Abstract
Dynamic mechanical input is believed to play a critical role in the development of functional musculoskeletal tissues. To study this phenomenon, cyclic uniaxial mechanical stretch was applied to engineered ligaments using a custom-built bioreactor and the effects of different stretch frequency, amplitude, and duration were determined. Stretch acutely increased the phosphorylation of p38 (3.5±0.74-fold), S6K1 (3.9±0.19-fold), and ERK1/2 (2.45±0.32-fold). The phosphorylation of ERK1/2 was dependent on time, rather than on frequency or amplitude, within these constructs. ERK1/2 phosphorylation was similar following stretch at frequencies from 0.1 to 1 Hz and amplitudes from 2.5% to 15%, whereas phosphorylation reached maximal levels at 10 min of stretch and returned toward basal within 60 min of stretch. Following a single 10-min bout of cyclic stretch, the cells remained refractory to a second stretch for up to 6 h. Using the phosphorylation of ERK1/2 as a guide, the optimum stretch paradigm was hypothesized to be 10 min of stretch at 2.5% of resting length repeated every 6 h. Consistent with this hypothesis, 7 days of stretch using this optimized intermittent stretch program increased the collagen content of the grafts more than a continuous stretch program (CTL=3.1%±0.44%; CONT=4.8%±0.30%; and INT=5.9%±0.56%). These results suggest that short infrequent bouts of loading are optimal for improving engineered tendon and ligament physiology.
Introduction
In industrialized societies, tendon and ligament (collectively referred to here as sinew) ruptures are occurring with greater frequency.1 In developing countries where more individuals are involved in life-long physical labor and regular exercise, sinew ruptures remain relatively uncommon. With inactivity, sinews lose collagen,2,3 and become brittle and stiff,4 making them prone to injury. In contrast, exercise increases the rate of collagen turnover,5 indicating that mechanical loading is of primary importance in the maintenance of sinew function.6 The increase in collagen turnover with exercise may be the result of the local production of growth factors, such as insulin-like growth factor-17,8 and transforming growth factor-β,5,7–9 which accumulate in the interstitial space around tendons following exercise. Exercise also increases lysyl oxidase expression, indicating that dynamic loading may increase crosslinking of collagen fibrils as well.9 Together, the increase in collagen turnover and improved enzymatic crosslinking should result in improved sinew function.
Our current understanding of how sinews respond to loading is limited largely because of the density of the extracellular matrix and the relative paucity of cells that comprise these tissues in the adult. To circumvent this problem, a number of two-dimensional (2D) cell culture models have been developed to study the effect of stretch on the phenotype and activity of fibroblasts. These studies have demonstrated that stretch produces a transient increase in collagen synthesis that is associated with the phosphorylation of focal adhesion kinase (FAK),10 transforming protein kinase of Rous sarcoma virus (p60src),11 and the Map kinases: JNK,12 ERK1/2,10,13 and p38.10 Most interestingly, inhibiting ERK1/2 activation using U0126 prevents the load-induced increase in both ERK1/2 phosphorylation and procollagen mRNA.14 This suggests that ERK1/2 phosphorylation is required and could serve as a marker for the increase in sinew function following stretch. However, 2D models cannot deliver a uniform uniaxial stretch to all cells and are not well suited to determine which pattern of stretch is optimal for sinew function as repeated long-term stretch results in cellular detachment. Further, cellular attachment to the external environment differs between 2D and 3D conditions and culturing in 2D can induce artificial polarity on nonpolar cells such as fibroblasts.15
We have recently developed a three-dimensional (3D) engineered sinew in which the cells produce a native 3D collagen matrix.16–19 This model has a number of advantages over traditional culture models. First, the cells are placed within a matrix that allows them to form in a manner similar to tendons in vivo.17 Second, using molded brushite anchors, the sinews can be stretched in a uniaxial manner using reverse-molded grips.18,20 This means that mechanical as well as biochemical data can be generated from the cultures. Third, as a fibrin matrix is used to engineer the sinews, the cells produce all of the collagen in the grafts. Therefore, collagen protein content can be measured and used as an indicator of interventions that result in anabolic changes within the sinew.
As ERK1/2 phosphorylation is necessary and required for the stretch-induced increase in collagen synthesis, the objective of this investigation was to determine the acute ERK1/2 response of tissue-engineered sinews to different frequency, amplitude, and duration of cyclic uniaxial stretch and then test the hypothesis that using a stretch paradigm that optimizes the activation of ERK1/2 would lead to increased collagen production in the tissue-engineered sinews.
Materials and Methods
Materials
SYLGARD (polydimethylsiloxane, type 184 silicone elastomer) is from Dow Chemical Company. The rabbit polyclonal antibody against total S6K1 (sc-11759) was from Santa Cruz Biotechnology. Rabbit polyclonal antibodies to total (3285) and phospho-FAK (3283) and monoclonal antibodies against phospho-S6K1 (9234), total (4695) and phospho-ERK1/2 (4370), total (2371) and phospho-p38 (4631), and total (4685) and phospho-Thr308 PKB (4056) were from Cell Signaling. The horseradish peroxidase-conjugated secondary antibodies and restore stripping buffer were from Perbio Science.
Brushite anchor formation
Individual anchors were designed as previously described19 using Solidworks software, and casting frames containing anchor molds were produced from polycarbonate in a Titan model T1 fusion deposition modeling (FDM) machine (StrataSys). The polycarbonate frames were used to produce a reverse mold using silicone glue (Dow Corning). Once set, the polycarbonate frame was removed from the silicone and this reverse mold was used to produce individual anchors. Individual anchors were formed as previously described.19 Breifly, brushite paste was spread into the silicone mold and centrifuged at 3700 rpm for 15 s (Eppendorf 5804R) before minutien insect pins (Fine Science Tools) were inserted into each anchor. The anchors were then left to set at room temperature overnight. Individual anchors were removed from the mold the following day and stored at room temperature until use.
Sinew formation
Tissue-engineered ligament constructs were engineered as previously described17 with modification.19 Briefly, two brushite anchors were pinned 12 mm apart in a Sylgard-coated 35 mm dish. After sterilizing the plate, 250,000 cells were plated on top or within a fibrin gel that was formed around the brushite anchors. The fibrin gel was made by adding 500 μL of growth media (Dulbecco's modified Eagles medium [DMEM] supplemented with 10% fetal bovine serum [FBS] and 1% penicillin) containing 10 U/mL thrombin (Calbiochem), 2 μL/mL aminohexanoic acid (200 mM), and 2 μL/mL aprotinin (10 mg/mL) solution to each dish and agitated to cover the surface of the plate. Two hundred microliters of fibrinogen (20 mg/mL; Sigma-Aldrich) was added dropwise and the resulting fibrin gel was left to polymerize at 37°C for 1 h. Primary rat Achilles tendon fibroblasts (PFB) were used for all acute stretch experiments involving western blotting because of the fact that the ERK1/2 antibodies did not react with chicken ERK. Primary chick tendon fibroblast (CTF) cells were used for long-term stretch experiments because of the fact that they form higher-quality constructs in a shorter period of time allowing higher throughput. Constructs from the PFB cells formed in an average of 7 days, whereas the CTF constructs formed in 3–4 days. Constructs were fed every 2–3 days with DMEM supplemented with 10% FBS and 1% penicillin. Ascorbic acid (AA) and proline (P) at a concentration of 50 μM were added to the culture media as stated for each individual experiment.
Stretching the sinews
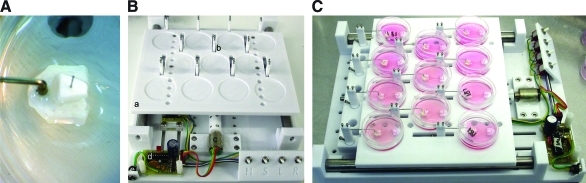
Mechanical stretch was performed using a uniaxial bioreactor, developed and built by Prof. Robert G. Dennis at the University of North Carolina, which has been described in detail elsewhere.21 Each bioreactor has space for 11 tissue-engineered constructs in 35 mm plates (Fig. 1B). One stepper motor (Fig. 1B, label c) controls the movement of all of the constructs, guaranteeing that each of the 11 constructs was subjected to the same mechanical stimulus during the stretch protocol. The constructs were attached to the bioreactor using reverse-molded grips (Fig. 1A) manufactured from polycarbonate in a Titan model T1 FDM machine (StrataSys). The bioreactors were placed in a standard tissue culture incubator, allowing the stretch experiments to be conducted at a temperature of 37°C and 5% CO2. The stretch protocol was microchip controlled (Fig. 1B, label d), allowing different stretch frequencies, amplitudes, and work-to-rest ratios to be programed using MPLAB IBE and a PICSTART Plus programmer (Microchip Technology, Inc.).
FIG. 1.
Reverse-molded grips and stretch bioreactor. (A) Ligaments were press fit into reverse-molded polycarbonate grips and loaded into (B) a stretch bioreactor composed of (a) 11 slots for 35 mm plates on the stretching frame (b) polycarbonate stretching posts to connect the constructs to (c) a single stepper motor controlled by (d) a microchip board that was (e) plugged into a standard 4.5 V power supply. (C) When fully loaded, each of the 11 constructs was subjected to the same mechanical stimulus. Color images available online at www.liebertonline.com/tea
Protein extraction, sodium dodecyl sulfate/polyacrylamide gel electrophoresis, and immunoblotting
All constructs were stretched at 1 week of formation. Following stretch, sinews were removed from their anchors at the appropriate time, washed twice in ice-cold phosphate-buffered saline, blotted dry, and then frozen in liquid nitrogen before being processed and analyzed. Frozen tissue samples were powdered on dry ice directly in 2 mL snap cap tubes. One hundred twenty-five microliters of fresh sucrose homogenization buffer (50 mM Tris [pH 7.5], 25 mM sucrose, 0.1 mM ethylenediaminetetraacetic acid, 0.1 mM ethylene glycol tetraacetic acid, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, 10 μg/mL aprotinin, 0.5 mM phenylmethanesulfonylfluoride, and 10 μg/mL leupeptin) was added to each sample and the tissue was homogenized using a handheld Polytron for 15 s. Samples were shaken for 30 min at 12,000 rpm and 4°C before centrifugation at 10,000 g for 5 min at 4°C. The supernatant was carefully removed and transferred to a new tube. Protein concentrations were determined using the DC protein assay kit (Biorad Laboratories) with bovine serum albumin as a standard. Equal aliquots of protein were solubilized in Laemmli sample buffer (LSB) and heated to 100°C for 5 min. Total lysates in LSB were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on a 10% gel using a constant current of 23 mA per gel for 50 min. Following electrophoresis, proteins were transferred to a Protran nitrocellulose membrane (Whatman) at 100 V for 1 h. The membrane was blocked for 1 h in 3% milk in Tris-buffered saline+0.1% Tween (TBST). Membranes were incubated overnight at 4°C with the appropriate primary antibody in TBST at 1:1000. The membrane was then washed three times in TBST before incubation for 1 h at room temperature with goat anti-rabbit IgG secondary antibody in 0.5% milk in TBST at 1:10,000. Antibody binding was then detected using an enhanced chemiluminescence detection kit (Millipore). Imaging and band quantification were carried out using a Chemi Genius Bioimaging Gel Doc System (Syngene).
Positive control for ERK1/2 phosphorylation
3T3 fibroblasts were grown in DMEM supplemented with 10% FBS and 1% penicillin in 95% air and 5% CO2 until ∼70% confluence was reached. Cells were treated with 1 μM Phorbol 12-myristate 13-acetate (Sigma-Aldrich) for 5 min before being collected and processed as described earlier.
Collagen content
The collagen content of the ligament constructs was determined using a hydroxyproline assay.22 Briefly, after mechanical testing, sinews were removed from their cement anchors and dried in an oven for 30 min at 110°C. Each sample was then weighed and hydrolyzed in 200 μL of 6 N HCl at 130°C for 3 h. The liquid was removed by allowing the HCl to evaporate for 30 min in a fume hood at 130°C. The resulting pellet was resuspended in 200 μL of hydroxyproline buffer. Samples were further diluted 1:8 in hydroxyproline buffer. One hundred fifty microliters of 0.05 M chloramine T solution was added to each sample, vortexed, and left at room temperature for 20 min. One hundred fifty microliters of aldehyde–perchloric acid solution was then added to each tube before the tubes were vortexed and incubated in a preheated water bath at 60°C for 15 min. Following incubation, tubes were left to cool for 10 min and then samples/standards were read at 550 nm on an Epoch Microplate Spectrophotometer (BioTek Instruments Ltd.). Hydroxyproline was converted to collagen using a factor of 13.34% as previously reported.23
Statistics
Data are presented as means±SEM. Differences in mean values were compared within groups and significant differences were determined by ANOVA with post hoc Tukey-Kramer HSD test using BrightStat (www.brightstat.com). The significance level was set at p<0.05.
Results
Acute activation of kinases following stretch
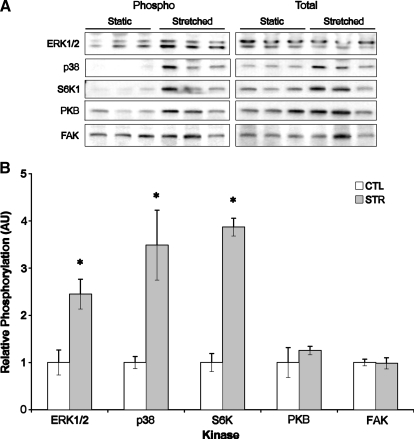
Mechanical stretch results in transient phosphorylation of p38 (3.5±0.74-fold), S6K1 (3.9±0.19-fold), and ERK1/2 (2.45±0.32-fold) in the tissue-engineered sinews (Fig. 2). These proteins were phosphorylated without an increase in focal adhesion kinase or protein kinase B/Akt phosphorylation. Since all of the kinases were activated over the same time frame and ERK1/2 phosphorylation is required for the effect of stretch on collagen synthesis,14 we focussed on the activation of ERK1/2 in the sinews as a marker of the positive effects of stretch.
FIG. 2.
Effect of stretch on the phosphorylation of different kinases within engineered sinews. (A) Representative western blots showing the phosphorylated and total amounts of ERK1/2, p38, S6K1, PKB/akt, and FAK following 10 min of stretch at 10% stretch amplitude. (B) Quantification of the relative phosphorylation (Phospho/Total) of the different kinases. *Significantly higher than unloaded constructs. Results are representative of three independent trials and presented as mean±SEM of n=6 for all groups.
Stretch frequency and ERK1/2 phosphorylation
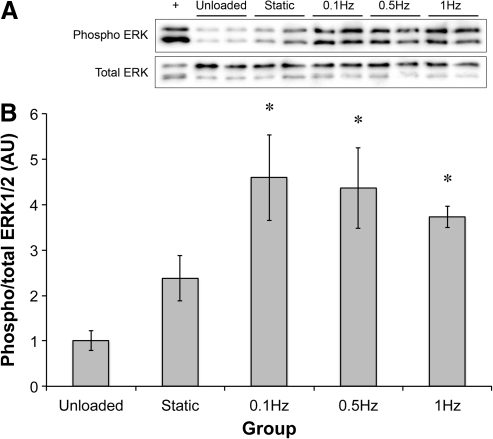
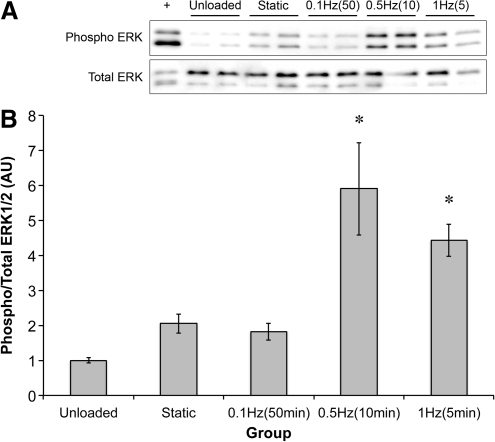
To establish the effect of stretch frequency, sinews were stretched for 10 min at 0.1, 0.5, or 1 Hz and the level of ERK1/2 phosphorylation was determined. Stretch increased ERK1/2 phosphorylation approximately fivefold over unloaded controls (2.32-fold over static controls) at all stretch frequencies (Fig. 3). No significant differences in ERK1/2 phosphorylation were observed between the dynamic stretch groups.
FIG. 3.
Effect of frequency of stretch on ERK1/2 phosphorylation. (A) Representative western blots showing the phosphorylation of ERK1/2 following 10 min of stretch at the stated frequency. (B) Quantification of the band intensities at the different frequencies. *Significantly higher than unloaded constructs. Results are representative of three independent trials and presented as mean±SEM of n=4 for all groups.
Stretch amplitude and ERK1/2 phosphorylation
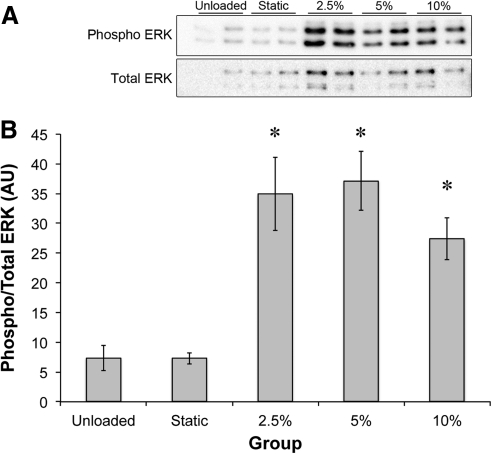
To determine the effect of the amplitude of stretch on ERK1/2 phosphorylation, sinews were stretched 2.5%, 5%, or 10% of their initial resting length at 0.5 Hz for 10 min and ERK1/2 phosphorylation was determined. Again, stretch increased ERK1/2 phosphorylation approximately fivefold in all groups (Fig. 4). No statistically significant differences were observed between the different stretch amplitude groups.
FIG. 4.
Effect of stretch amplitude on ERK1/2 phosphorylation. (A) Representative western blots showing the phosphorylation of ERK1/2 following 10 min of 0.5 Hz stretch at the stated stretch amplitude. (B) Quantification of the band intensities at the different amplitudes. *Significantly higher than unloaded group. Results are representative of five independent trials and presented as mean±SEM of n=4 for all groups.
Stretch duration
The effect of stretch duration was tested using stretch bouts each consisting of 300 stretches at 0.1, 0.5, and 1 Hz. Because of the different frequencies of stretch, the time taken to complete the 300 stretches varied between groups (0.1 Hz took 50 min; 0.5 Hz took 10 min; and 1 Hz took 5 min). Maximal ERK1/2 phosphorylation occurred at 0.5 Hz (Fig. 5), and this was significantly greater than in either the unloaded group (p=0.001), static group (p=0.008), or 0.1 Hz group (p=0.005). These data were interpreted as the cellular response to stretch being time dependent. To test this hypothesis, constructs were stretched at 1 Hz for 1, 5, 10, 15, 30, 45, or 60 min and the level of ERK1/2 phosphorylation was determined. In support of the time dependence of the response, ERK1/2 phosphorylation increased through 10 min and then progressively declined (Supplementary Fig. S1; Supplementary Data are available online at www.liebertonline.com/tea).
FIG. 5.
Effect of the number/duration of stretch on ERK1/2 phosphorylation. (A) Representative western blots showing the phosphorylation of ERK1/2 following 300 stretches at either 0.1, 0.5, or 1 Hz with the time taken to complete the bout in brackets. (B) Quantification of the band intensities for the different groups. *Significantly higher than unloaded, static, and 0.1 Hz groups. Results are representative of two independent trials and presented as mean±SEM of n=4 for all groups.
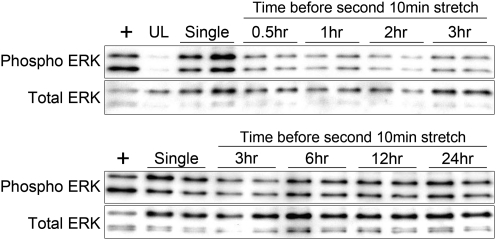
Recovery period length
The data above suggested that the optimal stretch duration was 10 min and that after that the cells became refractory. We next sought to determine how long the cells remained refractory before ERK1/2 could once more be fully activated. We first determined that once the stretch period was terminated, ERK1/2 phosphorylation rapidly returned to basal levels (within 30 min, data not shown). However, a second stretch stimulus 30 min following an initial bout of stretching did not result in ERK1/2 phosphorylation (Fig. 6), confirming that the cells remained refractory to stretch. The length of the refractory period was determined by extending the time before a second 10-min stretch (0.5, 1, 2, 3, 6, 12, or 24 h) and measuring ERK1/2 phosphorylation. The phosphorylation of ERK1/2 began to return following a 3 h rest period and had fully returned by 6 h (Fig. 6).
FIG. 6.
Determination of the refractory period for ERK1/2 phosphorylation. Representative western blots showing the phosphorylation of ERK1/2 following a single 10 min stretch (Single) or a second 10 min stretch following 0.5, 1, 2, 3, 6, 12, or 24 h of rest. Maximal ERK1/2 phosphorylation returns following a 6 h rest period. Results are representative of three independent trials.
Continuous and intermittent stretch and sinew collagen content
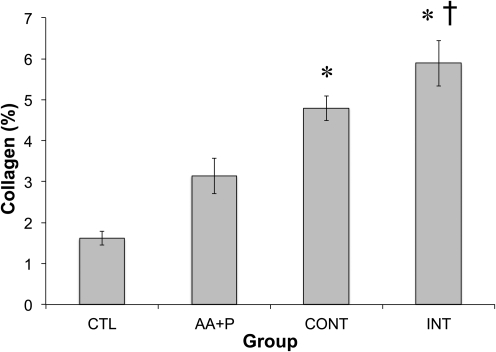
The data so far suggested that 10 min of stretch repeated every 6 h was optimal for maximizing ERK1/2 phosphorylation. To test whether this protocol improved sinew physiology, sinews were subjected to either a continuous stretch (CONT) stimulus at a frequency of 0.5 Hz and a strain of 2.5% for 7 days or an intermittent protocol (INT) in which the sinews received repeated 10 min bouts of 0.5 Hz and 2.5% strain followed by 6 h of rest over the 7 days. The 2.5% stretch amplitude was selected, as this is at the high end of the physiological range of motion for the anterior cruciate ligament (ACL)24 and all stretch amplitudes resulted in similar increases in ERK1/2 phosphorylation. As we have previously shown, adding AA and proline improved the collagen content of the sinews (CTL=1.6%±0.16%; AA+P=3.1%±0.44%; Fig. 7). Continuous stretching of the grafts also resulted in more collagen than control constructs (4.8%±0.30%), but was not significantly greater than the AA+P group. The intermittent stretch protocol resulted in a greater increase in collagen within the grafts (5.9%±0.56%) than both the control and AA+P groups. There was no difference in the mass of the constructs between groups (data not shown), indicating that four 10-min stretch periods separated by 6 h of rest is a stronger anabolic stimulus for sinews than a continuous stretch protocol.
FIG. 7.
Effect of continuous or intermittent stretch on chick tendon fibroblasts sinew collagen concentration. Static constructs were left untreated or were treated with 50 μM ascorbic acid+proline (AA+P). Dynamically loaded constructs were treated with both 50 μM AA+P and continuous (CONT) 2.5% strain at a frequency of 0.5 Hz for 7 days or treated with both 50 μM AA+P and intermittent stretch (INT), repeated 10 min bouts of 2.5% strain at a frequency of 0.5 Hz stretch separated by 6 h of rest for 7 days. *Significantly different than the no treatment group of the corresponding condition; †significantly different than the AA+P group. Results are representative of three independent trials and presented as mean±SEM of n=7 for all groups.
Discussion
In 3D engineered sinews with cells within a native collagen matrix, stretch activates ERK1/2 in a time-dependant, rather than a frequency or strain-dependant, manner. We have established that maximal activation of ERK1/2 occurs after 10 min of stretch, regardless of frequency or amplitude, and then decreases over time back to basal levels, indicating that tenocytes rapidly become refractory to stretch. The sensitivity to stretch returned completely following a rest period of 6 h. Using the dynamics of ERK1/2 activation, an intermittent stretch paradigm was designed based on the hypothesis that repeated elevation of ERK1/2 activity would lead to increased collagen synthesis. Consistent with this hypothesis, the optimized intermittent stretch protocol led to a greater increase in collagen content than continuous stretch. These data suggest that short bouts of stretch followed by significant rest periods produce the strongest anabolic stimulus for sinews.
Several kinases were transiently activated following acute stretch in our fibrin cast sinews. Specifically, the phosphorylation of ERK1/2, p38, and S6K1 transiently increased. This is consistent with previous work in both 2D and 3D, showing that stretch acutely activates a number of protein kinases in mesenchymal cells.10–14 The decision to concentrate solely on ERK1/2 activation was based on the important role that ERK1/2 plays in the stretch-activated increase in ColIA1 protein and gene expression14,25,26 and the quality of the ERK1/2 antibodies available for analysis. In an effort to elucidate the activation profile of ERK1/2 in the ligament constructs, constructs were subjected to cyclic uniaxial stretch for varying times from 1 min to 1 h. Maximal activation of ERK1/2 was seen to occur at 10 min of stretch (Figs. 5 and 6) with ERK1/2 phosphorylation returning toward basal within the hour. The transient activation profile of ERK1/2 described here in primary tendon fibroblasts has also been shown in other cell types such as dermal fibroblasts,27 bone marrow stromal cells,28 fetal rat cardiac fibroblasts,14 ACL-derived cells,25 and osteoblasts,29 with all studies reporting a maximal activation period between 5 and 15 min of stretch regardless of frequency, strain, or directional differences in the paradigm. It should be acknowledged that stretch-induced activation of ERK1/2 is not evident in all cell types. For example, Nishimura et al.30 showed that administering either a high-frequency or intermittent stretch regime to keratinocyte cells lead to no activation of the ERK1/2 or related p38 MAPK pathways.
The effect of strain amplitude on maximal ERK1/2 activation was also studied as we found that high-amplitude stretch (10%) of our native collagen matrices lead to a significant decrease in the collagen content of the 3D constructs (Supplementary Fig. S2). No significant differences were observed in ERK1/2 activation following 10 min of stretch (1 Hz) at 2.5%, 5%, 10% (Fig. 4), or 15% strain (data not shown). Consistent with the strain-independent activation of ERK1/2, others have shown that a wide range of strain magnitudes activate the ERK1/2 pathway. Rubin et al.28 used strain magnitudes between 0.5% and 8.5% strain and found that maximal activation of ERK1/2 occurs at the lowest strain magnitude in bone marrow stromal cells. Likewise, Jansen et al.29 achieved peak ERK1/2 phosphorylation using 0.4% strain in osteoblasts. In contrast, in skeletal muscle in vivo, ERK1/2 phosphorylation increases in a strain-dependent manner.31 Even though strain amplitude dependence has been demonstrated in some cell types, in fibroblasts the data suggest that ERK1/2 phosphorylation occurs equally with strain magnitudes of 0.5%,28 4%,13 10%,27 and 20%.14 These data strongly imply that any stretch amplitude results in maximal activation of ERK1/2 once a threshold strain value is reached, and further increases in strain have no additional effect on phosphorylation. A 2.5% strain amplitude was selected for the chronic and intermittent stretch protocols, as this is within the physiological range of strain of a ligament in vivo. During impact loading, the ACL elongates between 2% and 4%,24 whereas during normal activities such as stair climbing32 and bicycling33 the ACL undergoes ∼2.7% and 1.2%–2.1% strain, respectively. Further, the yield point of tendon/ligament is said to occur at around 5%–7%, with macroscopic failure occurring at 12%–15% strains.34,35 Consistent with microscopic damage occurring over 5% strain, our initial attempts to increase collagen in the grafts using 10% strain resulted in a decrease in graft collagen content (Supplementary Fig. S2, AA+P group). These data indicate that, at strain amplitudes greater than 5%, significant damage occurs within a developing collagen matrix.
In developing a chronic stretch protocol to maximize ERK1/2 activity, it was important not only to understand the dynamics of ERK1/2 activation, but also the length of the refractory period before stretch once again resulted in the maximal activation of ERK1/2. A second 10 min stretch was unable to activate ERK1/2 until at least 3 h after the first stretching bout. By 6 h, the activation of ERK1/2 by a second stretching bout was equivalent to that induced by the first bout (Fig. 6). As ERK1/2 phosphorylation is required for the stretch-induced increase in collagen synthesis,14,25 an intermittent stretch paradigm was developed using 10 min stretch followed by 6 h rest. Consistent with our hypothesis that maximizing ERK1/2 phosphorylation would lead to improved sinew collagen, 7 days of intermittent stretch (2.5% and 0.5 Hz) produced a greater increase in collagen than a continuous stretch paradigm. Stretching mesenchymal stem cell-seeded collagen sponges and gels with peak strains of 2.4% and 4% resulted in increased collagen type I and type III expression.36 However, whether this mRNA is translated into increased protein accumulation could not be determined in this system. In this way, using a noncollagenous scaffold has a distinct advantage. In fibrin constructs, collagen levels can be measured with ease as all of the collagen within the graft is produced endogenously. Consistent with our hypothesis, the intermittent strain paradigm leads to a significantly greater increase in collagen than a continuous stretch paradigm (Fig. 7). In contrast to the results presented here, Balestrini and Billiar37 have demonstrated that cyclic stretch ranging from 2% to 16% for a period of 8 days increases the collagen density of fibroblast-populated fibrin gels.37 However, it is important to note that the authors used a equibiaxial stretch technique, and unlike the present work, Balestrini and Billiar began their stretching regime only 24 h after cell seeding; therefore, very little endogenous matrix is produced prior to commencing their stretch regime. Saber et al.38 also observed an improvement in graft function with intermittent stretch when recellularized tendons were stretched for 5 days with a pattern of 1 h stretch at 0.017 Hz followed by 1 h of rest. However, they did not determine whether the collagen content of the graft was altered by their stretch paradigm and did not indicate how they chose these parameters of stretch.
Beyond the phosphorylation of ERK1/2, dynamic uniaxial stretch also activated other kinases, including p38, and S6K1, without altering the phosphorylation of PKB and FAK. The phosphorylation of S6K1 at Thr389 indicates mTORC1 (mammalian target of rapamycin complex 1) activation.39 mTORC1 is central to the control of protein synthesis by anabolic signals.39 The fact that mTORC1 is activated by stretch is completely consistent with stretch being a potent anabolic signal in ligaments. That mTORC1 activation occurs in the absence of PKB phosphorylation suggests that mTORC1 can be activated by the mechanical environment and does not require growth factors, as we have recently reported for muscle.40
The fact that the MAP kinases (ERK1/2 and p38) were activated in the absence of FAK phosphorylation differs from what has been reported for 2D stretch.10 Wang and his colleagues found a 2.75-fold increase in the phosphorylation of the Tyr397 residue of FAK in 3Y1 fibroblasts stretched atop a silicon membrane. Further, mutation of Tyr397 prevented the phosphorylation of both ERK1/2 and p38, indicating that the phosphorylation of FAK at Tyr397 was required for transduction of the mechanical signal.10 In contrast, we have shown that stretch of cells in 3D fibrin gels does not increase the phosphorylation of FAK. The decreased need for altered phosphorylation of FAK at Tyr397 in the ligament grafts likely represents a fundamental difference between the 2D and 3D environments. Tyr397 phosphorylated FAK does not associate with the 3D adhesion complex,15 indicating that the requirement for phosphorylation of FAK on Tyr397 in 2D cell culture might be an artifact of the culturing conditions. These data suggest that although FAK may have a role in transducing the mechanical signal, increased phosphorylation of Tyr397 is not required to perform that function.
The present work demonstrates that ERK1/2 phosphorylation is increased with both static and dynamic stretch, in a time, rather than frequency or strain amplitude- dependent manner. The time course of ERK1/2 phosphorylation showed that maximal activation occurred within 10 min of stretch and that a rest period of 6 h was required before an additional stretch bout could once more elicit maximal ERK1/2 phosphorylation. Combining these data to produce a long-term intermittent stretch protocol resulted in a greater increase in collagen content within the sinews than a continuous stretch paradigm. These data suggest that short bouts of low amplitude dynamic loading followed by rest periods of at least 6 h are optimal for improving tendon/ligament physiology.
Supplementary Material
Acknowledgments
The authors thank R.G. Dennis for his work in the development of the stretch bioreactor. The work was supported in part by a project grant from the Engineering and Physical Sciences Research Council (EP/E008925/1) and by a University of California Davis Health System, Vision Grant (54885).
Disclosure Statement
No competing financial interests exist.
References
- 1.Maffulli N. Waterston S.W. Squair J. Reaper J. Douglas A.S. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clin J Sport Med. 1999;9:157. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Loitz B.J. Zernicke R.F. Vailas A.C. Kody M.H. Meals R.A. Effects of short-term immobilization versus continuous passive motion on the biomechanical and biochemical properties of the rabbit tendon. Clin Orthop. 1989;244:265. [PubMed] [Google Scholar]
- 3.Vailas A.C. Deluna D.M. Lewis L.L. Curwin S.L. Roy R.R. Alford E.K. Adaptation of bone and tendon to prolonged hindlimb suspension in rats. J Appl Physiol. 1988;65:373. doi: 10.1152/jappl.1988.65.1.373. [DOI] [PubMed] [Google Scholar]
- 4.Arruda E.M. Calve S. Dennis R.G. Mundy K. Baar K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J Appl Physiol. 2006;101:1113. doi: 10.1152/japplphysiol.00612.2005. [DOI] [PubMed] [Google Scholar]
- 5.Heinemeier K. Langberg H. Olesen J.L. Kjaer M. Role of TGF-beta1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- 6.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- 7.Kjaer M. Langberg H. Heinemeier K. Bayer M.L. Hansen M. Holm L., et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand J Med Sci Sports. 2009;19:500. doi: 10.1111/j.1600-0838.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 8.Mackey A.L. Heinemeier K.M. Koskinen S.O. Kjaer M. Dynamic adaptation of tendon and muscle connective tissue to mechanical loading. Connective Tissue Res. 2008;49:165. doi: 10.1080/03008200802151672. [DOI] [PubMed] [Google Scholar]
- 9.Heinemeier K.M. Olesen J.L. Haddad F. Langberg H. Kjaer M. Baldwin K.M., et al. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J.G. Miyazu M. Matsushita E. Sokabe M. Naruse K. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen- activated protein kinase (MAPK) activation. Biochem Biophys Res Commun. 2001;288:356. doi: 10.1006/bbrc.2001.5775. [DOI] [PubMed] [Google Scholar]
- 11.Banes A.J. Tsuzaki M. Hu P. Brigman B. Brown T. Almekinders L., et al. PDGF-BB, IGF-I and mechanical load stimulate DNA synthesis in avian tendon fibroblasts in vitro. J Biomech. 1995;28:1505. doi: 10.1016/0021-9290(95)00098-4. [DOI] [PubMed] [Google Scholar]
- 12.Arnoczky S.P. Tian T. Lavagnino M. Gardner K. Schuler P. Morse P. Activation of stress-activated protein kinases (SAPK) in tendon cells following cyclic strain: the effects of strain frequency, strain magnitude, and cytosolic calcium. J Orthop Res. 2002;20:947. doi: 10.1016/S0736-0266(02)00038-4. [DOI] [PubMed] [Google Scholar]
- 13.MacKenna D.A. Dolfi F. Vuori K. Ruoslahti E. Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin- dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest. 1998;101:301. doi: 10.1172/JCI1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papakrivopoulou J. Lindahl G.E. Bishop J.E. Laurent G.J. Differential roles of extracellular signal-regulated kinase 1/2 and p38MAPK in mechanical load-induced procollagen alpha1(I) gene expression in cardiac fibroblasts. Cardiovasc Res. 2004;61:736. doi: 10.1016/j.cardiores.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 15.Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 16.Bayer M.L. Yeung C.Y. Kadler K.E. Qvortrup K. Baar K. Svensson R.B., et al. The initiation of embryonic-like collagen fibrillogenesis by adult human tendon fibroblasts when cultured under tension. Biomaterials. 2010;31:4889. doi: 10.1016/j.biomaterials.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapacee Z. Richardson S.H. Lu Y. Starborg T. Holmes D.F. Baar K., et al. Tension is required for fibripositor formation. Matrix Biol. 2008;27:371. doi: 10.1016/j.matbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Paxton J.Z. Donnelly K. Keatch R.P. Baar K. Grover L.M. Factors affecting the longevity and strength in an in vitro model of the bone-ligament interface. Ann Biomed Eng. 2010;38:2155. doi: 10.1007/s10439-010-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paxton J.Z. Grover L.M. Baar K. Engineering an in vitro model of a functional ligament from bone to bone. Tissue Eng Part A. 2010;16:3515. doi: 10.1089/ten.TEA.2010.0039. [DOI] [PubMed] [Google Scholar]
- 20.Paxton J.Z. Donnelly K. Keatch R.P. Baar K. Engineering the bone-ligament interface using polyethylene glycol diacrylate incorporated with hydroxyapatite. Tissue Eng Part A. 2009;15:1201. doi: 10.1089/ten.tea.2008.0105. [DOI] [PubMed] [Google Scholar]
- 21.Birla R.K. Huang Y.C. Dennis R.G. Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng. 2007;13:2239. doi: 10.1089/ten.2006.0359. [DOI] [PubMed] [Google Scholar]
- 22.Woessner J.F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 23.Neuman R.E. Logan M.A. The determination of hydroxyproline. J Biol Chem. 1950;184:299. [PubMed] [Google Scholar]
- 24.Yasuda K. Erickson A.R. Beynnon B.D. Johnson R.J. Pope M.H. Dynamic elongation behavior in the medial collateral and anterior cruciate ligaments during lateral impact loading. J Orthop Res. 1993;11:190. doi: 10.1002/jor.1100110206. [DOI] [PubMed] [Google Scholar]
- 25.Miyaki S. Ushida T. Nemoto K. Shimojo H. Itabashi A. Ochiai N., et al. Mechanical stretch in anterior cruciate ligament derived cells regulates type I collagen and decorin expression through extracellular signal-regulated kinase 1/2 pathway. Mater Sci Eng C. 2001;17:91. [Google Scholar]
- 26.Zhu J. Zhang X. Wang C. Peng X. Periprosthetic strain magnitude-dependent upregulation of type I collagen synthesis in human osteoblasts through an ERK1/2 pathway. Int Orthop. 2009;33:1455. doi: 10.1007/s00264-009-0735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura K. Blume P. Ohgi S. Sumpio B.E. Effect of different frequencies of tensile strain on human dermal fibroblast proliferation and survival. Wound Repair Regen. 2007;15:646. doi: 10.1111/j.1524-475X.2007.00295.x. [DOI] [PubMed] [Google Scholar]
- 28.Rubin J. Murphy T.C. Fan X. Goldschmidt M. Taylor W.R. Activation of extracellular signal-regulated kinase is involved in mechanical strain inhibition of RANKL expression in bone stromal cells. J Bone Miner Res. 2002;17:1452. doi: 10.1359/jbmr.2002.17.8.1452. [DOI] [PubMed] [Google Scholar]
- 29.Jansen J.H. Weyts F.A. Westbroek I. Jahr H. Chiba H. Pols H.A., et al. Stretch- induced phosphorylation of ERK1/2 depends on differentiation stage of osteoblasts. J Cell Biochem. 2004;93:542. doi: 10.1002/jcb.20162. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura K. Blume P. Ohgi S. Sumpio B.E. The effect of different frequencies of stretch on human dermal keratinocyte proliferation and survival. J Surg Res. 2009;155:125. doi: 10.1016/j.jss.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 31.Martineau L.C. Gardiner P.F. Insight into skeletal muscle mechanotransduction: MAPK activation is quantitatively related to tension. J Appl Physiol. 2001;91:693. doi: 10.1152/jappl.2001.91.2.693. [DOI] [PubMed] [Google Scholar]
- 32.Fleming B.C. Beynnon B.D. Renstrom P.A. Johnson R.J. Nichols C.E. Peura G.D., et al. The strain behavior of the anterior cruciate ligament during stair climbing: an in vivo study. Arthroscopy. 1999;15:185. doi: 10.1053/ar.1999.v15.015018. [DOI] [PubMed] [Google Scholar]
- 33.Beynnon B.D. Fleming B.C. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31:519. doi: 10.1016/s0021-9290(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 34.Lim H.C. Bae J.H. Wang J.H. Bae T.S. Kim C.W. Hwang J.H., et al. The biomechanical performance of bone block and soft-tissue posterior cruciate ligament graft fixation with interference screw and cross-pin techniques. Arthroscopy. 2009;25:250. doi: 10.1016/j.arthro.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 35.Wang I.E. Mitroo S. Chen F.H. Lu H.H. Doty S.B. Age-dependent changes in matrix composition and organization at the ligament-to-bone insertion. J Orthop Res. 2006;24:1745. doi: 10.1002/jor.20149. [DOI] [PubMed] [Google Scholar]
- 36.Juncosa-Melvin N. Matlin K.S. Holdcraft R.W. Nirmalanandhan V.S. Butler D.L. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007;13:1219. doi: 10.1089/ten.2006.0339. [DOI] [PubMed] [Google Scholar]
- 37.Balestrini J.L. Billiar K.L. Magnitude and duration of stretch modulate fibroblast remodeling. J Biomech Eng. 2009;131:051005. doi: 10.1115/1.3049527. [DOI] [PubMed] [Google Scholar]
- 38.Saber S. Zhang A.Y. Ki S.H. Lindsey D.P. Smith R.L. Riboh J., et al. Flexor tendon tissue engineering: bioreactor cyclic strain increases construct strength. Tissue Eng Part A. 2010;16:2085. doi: 10.1089/ten.TEA.2010.0032. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta S. Peterson T.R. Sabatini D.M. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamilton D.L. Philp A. MacKenzie M.G. Baar K. A limited role for PI(3,4,5)P3 regulation in controlling skeletal muscle mass in response to resistance exercise. PLoS One. 2010;5:e11624. doi: 10.1371/journal.pone.0011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.