Abstract
Contemporary clinical and basic neuroscience studies have increasingly implicated the anterior temporal lobe regions, bilaterally, in the formation of coherent concepts. Mounting convergent evidence for the importance of the anterior temporal lobe in semantic memory is found in patients with bilateral anterior temporal lobe damage (e.g. semantic dementia), functional neuroimaging and repetitive transcranial magnetic stimulation studies. If this proposal is correct, then one might expect patients with anterior temporal lobe resection for long-standing temporal lobe epilepsy to be semantically impaired. Such patients, however, do not present clinically with striking comprehension deficits but with amnesia and variable anomia, leading some to conclude that semantic memory is intact in resection for temporal lobe epilepsy and thus casting doubt over the conclusions drawn from semantic dementia and linked basic neuroscience studies. Whilst there is a considerable neuropsychological literature on temporal lobe epilepsy, few studies have probed semantic memory directly, with mixed results, and none have undertaken the same type of systematic investigation of semantic processing that has been conducted with other patient groups. In this study, therefore, we investigated the semantic performance of 20 patients with resection for chronic temporal lobe epilepsy with a full battery of semantic assessments, including more sensitive measures of semantic processing. The results provide a bridge between the current clinical observations about resection for temporal lobe epilepsy and the expectations from semantic dementia and other neuroscience findings. Specifically, we found that on simple semantic tasks, the patients’ accuracy fell in the normal range, with the exception that some patients with left resection for temporal lobe epilepsy had measurable anomia. Once the semantic assessments were made more challenging, by probing specific-level concepts, lower frequency/more abstract items or measuring reaction times on semantic tasks versus those on difficulty-matched non-semantic assessments, evidence of a semantic impairment was found in all individuals. We conclude by describing a unified, computationally inspired framework for capturing the variable degrees of semantic impairment found across different patient groups (semantic dementia, temporal lobe epilepsy, glioma and stroke) as well as semantic processing in neurologically intact participants.
Keywords: language processing, memory, semantic memory disorders, temporal lobe epilepsy
Introduction
Semantic memory encompasses a rich fund of general knowledge about the world, including our understanding of words, pictures, objects, sounds, faces and events (Rogers et al., 2004; Jefferies and Lambon Ralph, 2006; Patterson et al., 2007). It plays a critical role in many everyday verbal and non-verbal activities. Disruption of semantic memory through neurological disease or injury can, therefore, have serious consequences for patients’ daily lives. The degradation of semantic memory in semantic dementia and herpes simplex encephalitis is associated with bilateral damage to and hypometabolism of the anterior temporal lobes (Nestor et al., 2006; Noppeney et al., 2007; Rohrer et al., 2009; Mion et al., 2010). Consequently, behavioural data from these patients have suggested a model in which concepts are formed through the convergence of sensory, motor and verbal experience via an anterior temporal lobe, transmodal representational hub (Rogers et al., 2004), which licenses the formation of coherent concepts (Lambon Ralph et al., 2010b).
Although previously overlooked, there is now a growing consensus that this transmodal anterior temporal lobe hub contributes critically to semantic cognition (Patterson et al., 2007). This emerging view reflects a convergence of the established clinical data on semantic dementia, herpes simplex virus encephalitis, etc., with contemporary basic neuroscience studies. The multimodal, selective semantic impairment of semantic dementia can be mimicked in neurologically intact participants by applying repetitive transcranial magnetic stimulation to the lateral anterior temporal lobe (Pobric et al., 2007; Lambon Ralph et al., 2009; Pobric et al., 2010a). Indeed, by applying repetitive transcranial magnetic stimulation to either the transmodal anterior temporal lobe or modality-specific information-coding regions, it is possible to probe different parts of the ‘hub-and-spoke’ semantic architecture (Pobric et al., 2010b). Likewise, when using techniques that avoid (e.g. PET or magnetoencephalography) or correct for the various methodological issues associated with successful imaging of the anterior temporal lobe (Devlin et al., 2000; Visser et al., 2010b), studies find considerable bilateral anterior temporal lobe activation for multimodal semantic processing (Vandenberghe et al., 1996; Marinkovic et al., 2003; Sharp et al., 2004; Binney et al., 2010; Visser et al., 2010a; Visser and Lambon Ralph, 2011).
The resection for temporal lobe epilepsy puzzle
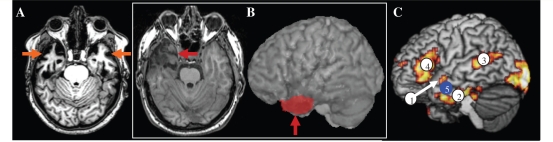
Despite this considerable convergent evidence implicating an important role for the anterior temporal lobe in semantic cognition, there remains a key puzzle and potential challenge to this view. One treatment for long-standing epilepsy with focal seizures in the temporal lobe is surgical resection. In standard ‘en bloc’ resection, part or all of the anterior temporal lobe (unilaterally) is removed. One example is shown in Fig. 1B. The resected area overlaps considerably with: (i) the core region of atrophy observed in semantic dementia (Fig. 1A; albeit the atrophy is bilateral, see below); (ii) the areas activated by normal participants when completing semantic tasks (example from Binney et al., 2010); and (iii) the target region in our previous repetitive transcranial magnetic stimulation studies (Fig. 1C; Pobric et al., 2007, 2010b; Lambon Ralph et al., 2009). Clinically, patients with resection for temporal lobe epilepsy (TLE) do not report comprehension impairment but do complain of significant anomia and amnesia. Consequently, it is sometimes concluded that semantic processing is entirely or largely spared following resection for TLE (Hickok and Poeppel, 2004; Kho et al., 2008; Simmons and Martin, 2009); a stance that could bring into question the necessity of the anterior temporal lobe in semantic cognition and could undermine the explanation of semantic impairment in semantic dementia, herpes simplex virus encephalitis, etc. This conclusion is premature, however, for three reasons:
Lack of data: clinical assessment tends to focus on naming and episodic memory, and rarely on comprehension (Giovagnoli et al., 2005). The same is true in the large neuropsychological published literature on TLE with and without resection. As noted above, many patients with resection for TLE complain of word-finding difficulties, which are confirmed by formal testing. The same is true in very mild semantic dementia and previous studies have demonstrated that this is driven by semantic impairment (Lambon Ralph et al., 2001). It is possible, therefore, that there is measurable semantic impairment in resection for TLE but there is a dearth of studies that investigate semantic processing in the literature (see below). Consequently resection for TLE and semantic impairment might be a case of ‘absence of evidence’ rather than ‘evidence of absence’.
Unilateral versus bilateral damage: although the affected area in resection for TLE and semantic dementia overlaps, one of the major neurological differences is that semantic dementia (as well as herpes simplex virus encephalitis, Alzheimer's disease, etc.) is a bilateral disease, whereas resection is only ever conducted unilaterally. Past investigations of semantic dementia have shown that the degree of semantic impairment is related to the extent of bilateral atrophy in this condition (Galton et al., 2001; Lambon Ralph et al., 2001). A previous study that compared patients with semantic dementia against those with unilateral temporal damage (of mixed aetiology including a subset of cases with resection for TLE) on the same standard semantic battery, found that unilateral damage generated minimal semantic impairment (Lambon Ralph et al., 2010a). These results have motivated our working hypothesis that semantic memory is bilaterally distributed across left and right anterior temporal lobes. This (a) might improve the robustness of the system to damage if there is some redundancy in the bilaterally distributed representations; and (b) would give a basis for plasticity-related reorganization. Consistent with this view, recent work with computational models of a bilateral semantic system has suggested several reasons why unilateral pathology might produce dramatically less severe impairments than bilateral damage (A. C. Schapiro et al., manuscript under revision).
Plasticity-related reorganization: the utility of studying resection for TLE for localization of function needs to be treated with caution for various plasticity-related reasons. A long-standing seizure history complicates attempts to generalize findings from patients with resection for TLE. This point is supported by at least three findings: (a) post-operative deficits of cognition/language tend to be more severe in patients with a later age of seizure onset (Hermann et al., 1999); (b) there is a significant change in the pattern of language-related white-matter pathways in patients with long-standing epilepsy (Powell et al., 2007); and (c) there is significant alteration in neurotransmitter function (Hammers et al., 2003). In the face of these neuroanatomical changes, semantic function may be shifted away from the seizure-related region, such that subsequent resection will have less dramatic consequences than an acute neurological event. In the limit, therefore, it is possible that resection will not produce any measurable semantic impairment because the tissue is no longer supporting this function. Secondly, after acute brain damage or neurosurgery (e.g. stroke, glioma), patients tend to demonstrate at least some degree of recovery—again suggesting a role of plasticity-related redistribution of function (Thiel et al., 2001, 2005; Duffau et al., 2003; Keidel et al., 2010). In keeping with this notion, one early study of semantic performance in resection for TLE found a negative correlation between time post-surgery and comprehension impairment (Wilkins and Moscovitch, 1978).
Figure 1.
The puzzle of semantic memory in resection for TLE. (A) An example axial MRI for a patient with semantic dementia, with clear bilateral anterior temporal lobe atrophy (orange arrows) underpinning the patient's demonstrable semantic impairment. (B) A comparable axial slice from a patient following anterior temporal lobe unilateral resection for TLE (red arrow). The red region on the lateral view shows the resected area. This overlaps with the anterior temporal lobe regions (1 and 2 in C) activated by normal subjects in our functional MRI semantic studies (Binney et al., 2010) and also with the region (5) that we have stimulated with repetitive transcranial magnetic stimulation in normal participants to produce a selective semantic effect (Pobric et al., 2007; 2010; Lambon Ralph et al., 2009).
As noted above, there is a considerable neuropsychological literature on the status of TLE and patients with resection for TLE but the majority of this is focused upon the patients’ episodic memory impairment and on their word-finding difficulties (anomia). To date the semantic status of patients with resection for TLE has rarely been systematically assessed using the type and breadth of semantic battery that has been adopted for other patient groups (e.g. semantic dementia, herpes simplex virus encephalitis, etc.; Bozeat et al., 2000; Adlam et al., 2006; Lambon Ralph et al., 2007). A handful of studies have assessed, however, specific aspects of semantic processing either directly or indirectly, yielding somewhat mixed results. Some studies have probed semantic memory in resection for TLE groups and found no evidence of semantic impairment on simple naming or comprehension tests (Hermann et al., 1994, 1995). Most studies have found, however, evidence of anomia after resection, which is more apparent in late-onset patients with TLE (Hermann et al., 1999), is more common in patients after left anterior temporal lobe resection (Martin et al., 1998; Seidenberg et al., 1998; Glosser et al., 2003), and appears to reflect an underlying semantic weakness (Bell et al., 2001; Antonucci et al., 2008; Drane et al., 2008). These reductions in word-finding also extend to verbal fluency tasks that have highlighted mild deficits after left or right anterior temporal lobe resection (Martin et al., 1990) and have detected semantically based deficits in patients with left and right TLE prior to resection (Tröster et al., 1995; N'Kaoua et al., 2001). Four investigations have probed more demanding, specific-level concepts in the form of famous face recognition and naming. Glosser et al. (2003) found that famous face naming was impaired in both left and right TLE or patients with resection for TLE, whilst the ability to provide information about famous people became impaired after resection in the right resection for TLE subgroup alone (Glosser et al., 2003). Three other studies found that patients with left TLE were impaired on famous face naming whilst cases with right TLE exhibited reduced ability in familiarity, identification and naming of famous people (Seidenberg et al., 2002; Viskontas et al., 2002; Drane et al., 2008). Similar results were obtained in the large-scale studies reported by Tranel and colleagues (2006, 2009) whose temporal polar groups contained a majority of patients with left versus right resection for TLE. One large-scale study of (non-resected) patients with TLE probed semantic function using a multi-modal semantic battery including naming, word–picture matching and semantic association judgements and object decisions (Giovagnoli et al., 2005). The investigation found that patients with left TLE scored significantly worse than controls on these measures, though the drop in performance only amounted to a few test items that would be too small a reduction to be clinically reliable at the level of individual patients. Very similar tests and results were used in a study of eight patients with left resection for TLE (Antonucci et al., 2008). In addition to the patients’ anomia on confrontational naming and fluency tests, Antonucci et al. (2008) found evidence of a mild underlying semantic impairment by using more challenging semantic measures (semantic association judgements and synonym judgements including lower frequency and more abstract items).
The purpose of the present study was to complete the first systematic and detailed investigation of semantic memory in patients with resection for chronic TLE. Our semantic battery included various expressive and receptive tasks that have been used previously with semantic dementia, herpes simplex virus encephalitis and other patient groups (Bozeat et al., 2000; Jefferies and Lambon Ralph, 2006; Lambon Ralph et al., 2007, 2010a), allowing us to compare the patients with resection for TLE directly to these other neurological groups. We were mindful, however, that the standard semantic battery tests might not be sufficiently sensitive given that (i) patients with TLE and patients with resection for TLE do not present clinically with striking comprehension impairments; and (ii) a previous study of patients with unilateral temporal damage (including a subset of resection for TLE cases) did not identify major semantic impairment using typical semantic battery assessments (indicating that semantic memory might be supported in a semi-redundant fashion through bilateral temporal representation: see above and Lambon Ralph et al., 2010a; A. C. Schapiro et al., manuscript under revision). Accordingly, we added a set of tasks that have proved to be more sensitive to the mild semantic impairment observed in very early cases of semantic dementia (Bozeat et al., 2000; Adlam et al., 2006) or in neurologically intact participants after left or right lateral anterior temporal lobe repetitive transcranial magnetic stimulation (Pobric et al., 2007, 2010a; Lambon Ralph et al., 2009). In very early semantic dementia (like resection for TLE), patients do not necessarily complain of impaired comprehension in the clinic (on the rare occasions that they present so early) but at this stage, their semantically driven anomia is already apparent, especially on graded tests of confrontational naming (Bozeat et al., 2000; Lambon Ralph et al., 2001; Adlam et al., 2006). Secondly, at all stages of the disease, the semantic dementia patients’ semantic impairment is most apparent for concepts that are: (i) less familiar/frequent; (ii) more abstract; and (iii) more specific (Warrington, 1975; Funnell, 1995; Jefferies et al., 2009; Hoffman and Lambon Ralph, 2011). As a result we probed abstract versus concrete concepts, high and low frequency words, and also the comprehension and naming of specific-level concepts (both faces and general concepts). Our previous investigations of repetitive transcranial magnetic stimulation to lateral anterior temporal lobe in neurologically intact participants confirmed this approach; repetitive transcranial magnetic stimulation has a relatively stronger effect on specific-level concepts, abstract concepts, etc. (Pobric et al., 2007, 2009) and also provided another important methodological insight for the current study. Specifically, the much weaker effect of repetitive transcranial magnetic stimulation shows itself primarily through reaction times rather than reduction in accuracy, so we measured the decision/response times of patients with resection for TLE in a number of the semantic assessments. Previous repetitive transcranial magnetic stimulation studies were also useful because we had developed difficulty-matched, non-semantic decision tasks to delineate generalized slowing of reaction times from selective slowing of semantic decisions. Again, we reused the most difficult of these non-semantic, timed assessments in the present study to investigate whether any slowing of semantic performance in the patients with resection for TLE reflected general, slowed processing or a more selective semantic inefficiency. The inclusion of reaction times as well as accuracy in the current study was also prompted by one of the first systematic investigations of semantic processing in patients with resection for TLE (Wilkins and Moscovitch, 1978). These authors found that semantic performance in resection for TLE was normal if the task was conducted without time limits but scores for all patients were outside of the normal range when trial duration was limited.
Materials and methods
Patients
Twenty patients with ‘en bloc’ resection for TLE (nine left and 11 right) were recruited from the epilepsy service at the Walton Centre NHS Foundation Trust (Liverpool, UK). Patients with developmental disorders, head injury, psychiatric history, stroke or glioma were excluded. Detailed background medical information for each patient is summarized in Table 1. All patients were in the chronic phase post-surgery [months post-surgery: mean = 35 (standard deviation = 19.9, min = 8)] and had long-standing epilepsy [age of diagnosis (years): mean = 13.1 (standard deviation = 10.1, min = 4)]. There was a non-significant trend for the left resection for TLE to be fewer months post-surgery than the right [left: mean = 30.3 (standard deviation = 18.6) versus right: mean = 43.0 (standard deviation = 20.6); t(18) = 1.43, P = 0.17]. Estimating from the histopathology samples, the volume of resected temporal lobe tissue varied across the cases [volume of resection (cm3): mean = 31.9 (standard deviation = 24.2, max = 92.0)]. The patients with left and right resection for TLE had equivalent volume resection [left: mean = 28.9 cm3 (standard deviation = 20.7) versus right: mean = 36.3 cm3 (standard deviation = 24.0); t(18) < 1]. In the majority of patients, analysis of these samples revealed gliosis and neuronal loss in the hippocampal region, consistent with a diagnosis of mesial temporal sclerosis. In line with the current neuropsychological literature, all patients complained of impaired episodic memory, word-finding difficulties and significant lethargy at the end of the day. No patient reported comprehension problems, even when asked directly, and the vast majority of patients had returned to full-time work or other occupations.
Table 1.
Background medical and biographical information
| Patient | Age | Months post-surgery | Years of education | Occupation | Age at diagnosis (years) | Seizure frequency | Pre-surgical scan report | Wada language test | Post-surgery issues | Volume resected (cm3) | Pathology report |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases with left temporal resection | |||||||||||
| SM | 24 | 21 | 21 | University student | 7 | Weekly+ | - | - | - | 55.5 | Marked loss of pyramid neurons and gliosis in CA1 and CA4 plus dentate thinning; subpial gliosis in temporal neocortex |
| DK | 49 | 17 | 18 | Senior operations manager | 45 | Biannually | MRI: abnormal left temporal lobe—possible cavernoma | - | - | 1.8 | Sections show clusters of dilated vessels of varying all thickness and calibre; surrounding gliosis and haemosiderin deposition |
| DL | 30 | 24 | 18 | Accounts assistant | 15 | Weekly | - | - | - | 68.5 | Isocortex and subcortical white matter without abnormalities; hippocampal formation - extensive neuronal loss and gliosis in sector CA1 and CA2 with moderate cell loss from the dentate fascia and CA4. |
| AW | 25 | 17 | 21 | Volunteer | 15 | Daily | MRI: bilaterally small hippocampi | - | Subdural haematoma evacuated | 20.5 | Isocortex and subcortical white matter without abnormalities; other fragments cannot be identified |
| SS | 28 | 8 | 16 | Packer | 15 | Weekly+ | - | - | Seizures came back in a cluster | 32.35 | Hippocampus shows focal dispersion, attenuation and loss of dentate gyrus neurons; scattered shrunken neurons and reactive astrocytes present |
| PW | 32 | 60 | 18 | - | 15 | Daily | MRI: reduced left hippocampal volume and high T2 signal | Left | - | 24.32 | Isocortex—no diagnostic features; hippocampal formation—gliosis associated with neuronal loss, particularly of the fascia dentata |
| MBW | 46 | 60 | 16 | Machinist | 22 | Weekly+ | MRI: reduced left hippocampal volume | - | - | 16.8 | Isocortex and subcortical white matter – subependymal gliosis, dystrophic calcification and ependymal canals; other fragments cannot be identified. |
| MF | 38 | 30 | 16 | Shop assistant | 5 | Monthly+ | MRI: reduced left hippocampal volume | Left | - | 24.5 | Isocortex and subcortical white matter without abnormalities; hippocampal formation - neuronal loss |
| MM | 32 | 36 | 18 | Accounts assistant | 13 | Weekly | MRI—no significant change; contrast enhancement—signal within the hippocampal and parahippocampal gyrus; MRS-NAA ratio is slightly reduced as compared with the contralateral side | Left | Atrophy of the left temporalis nerve | 16.25 | Isocortex without significant abnormalities; hippocampal formation, including parts of the end of folium, fascia dentata, pyramidal cell layer with evidence of gliosis and neuronal loss |
| Cases with right temporal resection | |||||||||||
| RT | 24 | 48 | 22 | Youth worker | 10 | Daily+ | - | Left | - | 27.64 | Isocortex and subcortical white matter without evidence of dysplastic changes; hippocampus—neuronal loss and gliosis from sector CA1 and, to a lesser extent CA2, CA3 and CA4. There is marginal granule cell loss from the dentate fascia |
| RC | 55 | 36 | 21 | Accountant | 5 | Monthly | - | - | - | 55.5 | Hippocampal formation—marked loss of pyramid neurons and gliosis in CA1 and CA4; thinning of dentate |
| JP | 32 | 36 | 21 | IT analyst | 16 | Daily+ | MRI: reduced right hippocampal gyrus and high signal | Left | - | 91.95 | Hippocampal formation—neuronal loss and gliosis are prominent in sectors CA1 and CA3, with neuronal loss from the dentate gyrus |
| NA | 27 | 74 | 16 | Distribution centre assistant | 19 | Weekly+ | - | Left | - | 40.16 | Hippocampal formation—loss and shrinkage of large pyramid neurons |
| MD | 39 | 17 | 18 | Butcher | 4 | Daily+ | MRI: right hippocampal atrophy, particularly in anterior region | Left | Left superior quadrantinopia | 52.5 | Temporal lobe—normal cortex and white matter; hippocampus—neuronal loss from the regions of CA1 and CA4 with associated gliosis |
| LL | 49 | 84 | 16 | Store keeper | 7 | Daily+ | MRI: hippocampal atrophy | Left | Left superior quadrantinopia | 24.08 | Hippocampal formation—severe focal loss of pyramidal neurons with corresponding gliosis; temporal lobe neocortex—no significant abnormalities; mild focal lymphocytic perivascular cuffing in neocortex and white matter |
| SW | 21 | 36 | 16 | Shop manager | 8 | Weekly+ | MRI: right hippocampal atrophy | - | - | 29.9 | Temporal lobe—normal cortex and white matter; hippocampus—neuronal loss and gliosis in CA1, CA3 and CA4 |
| CS | 42 | 17 | 18 | Mail line operator | 17 | Daily | MRI: foreign tissue lesion in the right hippocampus | Bilateral | Six months postoperative bleed | 0.144 | Rarefied ischaemic/post-haemorrhagic changes; no evidence of either tumour or a vascular malformation; no underlying pathological process |
| BB | 43 | 48 | 16 | Lab technician | 6 | Weekly | MRI: hippocampal asymmetry (right < left) | - | 20.525 | Hippocampus—shrinkage, increased eosinophia and loss of pyramidal neurons associated with gliosis; focal loss of neurones in the dentate gyrus | |
| PA | 28 | 36 | 21 | University student | 4 | Daily | MRI: decreased right hippocampal volume, with increased T2 relaxation time | Left | 22.5 | Isocortex, subcortical white matter—no evidence of neoplasia or dysplasia; hippocampal formation—neuronal and gliosis in sector CA1 associated with thinning of the fascia denta and mild gliosis of the end of folium | |
| MB | 32 | 41 | 16 | Nursing assistant | 10 | Weekly+ | MRI: hippocampal asymmetry right < left; hippocampal abnormalities bilaterally | Left | 34.5 | Isocortex and subcortical white matter—occasional focus of sclerosis with macrophages; hippocampus—extensive neuronal loss and gliosis on sectors CA1, CA3, CA4 and the dentate | |
Years of education = age when leaving formal education; plus sign indicates more than one event during the period noted, e.g. ‘weekly+’ indicates several seizures per week but less than ‘daily’. MRS-NAA = magnetic resonance spectroscopy-N-acetylaspartate.
Controls
The performance of patients with resection for TLE on the neuropsychological assessments was compared with the published normative data, where available. For the remaining tests and the timed assessments, their performance was compared with a group of 16 control participants. Given that the patients varied considerably across the case-series in terms of age (mean = 36.0, min = 24, max = 55 years) and education (age at leaving full-time education: mean = 18.5, min = 16, max = 22 years], there is no single obvious control group to compare them against and it would be logistically prohibitive to collect a control group for each patient. Consequently, we opted for a conservative method of comparing the patients with an older group of control participants (age: mean = 67.8, min = 62, max = 80 years; age at leaving full-time education: mean = 16.4, min = 10, max = 22 years). This choice was conservative in the sense that we could be confident that any impaired or slowed performance in the resection for TLE group was clinically significant (though it might reduce the sensitivity to subtle impairments—i.e. a type II error). As reported below, the latter potential problem did not arise (all patients were mildly impaired). In addition, for the timed synonym judgement test, we can compare the patients and older controls with the data from our previous repetitive transcranial magnetic stimulation explorations (e.g. Pobric et al.., 2007), which utilized exactly the same tasks. This is important because we know that vocabulary and general experience increases with age, which might boost semantic performance. The older controls mean decision times on this task were 2 s, whereas the younger repetitive transcranial magnetic stimulation participants were significantly faster in both the non-transcranial magnetic stimulation condition (1.62 s) and even after anterior temporal lobe repetitive transcranial magnetic stimulation (1.78 s), which had significantly slowed their decision times.
Assessment
The neuropsychological battery was designed to assess various aspects of general cognitive performance as well as semantic processing. Both simple and more challenging semantic assessments were included (see ‘Introduction’ section). Most patients were able to complete the entire battery within one or two 2-h testing sessions. In terms of general cognitive testing, we included the word and face subtests from the Camden Recognition Memory Battery (Warrington, 1996), forward and reversed digital span, copy and immediate recall of the Rey complex figure (Osterrieth, 1944) and the Raven's Coloured Progressive Matrices (Raven, 1962).
Three relatively simple semantic tasks were included to allow a direct comparison with semantic dementia. Two assessments (picture naming and spoken word–picture matching with 10 within-category choices) were drawn from the Cambridge Semantic Battery (Bozeat et al., 2000). We also included a non-verbal assessment of object action-to-picture matching in which the participant is asked to select which of the three semantically related tools is used with an action demonstrated by the examiner (Bozeat et al., 2002). Together, the three assessments covered verbal and non-verbal comprehension as well as simple expressive ability. All patients with mild to severe semantic dementia tend to perform below the normal range on these assessments (Bozeat et al., 2000; Adlam et al., 2006). Six additional, more sensitive semantic tasks were also included. Confrontational naming was assessed further through the Graded Naming Test (Warrington, 1997) and the Graded Faces Test (Thompson et al., 2004) both of which contain 30 psychometrically graded items probing the ability to name less familiar general objects or famous individuals. We included this famous face assessment because it requires identification of specific-level concepts (specific individuals) and because face recognition deficits are sometimes associated with right temporal pathology.
We also administered a 96-trial synonym judgement test. This three-alternative, forced-choice task requires participants to match a probe item to one of three alternatives that are presented simultaneously in both written and spoken forms (Jefferies et al., 2009). The test trials vary both frequency (high versus low) and imageability (high, medium, low) orthogonally (with 16 trials in each condition). It was a useful assessment to include in the current study for a variety of reasons: (i) it has proved to be a clinically sensitive test for semantic impairment across a variety of different patient groups (Jefferies and Lambon Ralph, 2006; Lambon Ralph et al., 2007; Jefferies et al., 2009); (ii) in its timed form, it is a sensitive assessment for detecting the effects of left or right lateral anterior temporal lobe repetitive transcranial magnetic stimulation in neurologically intact participants (Pobric et al., 2007, 2009; Lambon Ralph et al., 2009); and (iii) when used in functional MRI, it activates various regions within the anterior temporal lobe (Fig. 1C and Binney et al., 2010). The resection for TLE and control participants completed the timed version of this assessment. Specifically, they were asked to indicate their choice, by way of button press, as quickly and accurately as possible. In order to assess general speed of processing on complex (non-semantic) judgements, we also administered the difficulty-matched, number-decision task from our previous repetitive transcranial magnetic stimulation explorations (Pobric et al., 2007). The format of this test is the same as the synonym judgement task and participants are asked to pick which of the three alternative, double-digit numbers is closest in value to a probe number.
As an assessment of timed confrontational naming, we also asked the participants to complete a picture naming test containing 64 black and white pictures of everyday objects and animals (Lambon Ralph et al., 1998b). The pictures were presented on a computer screen simultaneously with a beep. The participants were asked to provide the name of the picture as quickly and accurately as possible. Their responses were recorded digitally. This recording was analysed offline in order to derive both the accuracy and speed of naming. In past studies, we have found that this method allows us to collect reliable naming/reading times from patients of all severities in a much more natural manner than through the use of a voice-key trigger because participants are able to respond freely.
Our final assessments of semantic processing utilized specific-level concepts to probe the integrity of finer semantic distinctions, which tend to be vulnerable to early semantic degradation in semantic dementia (Warrington, 1975; Adlam et al., 2006). Specific-level concepts from a variety of different categories were selected to ensure that the majority of normal participants were able to name and recognize each item. The picture naming version of these tests contains 22 items (each of which could be accurately named by >75% of the control participants) and the word–picture matching test contained 46 trials.
Results
The patients’ performance on the general cognitive testing is summarized in Table 2. As would be expected in resection for TLE, all patients demonstrated evidence of anterograde amnesia at least for verbal materials; 19/20 patients exhibited abnormal word recognition whilst recognition memory for unfamiliar faces was within the normal range except for one patient (Patient LL). The patients generally had good forward and backward digit span (except for Patients DK, MF and BB in the forward digit span and Patients MM, BB and PA in the backwards digit span). Similarly the patients demonstrated good performance on the Rey figure copy (except for Patients MM, RC and LL) and the immediate recall of the same figure (except for Patients DL and MB). All patients exhibited excellent performance on the Raven's Coloured Progressive Matrices.
Table 2.
Background neuropsychological data
| Max. | Control |
Left temporal lobe resection |
Right temporal lobe resection |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| score | Mean | Cut-off | SM | DK | DL | AW | SS | PW | MBW | MF | MM | RT | RC | JP | NA | MD | LL | SW | CS | BB | PA | MB | |
| Cognitive tasks | |||||||||||||||||||||||
| Camden Recognition Memory | |||||||||||||||||||||||
| Words (percentile) | - | - | - | 5 | 5 | <5 | 5 | 5 | <5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 25 | 5 | 5 | 5 | 5 | 5 | 5 |
| Faces (percentile) | - | - | - | 90 | 20 | 75 | 90 | 50 | 75 | 75 | 75 | 75 | 75 | 90 | 50 | 90 | 25 | 5 | 50 | 90 | 50 | 75 | 50 |
| Digit span: forwards | - | 6.8 | 5 | 5 | 4 | 7 | 6 | 6 | 5 | 6 | 4 | 5 | 6 | 6 | 8 | 7 | 5 | 6 | 6 | 7 | 3 | 5 | 7 |
| Digit span: backwards | - | 4.7 | 2.3 | 4 | 4 | 5 | 5 | 6 | 3 | 5 | 3 | 2 | 5 | 4 | 6 | 3 | 3 | 4 | 4 | 4 | 2 | 2 | 3 |
| Rey figure copy | 36 | 31.03 | 31 | 36 | 31 | 31 | 34 | 34 | 33 | 35 | 36 | 30 | 36 | 26 | 36 | 36 | 33 | 23 | 34 | 31 | 33 | 36 | 34 |
| Rey immediate recall | 36 | 18.3 | 9 | 24 | 19 | 5 | 17 | 17 | 18 | 17 | 17 | 12 | 31 | 15 | 21 | 24 | 17 | 9 | 23 | 23.5 | 12 | 16 | 1.5 |
| RCPM (percentile) | - | - | - | 95 | 95 | 90 | 95 | 95 | 95 | 90 | 95 | 95 | 95 | 95 | 95 | 95 | 90 | 50 | 95 | 90 | 95 | 90 | 75 |
| Semantic tasks | |||||||||||||||||||||||
| Naming | 64 | 62.3 | 59.1 | 62 | 60 | 59 | 63 | 61 | 59 | 60 | 64 | 53 | 62 | 62 | 63 | 64 | 62 | 61 | 61 | 63 | 63 | 61 | 60 |
| Word–picture matching | 64 | 63.8 | 63 | 64 | 64 | 62 | 64 | 64 | 64 | 64 | 62 | 60 | 64 | 64 | 64 | 64 | 64 | 63 | 64 | 64 | 64 | 64 | 63 |
| Object use: action-matching | 36 | 30.2 | 22 | 33 | 28 | 29 | 30 | 29 | 31 | 31 | 30 | 13 | 34 | 32 | 28 | 33 | 30 | 28 | 32 | 28 | 26 | 29 | 26 |
| Graded Faces Test | 30 | 21.5 | 13.1 | 11 | 15 | 9 | 10 | 7 | 14 | 21 | 15 | 10 | 14 | 24 | 21 | 18 | 23 | 15 | 17 | 14 | 19 | 9 | 16 |
| Graded Naming Test | 30 | 22.1 | 13.5 | 16 | 17 | 14 | 13 | 13 | 10 | 14 | 13 | 7 | 16 | 26 | 22 | 19 | 21 | 17 | 21 | 15 | 16 | 13 | 14 |
| Synonym judgement | 96 | 94.4 | 92.05 | 86 | 84 | 84 | 83 | 80 | 78 | 74 | 71 | 69 | 90 | 90 | 88 | 88 | 88 | 87 | 87 | 86 | 81 | 79 | 75 |
RCPM = Ravens Coloured Progressive Matrices; figures in bold fall below the control cut-off.
In line with the expectation derived from the current literature, the resection for TLE group's accuracy on the three simpler semantic tasks (naming, word–picture matching and object action-matching) was generally good; all patients with right resection for TLE performed in the normal range on these three measures. Some weakness was demonstrated by a minority of the cases with left resection for TLE (Patient DL failed naming and word–picture matching, Patient PW failed naming, Patient MF failed word–picture matching and Patient MM failed all three tasks).
In contrast, the more challenging semantic tasks revealed clear evidence for abnormality across all cases. First, on the more demanding naming tasks (Graded Naming Test, Graded Faces Test), the patients with left resection for TLE exhibited globally suppressed accuracy with 7/9 scoring below the normal cut-off on one or both tests. Replicating past studies (e.g. patients with TLE with unilateral temporal damage or left>right asymmetric semantic dementia: Martin et al., 1998; Seidenberg et al., 1998; Lambon Ralph et al., 2001, 2010a; Glosser et al., 2003), there was less pronounced anomia in the right resection cases (only Patient PA fell below the normal range). A 2 (face versus object naming) × 2 (left versus right resection) ANOVA confirmed the overall greater degree of anomia in left versus right cases [F(1,18) = 9.88, P = 0.006] but found no effect of material type [F(1,18) < 1] or interaction [F(1,18) < 1].
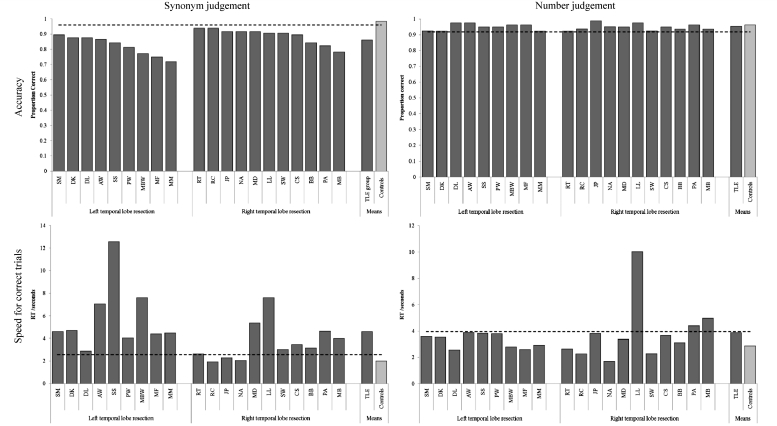
The 96-item synonym judgement test revealed abnormal semantic processing in all 20 patients. As can be seen in Table 1 and Fig. 2, all 20 cases fell below the control cut-off for accuracy on this test. In addition, decision times for the correct trials were also considerably and abnormally slowed: the patients’ mean decision time (4.6 s) was over twice that of the older controls (1.99 s). The same pattern was found at the individual level; all except three patients’ correct decision times fell outside the control range. This does not appear to reflect a generic effect or non-specific slowing; all 20 patients performed within the normal accuracy range on the difficulty-matched number decision task and 17/20 generated number decision times within the normal (older) control range.
Figure 2.
Comparison of timed synonym versus number judgements. Dashed lines denote the boundary of control performance (control mean −2 SD for accuracy, or control mean +2 SD for speed).
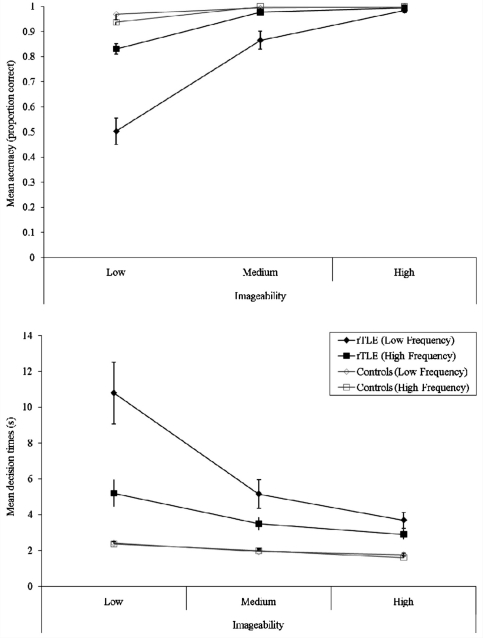
As noted in the ‘Introduction’, this assessment was included in part because it contains conditions with low frequency and more abstract words, which tend to be more sensitive to the presence of semantic impairment (Jefferies et al., 2009). Figure 3 confirms this pattern in the current resection for TLE group, in both accuracy and decision times. In terms of accuracy (Fig. 3), the patients only matched the control participants’ performance on the easiest items (high frequency, medium or high imageability items). For the lower frequency or least imageable words, the patients’ performance reduced (to 50%; per trial chance = 33%). A similar pattern was observed in the decision times for correct trials, though even on the easiest condition (high frequency, high imageability) the patients were considerably slower than the older controls. To confirm these patterns, the data were entered into a 2 (participant: patients versus controls) × 2 (frequency) × 3 (imageability) ANOVA. In terms of decision times (Fig. 3), the ANOVA confirmed a significant three-way interaction [F(2,56) = 12.1, P < 0.001]. Follow-up two-way ANOVA on each group separately found that the control group demonstrated a main effect of imageability [F(2,18) = 86.2, P < 0.001] but not of frequency [F(1,9) = 2.03, P = 0.2] or an interaction [F(2,18) = 2.97, P = 0.08], whereas the patients exhibited considerable imageability [F(2,38) = 24.4, P < 0.001] and frequency effects [F(1,19) = 21.6, P < 0.001] as well as an interaction [F(2,38) = 24.4, P < 0.001]. A very similar pattern was found for the accuracy data: there was a significant three-way interaction [group × frequency × imageability: F(2,56) = 12.4, P < 0.001], which stemmed from the control patients exhibiting an effect of imageability only [F(2,18) = 13.7, P < 0.001; frequency F(1,9) < 1, interaction F(2,18) = 1.6, P = 0.24)], whilst the patients were influenced substantially by both factors [frequency F(1,19) = 30.8, P < 0.001; imageability F(2,38) = 75.7, P < 0.001; interaction F(2,38) = 34.2, P < 0.001].
Figure 3.
Influence of frequency and imageability on synonym judgement performance. rTLE = resection for TLE.
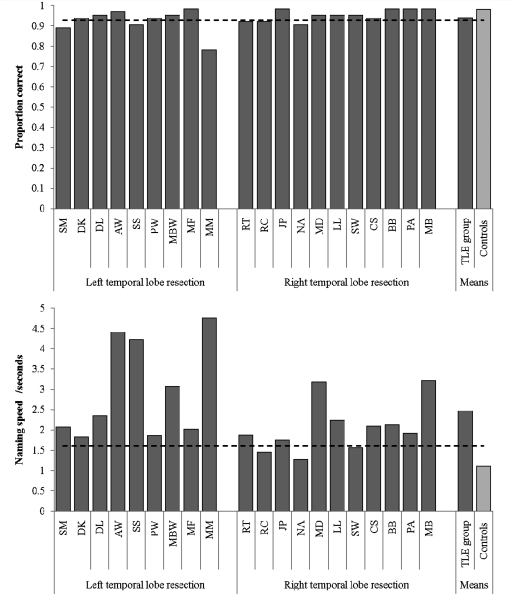
Given that the patients demonstrated considerably yet selectively slowed semantic performance on the synonym but not number judgement tasks (mirroring the pattern found in neurologically intact participants after left or right anterior temporal lobe repetitive transcranial magnetic stimulation: Pobric et al., 2007; Lambon Ralph et al., 2009), we revisited standard confrontation naming of basic-level concepts, instead measuring both accuracy and naming times (the simple naming test summarized in Table 2 used accuracy measures alone). The results are shown in Fig. 4 (accuracy in upper panel, naming speed for correct trials in lower panel). In terms of accuracy, this test replicated the earlier results (and those found in the current literature) of anomia in a minority of patients with resection for TLE (Patients SM, SS, MM and NA). In contrast, like the synonym judgement results, naming times were substantially and abnormally slow overall (mean = 2.5 s) in comparison with the older control group [mean = 1.1 s; t(28) = 4.13, P < 0.001], and abnormally slow naming times were observed in all bar three individual patients (Patients RC, NA and SW). In terms of laterality, the Graded Naming Test and Graded Faces Test assessments had revealed greater anomia in the left than right patients with resection for TLE (see above). This pattern was replicated on this basic-level naming test in terms of reaction times [left resection for TLE mean = 2.95 s (standard deviation = 1.20) versus right resection for TLE mean = 2.06 s (standard deviation = 0.63); t(18) = 2.13, P = 0.05].
Figure 4.
Performance on timed picture naming. Dashed line denotes the boundary of control performance (control mean −2 SD for accuracy, or control mean +2 SD for speed).
The weakened semantic performance in patients with resection for TLE was also evident on the two (untimed) tests that tapped specific-level concepts. Figure 5 shows that only five individuals’ accuracy in naming specific concepts fell into the normal control range (Patients SM, DL, AW, RT and RC) and, even on the receptive version of the task (word–picture matching), only half of the patients fell into the normal range (Patients SM, AW, PW, MBW, RT, RC, JP, MD, SW and BB). In summary, therefore, the semantic performance of patients with resection for TLE only appears to be ‘normal’ if relatively easy tasks, probing familiar concepts that use accuracy measures, are used. As soon as one of these assessment dimensions is changed (less familiar/imageable items, more specific concepts and/or reaction times) then semantic impairment in the majority, if not all, individuals is revealed.
Figure 5.
Performance on specific-level semantic concepts. Dashed line denotes the boundary of control performance (control mean −2 SD for accuracy, or control mean +2 SD for speed).
Finally, we explored the potential relationship between the degree of semantic impairment observed (synonym judgement, speed of naming, Graded Faces Test and Graded Naming Test) in each patient and the volume of resection (Table 1). The different measures of semantic performance correlated significantly with each other across the patient case-series (synonym judgement and naming speed: r = −0.51, P = 0.02; synonym judgement and Graded Naming Test: r = 0.77, P < 0.001, Graded Faces Test and Graded Naming Test: r = 0.50, P = 0.02). If all patients were included in the analysis, none of these tests correlated with volume resected (all P > 0.14). There were, however, two patients [Patients DK (left) and CS (right)] who had very minimal resected volumes noted in their histopathology reports, which may have skewed the data. When these two patients were excluded from the analyses, significant correlations were found with synonym judgement accuracy (ρ = 0.604, P = 0.004 one-tailed), Graded Naming Test (ρ = 0.606, P = 0.004 one-tailed) and naming speed (ρ = −0.401, P = 0.05 one-tailed).
Discussion
The purpose of this study was to provide one of the first systematic case-series investigations of semantic processing in patients with resection for TLE. The study had both clinical and basic science motivations. The considerable accumulated database on the status of semantic memory in semantic dementia, herpes simplex virus encephalitis and other patient groups with bilateral anterior temporal lobe damage indicates a pervasive multimodal semantic impairment (Bozeat et al., 2000; Coccia et al., 2004; Luzzi et al., 2007; Piwnica-Worms et al., 2010). The conclusion that the anterior temporal lobe is a crucial component for semantic memory has been bolstered by contemporary basic neuroscience studies utilizing magnetoencephalography, distortion-corrected functional MRI, PET or repetitive transcranial magnetic stimulation (Vandenberghe et al., 1996; Marinkovic et al., 2003; Sharp et al., 2004; Pobric et al., 2007, 2010b; Binney et al., 2010; Visser et al., 2010a; Visser and Lambon Ralph, 2011). Despite the overlap in lesion location (Fig. 1), patients with resection for TLE generally do not complain of comprehension difficulties in the clinic but tend to note their amnesia and anomia (particularly following left temporal lobe resection). These clinical observations have led some to conclude that patients with resection for TLE do not have a semantic impairment (Hickok and Poeppel, 2004; Kho et al., 2008; Simmons and Martin, 2009). The reality, however, is that the current literature contains a paucity of information on the status of semantic processing in patients with TLE with or without resection and the handful of studies that have probed semantic processing using a slightly more demanding assessment (e.g. specific concepts/individuals or time-limited semantic decisions) have found indications that semantic memory may be disrupted (Wilkins and Moscovitch, 1978; Glosser et al., 2003; Antonucci et al., 2008). Indeed, three studies have suggested that the anomia in patients with resection for TLE may itself reflect a semantic weakness (Bell et al., 2001; Antonucci et al., 2008; Drane et al., 2008), which would align directly with semantic dementia where the patients’ profound anomia is clearly linked to the underlying degradation of conceptual knowledge (Lambon Ralph et al., 2001).
The current study provides a bridge between the conclusions arising from the limited literature on semantic memory in resection for TLE and the established position for the crucial role of anterior temporal lobe in semantic processing arising from investigations of semantic dementia, herpes simplex virus encephalitis and contemporary neuroscience studies. The performance of the 20 patients with resection for TLE directly mirrors the current resection for TLE literature if we focus upon standard neuropsychological work-up, including simple clinical measures of semantic memory. Specifically, the patients present with amnesia for verbal materials, anomia in some patients (especially the cases with left resection for TLE) but no obvious comprehension impairment, through either clinical reports or formal testing. Likewise, these results also parallel investigations of patients with unilateral anterior temporal lobe damage of mixed aetiology—where naming impairment is observed following left anterior temporal lobe damage with minimal comprehension impairment (Tranel, 2009; Lambon Ralph et al., 2010a; Kemmerer et al., 2011). By transferring insights from semantic dementia and repetitive transcranial magnetic stimulation investigations, it is possible to derive more targeted and sensitive assessments. This is achieved by measuring either speed of semantic processing on the more simple assessments (e.g. probing basic-level familiar concepts) or extending the materials to include less familiar, more specific or more abstract concepts. The results of these targeted semantic assessments clearly demonstrate that semantic processing is abnormal and inefficient in patients with resection for TLE, although not to the same extent as most patients with semantic dementia (see below). Specifically, even on simple basic-level, familiar concepts, the patients with resection for TLE demonstrated reaction times that were around twice that of much older control participants—an observation that replicates Wilkins and Moscovitch's (1978) finding that semantic impairment is much more apparent in time-limited tests. As soon as a semantic assessment includes more challenging materials (more specific, more abstract or less familiar) then the patients’ reaction times slow even further and accuracy begins to decline—indicating that future, more sensitive clinical assessment of semantic processing in TLE/resection for TLE can be achieved by including these types of material (Antonucci et al., 2008). We should note here that the slowed semantic processing in patients with resection for TLE appears to be specific to semantic cognition given that the vast majority performed within normal limits on a demanding number decision task. In fact, the data from the resection for TLE group align very closely with the selective semantic processing results found in previous studies of repetitive transcranial magnetic stimulation to left or right anterior temporal lobe (Pobric et al., 2007; Lambon Ralph et al., 2009).
One final, important result from the current study was that we found a significant relationship between the volume of resected tissue and resultant semantic impairment. Again this fits with the expectations arising from the clinical and basic neuroscience research on the contribution that the anterior temporal lobe makes to semantic cognition, noted above. It also replicates the similar findings from a recent study of patients with semantic impairment following temporal lobe stroke (Tsapkini et al., 2011) and the relationship between the degree of bilateral anterior temporal lobe atrophy/hypometabolism and semantic impairment observed in semantic dementia (Galton et al., 2001; Mion et al., 2010).
We should also note that in this investigation we only studied the patients with resection for TLE post-surgery. One previous study of (non-resected) patients with TLE, which used a semantic assessment battery, found some mild semantic impairments (Giovagnoli et al., 2005), suggesting that semantic performance may not be entirely normal even before resection. Given long-standing epilepsy with resultant connectivity and neurotransmitter alteration (Hammers et al., 2003; Powell et al., 2007), it could be possible that some or all of the patients’ semantic deficit is present prior to resection because the seizure-affected part of the anterior temporal lobe system has been unable to contribute to the development of normal, detailed semantic representations, with the bulk of semantic memory being supported by the unaffected remainder of the temporal lobes, bilaterally. If correct, then the resection itself might not be the sole factor when considering the nature of semantic processing in patients with TLE with and without resection. These hypotheses could be tested in future studies by adopting the current sensitive semantic test battery in a comparison of pre- versus post-surgical patients with TLE.
We finish by considering the implications of the present findings for theories of the neural basis of semantic memory and, in particular, the role of the left and right anterior temporal lobe. Given the recent surge of studies on the anterior temporal lobe utilizing clinical and neuroscience methods, we start with a brief list of the key findings and then offer a unifying explanation for all these results, including those collected in the current study:
Once various methodological issues are taken into account (Visser et al., 2010b), functional neuroimaging studies of neurologically intact participants find bilateral, particularly inferolateral, anterior temporal lobe activation for semantic tasks across different modalities and types of concept (Vandenberghe et al., 1996; Marinkovic et al., 2003; Sharp et al., 2004; Rogers et al., 2006; Binney et al., 2010; Visser et al., 2010a; Visser and Lambon Ralph, 2011).
Patients with bilateral anterior temporal lobe pathology (e.g. semantic dementia, herpes simplex virus encephalitis, etc.) have an early and clear pan-modal semantic impairment leading to reduced accuracy on easy and hard semantic assessments unless the patients are extremely mild (Bozeat et al., 2000; Adlam et al., 2006). Irrespective of severity, all patients' performance is graded by frequency/familiarity, imageability and specificity (Warrington, 1975; Lambon Ralph et al., 1998a; Jefferies et al., 2009; Hoffman and Lambon Ralph, 2011).
Patients with unilateral temporal damage, even those with considerable lesions, can perform within the normal accuracy range on standard semantic battery assessments though many will show measureable anomia, especially after left temporal lobe damage and if probed with lower frequency items (Antonucci et al., 2008; Tranel, 2009; Lambon Ralph et al., 2010a; Kemmerer et al., 2011; Tsapkini et al., 2011).
Large-scale voxel-based lesion symptom mapping studies of stroke-related aphasic patients have demonstrated that lesions including the left superior, lateral anterior temporal lobe (centred on anterior superior temporal sulcus) are associated with the production of semantic naming errors, and that this correlation persists even when performance on challenging comprehension tests are partially out (Schwartz et al., 2009; Walker et al., 2011).
Patients with unilateral resection for TLE can also demonstrate very good accuracy on standard semantic tasks but, if the assessments extend to more demanding concepts (along the same dimensions that affect semantic dementia performance) or probe semantic processing speed then impairments become apparent (current study; Antonucci et al., 2008; Drane et al., 2008). In addition, it should be noted that the level of impairment in patients with unilateral resection for TLE only matches that observed in very mild semantic dementia and is not comparable with the degree of semantic deficit observed in most patients with semantic dementia.
Neurologically intact participants show a very similar, albeit milder, pattern to the current patients with unilateral resection for TLE—namely, selective yet mild pan-modal receptive and expressive semantic processing impairments—after left or right anterior temporal lobe repetitive transcranial magnetic stimulation (measured primarily in terms of slowed reaction times: Pobric et al., 2007, 2010a, b; Lambon Ralph et al., 2009).
Some patients with unilateral anterior temporal lobe resection for low-grade (i.e. slow-growing) glioma can perform well on a full range of semantic tasks, even those assessed using reaction times (Campanella et al., 2009; Bi et al., 2011). In contrast, those with high-grade (fast-growing) tumours exhibit reduced semantic accuracy (Campanella et al., 2009).
Verbal comprehension in patients with unilateral left temporal lobe lesions after stroke reflects not only the level of remaining anterior temporal lobe activation (Crinion et al., 2003) and the volume of damage (Tsapkini et al., 2011) but also the integrity of functional connectivity between left and right anterior temporal lobe (Warren et al., 2009).
There is at least one single-case study of extensive unilateral temporal damage leading to significant multimodal semantic impairment, matching that observed in moderate semantic dementia (Patient MP: Bub et al., 1988). Patient MP was initially studied for her surface dyslexia and became a standard and highly cited test case for computational models of reading. Her ‘pure’ surface dyslexia was accompanied by significant verbal and non-verbal semantic impairment as well as anomia (Bub et al., 1988; Patterson and Behrmann, 1997). Indeed, it is intriguing that Patient MP's set of impairments were similar to those observed in semantic dementia (multimodal semantic impairment, anomia and surface dyslexia: Patterson and Hodges, 1992; Woollams et al., 2007). Whilst her data provide an important example for current consideration, the information needs to be treated with some caution in that (a) only CT scan was available; (b) her left temporal lobe damage extended to subcortical and parietal regions (Bub et al., 1988; Patterson and Behrmann, 1997), and thus her semantic impairment may have been exacerbated by additional impairments of temporoparietal semantic control mechanisms (as observed in semantic aphasia: Head, 1926; Jefferies and Lambon Ralph, 2006); and (c) the damage was consequent on head injury and haematoma, which may have generated damage to other regions including the right temporal lobe.
Our working hypothesis and potential unifying explanation for this range of findings is informed by four computational models. First, the ‘hub-and-spoke’ model of semantic representation assumes that concepts are formed from the interaction of various modality-specific sources of information with an anterior temporal lobe transmodal representational hub (Rogers et al., 2004). This representational hub allows the various sources of specific information to be distilled into coherent concepts (Patterson et al., 2007; Lambon Ralph et al., 2010b). The Rogers et al. (2004) model was able to demonstrate how this framework functions and, when the anterior temporal lobe hub is impaired, how the model can reproduce the pan-modal semantic impairment observed in semantic dementia. Like previous models of semantic processing (Farah and McClelland, 1991), the hub-and-spoke framework exhibited ‘graceful’ degradation (a non-linear relationship between amount of damage and resultant semantic impairment, such that low levels of damage generate minimal decline in accuracy on semantic tasks) and its performance under damage was modulated by intrinsic characteristics such as frequency and specificity (because the intrinsically weaker representations for low frequency and specific knowledge are less robust to the effect of damage).
Secondly, the ‘no right to speak’ model was, perhaps, one of the first to assume that the semantic representational hub might be functionally unitary yet underpinned by the anterior temporal lobe bilaterally (Lambon Ralph et al., 2001). In addition, this model assumed that connectivity to left-lateralized speech production systems is stronger from the left anterior temporal lobe than from the right. Consequently, the degree of anomia for any level of semantic damage was much greater following left rather than right anterior temporal lobe damage. If one conceives of a hybrid of these two models, it is straightforward to imagine that a dual anterior temporal lobe hub would result in some representational redundancy between left and right components of the hub (A. C. Schapiro et al., manuscript under revision). As a result, the effects of unilateral damage might be partially compensated for by the intact contralateral representational system, whereas bilateral damage might degrade both representational systems so that semantic impairment is inescapable.
The importance of connectivity patterns has been further underlined by a recent neuroanatomically constrained computational model of normal and aphasic language performance (Ueno et al., 2011). Whilst retaining the insights from various computational frameworks of language, Ueno et al. (2011) also incorporated neuroanatomical information into the model's architecture such that it conformed to the contemporary neuroscience data in favour of dual language pathways (Parker et al., 2005; Hickok and Poeppel, 2007; Saur et al., 2008; Rauschecker and Scott, 2009). The model, therefore, provides a formal method for exploring the link between behaviour and neuroanatomy—licensing the simulation of aphasic data, voxel-based lesion symptom mapping results and functional neuroimaging data. Indeed, the voxel-based lesion symptom mapping data associating semantic naming errors with lesions extending to anterior superior temporal sulcus noted above (Schwartz et al., 2009; Walker et al., 2011) were formally simulated in this model.
The fourth and final observation from computational modelling is the demonstration that the time course of damage modulates the level of resultant impairment (Keidel et al., 2010). Based on important clinical studies of low- and high-graded glioma (Thiel et al., 2001, 2005; Duffau et al., 2003), Keidel et al. (2010) investigated the behaviour of a model in which learning proceeded simultaneously with simulated damage that increased either slowly (as in low-grade glioma) or rapidly (as in high-grade glioma). With slowly increasing damage, the model compensated better for the reduction in overall computational resources. In contrast, when the same level of damage was applied much more rapidly (like high-grade glioma) or instantaneously (like stroke or other acute neurological incident) then, even with post-damage recovery/learning, the model was only able to compensate partially and never re-attained the level of performance found in the low-grade glioma simulations.
With these observations in mind, the bilateral hub-and-spoke semantic framework might account for the clinical and neuroscience findings listed above in the following manner. Under normal circumstances both anterior temporal lobe hubs work collaboratively to support pan-modal semantic processing and thus both regions are activated by neurologically intact participants in functional neuroimaging studies. Mild levels of unilateral damage/interference (transcranial magnetic stimulation) reduce the overall level of computational efficiency and thus reaction times for semantic tasks become slowed. Partial redundancy in the representational structure coded in left and right hubs means that the effects of unilateral damage can be compensated, in part, by the normal interaction with the contralateral hub. If damage is bilateral or if the connectivity between the regions has also been compromised by brain damage, then no such compensation can occur and much more dramatic impairments are observed. It seems unlikely that left and right anterior temporal lobe representations are completely redundant given that, with sufficient unilateral damage, accuracy on intrinsically more demanding concepts (low frequency, abstract, specific level) becomes impaired. These patterns are found if the damage/neural interference is instantaneous or relatively fast. In contrast, if the damage is much more gradual in form (e.g. low-grade glioma), then plasticity-related, small iterative adjustments in the remaining bilateral system can maintain ‘normal’ performance and resection of the infiltrated region generates no behavioural impairment.
Finally, we note that the consistent, cross-aetiology finding that left temporal damage generates much greater levels of anomia than right temporal lesions, follows for the same reasons as those noted in the original computational simulations (Lambon Ralph et al., 2001). Given the greater connectivity from the left than the right anterior temporal lobe to left-lateralized speech production systems, naming ability (unlike other semantic tasks) is much more reliant upon the integrity of the left anterior temporal lobe. Thus even small levels of unilateral damage generate some degree of anomia. Because the anomia stems from damage to the semantic system, such patients are either unable to generate sufficient semantic input to drive successful speech production (thus generating omission or circumlocution errors), or they make semantically related naming errors (Antonucci et al., 2008; Lambon Ralph et al., 2010a). The fact that these patients often present as classical anomics (i.e. can provide good information about unnamed items) unless thoroughly tested with sensitive comprehension tests (Antonucci et al., 2008) may follow, in part, from the interactive support within the dual anterior temporal lobe hub: lateral support from the intact right anterior temporal lobe hub may improve the quality of the activated semantic representation overall (thus enhancing performance on semantic tasks or generating better, partial circumlocutions) but with little improvement in naming performance because it is primarily the (damaged) left anterior temporal lobe semantic region that can innervate speech production. These computational insights also provide an explanation for the association between aphasic semantic naming errors and lesions in the left anterior superior temporal sulcus (Schwartz et al., 2009; Walker et al., 2011) and, when constrained by neuroanatomical information, computational models are able to reproduce these important voxel-based lesion symptom mapping results (Ueno et al., 2011).
Funding
MRC programme grants (G0501632 and MR/J004146/1 to M.A.L.R.); NIH grant (R03 NS061164 to T.T.R.); Epilepsy Action (small project grant) to M.A.L.R., S.E. and G.A.B.
Acknowledgements
We thank all the patients and controls for their continued support of our research. We would also like to thank Argye Hillis and two anonymous reviewers for their constructive comments and suggestions on a previous version of this manuscript.
Glossary
Abbreviations
- TLE
temporal lobe epilepsy
References
- Adlam ALR, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–80. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Antonucci SM, Beeson PM, Labiner DM, Rapcsak SZ. Lexical retrieval and semantic knowledge in patients with left inferior temporal lobe lesions. Aphasiology. 2008;22:281–304. doi: 10.1080/02687030701294491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BD, Hermann BP, Woodard AR, Jones JE, Rutecki PA, Sheth R, et al. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15:434–43. doi: 10.1037//0894-4105.15.4.434. [DOI] [PubMed] [Google Scholar]
- Bi Y, Wei T, Wu C, Han Z, Jiang T, Caramazza A. The role of the left anterior temporal lobe in language processing revisited: evidence from an individual with ATL resection. Cortex. 2011;47:575–87. doi: 10.1016/j.cortex.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJM, Lambon Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex. 2010;20:2728–38. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Garrard P, Hodges JR. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–15. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Lambon Ralph MA, Patterson K, Hodges JR. When objects lose their meaning: what happens to their use? Cogn Affect Behav Neurosci. 2002;2:236–51. doi: 10.3758/cabn.2.3.236. [DOI] [PubMed] [Google Scholar]
- Bub D, Black S, Hampson E, Kertesz A. Semantic encoding of pictures and words: some neuropsychological observations. Cogn Neuropsychol. 1988;5:27–66. [Google Scholar]
- Campanella F, Mondani M, Skrap M, Shallice T. Semantic access dysphasia resulting from left temporal lobe tumours. Brain. 2009;132:87–102. doi: 10.1093/brain/awn302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M, Bartolini M, Luzzi S, Provinciali L, Lambon Ralph MA. Semantic memory is an amodal, dynamic system: evidence from the interaction of naming and object use in semantic dementia. Cogn Neuropsychol. 2004;21:513–27. doi: 10.1080/02643290342000113. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged during normal speech comprehension. Brain. 2003;126:1193–201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, et al. Susceptibility-induced loss of signal: comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242–55. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Capelle L, Denvil D, Sichez N, Gatignol P, Lopes M, et al. Functional recovery after surgical resection of low grade gliomas in eloquent brain: hypothesis of brain compensation. J Neurol Neurosurg Psychiatr. 2003;74:901–7. doi: 10.1136/jnnp.74.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, McClelland JL. A computational model of semantic memory impairment: modality specificity and emergent category specificity. J Exper Psychol Gen. 1991;120:339–57. [PubMed] [Google Scholar]
- Funnell E. Objects and properties: a study of the breakdown of semantic memory. Memory. 1995;3:497–518. doi: 10.1080/09658219508253162. [DOI] [PubMed] [Google Scholar]
- Galton CJ, Patterson K, Graham K, Lambon Ralph MA, Williams G, Antoun N, et al. Differing patterns of temporal atrophy in Alzheimer's disease and semantic dementia. Neurology. 2001;57:216–25. doi: 10.1212/wnl.57.2.216. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Erbetta A, Villani F, Avanzini G. Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia. 2005;43:1482–92. doi: 10.1016/j.neuropsychologia.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Glosser G, Salvucci AE, Chiaravalloti ND. Naming and recognizing famous faces in temporal lobe epilepsy. Neurology. 2003;61:81–6. doi: 10.1212/01.wnl.0000073621.18013.e1. [DOI] [PubMed] [Google Scholar]
- Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI. A PET study of 44 patients. Brain. 2003;126:1300–18. doi: 10.1093/brain/awg138. [DOI] [PubMed] [Google Scholar]
- Head H. London: Cambridge University Press; 1926. Aphasia and kindred disorders of speech. [DOI] [PubMed] [Google Scholar]
- Hermann B, Davies K, Foley K, Bell B. Visual confrontation naming outcome after standard left anterior temporal lobectomy with sparing versus resection of the superior temporal gyrus: a randomized prospective clinical trial. Epilepsia. 1999;40:1070–6. doi: 10.1111/j.1528-1157.1999.tb00821.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–45. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G, Dohan FC, Berry AD, Clement L, et al. Declarative memory following anterior temporal lobectomy in humans. Behav Neurosci. 1994;108:3–10. doi: 10.1037//0735-7044.108.1.3. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hoffman P, Lambon Ralph MA. Reverse concreteness effects are not a typical feature of semantic dementia: evidence for the hub-and-spoke model of conceptual representation. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq288. Advance Access published on February 1, 2011, doi:10.1093/cercor/bhq288. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–47. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- Jefferies E, Patterson K, Jones RW, Lambon Ralph MA. Comprehension of concrete and abstract words in semantic dementia. Neuropsychology. 2009;23:492–9. doi: 10.1037/a0015452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel JL, Welbourne SR, Lambon Ralph MA. Solving the paradox of the equipotential and modular brain: a neurocomputational model of stroke vs. slow-growing glioma. Neuropsychologia. 2010;48:1716–24. doi: 10.1016/j.neuropsychologia.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Kemmerer D, Rudrauf D, Manzel K, Tranel D. Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex. 2011 doi: 10.1016/j.cortex.2010.11.001. Advance Access published on December 16, 2010, doi:10.1016/j.cortex.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho KH, Indefrey P, Hagoort P, van Veelen CWM, van Rijen PC, Ramsey NF. Unimpaired sentence comprehension after anterior temporal cortex resection. Neuropsychologia. 2008;46:1170–8. doi: 10.1016/j.neuropsychologia.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Cipolotti L, Manes F, Patterson K. Taking both sides: do unilateral anterior temporal lobe lesions disrupt semantic memory? Brain. 2010a;133:3243–55. doi: 10.1093/brain/awq264. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Graham KS, Ellis AW, Hodges JR. Naming in semantic dementia - what matters? Neuropsychologia. 1998a;36:775–84. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Howard D, Nightingale G, Ellis AW. Are living and non-living category-specific deficits causally linked to impaired perceptual or associative knowledge? Evidence from a category-specific double dissociation. Neurocase. 1998b;4:311–38. [Google Scholar]
- Lambon Ralph MA, Lowe C, Rogers TT. Neural basis of category-specific semantic deficits for living things: evidence from semantic dementia, HSVE and a neural network model. Brain. 2007;130:1127–37. doi: 10.1093/brain/awm025. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological abstract evidence and a computational model. J Cogn Neurosci. 2001;13:341–56. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex. 2009;19:832–8. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci USA. 2010b;107:2717–22. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Snowden JS, Neary D, Coccia M, Provinciali L, Lambon Ralph MA. Distinct patterns of olfactory impairment in Alzheimer's disease, semantic dementia, frontotemporal dementia, and corticobasal degeneration. Neuropsychologia. 2007;45:1823–31. doi: 10.1016/j.neuropsychologia.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–97. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Loring DW, Meador KJ, Lee GP. The effects of lateralized temporal lobe dysfunction on normal and semantic word fluency. Neuropsychologia. 1990;28:823–9. doi: 10.1016/0028-3932(90)90006-a. [DOI] [PubMed] [Google Scholar]
- Martin RC, Sawrie SM, Roth DL, Gilliam FG, Faught E, Morawetz RB, et al. Individual memory change after anterior temporal lobectomy: a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39:1075–82. doi: 10.1111/j.1528-1157.1998.tb01293.x. [DOI] [PubMed] [Google Scholar]
- Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, et al. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–68. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- N'Kaoua B, Lespinet V, Barsse A, Rougier A, Claverie B. Exploration of hemispheric specialization and lexico-semantic processing in unilateral temporal lobe epilepsy with verbal fluency tasks. Neuropsychologia. 2001;39:635–42. doi: 10.1016/s0028-3932(00)00139-1. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Hodges JR. Declarative memory impairments in Alzheimer's disease and semantic dementia. Neuroimage. 2006;30:1010–20. doi: 10.1016/j.neuroimage.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Patterson K, Tyler LK, Moss H, Stamatakis EA, Bright P, et al. Temporal lobe lesions and semantic impairment: a comparison of herpes simplex virus encephalitis and semantic dementia. Brain. 2007;130:1138–47. doi: 10.1093/brain/awl344. [DOI] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d'une figure complexe. Arch Psychologie. 1944;30:205–550. [Google Scholar]
- Parker GJM, Luzzi S, Alexander DC, Wheeler-Kingshott CAM, Ciccarelli O, Lambon Ralph MA. Non-invasive structural mapping of two auditory-language pathways in the human brain. Neuroimage. 2005;24:656–66. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Patterson K, Behrmann M. Frequency and consistency effects in a pure surface dyslexic patient. J Exper Psychol: Hum Percept Perform. 1997;23:1217–31. doi: 10.1037//0096-1523.23.4.1217. [DOI] [PubMed] [Google Scholar]
- Patterson K, Hodges JR. Deterioration of word meaning: implications for reading. Neuropsychologia. 1992;30:1025–40. doi: 10.1016/0028-3932(92)90096-5. [DOI] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Piwnica-Worms KE, Omar R, Hailstone JC, Warren JD. Flavour processing in semantic dementia. Cortex. 2010;46:761–8. doi: 10.1016/j.cortex.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Amodal semantic representations depend on both anterior temporal lobes: evidence from repetitive transcranial magnetic stimulation. Neuropsychologia. 2010a;48:1336–42. doi: 10.1016/j.neuropsychologia.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Category-specific versus category-general semantic impairment induced by transcranial magnetic stimulation. Curr Biol. 2010b;20:964–8. doi: 10.1016/j.cub.2010.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric G, Lambon Ralph MA, Jefferies E. The role of the anterior temporal lobes in the comprehension of concrete and abstract words: rTMS evidence. Cortex. 2009;45:1104–10. doi: 10.1016/j.cortex.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobric GG, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci USA. 2007;104:20137–41. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CAM, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–21. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12:718–24. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JC. London: HK Lewis; 1962. Coloured progressive matrices: sets A, AB, B. [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, et al. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6:201–13. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, et al. The structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111:205–35. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–9. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry M-S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, et al. Anterior temporal involvement in semantic word retrieval: voxel-based lesion-symptom mapping evidence from aphasia. Brain. 2009;132:3411–27. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M, Griffith R, Sabsevitz D, Moran M, Haltiner A, Bell B, et al. Recognition and identification of famous faces in patients with unilateral temporal lobe epilepsy. Neuropsychologia. 2002;40:446–56. doi: 10.1016/s0028-3932(01)00096-3. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Hermann B, Wyler AR, Davies K, Dohan FC, Leveroni C. Neuropsychological outcome following anterior temporal lobectomy in patients with and without the syndrome of mesial temporal lobe epilepsy. Neuropsychology. 1998;12:303–16. doi: 10.1037//0894-4105.12.2.303. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJS. Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann Neurol. 2004;56:836–46. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A. The anterior temporal lobes and the functional architecture of semantic memory. J Int Neuropsychol Soc. 2009;15:645–9. doi: 10.1017/S1355617709990348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel A, Habedank B, Winhuisen L, Herholz K, Kessler J, Haupt WF, et al. Essential language function of the right hemisphere in brain tumor patients. Ann Neurol. 2005;57:128–31. doi: 10.1002/ana.20342. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, Ghaemi M, Kracht LW, Habedank B, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann Neurol. 2001;50:620–9. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- Thompson SA, Graham KS, Williams G, Patterson K, Kapur N, Hodges JR. Dissociating person-specific from general semantic knowledge: roles of the left and right temporal lobes. Neuropsychologia. 2004;42:359–70. doi: 10.1016/j.neuropsychologia.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D. The left temporal pole is important for retrieving words for unique concrete entities. Aphasiology. 2009;23:867–84. doi: 10.1080/02687030802586498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tröster AI, Warmflash V, Osorio I, Paolo AM, Alexander LJ, Barr WB. The roles of semantic networks and search efficiency in verbal fluency performance in intractable temporal lobe epilepsy. Epilepsy Res. 1995;21:19–26. doi: 10.1016/0920-1211(95)00002-r. [DOI] [PubMed] [Google Scholar]
- Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain. 2011;134:3094–3105. doi: 10.1093/brain/awr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno T, Saito S, Rogers TT, Lambon Ralph MA. Lichtheim 2: synthesising aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72:385–96. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Price C, Wise R, Josephs O, Frackowiak RSJ. Functional-anatomy of a common semantic system for words and pictures. Nature. 1996;383:254–6. doi: 10.1038/383254a0. [DOI] [PubMed] [Google Scholar]
- Viskontas IV, McAndrews MP, Moscovitch M. Memory for famous people in patients with unilateral temporal lobe epilepsy and excisions. Neuropsychology. 2002;16:472–80. [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, Parker GJ, Lambon Ralph MA. The inferior, anterior temporal lobes and semantic memory clarified: novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010a;48:1689–96. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph MA. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. J Cogn Neurosci. 2010b;22:1083–94. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Visser M, Lambon Ralph MA. Differential contributions of bilateral ventral anterior temporal lobe and left anterior superior temporal gyrus to semantic processes. J Cogn Neurosci. 2011;23:3121–31. doi: 10.1162/jocn_a_00007. [DOI] [PubMed] [Google Scholar]
- Walker GM, Schwartz MF, Kimberg DY, Faseyitan O, Brecher A, Dell GS, et al. Support for anterior temporal involvement in semantic error production in aphasia: new evidence from VLSM. Brain Lang. 2011;117:110–22. doi: 10.1016/j.bandl.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JE, Crinion JT, Lambon Ralph MA, Wise RJS. Anterior temporal lobe connectivity correlates with functional outcome after aphasic stroke. Brain. 2009;132:3428–42. doi: 10.1093/brain/awp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK. The selective impairment of semantic memory. Q J Exper Psychol. 1975;27:635–57. doi: 10.1080/14640747508400525. [DOI] [PubMed] [Google Scholar]
- Warrington EK. Hove: Psychology Press; 1996. Short Recognition Memory Test. [Google Scholar]
- Warrington EK. The Graded Naming Test: a restandardisation. Neuropsychol Rehabil. 1997;7:143–6. [Google Scholar]
- Wilkins A, Moscovitch M. Selective impairment of semantic memory after temporal lobectomy. Neuropsychologia. 1978;16:73–9. doi: 10.1016/0028-3932(78)90044-1. [DOI] [PubMed] [Google Scholar]
- Woollams AM, Lambon Ralph MA, Plaut DC, Patterson K. SD-squared: on the association between semantic dementia and surface dyslexia. Psychol Rev. 2007;114:316–39. doi: 10.1037/0033-295X.114.2.316. [DOI] [PubMed] [Google Scholar]