Abstract
We present a practical guide for the implementation of recently revised National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease (AD). Major revisions from previous consensus criteria are: (i) recognition that AD neuropathologic changes may occur in the apparent absence of cognitive impairment, (ii) an “ABC” score for AD neuropathologic change that incorporates histopathologic assessments of amyloid β deposits (A), staging of neurofibrillary tangles (B), and scoring of neuritic plaques (C), and (iii) more detailed approaches for assessing commonly co-morbid conditions such as Lewy body disease, vascular brain injury, hippocampal sclerosis, and TAR DNA binding protein (TDP)-43 immunoreactive inclusions. Recommendations also are made for the minimum sampling of brain, preferred staining methods with acceptable alternatives, reporting of results, and clinico-pathologic correlations.
INTRODUCTION
A consensus panel from the United States and Europe was convened recently to update and revise the 1997 [30] consensus guidelines for the neuropathologic evaluation of Alzheimer’s disease (AD). Here we summarize these consensus guidelines [29] and provide direction for their application.
CLINICO-PATHOLOGIC CORRELATION
“Alzheimer’s disease” refers to a constellation of cognitive and behavioral changes that are typical for patients who have substantial amounts of its hallmark lesions [6,39]. Similar neuropathologic changes, albeit usually at lower levels, also occur in individuals who did not show cognitive or behavioral impairments during life [54]. We recommend that neuropathologists adopt the term “AD neuropathologic change” and report on the presence and extent of lesions observed at autopsy regardless of the individual’s cognitive state, or even if cognitive state is not known.
AD neuropathologic change should be assessed in all cases of dementia. There are many other neurodegenerative disorders that can cause dementia in addition to those discussed here, and any may be co-morbid with AD neuropathologic change, especially in the elderly. We recommend that all lesions be documented for type and extent of co-morbidity in brains of individuals with AD neuropathologic change.
Multiple diseases in cerebrum can conspire to worsen cognitive symptoms; however, it often is difficult to judge the extent to which each disease observed at autopsy may have contributed to a given patient’s cognitive state. It is essential that the extent of AD neuropathologic change, as well as neuropathologic findings for any other disease(s) that may have contributed to cognitive impairment or dementia, be correlated with clinical, neuropsychological, neuro-imaging, and other laboratory data as part of the neuropathology report.
WORK-UP OF CASES
Autopsy should follow best practices and local regulations. Gross inspection of brain should include assessment of regional atrophy and blood vessels for cerebrovascular disease (CVD). We recommend a minimum sampling of brain regions in Table 1. All gross lesions also should be sampled. Each of the following sections indicates preferred and acceptable alternative methods for detection of neuropathologic changes. While we do not propose specific protocols, our recommendations are compatible with conclusions by others who have optimized reproducibility of some neuropathologic assessments across multiple sites [2-5,41]. We anticipate further efforts for increased harmonization and molecular specification. Finally, although organized for brain autopsy, these neuropathologic assessments can be applied to specimens from surgery; however, regional evaluation will be limited in biopsy specimens.
Table 1. Minimum recommended brain regions to be sampled and evaluated.
Each brain region should receive a hematoxylin and eosin (H&E) stain. In addition, regions are recommended for additional stains to reveal Alzheimer’s disease (AD) neuropathologic change and Lewy body disease (LBD). A tiered approach to assessment of Aβ/amyloid plaques and LBD is recommended to reflect their typically ordered appearance in brain. While NFTs also typically follow an ordered appearance in AD, we recommend wider screening to assist in capturing other tauopathies. H&E-stained sections can be used for evaluating vascular brain injury (VBI) in each region and hippocampal sclerosis (HS) as designated. We recommend that enumeration of microvascular lesions (MVLs) for clinico-pathologic correlations of cognitive impairment and dementia be limited to the six regions shaded green. Other lesions should be sampled as appropriate.
| AD Neuropathologic Change | LBD | VBI & HS | |||
|---|---|---|---|---|---|
| A | B | C | |||
| Region | Stain for Aβ/amyloid plaques [57] |
Stain for NFTs [14,15] |
Stain for NPs [41] |
Stain for LBs | H&E |
| Medulla including DMV | 1°: IHC or H&E 3 | VBI | |||
| Pons including LC | 1°: IHC or H&E 3 | VBI | |||
| Midbrain including SN | 3°: if 2° is + | 1°: IHC or H&E 3 | VBI | ||
| Cerebellar cortex and dentate n. | 3°: if 2° is + | VBI | |||
| Thalamus and subthalamic n.1 | MVL | ||||
| Basal ganglia at level of AC with basal nucleus of Meynert1 |
2°: if 1° is + | Consider4 | MVL | ||
| Hippocampus and EC1 | 2°: if 1° is + 2 | Yes | Consider4 |
2°: IHC in at least
one if 1° + |
HS |
| Cingulate, anterior | VBI | ||||
| Amygdala | 1°: IHC 3 | VBI | |||
| Middle frontal gyrus1 | 1° 2 | Yes | Yes |
2°: IHC in at least
one if 1° + |
MVL |
| Superior & middle temporal gyri1 | 1° 2 | Yes | Yes | MVL | |
| Inferior parietal lobule1 | 1° 2 | Yes | Yes | MVL | |
| Occipital cortex (BA 17 & 18)1 | Consider4 | Yes | Consider4 | MVL | |
| WM at ACA, MCA, and PCA watershed |
Consider4 | ||||
Consider taking bilateral sections if both cerebral hemispheres are available
Screen leptomeningeal and parenchymal vessels for cerebral amyloid angiopathy (CAA)
Screen for LBs with immunohistochemistry or H&E in brainstem and immunohistochemistry in amygdala. If positive, then expand immunohistochemistry for LBs in brainstem, limbic, and neocortical regions.
Consider performing noted stains in these regions; however, this is not necessary to perform ABC score.
Note: Stains for Aβ/amyloid plaques should be considered in other regions not needed for classification, such as in the precuneus or cingulate, as neuroimaging studies indicate that these sites are among the earliest to demonstrate retention of amyloid-binding molecules, a marker of fibrillar Aβ accumulation.
Abbreviations: DMV is dorsal motor nucleus of the vagus, LC is locus ceruleus, SN is substantia nigra, AC is anterior commissure, EC is entorhinal cortex, WM is white matter, NFTs is neurofibrillary tangles, NPs is neuritic plaques, LBs is Lewy bodies, ACA is anterior cerebral artery, MCA is middle cerebral artery, PCA is posterior cerebral artery, BA is Brodmann area.
AD NEUROPATHOLOGIC CHANGE
Senile plaques, which are extracellular deposits of the β-amyloid (Aβ) peptides, and neurofibrillary degeneration, which is best exemplified by neurofibrillary tangles (NFTs), are considered essential neuropathologic features of AD. Accumulation of Aβ plaques and NFTs follows distinct regional progressions across brain regions as AD advances. We recommend continued use of the staging scheme for neurofibrillary degeneration as described originally by Braak and Braak [14,15], reduced to four stages (Table 2) that improves inter-rater reliability [42]. We recommend a modified version of Thal phases of Aβ plaque accumulation [57], adapted to a four-point scale (Table 2). A subset of senile plaques called neuritic plaques appear closely associated with neuronal injury and are characterized by the occurrence of dystrophic neurites that frequently have phospho-tau immunoreactivity [19,36,37,55]. We recommend continued use of the Consortium to Establish a Registry for AD (CERAD) protocol for neuritic plaque scoring [41] (Table 2).
Table 2. “ABC” Score for AD Neuropathologic Change.
Method
Preferred method for β-amyloid (Aβ) plaques is immunohistochemistry for Aβ, and for neurofibrillary tangles (NFTs) is immunohistochemistry for tau or phospho-tau [14]. Other acceptable methods are Thioflavin S or sensitive silver histochemical stains [15]. It is important to stress that neuritic plaques need to be distinguished from Aβ deposits by special stains. Preferred methods for detection of neuritic processes in senile plaques are Thioflavin S or modified Bielschowsky stain [41]; immunohistochemical stains for neuritic processes, such as amyloid precursor protein, ubiquitin, neurofilament or phospho-tau, will identify specific, and partially overlapping, subtypes of dystrophic neurites that may differ in disease relevance [21].
Classification
AD neuropathologic change should be ranked along three parameters, Aβ plaque score (Figure 1) [57], Braak NFT stage (Figure 2; silver-based histochemistry [15] or phospho-tau immunohistochemistry [14]), and CERAD neuritic plaque score (Figure 3) [41] to obtain an “ABC score” (Table 2). Although cerebral amyloid angiopathy (CAA) is not considered in the “ABC” score, it is very commonly observed in cases with parenchymal Aβ plaques and should be evaluated and reported systematically as well as appreciated for its potential pathophysiological significance [56,61].
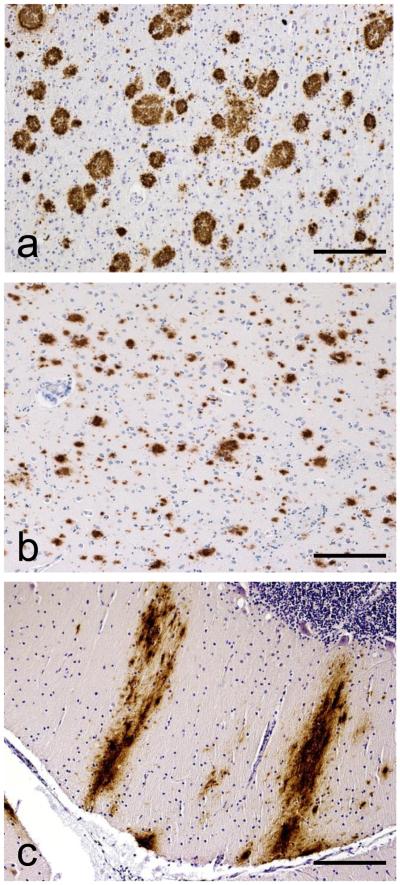
Figure 1. “ABC” Score for Alzheimer’s Disease Neuropathologic Change.
Immunohistochemical detection of Aβ plaques in (a) neocortex with as an example of “A1”, (b) neostriatum as an example of “A2”, and (c) molecular layer of cerebellum as an example of “A3”. Scale bars are 500 microns. Anti-Aβ was antibody 6F/3D (Novocastra, Newcastle, United Kingdom).
Figure 2. “ABC” Score for Alzheimer’s Disease Neuropathologic Change.
Immunohistochemical detection of neurofibrillary degeneration using phospho-tau antibody in the occipital cortex (Brodmann areas 17 and 18) as an example of “B3”. Scale bars equal (a) 150 microns and (b) 100 microns. PHF-1 antibody was generously provided by Dr. Peter Davies.
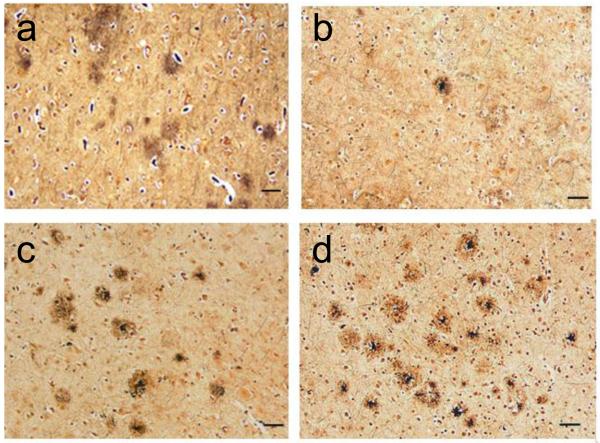
Figure 3. “ABC” Score for Alzheimer’s Disease Neuropathologic Change.
Bielschowsky stain of neocortex shows (a) diffuse plaques but not neuritic plaques as an example of “C0”, and increasing density of neuritic plaques as examples of (b) “C1” (1 to 5 neuritic plaques per 1 mm2), (c) “C2” (> 6 but < 20 neuritic plaques per 1 mm2), and (d) “C3” (> 20 neuritic plaques per 1 mm2). Scale bars equal 100 microns.
Reporting
For all cases, regardless of clinical history, reporting should follow the format of these examples: “Alzheimer Disease Neuropathologic Changes: A1, B0, C0” or “Alzheimer Disease Neuropathologic Changes: A3, B3, C3”. Using the system shown in Table 3, the ABC scores are transformed into one of four levels of AD neuropathologic change: Not, Low, Intermediate or High.
Table 3. “ABC” Score for Level of AD Neuropathologic Change.
AD neuropathologic change is evaluated with an “ABC” score (Table 2): Aβ/amyloid plaques (A), NFT stage (B), and neuritic plaque score (C). The combination of A, B, and C scores is designated as “Not”, “Low”, Intermediate” or “High” AD neuropathologic change. “Intermediate” or “High” AD neuropathologic change (black background) is considered sufficient explanation for dementia.
| AD Neuropathologic Change | B1 | |||
|---|---|---|---|---|
| A2 | C3 | 0 or 1 | 2 | 3 |
| 0 | 0 | Not4 | Not4 | Not4 |
| 1 | 0 or 1 | Low | Low | Low5 |
| 2 or 37 | Low | Intermediate | Intermediate5 | |
| 2 | Any C | Low6 | Intermediate | Intermediate5 |
| 3 | 0 or 1 | Low6 | Intermediate | Intermediate5 |
| 2 or 3 | Low6 | Intermediate | High | |
Aβ/amyloid plaque score should be determined by the method of Thal, et al. [57].
Neuritic plaque score should be determined by the method of CERAD [41].
Medial temporal lobe NFTs in the absence of significant Aβ or neuritic plaques occurs in older people and may be seen in individuals without cognitive impairment, with mild impairment, or with cognitive impairment from causes other than AD [44]. Consider other diseases when clinically or pathologically indicated.
Widespread NFTs with some Aβ/amyloid plaques but limited neuritic plaques is relatively infrequent and when it occurs, other diseases, particularly tauopathies, should be considered. Such cases may not fit easily into a specific Braak stage, which is intended for categorization of AD-type NFTs.
Higher levels of Aβ or neuritic plaques with low Braak stage should prompt consideration of contribution by co-morbidities like vascular brain injury, Lewy body disease, or hippocampal sclerosis. Also, consider additional sections as well as repeat or additional protocols to demonstrate other non-AD lesions.
High levels of neuritic plaques in setting of low Thal phase is a rare occurrence and should prompt reconsideration of neuritic vs. diffuse plaques, and the possible contribution of other diseases to cognitive impairment or dementia.
Clinico-pathologic correlations for individuals without cognitive impairment should indicate that it is possible for AD neuropathologic change to predate onset of symptoms by years [54]. For individuals with cognitive impairment at the time tissue was obtained, “Intermediate” or “High” level (Table 3 black background) of AD neuropathologic change should be considered adequate explanation of cognitive impairment or dementia, and should be reported with a final diagnosis of Alzheimer’s disease. “Low” level of AD neuropathologic change is not considered adequate explanation for cognitive impairment or dementia. In all cases with cognitive impairment, regardless of the extent of AD neuropathologic change, it is essential to determine the presence or absence, as well as extent, of other disease(s) that might have contributed to the clinical deficits. For cases with incomplete clinical history, higher levels of AD neuropathologic change typically are correlated with greater likelihood of cognitive impairment [29].
LEWY BODY DISEASE
Lewy body disease (LBD), including Parkinson’s disease and dementia with Lewy bodies (DLB), shares abnormal accumulation of α-synuclein within inclusions called LBs, as well as α-synuclein-immunoreactive neurites (so called “Lewy neurites”) and diffuse cytoplasmic immunoreactivity. LBs are frequent in the setting of moderate-to-severe levels of AD neuropathologic change [27,59], including some early-onset familial AD cases [32,33]. Given our focus on cognitive impairment and dementia rather than movement disorders [16], we recommend a modification of previous consensus guidelines [38] to classify LBD in five stages.
Method
LBs may be detected in neurons of medulla, pons and midbrain with hematoxylin and eosin (H&E)-stained sections; however, greater sensitivity can be achieved with immunohistochemistry for α-synuclein and this approach is strongly preferred [3,4,13]. Abnormal neuropil and neuronal cytoplasmic α-synuclein immunoreactivity are usually present with LBs but will not be apparent by H&E; in some instances, these changes occur in the absence of LBs.
Classification of all types of LBD should fall into a one of five categories following a modification of existing criteria (Figure 4) [16,38]: none, brainstem-predominant, limbic (transitional), neocortical (diffuse), or amygdala-predominant (Table 4). Although not part of this classification scheme, it is important to realize that LBD also occurs frequently in the olfactory bulb in older adults and should be sampled when possible [20].
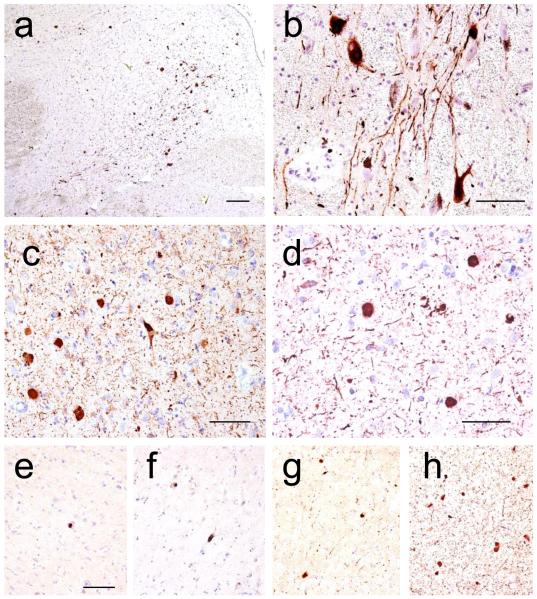
Figure 4. Lewy Body Disease Classification.
Immunohistochemical detection of α-synuclein in (a, scale bar 250 microns) dorsal motor nucleus of the vagus nerve and (b, scale bar 125 microns) substantia nigra neurons as examples of “Brainstem-predominant LBD”, (c, scale bar 50 microns) amygdala as an example of “Amygdala-predominant LBD”, (d, scale bar = 200 microns) anterior cingulate gyrus as an example of “Limbic (Transitional) LBD”, and (e - h, scale bar = 100 microns) superior temporal gyrus as an example of “Neocortical (diffuse) LBD” with varying levels of LBD severity: mild (e), moderate (f), severe (g), and very severe (h) [38]. Anti-alpha-synuclein was antibody KM51 (Novocastra, Newcastle, United Kingdom).
Table 4. Classification of Lewy Body Disease.
Results form the tiered approach to assessment of LBD in Table 1 should be as described here. Neocortical (diffuse) LBD is considered adequate explanation of dementia (shaded gray). It is important to note this classification is focused on the clinical context of cognitive impairment or dementia. LBD also occurs early in olfactory bulb, and even can occur outside of the brain.
| None | No LBs or related changes in IHC for α-synuclein |
|---|---|
| Brainstem-predominant | LBs in medulla, pons, or midbrain |
| Limbic (Transitional) | LBs in cingulate or entorhinal cortices, usually with brainstem involvement |
| Neocortical (Diffuse) | LBs in frontal, temporal, or parietal cortices usually with involvement of brainstem and limbic sites, which may include amygdala |
| Amygdala-predominant | LBs in amygdala with paucity of LBs in the above regions |
Reporting
For all cases, regardless of clinical history, reporting should follow the format of these examples: “Lewy body disease, limbic” or “Lewy body disease, amygdala-predominant”.
Clinico-pathologic correlation for individuals without cognitive impairment should indicate that, although much less common than AD neuropathologic change, LBD has been observed in individuals without apparent cognitive or motor deficit [1,49]; this may represent pre-clinical LBD [12,22,31]. For individuals with cognitive impairment, we recommend that “Neocortical LBD” be considered adequate explanation of cognitive impairment or dementia (Table 4 shaded gray); however, this does not preclude contribution from other diseases. “Brainstem-predominant LBD” in the setting of cognitive impairment should prompt consideration of other diseases. “Amygdala-predominant LBD” typically occurs in the context of advanced AD neuropathologic change [59]. For cases with incomplete clinical history, we note that “Neocortical LBD” is correlated with greater likelihood of cognitive impairment [1,11-13,22,31-33,38,46,49,52].
CEREBROVASCULAR DISEASE and VASCULAR BRAIN INJURY
CVD and vascular brain injury (VBI) [50] commonly are encountered in the brains with AD neuropathologic change [25]. We recommend reporting all macroscopic VBI and enumerating microvascular lesions or MVLs (microscopic infarcts/hemorrhages) in standard screening sections [26,51,53,60]. Diffuse white matter injury is a form of VBI but it is more difficult to judge objectively and is not specific to VBI.
Method
VBI, including MVLs (Figure 5), may occur in any region of brain but only those MVLs in the standardized sections (Table 1 shaded green) should be enumerated when correlating with cognitive impairment or dementia.
Figure 5. Microvascular Lesion.
Cerebral cortex stained with (a) hematoxylin and eosin or for (b) immunohistochemical detection of glial fibrillary acidic protein reveals a microvascular lesion (MVL). Scale bar = 500 microns. Anti-GFAP was antibody 6F2 (DAKO, Glostrup, Denmark).
Classification
All infarcts and hemorrhages should be documented including location, size, and age.
Reporting
Reporting should follow the format of these examples: “Cerebrovascular disease: Atherosclerosis, moderate, non-occlusive, affecting basilar artery, left internal carotid artery and middle cerebral artery; Arteriolosclerosis, severe, widespread involvement of hemispheric white matter” or “Vascular brain injury: Infarct in the territory of the left middle cerebral artery, remote, measuring 3 × 3 × 2 cm; Lacunar infarct, right anterior caudate, remote, measuring 0.5 × 0.3 × 0.2 cm; Microvascular lesions: 2 remote lesions detected on standard sections (right middle frontal gyrus and right inferior parietal lobule)”
Clinico-pathologic correlations for grossly visible infarcts or hemorrhages should follow classic neuropathologic approaches. Although there are some differences in approach, guidelines have emerged for clinico-pathologic correlation of MVLs: one MVL identified in standard sections of brain (Table 1 regions shaded green) is of unclear relationship to cognitive function, while multiple MVLs in these regions are associated with increased likelihood of cognitive impairment or dementia [24,52,62].
HIPPOCAMPAL SCLEROSIS and TAR DNA BINDING PROTEIN (TDP)-43 INCLUSIONS
Hippocampal sclerosis (HS) is defined by pyramidal cell loss and gliosis in CA1 and subiculum of the hippocampal formation that is out of proportion to AD neuropathologic change in the same structures [7]. HS can be observed in the context of AD neuropathologic change, frontotemporal lobar degeneration (FTLD), and VBI, likely reflecting a heterogeneous etiology. We recommend that HS be reported as present or absent.
TDP-43 immunoreactive inclusions are observed in the majority of cases of HS (Figure 6) [8,28,40,64], about one-half of cases with FTLD and ubiquitin inclusions with or without motor neuron disease, most sporadic cases of amyotrophic lateral sclerosis, and commonly in cases with AD neuropathologic change [8,9] or with LBD [43], as well as other neurodegenerative diseases [48]. With the exception of individuals with mutations in TARDBP, GRN, VCP or c9ORF72 [10,18], it is not clear whether TDP-43 proteinopathy in these other neurodegenerative diseases is a primary, secondary, or coincidental event [63].
Figure 6. TDP-43 Inclusions.
Immunohistochemical detection of TDP-43 in the dentate gyrus in a subject (a) without hippocampal sclerosis or (b) another case with hippocampal sclerosis that shows cytoplasmic inclusions in granule neurons that are (c) further highlighted by phospho-TDP-43. Scale bars equal 50 microns. Anti-TDP-43 was antibody 10782-2-AP (ProteinTech, Chicago IL, USA). Anti-phospho-TDP-43 was antibody TIP-PTD-M01 (pS409/410-1 from Cosmo Bio, Tokyo, Japan).
Method and Classification
HS should be evaluated by hematoxylin and eosin (H&E)-stained sections together with neurofibrillary tangle (NFT) stains. HS can be focal, thus its absence in the recommended screening section does not rule out the possibility of HS elsewhere in the hippocampal formation. If HS is present, further evaluation is indicated, including TDP-43 immunohistochemistry. If work up is negative for TDP-43 but there is other evidence to suggest FTLD, consider immunohistochemistry for phospho-tau, ubiquitin, or “fused in sarcoma” (FUS). In the absence of HS, screening for TDP-43 inclusions as part of evaluating AD neuropathologic change is of unclear value.
Reporting
HS should be reported as present or absent with a description of immunohistochemistry results.
Clinico-pathological correlations need to recognize that HS can occur in several different diseases and has varying clinical implications in different settings. Relatively isolated HS may occur in very old individuals, and in this context it is associated with TDP-43-immunoreactive inclusions and with cognitive impairment [45,47].
FRONTOTEMPORAL LOBAR DEGENERATION and PRION DISEASE
Both of these classes of neurodegenerative diseases are complex and beyond the scope of this summary. Nevertheless, each must be distinguished from AD neuropathologic change. We refer the reader to recent consensus guidelines for the neuropathologic evaluation of FTLD and its subtypes [17,34,35], and issues important in the distinction of AD neuropathologic change from those of some forms of FTLD [58]. Finally, not only can the neuropathologic changes of prion disease be co-morbid with AD, but some forms of prion disease can overlap with AD and need to be distinguished with special stains [23].
SUMMARY
The new consensus criteria recognize the continuum of AD neuropathologic change that underlies the progression of this disease from preclinical to dementia stage. The new consensus criteria also amplify methods for evaluating Aβ plaques, better define intermediate levels of AD pathologic change, and emphasize a structured approach to commonly co-morbid diseases.
Acknowledgments
Support was provided by the National Institute on Aging (AG05134, AG05136, AG03991, AG05681, AG24904, AG28383, AG10161, AG17917, AG10124, AG13854, AG19610, NS62684, AG16570, AG15819, AG12435, AG05131, AG016976, AG16570, AG15866 and AG18840) as well as the Alzheimer’s Association, Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Consortium), Deutsche Forschungsgemeinschaft (DFG TH-624-4-1) and Alzheimer Forschung Initiative (AFI #10810), the Nancy and Buster Alvord Endowment, and the Charles and Joanne Knight Alzheimer Research Initiative. We thank Dr. Joshua Sonnen for providing some photomicrographs. The authors extend their deepest thanks to Dr. Heiko Braak, Dr. Kelly Del Tredici, Dr. Nina Silverberg, Dr. Walter Kukull, Dr. Kathleen Montine, Dr. Cerise Elliott, Dr. Bill Thies, Dr. Maria C. Carrillo, and Ms. Sarah Monsell for their valuable input.
References
- 1.Adler CH, Connor DJ, Hentz JG, et al. Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord. 2010;25:642–6. doi: 10.1002/mds.22971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alafuzoff I, Arzberger T, Al-Sarraj S, et al. Staging of neurofibrillary pathology in Alzheimer’s disease: a study of the BrainNet Europe Consortium. Brain Pathol. 2008;18:484–96. doi: 10.1111/j.1750-3639.2008.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alafuzoff I, Parkkinen L, Al-Sarraj S, et al. Assessment of alpha-synuclein pathology: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2008;67:125–43. doi: 10.1097/nen.0b013e3181633526. [DOI] [PubMed] [Google Scholar]
- 4.Alafuzoff I, Pikkarainen M, Al-Sarraj S, et al. Interlaboratory comparison of assessments of Alzheimer disease-related lesions: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2006;65:740–57. doi: 10.1097/01.jnen.0000229986.17548.27. [DOI] [PubMed] [Google Scholar]
- 5.Alafuzoff I, Thal DR, Arzberger T, et al. Assessment of beta-amyloid deposits in human brain: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:309–20. doi: 10.1007/s00401-009-0485-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert MS, Dekosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amador-Ortiz C, Dickson DW. Neuropathology of hippocampal sclerosis. Handb Clin Neurol. 2008;89:569–72. doi: 10.1016/S0072-9752(07)01253-5. [DOI] [PubMed] [Google Scholar]
- 8.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–45. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–36. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 10.Aswathy PM, Jairani PS, Mathuranath PS. Genetics of frontotemporal lobar degeneration. Ann Indian Acad Neurol. 13:S55–62. doi: 10.4103/0972-2327.74246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beach TG, Adler CH, Lue L, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beach TG, Adler CH, Sue LI, et al. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–51. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beach TG, White CL, Hamilton RL, et al. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braak H, Alafuzoff I, Arzberger T, et al. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 17.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 20.Del Tredici K, Rub U, De Vos RA, et al. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–26. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 21.Dickson DW. The pathogenesis of senile plaques. J Neuropathol Exp Neurol. 1997;56:321–39. doi: 10.1097/00005072-199704000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Dickson DW, Fujishiro H, DelleDonne A, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–44. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 23.Dlouhy SR, Hsiao K, Farlow MR, et al. Linkage of the Indiana kindred of Gerstmann-Straussler-Scheinker disease to the prion protein gene. Nat Genet. 1992;1:64–7. doi: 10.1038/ng0492-64. [DOI] [PubMed] [Google Scholar]
- 24.Gorelick PB, Scuteri A, Black SE, et al. Vascular Contributions to Cognitive Impairment and Dementia: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–90. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–84. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatanpaa KJ, Blass DM, Pletnikova O, et al. Most cases of dementia with hippocampal sclerosis may represent frontotemporal dementia. Neurology. 2004;63:538–42. doi: 10.1212/01.wnl.0000129543.46734.c0. [DOI] [PubMed] [Google Scholar]
- 29.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & Dementia. 2011 doi: 10.1016/j.jalz.2011.10.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyman BT, Trojanowski JQ. Consensus recommendations for the postmortem diagnosis of Alzheimer disease from the National Institute on Aging and the Reagan Institute Working Group on diagnostic criteria for the neuropathological assessment of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56:1095–7. doi: 10.1097/00005072-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Jicha GA, Schmitt FA, Abner E, et al. Prodromal clinical manifestations of neuropathologically confirmed Lewy body disease. Neurobiol Aging. 2010;31:1805–13. doi: 10.1016/j.neurobiolaging.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leverenz JB, Fishel MA, Peskind ER, et al. Lewy body pathology in familial Alzheimer disease: evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol. 2006;63:370–6. doi: 10.1001/archneur.63.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–9. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–8. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masliah E, Mallory M, Deerinck T, et al. Re-evaluation of the structural organization of neuritic plaques in Alzheimer’s disease. J Neuropathol Exp Neurol. 1993;52:619–32. doi: 10.1097/00005072-199311000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Masliah E, Terry RD, Mallory M, et al. Diffuse plaques do not accentuate synapse loss in Alzheimer’s disease. Am J Pathol. 1990;137:1293–7. [PMC free article] [PubMed] [Google Scholar]
- 38.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 39.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miki Y, Mori F, Hori E, et al. Hippocampal sclerosis with four-repeat tau-positive round inclusions in the dentate gyrus: a new type of four-repeat tauopathy. Acta Neuropathol. 2009;117:713–8. doi: 10.1007/s00401-009-0531-2. [DOI] [PubMed] [Google Scholar]
- 41.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 42.Nagy Z, Yilmazer-Hanke DM, Braak H, et al. Assessment of the pathological stages of Alzheimer’s disease in thin paraffin sections: a comparative study. Dement Geriatr Cogn Disord. 1998;9:140–4. doi: 10.1159/000017038. [DOI] [PubMed] [Google Scholar]
- 43.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–9. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 44.Nelson PT, Abner EL, Schmitt FA, et al. Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:774–84. doi: 10.1097/NEN.0b013e3181aacbe9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson PT, Abner EL, Schmitt FA, et al. Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol. 2010;20:66–79. doi: 10.1111/j.1750-3639.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nelson PT, Kryscio RJ, Jicha GA, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127–33. doi: 10.1212/WNL.0b013e3181bacf9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–18. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauramaa T, Pikkarainen M, Englund E, et al. TAR-DNA binding protein-43 and alterations in the hippocampus. J Neural Transm. 2011;118:683–9. doi: 10.1007/s00702-010-0574-5. [DOI] [PubMed] [Google Scholar]
- 49.Saito Y, Ruberu NN, Sawabe M, et al. Lewy body-related alpha-synucleinopathy in aging. J Neuropathol Exp Neurol. 2004;63:742–9. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 50.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–47. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 51.Sonnen JA, Larson EB, Brickell K, et al. Different patterns of cerebral injury in dementia with or without diabetes. Arch Neurol. 2009;66:315–22. doi: 10.1001/archneurol.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sonnen JA, Larson EB, Crane PK, et al. Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol. 2007;62:406–13. doi: 10.1002/ana.21208. [DOI] [PubMed] [Google Scholar]
- 53.Soontornniyomkij V, Lynch MD, Mermash S, et al. Cerebral microinfarcts associated with severe cerebral beta-amyloid angiopathy. Brain Pathol. 2010;20:459–67. doi: 10.1111/j.1750-3639.2009.00322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 56.Thal DR, Griffin WS, de Vos RA, Ghebremedhin E. Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol. 2008;115:599–609. doi: 10.1007/s00401-008-0366-2. [DOI] [PubMed] [Google Scholar]
- 57.Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 58.Uchihara T, Nakamura A, Yamazaki M, et al. Different conformation of neuronal tau deposits distinguished by double immunofluorescence with AT8 and thiazin red combined with Gallyas method. Acta Neuropathol. 2001;102:462–6. doi: 10.1007/s004010100401. [DOI] [PubMed] [Google Scholar]
- 59.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–97. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vinters HV, Ellis WG, Zarow C, et al. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–45. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 61.Vonsattel JP, Myers RH, Hedley-Whyte ET, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages: a comparative histological study. Ann Neurol. 1991;30:637–49. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 62.White L. Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009;18:713–25. doi: 10.3233/JAD-2009-1178. [DOI] [PubMed] [Google Scholar]
- 63.Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease - a review. Int J Clin Exp Pathol. 2011;4:147–55. [PMC free article] [PubMed] [Google Scholar]
- 64.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–70. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]