Abstract
Human skeletal muscle respiratory chain defects restrict the ability of working muscle to extract oxygen from blood, and result in a hyperkinetic circulation during exercise in which oxygen delivery is excessive relative to oxygen uptake and oxygen levels within contracting muscle are abnormally high. To investigate the role of the muscle microcirculation in this anomalous circulatory response and possible implications for the regulation of muscle angiogenesis, we assessed muscle oxidative capacity during cycle exercise and determined capillary levels and distribution and vascular endothelial growth factor expression in quadriceps muscle biopsies in patients with mitochondrial myopathy attributable to heteroplasmic mitochondrial DNA mutations. We found that in patients with mitochondrial myopathy, muscle capillary levels were twice that of sedentary healthy subjects (3.0 ± 0.9% compared with 1.4 ± 0.3%, P < 0.001) despite the fact that oxygen utilization during peak cycle exercise was half that of control subjects (11.1 ± 4.0 ml/kg/min compared with 20.7 ± 7.9 ml/kg/min, P < 0.01); that capillary area was greatest in patients with the most severe muscle oxidative defects and was more than two times higher around muscle fibre segments with defective (i.e. cytochrome oxidase negative/succinic dehydrogenase-positive or ‘ragged-red’ fibres) compared with more preserved respiratory chain function; and that vascular endothelial growth factor expression paralleled capillary distribution. The increased muscle capillary levels in patients correlated directly (r2 = 0.68, P < 0.05) with the severity of the mismatch between systemic oxygen delivery (cardiac output) and oxygen utilization during cycle exercise. Our results suggest that capillary growth is increased as a result of impaired muscle oxidative phosphorylation in mitochondrial myopathy, thus promoting increased blood flow to respiration-incompetent muscle fibres and a mismatch between oxygen delivery and utilization during exercise. Furthermore, the finding of high capillary levels despite elevated tissue oxygen levels during exercise in respiration-deficient muscle fibres implies that mitochondrial metabolism activates angiogenesis in skeletal muscle by a mechanism that is independent of hypoxia.
Keywords: mitochondrial DNA defects, skeletal muscle, angiogenesis, regulation of oxygen delivery, oxidative metabolism
Introduction
Human genetic defects that impair oxidative phosphorylation within skeletal muscle profoundly alter the normal circulatory response to aerobic exercise. Specifically, they restrict the extraction of oxygen from blood and disrupt the normal close matching of increases in oxygen delivery by the circulation to oxygen utilization within contracting muscles during exercise (Taivassalo et al., 2003). In patients with mitochondrial myopathy attributable to mitochondrial or nuclear DNA mutations, the circulation during exercise is hyperkinetic, with oxygen delivery relative to utilization three times normal on average, and in the most severe oxidative defects, more than five times that of healthy humans. As a result, oxygen levels within contracting muscle and in venous effluent blood are abnormally high (Taivassalo et al., 2002; Grassi et al., 2007). The implications of this mismatch between oxygen delivery and utilization during exercise for the level and distribution of capillaries within skeletal muscle is unknown but is central to the understanding of the pathophysiology of this circulatory response to exercise in mitochondrial disease. More broadly, the relationship between mitochondrial dysfunction and capillary levels within skeletal muscle may illuminate the relative contribution of mitochondrial metabolism versus alterations in oxygen levels in the regulation of angiogenesis (capillary growth) in health and disease.
In healthy humans, skeletal muscle capillary levels correlate directly with muscle oxidative capacity and both are modulated by habitual levels of physical activity, increasing with regular aerobic exercise and decreasing in response to physical inactivity (Andersen and Henriksson, 1977; Klausen et al., 1981; Hepple, 1997, 2000; Mathieu-Costello and Hepple, 2002). The mechanism by which capillary growth within skeletal muscle is regulated is incompletely understood, but presumably is linked to patterns of oxidative energy demand as determined by habitual levels of physical activity. A major regulator of angiogenesis is hypoxia, where in the setting of reduced cellular oxygen levels, the normal constitutive degradation of hypoxia-inducible factor-1α (HIF-1α) is inhibited and expression levels of HIF-1-regulated transcription factors, including vascular endothelial growth factor (VEGF), are increased (Semenza, 1998; Pugh and Ratcliffe, 2003; Tang et al., 2004). Exercise in healthy subjects increases the extraction of available oxygen relative to delivery as a result of an increased rate of mitochondrial oxidative phosphorylation, so muscle pO2 falls from ∼34 mmHg at rest to 3–5 mmHg with moderate exercise (Richardson et al., 2006). Accordingly, the exercise-related decrease in oxygen levels in active skeletal muscle has been considered to be an important mechanism by which regular physical activity promotes increased capillary density within working muscle (Adair et al., 1990; Breen et al., 1996; Gustafsson et al., 1999).
However, recent work has suggested that angiogenesis in skeletal muscle may also occur independently of the canonical HIF-1α pathway (Arany et al., 2008) through a peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α)-mediated mechanism. Specifically, experiments have revealed a parallel upregulation of VEGF expression and capillary levels in mice overexpressing PGC-1α without changes in HIF-1α expression or protein levels (Arany et al., 2008). To date, there are no data to implicate a hypoxia-independent mechanism in the regulation of angiogenesis in humans.
In order to clarify the influence of mitochondrial disease upon the network of capillaries within skeletal muscle and implications for disruption of the normal relationship between oxygen delivery and utilization in exercise, we determined quadriceps capillary levels and distribution in patients with mitochondrial myopathy attributable to heteroplasmic mitochondrial DNA mutations as well as in healthy, sedentary controls and correlated these results with oxygen utilization and circulatory responses during cycle exercise.
Materials and methods
Study population
Nine patients (two males, seven females; 38.1 ± 12.0 years; body mass index = 22.2 ± 3.2) with mitochondrial myopathy attributable to a heteroplasmic mitochondrial DNA defect based on molecular or histological evidence (Table 1) were studied. All patients presented with moderate to severe exercise intolerance, most of whom (Patients 2, 4, 5, 6, 8 and 9) had prominent dyspnoea and tachycardia at low levels of physical activity. Nine highly sedentary healthy controls (two males, seven females; 38.2 ± 15.1 years; body mass index = 27.24 ± 3.8) were also studied. Subjects gave their written consent to participate in the study, which was approved by the institutional review boards of the University of Texas Southwestern Medical Centre and Texas Health Presbyterian Hospital of Dallas. Exercise testing and a muscle biopsy were performed in each subject.
Table 1.
Characterization of patients with mitochondrial myopathy
| Patient | Age/sex | Mitochondrial DNA mutation | Histochemistry findings |
|---|---|---|---|
| Patient 1 | 25/F | Single 5 kB deletion | Ragged-red fibres and cytochrome oxidase-negative fibres |
| ▴ | |||
| Patient 2 | 20/F | tRNA Leu A3243G | Ragged-red fibres and cytochrome oxidase-negative fibres |
| • | |||
| Patient 3 | 32/F | Single 5 kB deletion | Ragged-red fibres and cytochrome oxidase-negative fibres |
| ☆ | |||
| Patient 4 | 53/F | Cyt b G14846A (Andreu et al., 1999) | Ragged-red fibres and cytochrome oxidase-positive fibres |
| ♦ | |||
| Patient 5 | 53/M | tRNA Trp T5543C (Anitori et al., 2005) | Ragged-red fibres and cytochrome oxidase-negative fibres |
| △ | |||
| Patient 6 | 49/F | tRNA Ser double mutation (C7472ins and 7472A>C) (Swalwell et al., 2008) | Ragged-red fibres and cytochrome oxidase-negative fibres |
| ▪ | |||
| Patient 7 | 32/F | Single 4.3 kB deletion | Ragged-red fibres and cytochrome oxidase-negative fibres |
| ▽ | |||
| Patient 8 | 38/M | ND4 G11832A (Andreu et al., 1999) | Ragged-red fibres and cytochrome oxidase-positive fibres |
| □ | |||
| Patient 9 | 41/F | Unidentified (Haller et al., 1989) | Ragged-red fibres and cytochrome oxidase-negative fibres |
| ◊ |
F = female; M = male.
Exercise testing
Cycle ergometry testing was performed to determine peak capacity for oxygen (O2) utilization (VO2), O2 delivery (cardiac output, Q) and O2 extraction (systemic a-vO2 difference) as previously described (Taivassalo et al., 2003). Briefly, the workload was increased by 5–10 W (for patients) or 10–20 W (for controls) increments every 1–2 min until the subject reached maximal heart rate (220−age) or exhaustion, usually between 8 and 12 min of exercise. Gas exchange and cardiac output were determined at rest, during one or more submaximal workloads and with peak exercise. Expired air was collected in Douglas bags for 120 s at rest and 60 s during exercise for determination of VO2. The fractions of O2, CO2 and N2 in each bag were analysed with a Marquette 1100 Medical Gas Analyser and ventilation was measured with a Tissot spirometer for calculation of VO2. Q was measured utilizing acetylene rebreathing in which the rate of disappearance of C2H2 from a rebreathing bag is proportional to pulmonary blood flow and Q. Determination of VO2 and Q allows for the calculation of systemic arteriovenous oxygen difference, indicated by the Fick equation: VO2 = Q × systemic a-vO2 difference.
In order to assess the relationship between O2 delivery and uptake for each subject, the increase in cardiac output relative to the increase in oxygen utilization (ΔQ/ΔVO2) was calculated from the slope of the linear regression between Q and VO2 from rest, submaximal and peak exercise data. In healthy individuals, irrespective of age, sex, body weight or level of conditioning, there is a near 1:1 relationship between O2 delivery and utilization as indicated by the fact that Q increases ∼5 litres for each litre of increase in O2 consumption (ΔQ/ΔVO2  5) from rest to maximal exercise. When VO2 is limited by impaired muscle oxidative phosphorylation, ΔQ/ΔVO2 is characteristically exaggerated (Taivassalo et al., 2003). Heart rate was continuously monitored during rest and exercise with a 12-lead electrocardiogram (Quinton 3040 ECG monitor).
5) from rest to maximal exercise. When VO2 is limited by impaired muscle oxidative phosphorylation, ΔQ/ΔVO2 is characteristically exaggerated (Taivassalo et al., 2003). Heart rate was continuously monitored during rest and exercise with a 12-lead electrocardiogram (Quinton 3040 ECG monitor).
Muscle needle biopsy
A biopsy of the mid-portion of the vastus lateralis muscle was obtained using the percutaneous Bergstrom technique. A sample of muscle (∼100 mg) was immediately transversely oriented and frozen in isopentane cooled by liquid nitrogen for histological and immunohistochemical analysis. Serial transverse sections (6–10 µm each) were stained sequentially for: (i) fibre type using the alkaline (pH 10.5) ATPase to distinguish Type 1 and 2 fibres; (ii) respiration-incompetent muscle fibres using either the cytochrome oxidase/succinic dehydrogenase sequential assay (Taylor et al., 2004) to identify cytochrome c oxidase-deficient fibres or modified Gomori trichrome staining to detect ragged-red fibres in patients with mutations that preserve cytochrome oxidase expression (one patient with a cytochrome b mutation and one with an ND4 mutation); (iii) capillaries using a monoclonal antibody to the capillary endothelium marker CD31 (Chemicon; 1:100 dilution); and (iv) VEGF using a mouse anti-VEGF monoclonal antibody (Chemicon; 1:200 dilution).
Muscle morphological analysis
For each biopsy, serial sections were examined in three random and non-overlapping fields and photographed under light microscopy (Nikon Microphot and DXM1200F camera) using Axiovision software. Muscle morphometry and quantification of capillary-to-muscle area was performed using ImageJ software (NIH Image). For each subject, approximately 250 fibres were analysed in total in the three fields at ×10 magnification. Within each field, the number of fibres (as per Gundersen, 1977), percentage of Types 1 and 2 fibres, average fibre area and capillary levels defined as percentage capillary area (capillary area/muscle fibre + capillary area) were determined.
In order to assess the distribution of capillary levels between respiration-deficient and more normal fibres, 18 single fibres were analysed per patient. In each case, nine respiration-deficient (cytochrome oxidase-deficient or ragged-red) fibres based on histological staining for Gomori trichrome or cytochrome oxidase/succinic dehydrogenase and nine normal (cytochrome oxidase-positive or non-ragged-red fibres) fibres of similar size were first randomly selected by an investigator (R.G.H. and T.T.) based solely on cytochrome oxidase/succinic dehydrogenase or Gomori trichrome results. Subsequently for each fibre, the percentage capillary area and fibre perimeter were determined by another investigator (K.A.) using CD31 immunostaining and Imag J software.
Statistical analysis
For comparison of exercise physiological and muscle biopsy data, unpaired t-tests were performed to determine significant differences between the patients with mitochondrial myopathy and control group means. Similar analysis was used to detect differences within the mitochondrial myopathy group between the cytochrome oxidase-deficient or ragged-red fibres and normal (cytochrome oxidase-positive and non-ragged-red) fibres. Differences were considered statistically significant when P < 0.05. Linear regression analysis was performed to determine the strength of the relationship between variables: peak VO2 and percentage capillary area in controls; ΔQ /ΔVO2 and percentage capillary area; and percentage cytochrome oxidase-deficient ragged-red fibres and percentage capillary area in the patients with mitochondrial myopathy.
Results
Comparison between controls and patients with mitochondrial myopathy
Oxygen delivery and utilization during exercise
Maximal oxygen utilization in patients with mitochondrial myopathy was approximately half that of sedentary controls (Table 2). This limitation in peak exercise capacity was attributable to impaired muscle oxidative phosphorylation as marked by a severely attenuated ability for oxygen extraction (low peak a-vO2 difference) in patients with mitochondrial myopathy compared with sedentary controls (Table 2). Peak heart rates achieved at the end of cycling exercise did not differ between the two groups (mitochondrial myopathy = 160 ± 16 beats/min; controls = 161 ± 23 beats/min). Patients with mitochondrial myopathy also demonstrated a hyperkinetic circulatory response to exercise in which the average increase in systemic oxygen delivery (cardiac output) relative to oxygen utilization (ΔQ/ΔVO2) was almost 3-fold that of healthy controls. One patient (Patient 4) harbouring a cytochrome b mutation (Andreu et al., 1999a, b) with severe exercise intolerance was determined to be a statistical outlier with respect to ΔQ/ΔVO2 (Hedges and Olkin, 1985) and was not included in the mitochondrial myopathy group average or in the regression analysis. This patient has been repeatedly studied in our laboratory with ΔQ/ΔVO2 ranging from 45–59.
Table 2.
Peak capacity for oxygen utilization and delivery in patients with mitochondrial myopathy and healthy controls
| Group | VO2 peak (ml/kg/min) | Peak a-vO2 difference (ml/dl) | ΔQ/ΔVO2 |
|---|---|---|---|
| Controls | 20.7 ± 7.9 (9.5–31.9) | 12.6 ± 2.3 (9.3–16.0) | 5.9 ± 1.2 (4.7–7.7) |
| Patients with mitochondrial myopathy | 11.1 ± 4.0* (5.6–18.7) | 5.9 ± 2.3* (3.1–8.9) | 14.4 ± 5.1** (9.2–25.0)a |
a Value for Patient 4 not included in average and range presented (see text). Group average ± SD and range of values are provided.
*P < 0.01; **P < 0.001.
Muscle biopsy findings
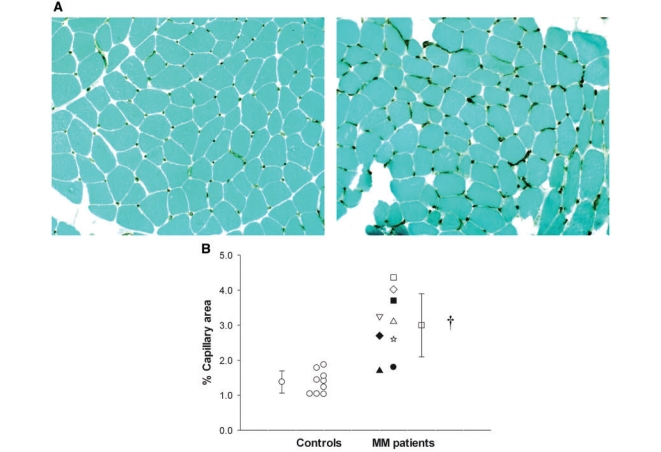
The total number of fibres and distribution of fibre types analysed in three separate areas of the biopsy for each subject was similar between patients with mitochondrial myopathy and controls (Table 3). A trend towards increased mean fibre area in controls did not reach statistical significance (P = 0.053). In contrast, capillary levels were significantly higher in patients with mitochondrial myopathy. Although a reduction in fibre size alone could increase the percentage capillary area, the trend to smaller fibres in patients could account for only a small fraction of the >2-fold greater per cent area occupied by capillaries in patients compared with controls. Figure 1A compares an individual patient with mitochondrial myopathy (Patient 9 in Table 1) to a sedentary healthy female control of similar age. Despite the fact that oxygen utilization in cycle exercise was less than half that of the control (mitochondrial myopathy patient peak VO2 = 8.9 ml/kg/min; control subject = 18.8 ml/kg/min), the percentage capillary area in muscle was almost 3-fold higher (mitochondrial myopathy = 4.0%; control = 1.4%). A mismatch between oxygen utilization and delivery in the patient with mitochondrial myopathy was indicated by a hyperkinetic circulation during cycle exercise in which the increase in cardiac output relative to oxygen utilization was 3-fold greater than normal (mitochondrial myopathy patient ΔQ/ΔVO2 = 15; control subject ΔQ/ΔVO2 = 5) and peak oxygen extraction was severely restricted (peak a-vO2 difference in patient with mitochondrial myopathy = 5.6; control = 14.0 ml/dl).
Table 3.
Muscle morphological analysis in patients with mitochondrial myopathy and healthy controls
| Group | Number of fibres analysed (per subject) | Type I (%) | Type II (%) | Mean fibre area (µm2) | Capillary area (%) |
|---|---|---|---|---|---|
| Controls | 243 ± 54 | 46.5 ± 10.5 | 53.5 ± 10.4 | 4397 ± 930 | 1.4 ± 0.3 |
| Patients with mitochondrial myopathy | 274 ± 44 | 49.5 ± 16.0 | 50.5 ± 16.1 | 3509 ± 872 | 3.0 ± 0.9** |
**P < 0.001.
Figure 1.
Capillary levels within skeletal muscle of patients with mitochondrial myopathies compared with healthy, age-matched and sedentary controls. (A) Representative images in a female control (left) and a female patient (patient 9 in Table 1) with mitochondrial myopathy (MM, right), highlighting greater capillary levels (immunostaining utilizing antibody to CD31) surrounding muscle fibres in the patient with mitochondrial myopathy. Images at ×10 magnification. (B) Group means ± SD and the distribution of per cent capillary area in individual controls and patients with mitochondrial myopathy. *P < 0.001. ▴ = Patient 1; • = Patient 2; ☆ = Patient 3; ♦ = Patient 4; △ = Patient 5; ▪ = Patient 6; ▽ = Patient 7; □ = Patient 8; and ◊ = Patient 9.
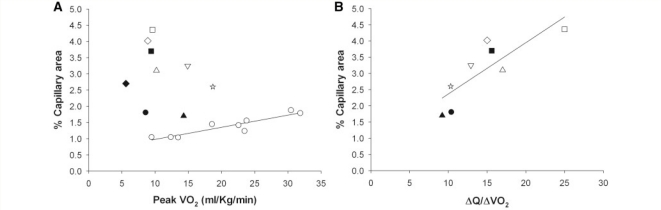
The mean capillary area of patients with mitochondrial myopathy (3.0 ± 0.92%) was twice that of control subjects (1.5 ± 0.30%, P < 0.001; Fig. 1B) despite the patients’ restricted oxidative capacity. The range of percentage capillary area among the patients with mitochondrial myopathy was also greater than that in the controls (1.7–4.4% versus 1.0–1.9%; Fig. 1B). In control subjects, capillary area correlated directly with oxidative capacity during cycle exercise (r2 = 0.85, P < 0.01) with differences in aerobic fitness accounting for differences in capillary levels (Fig. 2A). Additionally, in control subjects, peak capacity for O2 extraction within muscle (a-vO2 difference) was positively correlated with capillary area (r2 = 0.70, P < 0.05) (data not shown). In contrast, in patients with mitochondrial myopathy muscle capillary levels did not correlate with oxidative capacity or oxygen extraction. In all patients with mitochondrial myopathy, capillary areas were higher than would be predicted for a given level of peak VO2 and the highest capillary levels were detected in patients with the lowest oxidative capacities (Fig. 2A). Furthermore, high levels of capillaries in patients with mitochondrial myopathy correlated directly (r2 = 0.68, P < 0.05) with the level of mismatch between oxygen delivery and utilization as indicated by the ΔQ/ΔVO2 relationship in exercise, with higher capillary levels associated with more exaggerated oxygen delivery relative to oxygen uptake in exercise (Fig. 2B).
Figure 2.
Relationship between quadriceps muscle-capillary levels and oxygen utilization (VO2) and between capillary levels and oxygen delivery relative to oxygen utilization (ΔQ/ΔVO2) during cycle exercise. (A) Per cent capillary area is linearly related to peak VO2 (r2 = 0.85, P < 0.01) in healthy controls (open circles), whereas no such correlation exists in patients with mitochondrial myopathy, and in all patients, capillary levels are high relative to VO2. (B) In patients with mitochondrial myopathy, high capillary levels correlate directly (r2 = 0.68, P < 0.05) with the degree of mismatch between the increase in oxygen delivery relative to the increase in oxygen utilization (ΔQ/ΔVO2) during exercise. Data from Patient 4 is excluded (see main text) ▴ = Patient 1; • = Patient 2; ☆ = Patient 3; ♦ = Patient 4; △ = Patient 5; ▪ = Patient 6; ▽ = Patient 7; □ = Patient 8; and ◊ = Patient 9.
Muscle capillary distribution in patients with mitochondrial myopathy
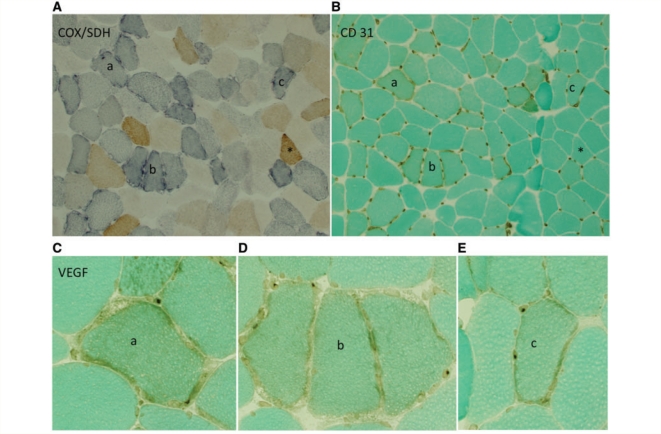
The level of capillaries around single fibres was analysed in eight patients in whom more normal fibres could be differentiated from respiration-deficient fibres as based on histochemical staining. Six of these patients exhibited cytochrome oxidase-deficient fibres on the basis of cytochrome oxidase/succinic dehydrogenase staining, where fibres containing functional cytochrome oxidase and lower levels of mitochondrial DNA mutation appear brown and fibres that are cytochrome oxidase-deficient attributable to high levels of mitochondrial DNA mutation appear blue (Fig. 3A). In the two patients with protein-coding defects affecting Complex I or Complex III, respiration-deficient fibres were determined as ragged-red fibres based on the modified Gomori trichrome and ragged-blue fibres based on the succinic dehydrogenase stain. In one patient (Patient 9, a 41-year-old female with an unidentified presumed mitochondrial DNA mutation, Haller et al., 1989), >95% of fibres were oxidatively deficient by criterion of cytochrome oxidase/succinic dehydrogenase staining and were not included in the distribution analysis.
Figure 3.
Capillaries are most abundant around respiration-deficient fibres within the muscle of patients with mitochondrial myopathy. (A) Serial sections within Patient 5 illustrate cytochrome oxidase-positive (brown) and respiration-deficient, cytochrome oxidase-negative, succinic dehydrogenase-positive (intense blue) fibres. (B) Serial section with CD31 immunostaining reveals increased capillaries around cytochrome oxidase-negative compared with cytochrome oxidase-positive fibres. (C–E) VEGF immunostaining shows more intense staining of endothelial perinuclei surrounding individual cytochrome oxidase-deficient fibres. Individual cytochrome oxidase-negative fibres in the serial section are marked as a, b and c; a single cytochrome oxidase-positive fibre is marked with an asterisk. Images in A and B = ×10 magnification, C–E = ×40 magnification. COX = cytochrome oxidase; SDH = succinic dehydrogenase.
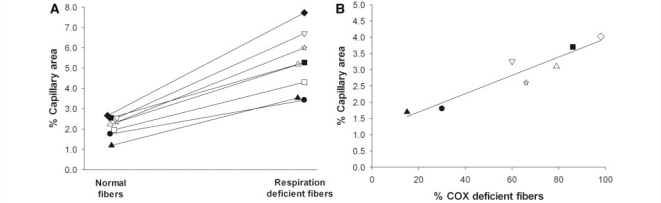
The differences in capillary distribution among respiration-deficient and normal fibres is illustrated in the quadriceps muscle biopsy of a single patient with mitochondrial myopathy with a tRNAtrp T5543C mutation (Anitori et al., 2005) (Fig. 3). As illustrated in serial muscle sections, fibres staining intensely blue had a correspondingly increased compliment of capillaries compared with fibres staining brown. This was a consistent finding in each of the eight patients with mitochondrial myopathy. The mean percentage capillary area was almost 2.5-fold higher in respiration-deficient compared with more normal fibres (5.4 ± 1.5%, range 3.4–7.7% versus 2.2 ± 0.5%, range 1.2–2.7%, P < 0.001) (Fig. 4A). There was no difference in fibre perimeter between these two groups (respiration normal = 226.7 ± 35.5; respiration deficient = 239.5 ± 41.6 µm). Furthermore, the proportion of Type 1 and Type 2 fibres did not differ between the normal (Type 1 = 60 fibres, Type 2 = 12 fibres) and respiration-deficient (Type 1 = 62 fibres, Type 2 = 10 fibres) groups.
Figure 4.
Comparison of percentage capillary area among respiratory-deficient and more normal fibres within skeletal muscle of individual patients with mitochondrial myopathy. (A) In each patient, respiratory-deficient (cytochrome oxidase-negative or ragged-red) fibres contain significantly higher (P < 0.001) levels of capillaries compared with more normal fibres. (B) Percentage capillary area increases in direct proportion to the percentage of cytochrome oxidase-negative fibres in the seven patients whose mutation affects cytochrome oxidase activity (r2 = 0.90; P < 0.01). COX = cytochrome oxidase. ▴ = Patient 1; • = Patient 2; ☆ = Patient 3; ♦ = Patient 4; △ = Patient 5; ▪ = Patient 6; ▽ = Patient 7; □ = Patient 8; and ◊ = Patient 9.
Among patients whose mitochondrial DNA mutation affected Complex IV activity, the proportion of cytochrome oxidase-deficient fibres correlated directly with the overall percentage capillary area within their muscle (r2 = 0.90, P < 0.01, Fig. 4B).
Localization of VEGF protein was assessed immunohistochemically and compared with serial sections stained for cytochrome oxidase/succinic dehydrogenase and CD31. The distribution of more intense staining paralleled CD31 immunostaining with greater VEGF immunostaining surrounding respiration-deficient compared with normal fibres (Fig. 3C). Intense VEGF immunostaining was not detected in control muscle.
Discussion
Analysis of capillaries in skeletal muscle of patients with heteroplasmic mitochondrial DNA mutations revealed: (i) overall capillary levels are greater compared with age- and sex-matched sedentary control subjects; (ii) the normal relationship between muscle oxidative capacity and capillary levels is disrupted with an abnormally high capillary area relative to peak VO2 in cycle exercise in all patients; (iii) within skeletal muscle of patients, capillary levels are most abundant around cytochrome oxidase-negative or ragged-red fibres and overall capillary area correlates directly with the percentage of cytochrome oxidase-negative fibres; and (iv) the increase in muscle capillary levels correlates directly with the severity of the mismatch between systemic oxygen delivery (cardiac output) and oxygen utilization during cycle exercise. These results suggest that impaired mitochondrial metabolism increases the compliment of capillaries around respiration-incompetent muscle fibres, thus promoting increased blood flow to these fibre segments to account for the characteristic mismatch between oxygen delivery and utilization in severe mitochondrial myopathy. They also provide evidence that impaired oxidative phosphorylation drives angiogenesis in skeletal muscle independently of hypoxia.
Capillaries surrounding muscle fibres constitute the ultimate point of transfer of oxygen from the circulation to respiring muscle mitochondria. Muscle capillary levels in healthy humans are closely related to muscle oxidative capacity (Andersen and Henriksson, 1977; Ingjer, 1978) consistent with the linear relationship between peak oxygen utilization in cycle exercise and quadriceps capillary levels in our control subjects. Regular aerobic training is associated with an increase in capillary levels to match increased delivery of oxygen to increased capacity for muscle oxidative phosphorylation, thus maintaining the tight, virtually 1:1 coupling of oxygen delivery by the circulation to oxygen utilization by working muscle that is characteristic of healthy humans.
The fact that the normal distribution of muscle capillaries is disrupted by mitochondrial defects was recognized in morphological studies that described an increase in capillary abundance around ragged-red fibres in patients with mitochondrial myopathy associated with progressive external ophthalmoplegia and Kearns–Sayre syndrome almost 25 years ago (Stadhouders and Sengers, 1987); and increased capillaries surrounding ragged-red fibres in patients with inclusion body myositis was reported >30 years ago (Carpenter et al., 1978). Our study confirms and extends these early observations. We found that capillary area in quadriceps muscle was high relative to muscle oxidative capacity, as assessed in cycle exercise, in all patients with mitochondrial myopathy and that the distribution of capillaries was most abundant around respiration-deficient muscle fibre segments, i.e. cytochrome oxidase-negative or ragged-red fibres. Furthermore, the overall abundance of muscle capillaries correlated directly with the percentage of respiration-deficient (cytochrome oxidase-negative or ragged-red) fibres. Accordingly, in contrast to the relationship that prevails in healthy humans, capillary area was greatest in patients with the most profoundly limited muscle oxidative capacity. This suggests that the increase in capillary levels around respiration-deficient muscle fibres in mitochondrial myopathy preferentially directs oxygen delivery to respiration-deficient fibres during exercise and thus promotes the mismatch between oxygen delivery and utilization that is characteristic of severe mitochondrial myopathies (Haller et al., 1989, 1991; Taivassalo et al., 2003). This conclusion is strongly supported by our finding of a direct correlation between capillary abundance and the level of exaggerated circulatory response to exercise in mitochondrial myopathy (Fig. 2B). Our previous studies have linked the magnitude of the mismatch between systemic oxygen delivery and utilization during exercise to severity of impaired muscle-oxidative phosphorylation (Taivassalo et al., 2003). We now demonstrate that an important element of this anomalous circulatory response is a maladaptive increase in capillary abundance that parallels the severity of the defect in oxidative phosphorylation and promotes futile perfusion of muscle fibres that are unable to utilize oxygen. Patients with less severe defects have a more normal circulatory response to exercise (Taivassalo et al., 2003) and would therefore be expected to have a more normal complement of capillaries relative to oxidative capacity. In keeping with this notion, Jeppesen et al. (2006) studied patients with mitochondrial myopathy with more preserved oxidative capacity and found a more normal abundance of muscle capillaries.
This increase in capillaries among respiration-deficient muscle fibres implies that impaired muscle oxidative phosphorylation promotes angiogenesis independently of hypoxia. This conclusion is supported by the fact that the respiration-incompetent muscle fibre segments as identified histologically would be unable to increase the extraction of oxygen from blood and that increased capillary numbers and resulting increased blood flow to these fibres during exercise would promote anomalously high-muscle oxygen levels during exercise. Experimental results confirm that muscle oxygen levels remain high or actually increase during exercise in mitochondrial myopathy as assessed by near-infrared spectroscopy (Bank and Chance, 1994; Grassi et al., 2007) and by the finding of anomalously high venous oxygen levels draining active muscle where the level of arterialization of venous blood correlates directly with the magnitude of exaggerated systemic oxygen delivery during exercise (Taivassalo et al., 2002). Hypoxia is a key regulator of angiogenesis as mediated by HIF-1α. Constitutively expressed HIF-1α, under conditions of normal tissue oxygen levels, is degraded after hydroxylation of specific proline residues by an ubiquitin-mediated mechanism. Under conditions of low oxygen tensions, proline hydroxylases are inhibited, and HIF-1α levels are increased, thereby promoting HIF-related gene expression, including VEGF, to augment angiogenesis.
Our finding that capillary abundance and VEGF expression are increased despite high muscle oxygen levels during exercise implies that an alternate mechanism is operative to increase capillary abundance around respiration-deficient muscle fibres. Recently, Arany et al. (2008) have shown that PGC-1α augments angiogenesis by increasing VEGF levels independently of HIF-1α via PGC-1α-mediated coactivation of the orphan nuclear receptor oestrogen-related receptor-α (ERRα). Since PGC-1α is induced by aerobic exercise, this signalling pathway may thus promote a coordinated increase in mitochondrial numbers and an increase in capillary abundance to augment the delivery of oxygen that is needed for enhanced rates of oxidative phosphorylation (Arany et al., 2008). The role of PGC-1α in augmenting mitochondrial biogenesis in skeletal muscle is well established (Lin et al., 2005); so this signalling mechanism is presumably operative in the mitochondrial proliferation that is typical of ragged-red fibres in response to impaired cellular energy production in mitochondrial myopathies. In support of this notion, we have previously reported an increase in PGC-1α in patients with mitochondrial myopathy attributable to mitochondrial DNA mutations (Adhihetty et al., 2007).
Accordingly, we consider these results to provide strong evidence that a central mechanism of the mismatch between oxygen delivery and oxygen utilization during exercise in mitochondrial myopathy is attributable to the increase in capillary abundance that occurs surrounding respiration-incompetent muscle fibres. We further postulate that the increase in capillary abundance, like the increase in mitochondrial numbers, is mediated by PGC-1α as activated by the cellular oxidative energy crisis that occurs when the mitochondrial respiratory chain is defective and oxidative energy demand exceeds the capacity for muscle oxidative phosphorylation.
While our study focused upon the implications of mitochondrial disease for capillary abundance within skeletal muscle, it should be noted that vascular proliferation is a feature of mitochondrial defects in other tissues, notably in the CNS. Vascular proliferation is a characteristic pathological feature of Leigh's encephalopathy due to a variety of mitochondrial defects that severely impair oxidative phosphorylation in the developing nervous system (Brown and Squier, 1996; DiMauro and Schon, 2008). Accordingly, we suggest that capillary proliferation driven by impaired oxidative phosphorylation may represent a common consequence of mitochondrial disease in highly oxidative tissues.
In summary, our results indicate that excess oxygen delivery relative to oxygen utilization in mitochondrial myopathy is paralleled by a preferential increase in capillaries around respiration-deficient muscle fibres in response to impaired oxidative phosphorylation, thus promoting futile delivery of oxygen. These findings imply that intact mitochondrial function is critical for maintaining normal circulation during exercise and for a normal circulatory adaptation to changes in habitual levels of physical activity. While the mitochondrial signalling mechanism(s) remain to be discovered, we postulate that the cellular phosphorylation potential ([ATP]/([ADP][Pi]) or closely related indicators of mitochondrial energy demand are involved.
Funding
The National Institutes of Health [National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS); RO1-AR050597 to R.G.H.]; the Muscular Dystrophy Association USA (to R.G.H. and T.T.); the Natural Sciences and Engineering Council of Canada (NSERC; to T.T.); the Canadian Institutes of Health Research (to T.T.).
Glossary
Abbreviations
- HIF-1α
hypoxia-inducible factor
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator-1α
- VEGF
vascular endothelial growth factor
References
- Adair TH, Gay WJ, Montani JP. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol. 1990;259(3 Pt 2):R393–404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- Adhihetty PJ, Taivassalo T, Haller RG, Walkinshaw DR, Hood DA. The effect of training on the expression of mitochondrial biogenesis- and apoptosis-related proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab. 2007;293:E672–80. doi: 10.1152/ajpendo.00043.2007. [DOI] [PubMed] [Google Scholar]
- Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–90. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu AL, Hanna MG, Reichmann H, Bruno C, Penn AS, Tanji K, et al. Exercise intolerance due to mutations in the cytochrome b gene of mitochondrial DNA. N Engl J Med. 1999a;341:1037–44. doi: 10.1056/NEJM199909303411404. [DOI] [PubMed] [Google Scholar]
- Andreu AL, Tanji K, Bruno C, Hadjigeorgiou GM, Sue CM, Jay C, et al. Exercise intolerance due to a nonsense mutation in the mtDNA ND4 gene. Ann Neurol. 1999;45:820–3. doi: 10.1002/1531-8249(199906)45:6<820::aid-ana22>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Anitori R, Manning K, Quan F, Weleber RG, Buist NR, Shoubridge EA, et al. Contrasting phenotypes in three patients with novel mutations in mitochondrial tRNA genes. Mol Genet Metab. 2005;84:176–88. doi: 10.1016/j.ymgme.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- Bank W, Chance B. An oxidative defect in metabolic myopathies: diagnosis by noninvasive tissue oximetry. Ann Neurol. 1994;36:830–7. doi: 10.1002/ana.410360606. [DOI] [PubMed] [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–61. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Brown GK, Squier MV. Neuropathology and pathogenesis of mitochondrial diseases. J Inherit Metab Dis. 1996;19:553–72. doi: 10.1007/BF01799116. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Karpati G, Heller I, Eisen A. Inclusion body myositis: a distinct variety of idiopathic inflammatory myopathy. Neurology. 1978;28:8–17. doi: 10.1212/wnl.28.1.8. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- Grassi B, Marzorati M, Lanfranconi F, Ferri A, Longaretti M, Stucchi A, et al. Impaired oxygen extraction in metabolic myopathies: detection and quantification by near-infrared spectroscopy. Muscle Nerve. 2007;35:510–20. doi: 10.1002/mus.20708. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ. Notes on the estimation of the numerical density of arbitrary profiles; the edge effect. J Microsc. 1977;111 (Pt 2):219–23. [Google Scholar]
- Gustafsson T, Puntschart A, Kaijser L, Jansson E, Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276(2 Pt 2):H679–85. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- Haller RG, Henriksson KG, Jorfeldt L, Hultman E, Wibom R, Sahlin K, et al. Deficiency of skeletal muscle succinate dehydrogenase and aconitase. Pathophysiology of exercise in a novel human muscle oxidative defect. J Clin Invest. 1991;88:1197–206. doi: 10.1172/JCI115422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller RG, Lewis SF, Estabrook RW, DiMauro S, Servidei S, Foster DW. Exercise intolerance, lactic acidosis, and abnormal cardiopulmonary regulation in exercise associated with adult skeletal muscle cytochrome c oxidase deficiency. J Clin Invest. 1989;84:155–61. doi: 10.1172/JCI114135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. Procedure to identify outliers in regression models. New York: Harcourt Brace Jovanovich Publishers; 1985. [Google Scholar]
- Hepple RT. Skeletal muscle: microcirculatory adaptation to metabolic demand. Med Sci Sports Exerc. 2000;32:117–23. doi: 10.1097/00005768-200001000-00018. [DOI] [PubMed] [Google Scholar]
- Hepple RT, Mackinnon SL, Goodman JM, Thomas SG, Plyley MJ. Resistance and aerobic training in older men: effects on VO2peak and the capillary supply to skeletal muscle. J Appl Physiol. 1997;82:1305–10. doi: 10.1152/jappl.1997.82.4.1305. [DOI] [PubMed] [Google Scholar]
- Ingjer F. Maximal aerobic power related to the capillary supply of the quadriceps femoris muscle in man. Acta Physiol Scand. 1978;104:238–40. doi: 10.1111/j.1748-1716.1978.tb06273.x. [DOI] [PubMed] [Google Scholar]
- Jeppesen TD, Schwartz M, Olsen DB, Wibrand F, Krag T, Duno M, et al. Aerobic training is safe and improves exercise capacity in patients with mitochondrial myopathy. Brain. 2006;129(Pt 12):3402–12. doi: 10.1093/brain/awl149. [DOI] [PubMed] [Google Scholar]
- Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–70. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Mathieu-Costello O, Hepple RT. Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev. 2002;30:80–4. doi: 10.1097/00003677-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG. Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol. 2006;571(Pt 2):415–24. doi: 10.1113/jphysiol.2005.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–94. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- Stadhouders AM, Sengers RC. Morphological observations in skeletal muscle from patients with a mitochondrial myopathy. J Inherit Metab Dis. 1987;10(Suppl. 1):62–80. doi: 10.1007/BF01812848. [DOI] [PubMed] [Google Scholar]
- Swalwell H, Blakely EL, Sutton R, Tonska K, Elstner M, He L, et al. A homoplasmic mtDNA variant can influence the phenotype of the pathogenic m.7472Cins MTTS1 mutation: are two mutations better than one? Eur J Hum Genet. 2008;16:1265–74. doi: 10.1038/ejhg.2008.65. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Abbott A, Wyrick P, Haller RG. Venous oxygen levels during aerobic forearm exercise: an index of impaired oxidative metabolism in mitochondrial myopathy. Ann Neurol. 2002;51:38–44. doi: 10.1002/ana.10027. [DOI] [PubMed] [Google Scholar]
- Taivassalo T, Jensen TD, Kennaway N, DiMauro S, Vissing J, Haller RG. The spectrum of exercise tolerance in mitochondrial myopathies: a study of 40 patients. Brain. 2003;126(Pt 2):413–23. doi: 10.1093/brain/awg028. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Gerber HP, Ferrara NM, Wagner PD. Capillary regression in vascular endothelial growth factor-deficient skeletal muscle. Physiol Genomics. 2004;18:63–9. doi: 10.1152/physiolgenomics.00023.2004. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Schaefer AM, Barron MJ, McFarland R, Turnbull DM. The diagnosis of mitochondrial muscle disease. Neuromuscul Disord. 2004;14:237–45. doi: 10.1016/j.nmd.2003.12.004. [DOI] [PubMed] [Google Scholar]