it might be more convenient to use in situ synthesized peptides on planar microarrays for the mapping effort to limit the synthesis of individual peptides to the epitopes identified by high-density peptide arrays. Recently, we have obtained high-quality, reproducible results from such an approach with synthetic peptides of the length 10–20 amino acids generated by photo-activated in situ chemical synthesis on a microarray with more than 100,000 available spots (Rockberg, Schaffer and Uhlen, unpublished). Alternatively, it is also possible to use a bacterial display method,20 in which the epitopes are mapped using cell sorting and sequencing of the inserts of bacterial cells displaying various fragments of the antigen on the surface. Together, these methods provide a powerful toolbox for mapping the binding sites of antibodies to enable the generation of monospecific antibodies as described in this article.
It is noteworthy that the method described here provides a strategy to produce monoclonal antibodies in a systematic manner based on the information generated by prior analysis of the functionality of a polyclonal antibody. Several monospecific antibodies generated from a single polyclonal antibody can be tested in relevant application-specific assays and antibodies towards the epitopes showing good functionality across these assays can subsequently be used as a lead of peptide selection to generate monoclonal antibodies. Here, the results from the monospecific antibodies were used to guide the generation of the monoclonal antibodies towards human RBM3. Synthetic peptides corresponding to the epitopes, shown to give functional monospecific antibodies, were used for the immunization to generate hybridoma cells, but alternatively one could also use the original antigen for the immunization and then use the peptide as a tool for screening antibodies to suitable epitopes during the hybridoma selection.
Here, we have used the word monospecific or epitope-specific antibody to describe the fraction of the polyclonal antibody obtained after affinity purification using a synthetic peptides corresponding to a single epitope. We are aware that the word monospecific has been used earlier by us21 to describe an affinity-purified polyclonal using the antigen as ligand, but since these antibodies recognize several epitopes, we suggest that these type of antibodies should instead be called antigen-purified polyclonal antibodies, while the term monospecific antibody should be restricted to polyclonal antibodies specific for a single epitope. This applies to both epitope purified and peptide immunized polyclonal antibodies.
In summary, we describe a method for generation of monospecific antibodies based on epitope-specific affinity purification of polyclonal antibodies. The study demonstrates that specificity and functionality of the various epitopes from a single polyclonal antibody differs across various application platforms and the method can thus be used to facilitate the establishment of suitable binding sites to generate specific antibodies with low cross-reactivity across the human proteins. The results suggest that this method is an attractive tool to systematically explore the human proteome, including efforts to use the information from polyclonal antibodies to guide the generation of a second wave of monoclonal antibodies or other renewable antibodies, such as aptamers,22,23 recombinant antibody fragments,24 or other protein scaffolds.25 The generation of epitope-specific antibodies from polyclonal antibodies is thus an attractive method for large-scale efforts to characterize the human proteome by antibodies.
Material and Methods
Generation of polyclonal sera
Antigens were designed using the software PRESTIGE.26 Gene fragments were amplified from a pool of RNA isolated from human tissues and cloned into a vector and expressed in Escherichia coli. The purified and validated recombinant protein fusions were used for immunization of New Zealand White rabbits.27
Epitope mapping of polyclonal sera
Polyclonal sera were mapped using 15mer peptides as described previously.28
Affinity purification of polyclonal sera
Six hundred nanomoles of each biotinylated peptide (provided by Sigma-Aldrich, St Louis, MO) was applied to 1 mL HiTrap™ Streptavidin HP columns (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) for binding. Approximately, 8–12 mL of sera was purified on a ÄKTAxpress™ (GE Healthcare) liquid chromatography system on respective columns in a serial manner as indicated by Figures 1–5. After sample loading, the columns were washed and eluted in parallel to obtain separate monospecific antibody fractions. The eluted monospecific antibodies were epitope mapped as described above.
Western blot
Antibodies were analyzed by running approximately 15 μg of total protein lysate from respective cell line (RT-4, U-251 MG, Caco-2) or recombinant protein (CNDP1 (NM_032649), Origene, Rockville, MD) on precast 10–20% Criterion™ SDS-PAGE gradient gels (Bio-Rad Laboratories, Hercules, CA). The proteins were separated under reducing conditions, followed by electroblotting to PVDF membranes using Criterion GelTM Blotting Sandwiches (Bio-Rad Laboratories, Hercules, CA), all according to the manufacturer's recommendations. The SDS-PAGE gel was stained using GelCode® Blue Stain Reagent (Pierce, Rockford, IL) and the membranes were blocked (5% dry milk, 0.5% Tween20, 1× TBS; 0.1M Tris-HCl, 0.5M NaCl) for 1 h at RT prior to addition of antibodies. After incubation for 1 h with the primary antibodies, diluted 1/250–3000 in blocking buffer, the membranes were washed 4× 5 min in 1× TBS with 0.05% Tween20. The secondary HRP-conjugated swine anti-rabbit or anti-mouse antibody (DakoCytomation, Glostrup, Denmark) was diluted 1/3000 in blocking buffer and incubated for 1 h before a final round of washing to remove unbound material. Chemiluminescence detection was carried out using a Chemidoc CCD-camera system (Bio-Rad Laboratories) with SuperSignal® West Dura Extended Duration Substrate (Pierce) according to the manufacturer's protocol.
Immunohistochemistry
Automated immunohistochemistry was performed on 4 μm sections from tissue microarrays with human tissue from formalin fixed paraffin embedded specimens. Glass slides were baked at 50°C over night, deparaffinized in xylene, hydrated in graded ethanol, and blocked for endogenous peroxidase in 0.3% hydrogen peroxide in an Autostainer XL (Leica Microsystems, Wetzlar, Germany) before antigen retrieval was performed by boiling for 4 min at 125°C in citrate buffer, pH 6.0 (Thermo Fisher Scientific, Waltham, MA) in a Decloaking chamber (Biocare Medical, Walnut Creek, CA). Slides were immunostained in an automated staining instrument, Lab Vision Autostainer 480 (Thermo Fisher Scientific, Waltham, MA). Primary antibodies, diluted in UltraAb Diluent (Thermo Fisher Scientific, Waltham, MA), were incubated on the slides for 30 min at room temperature. For detection, the slides were incubated with secondary anti-rabbit antibodies conjugated to a horse radish peroxidase labeled polymer, UltraVision LP detection system (Thermo Fisher Scientific, Waltham, MA), for 30 min at room temperature. To develop the slides, DAB (diaminobenzidine) Plus Chromogen (Thermo Fisher Scientific, Waltham, MA) was diluted 1:25 and incubated with tissue sections for 10 min at room temperature. Counterstaining was performed using Mayers hematoxylin (Histolab, Gothenburg, Sweden) for 5 min. The slides were then rinsed in lithium carbonate water diluted 1:5, tap water, and dehydrated in graded ethanol and Tissue-Tek Tissue-Clear (Sakura Finetek, Tokyo, Japan) before mounting them with Pertex (Histolab, Gothenburg, Sweden). Scanning of the slides was performed using a Scanscope XT scanner (Aperio, Vista, CA).
Immunofluorescence
The cell lines used in this study were a glioblastoma cell line U-251 MG provided by Prof. Bengt Westermark, Uppsala University (Uppsala, Sweden), a human epithelial colorectal adenocarcinoma cell line Caco-2 (DSMZ, GmbH Germany), and an osteosarcoma cell line U-2 OS (ATCC-LGC Promochem, Borås, Sweden). Cells were grown in 37°C in a 5% CO2 environment in culture media recommended by the provider with the addition of 10% fetal bovine serum (FBS). For immunofluorescent experiments, 96-well glass bottom plates (Whatman Inc, Florham Park, USA) were coated with 12.5 μg/mL fibronectin for 1 h at room temperature before 15,000 cells were seeded per well and allowed to attach for 6 h before fixation. Cells were washed with ice-cold phosphate-buffered saline, PBS, (8 mM Na2HPO4, 2 mM NaH2PO4, 150 mM NaCl, pH 7.2) before fixation and permeabilization. The cells were treated for 15 min with freshly prepared ice-cold 4% paraformaldehyde (PFA) in growth media supplemented with 10% FBS. For permeabilization, 50 μL of 0.1% (w/v) of Triton X-100 in PBS was added three times for 5 min per round. Primary antibody was added at a concentration of 2 μg/mL together with mouse anti-tubulin as well as chicken anti-calreticulin dissolved to 1 μg/mL in blocking buffer (PBS supplemented with 4% FBS) and incubated over night in 4°C. After washing, secondary antibodies (goat anti-rabbit Alexa 488, goat anti-mouse Alexa 555, and goat anti-chicken Alexa 647, all from Invitrogen) diluted to 1 μg/mL were added in blocking buffer and incubated for 90 min at room temperature. 300 nM of the nuclear marker DAPI was added for 5 min before the wells were washed with PBS. Finally, wells were filled with PBS containing 78% glycerol prior to imaging.
Image acquisition and image processing
Images were acquired with a Leica SP5 Laser confocal microscope (Leica Microsystems, Mannheim, Germany) with a ×63 1.4 N/A oil immersion objective and the following scanning settings: 16 bit, 600 Hz, line average 2, and a pixel size of 0.08 μm × 0.08 μm. For each antibody (targeting RBM3, ANLN, and SATB2), the laser power and detector gain was adjusted to the antibody (the polyclonal or any of the monospecific) showing the most intense staining. Settings were then fixed during imaging of all antibodies to the same target protein, thus enabling relative signal intensity. Images were cropped and smoothed in ImageJ (http://imagej.nih.gov/ij/, US National Institutes of Health, Bethesda, MD) and a rainbow RGB look up table (LUT) was applied to better visualize differences in staining intensity between the antibodies used.
SiRNA knockdown of ANLN protein expression
Two ANLN targeting Silencer Select siRNAs s28983 and s28984, and as a negative control a scrambled siRNA s229174 (all from Ambion), were used for protein knock down experiments in a solid phase set-up. Each siRNA was coated together with lipofectamine 2000 (Invitrogen) and lyophilized into separate wells in 96-well glass bottom plates.29 2500 U-2 OS cells per well were then seeded from a 70% confluent culture, and continuously transfected for 72 h before fixation and immunostaining. Images were acquired automatically using a Leica SP5 laser-scanning confocal microscope and the Leica Matrix Screener v.s 2.3.0 software (Leica Microsystems, Mannheim, Germany). A ×10 N/A 0.3 objective was used with the following scanning settings: 8 bit, 600 Hz, line average 1, and a pixel size of 0.75 μm × 0.75 μm. Laser and detection gain was adjusted to the negative control before imaging was initiated and images were acquired at four different positions in each siRNA well. The microtubule antibody staining was used for contrast-based autofocusing prior to sequential scanning of the DAPI, microtubule- and ANLN antibody staining of each position. Quantitative image analysis was performed using the program Cell Profiler30 where the integrated fluorescence intensity of the ANLN staining was normalized to the microtubule antibody staining for each cell in each of the siRNA populations (siRNA1, siRNA2, and scrambled). The distribution of normalized ANLN fluorescence intensities within each population and the median from siRNA1 and siRNA2, respectively, was compared to the scrambled siRNA to calculate the relative fluorescence intensity (RFI). A P-value for significance testing was also calculated using a Mann–Whitney test (Stadler et al., unpublished).
Sandwich immunoassays
One polyclonal and 5 monospecific antibodies targeting CNDP1 were employed both as capture and detection reagents in a multiplexed bead based sandwich immunoassay. All six antibodies were coupled to magnetic and carboxylated beads (MagPlex, Luminex Corp.) and validated for coupling efficiency both as previously described.15 All six antibodies were also labeled with biotin (NHS-PEG4-Biotin, Pierce) at a 50-fold molar excess over 2 h at 4°C and stored with adding Tris-HCl (pH 8.0). For the assay, plasma was diluted 1:10 with 1× PBS, spiked with 0–1 μg/mL recombinant CNDP1 (Origene), and diluted 1:50 in a buffer composed of 0.5% (w/v) polyvinylalcohol and 0.8% (w/v) polyvinylpyrrolidone in 0.1% casein (all Sigma) in 1× PBS supplemented with rabbit IgG (0.5 mg/mL, Bethyl Laboratories). Samples were heat treated at 56°C for 30 min before 45 μL were combined with 5 μL of the 6-plex bead array. Incubation took place in a 96-well half area plate (Greiner Bio-One) and for 16 h at RT under permanent shaking. Beads were washed with 3× 100 μL of PBST (1× PBS, pH 7.4, 0.05% Tween20) with plates hosted on a magnet (LifeSep96F, Dexter Magnet Technologies). Detection antibodies were added at 1 μg/mL in PBS and incubated for 1 h at RT under permanent shaking. The beads were washed, and PBS with R-phycoerythrin labeled streptavidin (0.5 μg/mL, Invitrogen) was added, incubated for 20 min, and beads were washed before a final 100 μL PBST were added. Beads were measured using a LX200 instrument (Luminex Corp.) to record median fluorescence intensities (MFI) from at least 50 events per bead identity. An apparent detection limit was assessed for each antibody pair via a five-parametric logistic regression as the concentration of spiked antigen corresponding to MFI values 30% above background. The detection antibodies with favored detection limits were confirmed during a second experiment that included technical replicates and to determine the detection limits as 3× standard deviation above the background.
Generation of monolclonal antibodies
Peptides CTQRSRGFGFITFTNP, CTQRSRGFGFITFTNPEHASV, CRDYNGRNQGGYDRYS, and CRDYNGRNQGGYDRYSGGNY were used for production of monoclonal antibodies by Absea Biotechnology Ltd., Beijing, China.
Acknowledgments
The authors would like to acknowledge the entire staff of the Human Protein Atlas project and Martin Hjelmare for preparing the siRNA transfection plates.
Supplementary material
References
- 1.Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- 2.Berglund L, Bjoerling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CAK, Persson A, Ottosson J, Wernerus H, Nilsson P, Lundberg E, Sivertsson A, Navani S, Wester K, Kampf C, Hober S, Ponten F, Uhlen M. A genecentric human protein atlas for expression profiles based on antibodies. Mol Cell Proteomics. 2008;7:2019–2027. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 3.Uhlen M, Graslund S, Sundstrom M. A pilot project to generate affinity reagents to human proteins. Nat Methods. 2008;5:854–855. doi: 10.1038/nmeth1008-854. [DOI] [PubMed] [Google Scholar]
- 4.Gloriam DE, Orchard S, Bertinetti D, Bjorling E, Bongcam-Rudloff E, Borrebaeck CAK, Bourbeillon J, Bradbury ARM, de Daruvar A, Dubel S, Frank R, Gibson TJ, Gold L, Haslam N, Herberg FW, Hiltke T, Hoheisel JD, Kerrien S, Koegl M, Konthur Z, Korn B, Landegren U, Montecchi-Palazzi L, Palcy S, Rodriguez H, Schweinsberg S, Sievert V, Stoevesandt O, Taussig MJ, Ueffing M, Uhlen M, van der Maarel S, Wingren C, Woollard P, Sherman DJ, Hermjakob H. A community standard format for the representation of protein affinity reagents. Mol Cell Proteomics. 2010;9:1–10. doi: 10.1074/mcp.M900185-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bjorling E, Lindskog C, Oksvold P, Linne J, Kampf C, Hober S, Uhlen M, Ponten F. A web-based tool for in silico biomarker discovery based on tissue-specific protein profiles in normal and cancer tissues. Mol Cell Proteomics. 2008;7:825–844. doi: 10.1074/mcp.M700411-MCP200. [DOI] [PubMed] [Google Scholar]
- 6.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, Zwahlen M, Kampf C, Wester K, Hober S, Wernerus H, Bjorling L, Ponten F. Towards a knowledge-based human protein atlas. Nat Biotechnol. 2010;28:1248–1250. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 7.Kiermer V. Antibodypedia. Nat Methods. 2008;5:860. [Google Scholar]
- 8.Larsson K, Eriksson C, Schwenk JM, Berglund L, Wester K, Uhlen M, Hober S, Wernerus H. Characterization of PrEST-based antibodies towards human Cytokeratin-17. J Immunol Methods. 2009;342:20–32. doi: 10.1016/j.jim.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Geysen HM, Rodda SJ, Mason TJ, Tribbick G, Schoofs PG. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987;102:259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- 10.Jogi A, Brennan DJ, Ryden L, Magnusson K, Ferno M, Stal O, Borgquist S, Uhlen M, Landberg G, Pahlman S, Ponten F, Jirstrom K. Nuclear expression of the RNA-binding protein RBM3 is associated with an improved clinical outcome in breast cancer. Mod Pathol. 2009;22:1564–1574. doi: 10.1038/modpathol.2009.124. [DOI] [PubMed] [Google Scholar]
- 11.Ehlen A, Brennan DJ, Nodin B, O'Connor DP, Eberhard J, Alvarado-Kristensson M, Jeffrey IB, Manjer J, Brandstedt J, Uhlen M, Ponten F, Jirstrom K. Expression of the RNA-binding protein RBM3 is associated with a favourable prognosis and cisplatin sensitivity in epithelial ovarian cancer. J Transl Med. 2010;8:78. doi: 10.1186/1479-5876-8-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magnusson K, de Wit M, Brennan DJ, Johnson LB, McGee SF, Lundberg E, Naicker K, Klinger R, Kampf C, Asplund A, Wester K, Gry M, Bjartell A, Gallagher WM, Rexhepaj E, Kilpinen S, Kallioniemi OP, Belt E, Goos J, Meijer G, Birgisson H, Glimelius B, Borrebaeck CA, Navani S, Uhlen M, O'Connor DP, Jirstrom K, Ponten F. SATB2 in combination with cytokeratin 20 identifies over 95% of all colorectal carcinomas. Am J Surg Pathol. 2011;35:937–948. doi: 10.1097/PAS.0b013e31821c3dae. [DOI] [PubMed] [Google Scholar]
- 13.Oegema K, Savoian MS, Mitchison TJ, Field CM. Functional analysis of a human homologue of the Drosophila actin binding protein anillin suggests a role in cytokinesis. J Cell Biol. 2000;150:539–552. doi: 10.1083/jcb.150.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki C, Daigo Y, Ishikawa N, Kato T, Hayama S, Ito T, Tsuchiya E, Nakamura Y. ANLN plays a critical role in human lung carcinogenesis through the activation of RHOA and by involvement in the phosphoinositide 3-kinase/AKT pathway. Cancer Res. 2005;65:11314–11325. doi: 10.1158/0008-5472.CAN-05-1507. [DOI] [PubMed] [Google Scholar]
- 15.Schwenk JM, Igel U, Neiman M, Langen H, Becker C, Bjartell A, Ponten F, Wiklund F, Gronberg H, Nilsson P, Uhlen M. Toward next generation plasma profiling via heat-induced epitope retrieval and array-based assays. Mol Cell Proteomics. 2010;9:2497–2507. doi: 10.1074/mcp.M110.001560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teufel M, Saudek V, Ledig J-P, Bernhardt A, Boularand S, Carreau A, Cairns NJ, Carter C, Cowley DJ, Duverger D, Ganzhorn AJ, Guenet C, Heintzelmann B, Laucher V, Sauvage C, Smirnova T. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J Biol Chem. 2003;278:6521–6531. doi: 10.1074/jbc.M209764200. [DOI] [PubMed] [Google Scholar]
- 17.Christmann A, Wentzel A, Meyer C, Meyers G, Kolmar H. Epitope mapping and affinity purification of monospecific antibodies by Escherichia coli cell surface display of gene-derived random peptide libraries. J Immunol Methods. 2001;257:163–173. doi: 10.1016/s0022-1759(01)00461-6. [DOI] [PubMed] [Google Scholar]
- 18.Barlow DJ, Edwards MS, Thornton JM. Continuous and discontinuous protein antigenic determinants. Nature. 1986;322:747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- 19.Van Regenmortel MHV. Mapping epitope structure and activity: from one-dimensional prediction to four-dimensional description of antigenic specificity. Methods. 1996;9:465–472. doi: 10.1006/meth.1996.0054. [DOI] [PubMed] [Google Scholar]
- 20.Rockberg J, Lofblom J, Hjelm B, Uhlen M, Stahl S. Epitope mapping of antibodies using bacterial surface display. Nat Methods. 2008;5:1039–1045. doi: 10.1038/nmeth.1272. [DOI] [PubMed] [Google Scholar]
- 21.Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg M, Andersson AC, Kampf C, Persson A, Szigyarto CAK, Ottosson J, Bjorling E, Hober S, Wernerus H, Wester K, Ponten F, Uhlen M. Towards a human proteome atlas: high-throughput generation of mono-specific antibodies for tissue profiling. Proteomics. 2005;5:4327–4337. doi: 10.1002/pmic.200500072. [DOI] [PubMed] [Google Scholar]
- 22.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 23.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 24.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 25.Skerra A. Engineered protein scaffolds for molecular recognition. J Mol Recognit. 2000;13:167–187. doi: 10.1002/1099-1352(200007/08)13:4<167::AID-JMR502>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 26.Berglund L, Björling E, Jonasson K, Rockberg J, Fagerberg L, Al-Khalili Szigyarto C, Sivertsson Å, Uhlen M. A whole-genome bioinformatics approach to selection of antigens for systematic antibody generation. Proteomics. 2008;8:2832–2839. doi: 10.1002/pmic.200800203. [DOI] [PubMed] [Google Scholar]
- 27.Agaton C, Falk R, Guthenberg IH, Gostring L, Uhlen M, Hober S. Selective enrichment of monospecific polyclonal antibodies for antibody-based proteomics efforts. J Chromatrogr A. 2004;1043:33–40. doi: 10.1016/j.chroma.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Hjelm B, Fernandez CD, Lofblom J, Stahl S, Johannesson H, Rockberg J, Uhlen M. Exploring epitopes of antibodies toward the human tryptophanyl-tRNA synthetase. N Biotechnol. 2010;27:129–137. doi: 10.1016/j.nbt.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Erfle H, Neumann B, Rogers P, Bulkescher J, Ellenberg J, Pepperkok R. Work flow for multiplexing siRNA assays by solid-phase reverse transfection in multiwell plates. J Biomol Screen. 2008;13:575–580. doi: 10.1177/1087057108320133. [DOI] [PubMed] [Google Scholar]
- 30.Carpenter AE, Jones TR, Lamprecht MR, Clarke C, Kang IH, Friman O, Guertin DA, Chang JH, Lindquist RA, Moffat J, Golland P, Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.