Abstract
Despite the great potential of cell therapy for ischemic disease, poor cell survival after engraftment in ischemic tissue limits its efficacy. Here we tested a hypothesis that three-dimensionally grafted human umbilical vein endothelial cell (HUVEC) spheroids would exhibit improved angiogenic efficacy following transplantation into mouse ischemic limbs compared with HUVECs prepared by conventional two-dimensional monolayer culture. One day after surgical induction of hindlimb ischemia in athymic mice, HUVECs cultured in monolayer or HUVEC spheroids were transplanted intramuscularly into ischemic limbs. Four weeks after the treatment, in the spheroid HUVEC transplantation group, we observed increased hypoxia-inducible factor-1α expression, decreased apoptosis, and increased HUVEC survival in the ischemic tissue compared with the monolayer HUVEC transplantation group. Transplantation of HUVEC spheroids also resulted in enhanced and prolonged secretion of paracrine factors as well as enhanced expression of factors involved in the recruitment of circulating angiogenic progenitor cells. In summary, transplantation of HUVECs as spheroids enhanced cell survival, increased paracrine factor secretion, and showed a potential as a therapeutic method to treat ischemic tissue damages by promoting angiogenesis.
Introduction
Cell therapy holds great potential for therapeutic angiogenesis in the treatment of ischemic diseases. For example, transplanted endothelial cells (ECs) are incorporated into newly formed capillaries or arterioles, where they enhance neovascularization after ischemic injury.1,2 Incorporated ECs may also promote neovascularization by releasing factors that act in a paracrine manner to support local angiogenesis.2,3 However, despite the great potential of cell therapy for treating ischemic diseases, poor cell survival after engraftment in ischemic tissue limits its efficacy.4–6 Because of nutritional and oxygen limitations in avascular tissues, transplanted ECs could be exposed to hypoxia after transplantation and therefore prone to undergo ischemic apoptosis.7 Formation of spheroid could be a solution to such ischemic apoptosis of ECs after transplantation into ischemic tissues.
Spheroid made of ECs has been reported to prevent EC apoptosis and stabilize cells by preserving cell–cell contacts that are required to survive.8 Further, it has been reported that the transplantation of EC spheroids in combination with Matrigel–fibrin matrix containing vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) induced formation of human vasculature in mice.9 However, although these studies involved use of human umbilical vein endothelial cell (HUVEC) spheroids, either they did not examine the therapeutic efficacy of the spheroids in treating ischemic disease8 or they did use the spheroids with additional cell-supporting components such as scaffolds or growth factors,9 making it difficult to see the effect of spheroids itself in treating ischemic diseases.
Consequently, in the present study, we tested the hypothesis that HUVEC spheroids itself could improve engraftment and angiogenic efficacy following transplantation into mouse ischemic hindlimbs compared with HUVECs prepared by conventional two-dimensional monolayer culture. It has been reported that a hypoxic condition is established in the core of spheroids.10 Thus, it is also possible that HUVEC spheroids could create an oxygen- and nutrient-limited microenvironment beneath the surface of the spheroid cell layer that could consequently reprogram the cells to the hypoxic conditions typical of ischemic tissues by inducing the expression of factors such as hypoxia-inducible factor-1α (HIF-1α) to survive the hypoxia.
HIF-1α could also enhance the viability of HUVECs transplanted into ischemic tissues by inducing expression of VEGF,11 an angiogenic and antiapoptotic factor. Transplantation of HUVECs as spheroids could also enhance engraftment by avoiding the occurrence of anoikis, apoptotic events observed in anchorage-dependent cells that are detached from the surrounding extracellular matrix (ECM). Interactions between cells and the ECM provide essential signals for cell growth or survival.12,13 Therefore, cells cultured in monolayer and detached from the surrounding ECM by proteolytic enzyme treatment may undergo anoikis because of a loss of survival signals.14,15 In contrast, HUVEC spheroids can maintain the organization of the ECM, because they do not require enzyme treatment for cell harvesting from culture and avoidance of anoikis. Here, we investigated whether transplantation of HUVECs as spheroids enhances engraftment, paracrine secretion of angiogenic factors, angiogenesis, and limb survival in a mouse ischemic hindlimb model compared with transplantation of monolayer-cultured HUVECs.
Materials and Methods
HUVEC culture
HUVECs were purchased (Lonza) and subcultured in endothelial growth medium-2 (EGM-2; Lonza) supplemented with 100 units/mL of penicillin (Gibco BRL) and 100 μg/mL of streptomycin (Gibco BRL) with a 1:5 splitting ratio. For hypoxic conditions, cells were cultured under 1% oxygen. Normoxia condition was 20% oxygen.
Culture and characterization of HUVEC spheroids
To generate spheroids, 10 mL of HUVEC suspension (6.0×105 cells/mL) in EGM2 was placed in a siliconized spinner flask (Bellco) followed by stirring at 70 rpm for 3 days. Sigmacote (Sigma) was applied to the siliconized spinner flask to prevent cell adhesion to the wall of spinner flask and allowed to dry for a minimum of 24 h. The medium was exchanged every other day. Spheroids were formed at day 3. The average number of spheroids (per mL) in the suspension was counted using a hemocytometer. Spheroids were collected by settling down 1 mL of spheroid suspension for 3 min and discarding the supernatant. The settled down spheroids were dissociated into single cells with 0.1% (w/v) trypsin (Sigma), and the number of cells in spheroids was determined using hemocytometer. All experiments were performed using HUVECs after five passages. HUVEC spheroids were examined with scanning electron microscopy as previously described.16
Mouse hindlimb ischemia induction
Hindlimb ischemia was induced in 4-week-old female athymic mice (20–25 g body weight; Orient) as previously described.17,18 Briefly, 4-week-old, female athymic mice (20 g body weight; Jungang Lab Animal) were anesthetized with xylazine (10 mg/kg) and ketamine (100 mg/kg). The femoral artery and its branches were ligated through a skin incision using 5-0 silk suture (Ethicon). The external iliac artery and all of the above arteries were then ligated. The femoral artery was excised from its proximal origin as a branch of the external iliac artery to the distal point where it bifurcates into the saphenous and popliteal arteries.
Treatment of limb ischemia and analysis
One day after ischemia induction surgery, the mice were divided randomly into four groups (n=8 for each group for each time point, a total of 24 animals per group). Cells to be transplanted (both monolayer and spheroids) were cultured under normoxic condition. Monolayer-cultured and trypsinized HUVECs or spheroid HUVECs (1.0×107 cells suspended in 0.2 mL of phosphate-buffered saline per mouse) were injected intramuscularly into the gracilis muscle of the medial thigh. Spheroids for implantation were collected following culture by stopping agitation for 3 min and collecting spheroids that settled down without use of trypsin. The physiological status of ischemic limbs was followed for 4 weeks after treatment. Untreated mice served as a negative control. Mice that did not undergo surgical procedure served as a positive control. All animals received humane care in compliance with “the Institute of Laboratory Animal Resources of Seoul National University” (Institutional Animal Care and Use Committee No. SNU-100203-3).
Histology
Ischemic limb muscles were harvested 28 days after the treatment, fixed in 10% (v/v) buffered formaldehyde, dehydrated with a graded ethanol series, and embedded in paraffin. The specimens were sliced into 4 μm sections and stained with hematoxylin and eosin to examine muscle degeneration and tissue inflammation. Masson's trichrome collagen staining was performed to assess tissue fibrosis in the ischemic regions.
Immunohistochemistry
Ischemic limb muscles harvested 3 and 28 days after treatment were embedded in optimized cutting temperature compound (O.C.T., TISSUE-TEK® 4583; Sakura Finetek USA, Inc.), frozen, and cut into 10-μm-thick sections at −22°C. All samples were completely sectioned, and six slides were selected from both the middle and end parts of each sample. To detect transplanted human cells, the sections were immunofluorescently stained with anti-human nuclear antigen antibody (HNA; Chemicon). For staining of the capillaries and arterioles in ischemic regions, the sections were immunofluorescently stained with anti-von Willebrand factor (vWF; Abcam) and anti-smooth muscle (SM) α-actin (Abcam) antibodies, respectively. vWF-positive vessels with large (≥100 μm) diameters were omitted from capillary counting. Primary antibodies against human-specific VEGF (Santa Cruz Biotechnology) and FGF2 (BD Transduction Laboratories) were used to examine the production of human angiogenic factors in the ischemic tissues. Primary antibody against caspase-3 (Abcam) was also used for immunofluorescence staining of ischemic tissue. Staining signals were visualized with rhodamine- or FITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). The sections were counterstained with 4,6-diamidino-2-phenylindole (Vector Laboratories) and examined using a fluorescence microscope (Nikon TE2000) with 10× (0.3 NA) and 20× (0.45 NA) objectives.
Reverse transcription–polymerase chain reaction
Samples were homogenized and lysed in TRIzol reagent (Invitrogen). Total RNA was extracted with chloroform (Sigma) and precipitated with 80% (v/v) isopropanol (Sigma). After the supernatant was removed, the RNA pellet was washed with 75% (v/v) ethanol, air-dried, and dissolved in 0.1% (v/v) diethyl pyrocarbonate-treated water (Sigma). RNA concentration was determined by measuring absorbance at 260 nm using a spectrophotometer. Reverse transcription was performed using 5 μg of pure total RNA and SuperScript™ II reverse transcriptase (Invitrogen), and the synthesized cDNA was amplified by polymerase chain reaction (PCR). PCR was carried out for 35 cycles of denaturation (94°C, 30 s), annealing (58°C, 45 s), and extension (72°C, 45 s), with a final extension at 72°C for 10 min. PCR products were visualized by electrophoresis on a 1.5% (w/v) agarose gel with ethidium bromide staining and were analyzed using a gel documentation system (Gel Doc 1000; Bio-Rad). β-Actin served as the internal control.
Western blot
The retrieved ischemic limb tissues were lysed using a Dounce homogenizer (50 strokes, 4°C) in ice-cold lysis buffer (15 mM Tris HCl [pH 8.0], 0.25 M sucrose, 15 mM NaCl, 1.5 mM MgCl2, 2.5 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol, 2 mM NaPPi, 1 μg/mL of pepstatin A, 2.5 μg/mL of aprotinin, 5 μg/mL of leupeptin, 0.5 mM phenylmethylsulfonyl fluoride, 0.125 mM Na3VO4, 25 mM NaF, and 10 μM lactacystin). Protein concentration was determined using bicinchoninic acid protein assay (Pierce Biotechnology). Equal protein concentrations from each sample were mixed with Laemmli sample buffer, loaded, and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% (v/v) resolving gel. Proteins separated by SDS-PAGE were transferred to Immobilon-P membrane (Millipore Corp.) and then probed with antibodies against phospho-AKT (pAKT, Abcam), FOXO-3α (GenWay Biotech Inc.), caspase-9 (Cell Signaling), phospho-extracellular signal-regulated kinase (pERK; Invitrogen), phospho-mitogen activated protein kinase (pMAPK; Lifespan), BAD (Lifespan), intercellular adhesion molecule (ICAM; Abcam), vascular cell adhesion molecule (VCAM; Abcam), pericyte marker (NG2; Santa Cruz Biotechnology), and matrix metalloproteinase-2 (MMP-2; Life Span Bioscience) for 1 h at room temperature. The membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. The blots were developed using an enhanced chemiluminescence detection system (Amersham Bioscience). Luminescence was recorded on X-ray film (Fuji super RX; Fujifilm Medical Systems), and bands were imaged using a densitometer (Model GS-690; BioRad).
Statistical analysis
Quantitative data were expressed as mean±standard deviation. For limb loss/salvage, nonparametric Fisher's exact test was performed using SAS/STAT® software (SAS Institute, Inc.). Student's t-test was used to determine significant difference between two groups (p<0.05). For statistical analysis of more than two groups, the one-way ANOVA test using Bonferroni post hoc analysis was performed using OriginPro 8 SR4 software (version 8.0951; OriginLab Corporation). A p-value of <0.05 was considered statistically significant.
Results
Preconditioning HUVECs for resistance to ischemic conditions
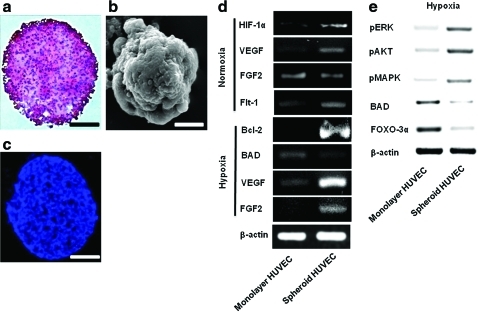
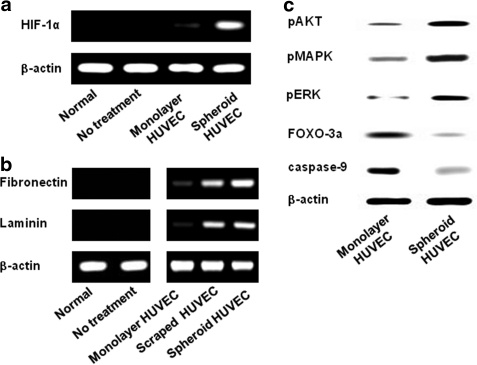
Three days after HUVEC spheroid culturing, the structure of generated HUVEC spheroids showed high cellularity and a low apoptotic core (Fig. 1a–c). HUVEC spheroids were preconditioned to survive in hypoxic conditions. The expression levels of HIF-1α, a hypoxia-induced survival factor, as well as that of VEGF, an angiogenic growth factor, were increased in the HUVEC spheroid culture compared with the HUVEC monolayer culture (Fig. 1d). Under hypoxic culture conditions (1% O2), HUVEC spheroids downregulated the expression of BAD, a proapoptotic gene, while upregulating that of Bcl-2, an antiapoptotic gene, compared with monolayer HUVECs (Fig. 1d). The cell survival regulator proteins pAKT, pMAPK, and pERK were upregulated, whereas FOXO-3α protein, a proapoptotic factor, was downregulated in HUVEC spheroids compared with monolayer HUVECs (Fig. 1e). FGF2 expression was upregulated compared with monolayer HUVECs only when HUVEC spheroids were cultured under hypoxic conditions (Fig. 1d). HUVECs were transplanted intramuscularly into mouse ischemic hindlimbs as spheroids (spheroid HUVECS) or dissociated cells (monolayer HUVECS). Transplantation of HUVECs as spheroids showed dramatically increased expression of human HIF-1 compared with transplantation of monolayer HUVECs (Fig. 2a). HUVEC spheroids could prevent the apoptosis caused by a lack of cell–ECM interaction (i.e., anoikis). Large portions of fibronectin and laminin were lost by preparing HUVECs in monolayer culture and harvesting with trypsinization (monolayer HUVECs) compared with HUVEC spheroids (Fig. 2b). In addition, HUVEC spheroids had well-preserved ECMs compared with monolayer HUVECs.
FIG. 1.
Representative properties of HUVEC spheroids. Formation of HUVEC spheroids in vitro (a–c) and alteration in gene expression by spheroid formation in vitro (d) at 3 days of culture. (a) H&E staining. Scale bar=100 μm. (b) Scanning electron microscopy image. Scale bar=100 μm. (c) TUNEL staining. Scale bar=100 μm. (d) RT-PCR and (e) western blot analysis. HUVEC, human umbilical vein endothelial cell; H&E, hematoxylin and eosin; RT-PCR, reverse transcription–polymerase chain reaction. Color images available online at www.liebertonline.com/tea
FIG. 2.
Enhanced expression of cell survival factors and preservation of HUVEC ECMs by transplantation of HUVEC spheroids into ischemic tissues. (a) RT-PCR analysis of human-specific HIF-1α expression in ischemic tissue at 1 day after transplantation of HUVEC spheroids or monolayer HUVECs. (b) RT-PCR analysis to examine human ECM components (fibronectin and laminin) present in ischemic tissues at 1 day after transplantation. (c) Western blot analysis for the cell survival regulators pAKT, pMAPK, and pERK and for the proapoptotic factors FOXO-3α and caspase-9 of monolayer HUVECs and HUVEC spheroids at 3 days after transplantation. HIF-1α, hypoxia-inducible factor-1α; ECM, extracellular matrix; pMAPK, phospho-mitogen activated protein; pERK, phospho-extracellular signal-regulated kinase; pAKT, phospho-AKT.
Enhanced survival of HUVECs in ischemic tissue by grafting as spheroids
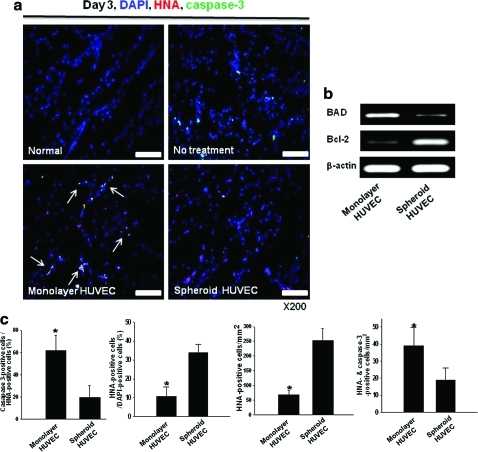
Expression of the cell survival regulators pAKT, pMAPK, and pERK was increased in HUVEC spheroids compared with monolayer HUVECs following transplantation into mouse ischemic limbs (Fig. 2c). Transplantation of HUVECs into mouse ischemic limbs as spheroids improved the survival of HUVECs. The expression of the proapoptotic factors FOXO-3α and caspase-9 was downregulated by transplantation of HUVECs as spheroids (Fig. 2c). Immunofluorescent staining for caspase-3 in ischemic muscle at 3 days after transplantation showed that apoptosis was significantly decreased (p<0.05) when HUVECs were grafted as spheroids than as monolayer cells (Fig. 3a, c). Reverse transcription (RT)–PCR analysis using human-specific primers showed that mRNA expression of the antiapoptotic factor Bcl-2 was increased by HUVEC spheroid transplantation compared with monolayer HUVEC transplantation, whereas expression of the proapoptotic factor BAD was reduced (Fig. 3b). HUVEC survival was also significantly greater in spheroid transplantation than in monolayer cell transplantation (Fig. 3c).
FIG. 3.
Enhanced HUVEC survival in ischemic limb muscle by grafting as spheroids. (a) Immunofluorescent staining for caspase-3 (green) of ischemic limb tissues retrieved at 3 days after HUVEC grafting. HUVECs were double-stained with HNA (red). Apoptosis of transplanted HUVECs (arrows) was reduced in the spheroid group. Scale bars=100 μm. (b) RT-PCR analysis for human proapoptotic (BAD) and antiapoptotic (Bcl-2) factor at 3 days after HUVECs grafting. (c) The ratio of caspase-3-positive cells (apoptotic cells) to HNA-positive cells (transplanted HUVECs) and the ratio of HNA-positive cells (transplanted cells) to 4,6-diamidino-2-phenylindole-positive cells (total cells) in the ischemic region (*p<0.05). HNA-positive cells and HNA/caspase-3 double-positive cells were counted as well. HNA, human nuclear antigen. Color images available online at www.liebertonline.com/tea
Enhanced and prolonged secretion of angiogenic growth factors from HUVECs in ischemic tissue by grafting as spheroids
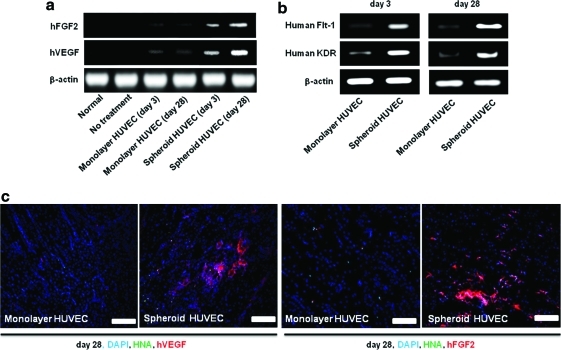
Transplantation of HUVECs as spheroids into ischemic tissues enhanced paracrine secretion of angiogenic growth factors from the transplanted HUVECs and prolonged the secretion period. RT-PCR analysis using human-specific primers indicated that the expression of human angiogenic growth factors (VEGF and FGF2) that were secreted from the transplanted HUVECs was much higher in HUVEC spheroids than in monolayer HUVECs at both 3 and 28 days after transplantation (Fig. 4a). Double immunofluorescence staining for HNA, VEGF, and FGF2 indicated that secretion of human angiogenic growth factors from HUVECs was sustained until at least 28 days after spheroid transplantation, whereas monolayer HUVECs secreted a smaller amount of angiogenic growth factors at day 28 (Fig. 4c). RT-PCR for human-specific Flt-1 and KDR showed that the expression of EC markers related to angiogenic growth factor activation19,20 was increased in HUVEC spheroids transplanted group compared with that in monolayer HUVEC transplanted group at both day 3 and 28 (Fig. 4b).
FIG. 4.
Enhanced and prolonged secretion of angiogenic growth factors from HUVECs in ischemic limb muscle by grafting as spheroids. RT-PCR analysis using human-specific primers for (a) VEGF and FGF2 and (b) angiogenic growth factor activation marker at 3 and 28 days after transplantation. (c) Double-immunofluorescent staining for HNA and the human-specific angiogenic growth factors VEGF and FGF2 (red) of ischemic limb tissues at 28 days after HUVECs (green). Scale bars=200 μm. VEGF, vascular endothelial growth factor; FGF2, fibroblast growth factor 2. Color images available online at www.liebertonline.com/tea
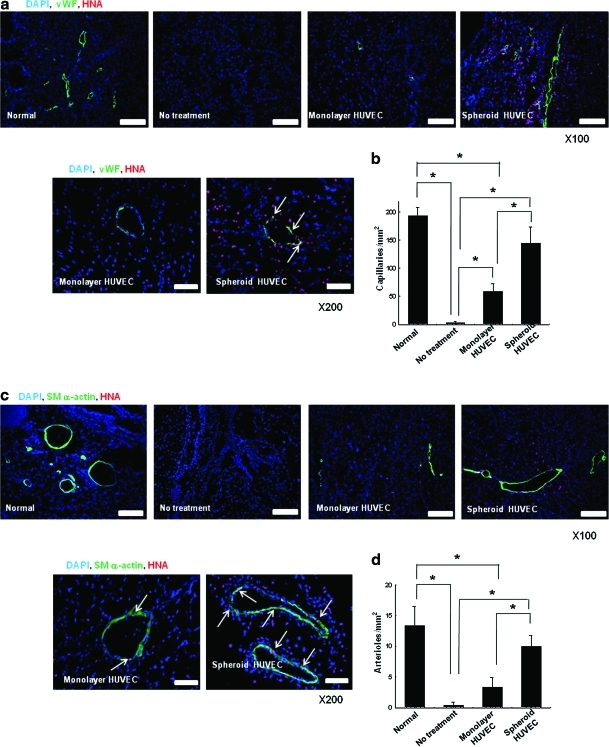
Enhanced angiogenesis by transplantation of HUVECs as spheroids
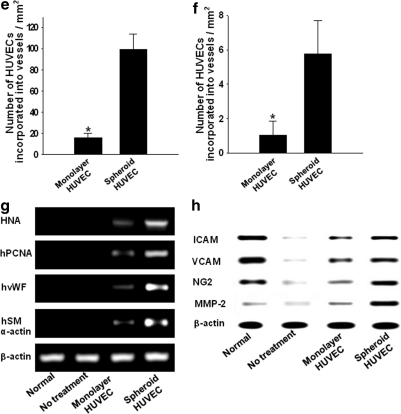
Transplantation of HUVECs as spheroids enhanced angiogenesis in mouse ischemic limb tissues compared with transplantation of monolayer HUVECs. Immunofluorescence staining for mouse vWF (Fig. 5a) and quantification of capillary density (Fig. 5b) revealed that transplantation of HUVEC spheroids significantly enhanced (p<0.05) capillary formation, compared with the transplantation of monolayer HUVECs and the no-cell transplantation (no treatment) group. However, as the used vWF antibodies can cross-react with human vWF, we were not able to quantify how many of the vWF-stained cells were of mouse or human origin. Immunofluorescence staining for mouse SM α-actin (Fig. 5c) along with quantification of arteriole density (Fig. 5d) showed that arteriole formation was also enhanced significantly (p<0.05) by transplantation of HUVECs as spheroids compared with both the monolayer-cultured HUVEC transplantation and the no-cell transplantation. A higher number of transplanted HUVECs (HNA-positive cells) were found in the vicinity as well as on the capillaries and arterioles of ischemic tissues receiving HUVEC spheroids, compared with tissues receiving monolayer HUVECs (Fig. 5a, c, e, and f). HUVECs transplanted as spheroids were incorporated with higher frequency into microvessels compared with monolayer HUVECs (Fig. 5e, f). HUVECs transplanted as spheroids exhibited higher cell survival, cell proliferation activity, and vascular differentiation compared with HUVECs transplanted as dissociated cells (Fig. 5g). Transplantation of HUVEC spheroids resulted in the increased expression of ICAM, VCAM, NG2, and MMP-2 in host cells in the ischemic region (Fig. 5h).
FIG. 5.
Enhanced angiogenesis by transplantation of HUVECs as spheroids. Double-immunofluorescent staining for HNA and mouse-specific (a) vWF and (c) SM α-actin of the ischemic hindlimb tissues retrieved at 28 days after HUVEC transplantation. Scale bars indicate 200 μm at 100×and 100 μm at 200×. The quantification of (b) capillary density and (d) arteriole density in the ischemic region (*p<0.05 between groups compared). Quantification of the number of HUVECs incorporated into (e) capillaries and (f) arterioles in the ischemic region (*p<0.05). (g) RT-PCR analysis of human-specific vWF, SM α-actin, HNA, and proliferating cell nucleus antigen of the ischemic hindlimb tissues at 28 days after transplantation. (h) Western blot analysis for ICAM, VCAM, NG2, and MMP-2 in the ischemic hindlimb tissues at 28 days after transplantation. White arrow indicates double stained cells (vWF+/HNA+, or SM α-actin+/HNA+). vWF, von Willebrand factor; SM, smooth muscle; ICAM, intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; MMP, matrix metalloproteinase. Color images available online at www.liebertonline.com/tea
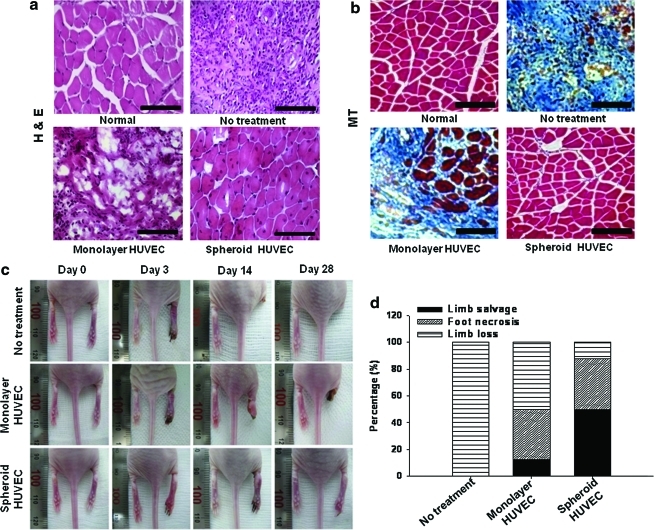
Effect of transplantation of HUVECs as spheroids on ischemic limb salvage
Next, it was determined whether or not transplantation of HUVECs as spheroids improved the physiological status of the ischemic limbs. The mice with hindlimb ischemia in the no-treatment group showed extensive muscle degeneration and inflammation in the ischemic region (Fig. 6), resulting in rapid limb necrosis at day 3 and complete limb loss via autoamputation by day 28 (Fig. 6c, d). All mice in the no-treatment group underwent complete limb loss (100%) (Fig. 6d). Transplantation of monolayer HUVECs decreased limb necrosis (Fig. 6c, d), but the limbs still showed muscle degeneration and inflammation in the ischemic region (Fig. 6); 50% of the mice ultimately underwent limb loss at 28 days (Fig. 6d). Most mice receiving HUVEC spheroids exhibited limb salvage (50%) or mild limb necrosis (37.5%), although 1 in 8 mice lost limbs (12.5%) (Fig. 6d). Nevertheless, Fisher's exact test indicated that there was no statistically significant difference between monolayer and spheroid treatments (p=0.223).
FIG. 6.
Histological analysis and physiological status of the hindlimbs at 28 days after the treatment. (a) H&E staining and (b) Masson's trichrome staining. Blue region indicates fibrosis. Scale bars=100 μm. (c) Photographs of mouse hindlimbs at days 0, 3, 14, and 28 after transplantation. Photomicrographs and photographs are representative of each group. (d) Physiological status of the ischemic hindlimbs at 28 days after transplantation. Color images available online at www.liebertonline.com/tea
Discussion
Grafting HUVECs as spheroids could be advantageous for treating ischemic diseases. Our data indicated that various cell survival signals such as ERK, AKT, and MAPK can be upregulated by spheroid formation without any chemical or genetic treatments. This can make HUVEC spheroids more proliferative and resistant to an ischemic environment, thereby improving their antiapoptotic capacity. Increased paracrine angiogenic factor secretion of HUVEC spheroids makes them more potent in inducing angiogenesis. We report here that grafting of HUVECs as spheroids could provide an efficient tool that improves the therapeutic efficacy of HUVEC transplantation for treating ischemic diseases.
It has been reported that, in the core of spheroids, a hypoxic condition is established.10 Thus, it is also feasible that the cells in the core of HUVEC spheroid are exposed to a condition similar to hypoxia. In fact, we observed a significantly increased HIF-1α in the HUVEC spheroids even under normoxic condition, as well as in the ischemic hind limb treated with HUVEC spheroids, supporting our speculation. HIF-1α is known to stimulate the production of angiogenic growth factors as well as antiapoptotic signaling cascades.3,11,21,22 Thus, we speculate that the increased expression and secretion of angiogenic growth factors such as VEGF and FGF2 (Fig. 4a, c), even up to 4 weeks after transplantation, observed in the HUVEC spheroid group could be, at least partially, due to the increased HIF-1α expression (Figs. 1d and 2d).
VEGF is known to regulate the expression of receptors (Flt-1 and KDR) as well as their signaling.23,24 The activation of these receptors could initiate signaling cascades that promote endothelial cell proliferation and angiogenic potential.19 A higher level of human PCNA expression observed in mouse ischemic tissues that received HUVEC spheroids indicates that in vivo proliferation of transplanted HUVECs could have been improved by grafting HUVECs as spheroids. Additionally, these increased growth factors, in turn, could have activated cell survival signaling molecules (ERK, AKT, and MAPK) by facilitating their phosphorylation,23–29 resulting in deactivation of proapoptotic factors such as BAD and FOXO-3α, as we observed in the present study. In fact, the number of caspase-3-positive apoptotic human cells was significantly lowered in the HUVEC spheroid group compared with that in the monolayer HUVEC group, indicating that culturing HUVECs as spheroids can increase antiapoptotic capacity of the cells so that the survival of the transplanted cells in the ischemic tissues can be improved.
Another possible explanation for the increased cell survival of the HUVECs transplanted as spheroids involves anoikis, a type of apoptosis induced by the lack of survival signals originating from the cell–matrix interaction.15 Anoikis is usually observed in monolayer cells during cell transplantation.30,31 As proteolytic enzyme treatment for the harvesting of monolayer-cultured cells for passaging or transplantation degrades ECM proteins such as fibronectin or laminin, the cells may lose their substrates as well as survival signals generated via cell–matrix interactions. Consequently, it is possible that trypsinized cells for transplantation undergo anoikis once they are transplanted, lowering the efficacy of cell transplantation. Cell death by anoikis could be more severe when cells are transplanted to tissues in a harsh environment such as ischemia. Culturing and grafting HUVECs as spheroids avoided the harsh enzymatic treatment so that inherent ECM such as fibronectin and laminin could be preserved as observed in the present study. This well-preserved ECM can prevent anoikis by maintaining interactions between cells and the ECM.
Spheroid HUVEC therapy could promote mobilization of host cells for angiogenesis. Overexpression of adhesion molecules such as ICAM-1 in ischemic regions might further facilitate angiogenesis through recruitment of circulating endothelial progenitor cells.32 Enhanced NG-2 expression demonstrates that HUVEC spheroids promote the recruitment of host pericytes to ischemic regions. Mobilized pericytes can support vessel formation and also stabilize the newly generated vasculature.33 It was reported that MMP-2 contributes to angiogenesis by breaking down vessel basement membranes and generating paths for host cell mobilization.34 Thus, MMP-2 expression enhanced by HUVEC spheroid therapy might induce various types of host cells (e.g., pericytes, smooth muscle cells, endothelial progenitors, and endothelial cells) to migrate toward ischemic regions, which would support blood vessel formation and maturation.
Conclusion
The results of the present study suggests that the grafting HUVEC spheroids can provide a simple, reliable, and effective way to promote angiogenesis in ischemic tissue without any other cell-supporting components such as scaffolds or growth factors. The transplantation of HUVECs as spheroids may be clinically relevant and easily applicable for the treatment of ischemic diseases including myocardial or cerebral ischemia.
Acknowledgments
This work was supported by a grant (No. A050082) from the Korean Health 21 R&D Project, Ministry of Health, Welfare, and Family Affairs, and a grant (2010-0020352) from the National Research Foundation of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Chekanov V. Akhtar M. Tchekanov G. Dangas G. Shehzad M.Z. Tio F. Adamian M. Colombo A. Roubin G. Leon M.B. Moses J.W. Kipshidze N.N. Transplantation of autologous endothelial cells induces angiogenesis. Pacing Clin Electrophysiol. 2003;26:496. doi: 10.1046/j.1460-9592.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim E.J. Li R.K. Weisel R.D. Mickle D.A. Jia Z.Q. Tomita S. Sakai T. Yau T.M. Angiogenesis by endothelial cell transplantation. J Thorac Cardiovasc Surg. 2001;122:963. doi: 10.1067/mtc.2001.117623. [DOI] [PubMed] [Google Scholar]
- 3.Calvani M. Rapisarda A. Uranchimeg B. Shoemaker R.H. Melillo G. Hypoxic induction of an HIF-1alpha-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107:2705. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M. Methot D. Poppa V. Fujio Y. Walsh K. Murry C.E. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33:907. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y. Yasuda T. Weisel R.D. Li R.K. Enhanced cell transplantation: preventing apoptosis increases cell survival and ventricular function. Am J Physiol Heart Circ Physiol. 2006;291:H939. doi: 10.1152/ajpheart.00155.2006. [DOI] [PubMed] [Google Scholar]
- 6.Hu X. Yu S.P. Fraser J.L. Lu Z. Ogle M.E. Wang J.A. Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infracted heart function via enhanced survivial of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 7.Stempien-Otero A. Karsan A. Cornejo C.J. Xiang H. Eunson T. Morrison R.S. Kay M. Winn R. Harlan J. Mechanisms of hypoxia-induced endothelial cell death. J Biol Chem. 1999;274:8039. doi: 10.1074/jbc.274.12.8039. [DOI] [PubMed] [Google Scholar]
- 8.Korff T. Augustin H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alajati A. Laib A.M. Weber H. Boos A.M. Bartol A. Ikenberg K. Korff T. Zentgraf H. Obodozie C. Graeser R. Christian S. Finkenzeller G. Stark G.B. Heroult M. Augustin H. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 10.Land S.C. Rae C. iNOS initiates and sustains metabolic arrest in hypoxic lung adenocarcinoma cells: mechanism of cell survival in solid tumor core. Am J Physiol Cell Physiol. 2005;289:C918. doi: 10.1152/ajpcell.00476.2004. [DOI] [PubMed] [Google Scholar]
- 11.Pugh C.W. Ratcliffe P.J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 12.Chen C.S. Mrksich M. Huang S. Whitesides G.M. Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 13.Lelièvre S.A. Weaver V.M. Nickerson J.A. Larabell C.A. Bhaumik A. Petersen O.W. Bissell M.J. Tissue phenotype depends on reciprocal interactions between the extracellular matrix and the structural organization of the nucleus. Proc Natl Acad Sci USA. 1998;95:14711. doi: 10.1073/pnas.95.25.14711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frisch S.M. Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisch S.M. Screaton R.A. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 16.Bhang S.H. Cho S.W. Lim J.M. Kang J.M. Lee T.J. Yang H.S. Song Y.S. Park M.H. Kim H.S. Yoo K.J. Jang Y. Langer R. Anderson D.G. Kim B.S. Locally-delivered growth factor enhances the angiogenic efficacy of adipose-derived stromal cells transplanted to ischemic limbs. Stem Cells. 2009;27:1976. doi: 10.1002/stem.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhang S.H. Cho S.W. La W.G. Lee T.J. Yang H.S. Sun A.Y. Baek S.H. Rhie J.W. Kim B.S. Angiogenesis in ischemic tissue produced by spheroid grafting of human adiopose-derived stromal cells. Biomaterials. 2011;32:2734. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 18.Cho S.W. Moon S.H. Lee S.H. Kang S.W. Kim J. Lim J.M. Kim H.S. Kim B.S. Chung H.M. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 19.Wu L.W. Mayo L.D. Dunbar J.D. Kessler K.M. Baerwald M.R. Jaffe E.A. Wang D. Warren R.S. Donner D.B. Utilization of distinct signaling pathways by receptors for vascular endothelial cell growth factor and other mitogens in the induction of endothelial cell proliferation. J Biol Chem. 2000;275:5096. doi: 10.1074/jbc.275.7.5096. [DOI] [PubMed] [Google Scholar]
- 20.Ye L. Haider H.K. Jiang S.J. Sim E.K. Therapeutic angiogenesis using vascular endothelial growth factor. Asian Cardiovasc Thorac Ann. 2004;12:173. doi: 10.1177/021849230401200221. [DOI] [PubMed] [Google Scholar]
- 21.Bernaudin M. Nedelec A.S. Divoux D. MacKenzie E.T. Petit E. Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;4:393. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Ma D. Lim T. Xu J. Tang H. Wan Y. Zhao H. Hossain M. Maxwell P.H. Maze M. Xenon preconditioning protects against renal ischemic-reperfusion injury via HIF-1alpha activation. J Am Soc Nephrol. 2009;20:713. doi: 10.1681/ASN.2008070712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Vries C. Escobedo J.A. Ueno H. Houck K. Ferrara N. Williams L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 24.Nunez G. del Peso L. Linking extracellular survival signals and the apoptotic machinery. Curr Opin Neurobiol. 1998;8:613. doi: 10.1016/s0959-4388(98)80089-5. [DOI] [PubMed] [Google Scholar]
- 25.Cai J. Ahmad S. Jiang W.G. Huang J. Kontos C.D. Boulton M. Ahmed A. Activation of vascular endothelial growth factor receptor-1 sustains angiogenesis and Bcl-2 expression via the phosphatidylinositol 3-kinase pathway in endothelial cells. Diabetes. 2003;52:2959. doi: 10.2337/diabetes.52.12.2959. [DOI] [PubMed] [Google Scholar]
- 26.Hutchings H. Ortega N. Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration and survival through integrin ligation. FASEB J. 2003;17:1520. doi: 10.1096/fj.02-0691fje. [DOI] [PubMed] [Google Scholar]
- 27.Marti H.H. Risau W. Systemic hypoxia changes the organspecific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95:15809. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munoz-Chapuli R. Quesada A.R. Medina M.A. Angiogenesis and signal transduction in endothelial cells. Cell Mol Life Sci. 2004;61:2224. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nor J.E. Christensen J. Mooney D.J. Polverini P.J. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchentouf M. Benabdallah B.F. Rousseau J. Schwartz L.M. Tremblay J.P. Induction of anoikis following myoblast transplantation into SCID mouse muscles requires the Bit1 and FADD pathways. Am J Transplant. 2007;7:1491. doi: 10.1111/j.1600-6143.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 31.Grossmann J. Molecular mechanisms of “detachment-induced apoptosis-Anoikis.”. Apoptosis. 2002;7:247. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- 32.Yoon C.H. Hur J. Oh I.Y. Park K.W. Kim T.Y. Shin J.H. Kim J.H. Lee C.S. Chung J.K. Park Y.B. Kim H.S. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Artheioscler Thromb Vasc Biol. 2006;26:1066. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 33.Gaengel K. Genové G. Armulik A. Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 34.Kvanta A. Sarman S. Fagerholm P. Seregard S. Steen B. Expression of matrix metalloproteinase-2 (MMP-2) and vascular endothelial growth factor (VEGF) in inflammation-associated corneal neovascularization. Exp Eye Res. 2000;70:419. doi: 10.1006/exer.1999.0790. [DOI] [PubMed] [Google Scholar]