Abstract
Purpose of review
Artemisinin-based combination therapies (ACTs) have been deployed globally with remarkable success for more than 10 years without having lost their malaria treatment efficacy. However, recent reports from the Thai–Cambodian border reveal evidence of emerging resistance to artemisinins. The latest published clinical and molecular findings are summarized herein.
Recent findings
Clinical studies have identified delayed parasite clearance time as the most robust marker of artemisinin resistance. Resistance has only been documented from Southeast Asia and has been observed in isolates that show no significant decrease in drug susceptibility in vitro. Genetic investigations have yet to uncover robust molecular markers. In-vitro studies have identified parasite quiescence or dormancy mechanisms that protect early ‘ring-stage’ intra-erythrocytic parasites against short-term artemisinin exposure. This might be achieved by reducing the rate of hemoglobin degradation, important for artemisinin bioactivation.
Summary
Should ACTs fail, no suitable alternatives exist as first-line treatments of P. falciparum malaria. Intensified efforts are essential to monitor the spread of resistance, define therapeutic and operational strategies to counter its impact, and understand its molecular basis. Success in these areas is critical to ensuring that recent gains in reducing the burden of malaria are not lost.
Keywords: artemisinin-based combination therapy, drug resistance, malaria, molecular markers, parasite clearance times, Plasmodium falciparum, recrudescence, treatment failure
Introduction
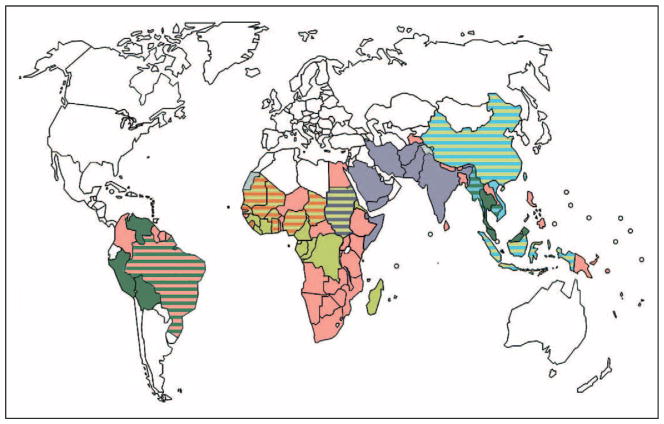
In 2006, the WHO officially recommended that artemisinin (ART)-based combination therapies (ACTs) be adopted as first-line treatment of uncomplicated malaria caused by Plasmodium falciparum [1]. This recommendation came in response to the global spread of resistance to the former first-line antimalarials chloroquine and sulfadoxine–pyrimethamine [2,3•]. The global implementation of ACTs (Fig. 1) [1], along with vector control measures including insecticide-treated bednets and indoor residual spraying, has helped to considerably reduce the disease burden in many endemic countries. In the past decade, over 40 countries have reported a greater than two-fold reduction in malaria cases (http://www.who.int/malaria/world_malaria_report_2010/en/index.html). Despite these achievements, malaria continues to have a devastating impact, with an estimated 780 000 deaths and over 225 million cases in 2009. The greatest burden falls on young children residing in sub-Saharan Africa [4••].
Figure 1. Current global distribution of artemisinin-based combination therapies as the first-line treatment of uncomplicated falciparum malaria.
This distribution was collated from maps of country-wide artemisinin-based combination therapy use, as published in [1].
 , Artemether–lumefantrine;
, Artemether–lumefantrine;
 , dihydroartemisinin–piperaquine;
, dihydroartemisinin–piperaquine;
 , artesunate–amodiaquine;
, artesunate–amodiaquine;
 , artesunate–mefloquine;
, artesunate–mefloquine;
 , artesunate–sulphadoxine/pyrimethamine.
, artesunate–sulphadoxine/pyrimethamine.
ART, long used in Chinese traditional medicine, was found in the early 1970s to have potent antimalarial activity [5,6]. This was soon followed by its chemical identification as a sesquiterpene peroxide and the development of methods to produce the derivatives artesunate, artemether (ATM), and the active metabolite dihydroartemisinin (DHA). Defining properties of these ART derivatives include their ability to very rapidly decrease numbers of asexual blood stage parasites, their activity against the broadest range of intra-erythrocytic developmental stages (including action on the immature ring-stage forms as well as the more mature trophozoite stages) of all known antimalarial drugs, and their ability to inhibit the development of immature sexual stage parasites (gametocytes). This gametocytocidal activity acts to reduce the transmission of ART-treated Plasmodium parasites to their Anopheles mosquito vector [7]. ARTs have also shown an excellent safety profile in humans. Their major caveat, however, is their very short half-life in plasma, typically on the order of 1–3 h [8,9]. Clinically, ART monotherapy is only curative when administered for 7 days, and recrudescence (i.e. the reappearance of asexual blood stage parasites during the follow-up period) is common [10,11•]. ART derivatives have, therefore, been combined with longer lasting partner drugs, which include lumefantrine (combined with ATM and globally the most widely used ACT), amodiaquine (typically paired with artesunate), mefloquine (combined with artesunate), sulfadoxine–pyrimethamine (with artesunate), piperaquine (combined with DHA), and pyronaridine (currently being registered in combination with artesunate) (Fig. 1) [12]. These combinations typically provide radical cure with 3-day treatments and help protect against the emergence of resistance to the ART derivative [13••,14•].
Evidence for the emergence of artemisinin resistance
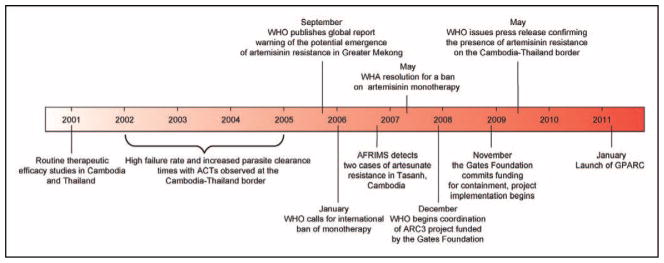
Preliminary indications of clinical treatment failures with ACTs came from observational studies in the early 2000s of artesunate–mefloquine use near the Thai–Cambodian border (Fig. 2) [1,15,16]. It was unclear, however, whether this resulted from resistance to artesunate or its partner drug, or other host or pharmacological factors. A subsequent report from western Cambodia by Noedl et al. in 2008 [17] identified two patients with clear evidence of artesunate-resistant infections. These patients recrudesced following 7 days of artesunate monotherapy (4 mg/kg per day) and showed delayed parasite clearance times (133 and 95 h, as compared with a median of 52 h for patients that were cured) despite having adequate drug levels. These times represent the interval from the start of treatment to the time the parasitemia (i.e. the percentage of parasitized erythrocytes) falls below the lower limit of parasite detection by light microscopy (approximately 0.001%). Ex-vivo drug dose–response measurements revealed that the patient isolates with prolonged parasite clearance times had DHA half maximal inhibitory concentration (IC50) values that were four times the geometric mean for cured patients [18•].
Figure 2. Schematic representation of recent events relating to the emergence of and response to artemisinin resistance.
AFRIMS, Armed Forces Research Institute of Medical Sciences; ARC3, artemisinin resistance project: pilot studies to confirm, characterize and plan for containment; the Gates Foundation, Bill & Melinda Gates Foundation; GPARC, Global Plan for Artemisinin Resistance Containment; WHA, World Health Assembly; WHO, World Health Organization. Reproduced with permission from [1].
Compelling evidence supporting the emergence of ART resistance came from the study published mid-2009 by Dondorp et al. [19], showing delayed parasite clearance rates in Pailin, western Cambodia, compared with Wang Pha, northwestern Thailand, following artesunate monotherapy or artesunate–mefloquine combination therapy. Median parasite clearance times between Wang Pha and Pailin were prolonged from 48 to 72 h in patients treated with 2 mg/kg artesunate and from 54 to 84 h with 4 mg/kg artesunate with mefloquine. These differences could not be attributed to drug pharmacokinetics or differences in age between these two study populations. In-vitro drug susceptibilities, as measured by DHA and artesunate IC50 values, were comparable between these two populations. One concern with this study was the higher parasite density at baseline in the Pailin group [20]; this was rebutted by Dondorp et al. [19] as not affecting the core findings. It is important to stress that this indication of emerging resistance did not substantially reduce clinical efficacy, with cure rates continuing to exceed 90%.
Factors implicated in the emergence of ART resistance in western Cambodia include 30 years of ART monotherapy (now officially banned), drugs that were substandard and/or used suboptimally, and possibly parasite genetic backgrounds that favor the emergence of multi-drug resistance [21••,22,23]. Further heritability studies with isolates from Pailin calculated that 56% of the variability in parasite clearance rates could be attributed to parasite genetics, suggesting that resistance determinants could disseminate geographically under drug selection [24•]. Another factor likely to contribute is the very low rate of transmission, resulting in insufficient immunity to eliminate parasites that might have survived drug treatment, thus increasing selection pressure [25].
Adding to the concerns of diminished ART efficacy, the Thai–Cambodian border has historically been a focal point for multidrug resistance in P. falciparum [26•]. Parasites from this region were among the first to develop resistance to chloroquine, sulfadoxine–pyrimethamine, and mefloquine, and in the case of the two former drugs this resistance subsequently spread to Africa [27–30]. Accordingly, intense efforts are being pursued throughout the Greater Mekong Subregion (GMS), composed of Cambodia, Laos, Myanmar, Thailand, Vietnam, and the Yunnan Province of China, to monitor for the emergence and spread of ART resistance [31••]. Data from a longitudinal study of over 3200 patients along the Thai–Myanmar border showed continued efficacy of a 3-day course of artesunate–mefloquine (over the period 1995–2007), with 96% of patients being cleared of parasitemia on day 3 [32]. Nevertheless, the study observed a small but significant increase in parasite clearance times, associated with a small increase in treatment failure rates (i.e. parasite recrudescence during a 42-day follow-up period). These increases were not accompanied by significant reductions in artesunate sensitivity in vitro. Slower parasite clearance was also associated with increased gametocyte carriage, thereby increasing the risk of transmission of drug-tolerant parasites. To date, ARTs remain highly effective in Vietnam [33], where clinical outcomes are being closely monitored.
Active surveillance for the emergence of ART resistance is also being pursued globally, including efforts outlined in the WHO Global Plan for Artemisinin Resistance Containment (http://www.who.int/malaria/publications/atoz/9789241500838/en/index.html) and activities coordinated by the WorldWide Antimalarial Resistance Network [34•]. A recent report by Stepniewska et al. [11•] collected parasite clearance data from over 18 000 patients, in 25 different countries ranging from low to high transmission. This meta-analysis confirmed the earlier suggestion from western Cambodia [19] that elevated parasite clearance time represents the most robust marker of ART resistance. The study reported that parasite clearance rate is largely determined by parasite density on admission and found that the proportion of patients with positive blood smears on day 3 could be used as a simple measure of artemisinin susceptibility in vivo. The report concluded that ART resistance in a given area is highly unlikely if at least 97% of patients presenting with a parasite density of less than 100 000 infected erythrocytes per microliter and given a 3-day course of ACTs are smear-negative on day 3. Of note, emerging resistance to ARTs appears to be largely restricted to the GMS, and no definitive evidence has yet been reported from Africa [35].
The intense logistical requirements to clinically monitor ART resistance have led several teams to explore in-vitro assays as a surrogate of resistance. Indeed, reduced in-vitro susceptibility has been closely associated with an increased risk of treatment failure for all other classes of antimalarial drugs [36]. Evidence in favor of the applicability of in-vitro studies to ART resistance comes from the work by Noedl et al. [17], cited above, showing elevated DHA IC50 values in patients that failed artesunate monotherapy. Longitudinal studies conducted by the Cambodian National Malaria Control Program also showed increased geometric means of artesunate IC50 values in isolates from western Cambodia compared with eastern Cambodia. IC50 values were significantly higher in patients that failed artesunate–mefloquine therapy [37•]. Furthermore, this study reported a recent increase in artesunate IC50 values in genetically distinct isolates from eastern Cambodia. Other studies from northwestern Thailand reported an approximately 10-fold decrease in artesunate in-vitro susceptibility over a 10-year period; however, no clinical assessments were conducted [38]. Nonetheless, the work from Pailin provides compelling evidence that the clinical surrogate marker of parasitemia on day 3 (after initiation of treatment) did not translate into reduced ART susceptibility in vitro [21••]. Further studies are required to assess to what extent in-vitro assays can predict the emergence of ART resistance.
Recent advances in defining artemisinin mode of action and mechanisms of resistance
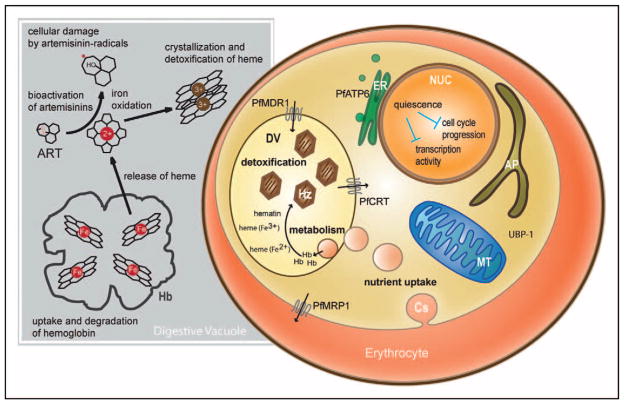
The mechanism by which ARTs exert their antimalarial action has long remained enigmatic (Fig. 3) [39•,40•,41–45,46••,47•–49•]. Investigations with ART derivatives have defined a critical requirement for the endoperoxide moiety that is generally believed to produce active compound upon interaction with intracellular iron. The likely source of this iron is in Fe-protoporphyrin IX that is released as a result of proteolytic degradation of host hemoglobin, which is imported into the acidic digestive vacuole of the intra-erythrocytic parasite (Fig. 3) [50]. Several mechanisms of ART action have been proposed, including oxidative damage to parasite membranes or inactivation of parasite proteins [51,52]. One candidate parasite protein that has received considerable scrutiny is the calcium-dependent endoplasmic reticulum-resident ATPase PfATP6, which can be inhibited by ART when expressed in Xenopus laevis frog oocytes. Mutations were identified that abolished this inhibition or that were associated with reduced ART susceptibility in P. falciparum field isolates [41]. A recent study, however, found no significant increase in IC50 values for ART derivatives in recombinant P. falciparum cell lines expressing mutant vs. wild-type pfatp6, although a subset of dose–response assays was suggestive of reduced susceptibility in mutant parasites [53].
Figure 3. Depiction of an intra-erythrocytic Plasmodium falciparum parasite showing proteins and biological processes implicated in artemisinin action.
Several parasite proteins have been implicated in decreased susceptibility to artemisinins (ARTs), including PfATP6 (proposed to be in the endoplasmic reticulum [41]), PfMDR1 on the digestive vacuole [42], PfMRP1 on the parasite plasma membrane [43], and UBP-1 whose ortholog in Plasmodium chabaudi is associated with ART resistance [44]. The digestive vacuole protein PfCRT is also indicated as mutations that confer chloroquine resistance have been shown to significantly increase susceptibility to ARTs [45]. Host hemoglobin is delivered via cytostomes to the digestive vacuole, wherein it is proteolytically degraded. This liberates iron-heme (Fe-protoporphyrin IX) moieties, with subsequent oxidation of iron. Iron-heme is detoxified via its incorporation into hemozoin crystals. Iron-heme is thought to activate ARTs via interaction with the endoperoxide bridge, with the resulting ART radicals causing cellular damage [46••]. Investigations of field isolates and drug-pressured laboratory lines have implicated quiescence or dormancy of early ring-stage parasites in resistance to ART action [47•–49•]. ART, artemisinin; AP, apicoplast; Cs, cytostome; DV, digestive vacuole; ER, endoplasmic reticulum; Hb, hemoglobin; Hz, hemozoin; MT, mitochondria; NUC, nucleus.
Multiple investigations have focused on pfmdr1, which encodes an ATP-binding cassette (ABC) transporter resident on the digestive vacuole and can vary via point mutations or copy number [42]. Field studies and laboratory investigations demonstrate that pfmdr1 gene amplification is associated with an increased risk of parasite recrudescence following mefloquine or artesunate–mefloquine treatment, and in-vitro resistance to mefloquine accompanied by reduced susceptibility to ART [36,54,55]. Related studies have found that in some strains of P. falciparum, selection for resistance to ART or its derivative artelinic acid was accompanied by amplification of pfmdr1 [56,57]. Removal of drug pressure frequently resulted in deamplification of this gene and attenuation of the resistance phenotype. Genetic studies of drug-pressured rodent Plasmodium chabaudi parasites also identified amplification of the mdr1 ortholog, which was associated with rodent parasite resistance to ART and the partner drugs mefloquine and lumefantrine [58]. The pfmdr1 polymorphisms have also been associated with diminished ART susceptibility: in a recent study of field isolates from the Thai–Myanmar border, novel polymorphisms in this gene (and the related ABC transporter gene pfmrp1) were associated with reduced in-vitro susceptibility to ART and DHA [59]. The pfmdr1 gene may, therefore, play a role in reducing parasite susceptibility to some ACTs. However, this is not thought to constitute a major determinant of delayed parasite clearance, as evidenced by recent genotyping of pfmdr1, pfatp6, and ubp-1 (a gene associated with resistance from ART selection studies in P. chabaudi [44,60]). This genotyping study found no association between these genes and P. falciparum clearance rates in patients from western Cambodia and northwestern Thailand [61•].
Genome-wide studies are also being actively pursued to identify candidate determinants of ART susceptibility, including linkage analysis of a P. falciparum genetic cross that identified several regions of the genome (including pfmdr1) that were associated with decreased ART susceptibility [62]. Recently, drug susceptibility profiles for over 2800 compounds in 61 P. falciparum lines were used to map response phenotypes to genetic loci [63••]. This identified 15 genes associated with P. falciparum responses to ART and mefloquine, many of which had not been previously identified, making these important candidates to examine in patient isolates showing delayed responses to ACTs. Gene disruption studies have also implicated PfMRP1, located on the asexual blood stage parasite’s plasma membrane, as a minor modulator of ART potency [43]. As an alternative approach, field isolates from Pailin and neighboring sites in the GMS were briefly cultured ex vivo and RNA was prepared from parasites harvested regularly throughout a 48-h period (corresponding to one intra-erythrocytic developmental cycle). Transcriptional analysis of time-matched samples revealed altered regulation profiles for several hundred genes in the isolates with delayed parasite clearance times [49•,64•]. Those isolates revealed downregulation of several metabolic and cellular pathways (including glycolysis, redox regulation, and glutathione synthesis) early in the cycle, and a lesser degree of upregulation of pathways including protein synthesis in later stages. These complex studies involved a small sample size and could only be performed once as isolates were cultured directly from patient blood. Additional experiments are required to provide further evidence that these broad transcriptional changes represent an underlying molecular basis of reduced ART susceptibility. Preliminary transcriptome studies were also applied to a P. falciparum line selected for tolerance to very high concentrations of ART following continuous exposure to increasing ART doses over a 3-year period [47•]. These studies reported a number of differentially expressed genes in the resistant line compared with its parental control, including genes encoding a heat shock protein and a cell cycle regulator. However, only two time points were selected over a single 48-h cycle, precluding accurate matching of time points. The selection of ART-tolerant lines in several laboratories and their ongoing molecular characterization should provide important new insights into general mechanisms of ART resistance or tolerance in P. falciparum parasites in the near future.
In summary, molecular studies on the basis of ART resistance have identified some genes associated with reduced ART susceptibility in vitro (most notably pfmdr1), but researchers have yet to define common genetic determinants that can account for prolonged parasite clearance times in drug-treated patients. This gap in knowledge can be addressed through expanded genome-wide association studies with large numbers of clinically characterized field isolates, transcriptome and metabolomic studies on highly synchronized cultured parasites, and confirmation of candidates using transfection and allelic exchange techniques. Adding to the complexity of finding these determinants is the difficulty in defining markers of clinical or in-vitro resistance. It is conceivable that single determinants of resistance will not be found, and that clinical resistance involves multi-factorial molecular processes that achieve little more than to permit parasites to survive the initial onslaught of ART action and to resume growth once the levels of these very short-lived drugs have dropped below therapeutic concentrations. One mechanism by which P. falciparum might survive is a recently described dormancy trait whereby small numbers of parasites can abruptly arrest their intra-erythrocytic ring-stage development after a single exposure to DHA and remain dormant for several days to weeks before resuming normal growth rates [48•]. This is consistent with a similar quiescence trait observed in ART-pressured ring-stage parasites selected for tolerance to high concentrations of drug [47•]. Recent mechanistic investigations provide evidence that parasites might achieve a state of dormancy by reducing the rate of hemoglobin degradation in ring-stage parasites. This would effectively reduce the amount of hemoglobin products available to potentiate ART activity and enable stalled ring-stage parasites to survive short-lasting drug exposure [46••]. This dormancy–recovery phenomenon is not thought to contribute to the initial slowing of parasite clearance following treatment with ACTs or artesunate monotherapy, but might help explain the high rates of recrudescence observed following ART monotherapy [65]. Mathematical modeling of parasite responses to treatments conducted in Cambodia and Thailand suggest that prolonged parasite clearance times are likely to reflect reduced susceptibility of ring-stage parasites, via cellular mechanisms yet to be elucidated [66•].
Conclusion
The evidence for the emergence of resistance to ARTs in South-east Asia is compelling and demands aggressive intervention measures. Modeling studies using parasite responses to ACT treatment in western Cambodia argue that as intervention measures decrease the local parasite pool, the relative proportion of resistant parasites will increase [6]. These authors concluded that the spread of resistance can only be halted by eliminating malaria in this region. Proposals to counter resistance include mass drug administration, mass screen and treat campaigns including the use of rapid diagnostic tests, the use of newer ACTs such as DHA–piperaquine or artesunate–pyronaridine that have favorable efficacy and compliance characteristics, and improved case detection and treatment [67•–69•,70]. Vector control measures are also critical and need to include careful monitoring for the emergence and spread of insecticide resistance [71••]. Additional efforts include the Affordable Medicines Facility-malaria (AMFm), an innovative financing mechanism to expand access to affordable ACTs [72,73•]. Defining the molecular basis of resistance will also be critical to effectively monitor for the spread of resistance. With precious few drugs available to replace ARTs should they fail, the importance of countering resistance cannot be underestimated.
Key points.
Artemisinin-based combination therapies have been adopted worldwide as official first-line policy for the treatment of Plasmodium falciparum malaria. These combine a fast-acting but short-lived artemisinin derivative with one of several longer-lasting partner drugs.
Evidence of emerging resistance to artemisinin derivatives has been gathered from clinical studies in western Cambodia, a known source of multidrug-resistant P. falciparum.
Resistance manifests as prolonged parasite clearance time, as evidenced by microscopically detectable blood stage infections on day 3 after initiation of treatment.
Intense efforts are being pursued worldwide to confirm, characterize, and contain resistance to artemisinins. The urgency of these efforts is increased by the paucity of alternative antimalarials should artemisinins fail.
Molecular markers have yet to be defined. Nevertheless, evidence is mounting that the ability of early ring-stage intra-erythrocytic parasites to resist artemisinin action, possibly including mechanisms of quiescence or dormancy, might contribute to prolonged clearance times and/or parasite recrudescence after treatment.
Acknowledgments
The authors thank Dr Pascal Ringwald, from the Global Malaria Program at the WHO, for kindly providing figures. D.A.F. gratefully acknowledges funding from the National Institutes of Health (R01 AI079709) and the Medicines for Malaria Venture. C.O.B. gratefully acknowledges funding from the Howard Hughes Medical Institute through their Medical Fellows Program.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 615).
- 1.World Health Organization. WHO global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva, Switzerland: WHO Press; [[accessed 1 August 2010].]. http://www.who.int/malaria/publications/atoz/9789241500470/en/index.html. [Google Scholar]
- 2.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 3•.Lin JT, Juliano JJ, Wongsrichanalai C. Drug-resistant malaria: the era of ACT. Curr Infect Dis Rep. 2010;12:165–173. doi: 10.1007/s11908-010-0099-y. A review of ACTs, emerging resistance to ART, and resistance surveillance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Crawley J, Chu C, Mtove G, Nosten F. Malaria in children. Lancet. 2010;375:1468–1481. doi: 10.1016/S0140-6736(10)60447-3. An outstanding clinical review that covers epidemiology and disease burden, clinical presentation, diagnosis, treatment, prevention through prophylaxis, vector control, and approaches to developing a malaria vaccine. [DOI] [PubMed] [Google Scholar]
- 5.White NJ. Qinghaosu (artemisinin): the price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 6.Maude RJ, Pontavornpinyo W, Saralamba S, et al. The last man standing is the most resistant: eliminating artemisinin-resistant malaria in Cambodia. Malar J. 2009;8:31. doi: 10.1186/1475-2875-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price RN, Nosten F, Luxemburger C, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet. 1996;347:1654–1658. doi: 10.1016/s0140-6736(96)91488-9. [DOI] [PubMed] [Google Scholar]
- 8.Eastman RT, Fidock DA. Artemisinin-based combination therapies: a vital tool in efforts to eliminate malaria. Nat Rev Microbiol. 2009;7:864–874. doi: 10.1038/nrmicro2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jambou R, Le Bras J, Randrianarivelojosia M. Pitfalls in new artemisinin-containing antimalarial drug development. Trends Parasitol. 2011;27:82–90. doi: 10.1016/j.pt.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Li GQ, Guo XB, Fu LC, et al. Clinical trials of artemisinin and its derivatives in the treatment of malaria in China. Trans R Soc Trop Med Hyg. 1994;88 (Suppl 1):S5–S6. doi: 10.1016/0035-9203(94)90460-x. [DOI] [PubMed] [Google Scholar]
- 11•.Stepniewska K, Ashley E, Lee SJ, et al. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis. 2010;201:570–579. doi: 10.1086/650301. An analysis of parasite clearance data from 18 699 patients with falciparum malaria from low, medium, or high malaria transmission settings. The report concluded that the result of the blood smear on day 3 was a good predictor of subsequent treatment failure and provided a simple screening measure for ART resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells TN, Alonso PL, Gutteridge WE. New medicines to improve control and contribute to the eradication of malaria. Nat Rev Drug Discov. 2009;8:879–891. doi: 10.1038/nrd2972. [DOI] [PubMed] [Google Scholar]
- 13••.Vinetz JM, Clain J, Bounkeua V, et al. Chemotherapy of malaria. In: Brunton L, Chabner B, Knollman B, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 12. New York, NY: McGraw Hill Medical; 2011. pp. 1383–1418. A textbook summary of malaria chemotherapy, including treatment and prophylaxis, with a review of the history, chemistry, mode of action, mechanisms of resistance, therapeutic use, toxicity, and contraindications of each antimalarial drug in clinical use. [Google Scholar]
- 14•.Hastings I. How artemisinin-containing combination therapies slow the spread of antimalarial drug resistance. Trends Parasitol. 2011;27:67–72. doi: 10.1016/j.pt.2010.09.005. A theoretical discussion of antimalarial drug resistance and how this is affected by the use of ACTs. [DOI] [PubMed] [Google Scholar]
- 15.Vijaykadga S, Rojanawatsirivej C, Cholpol S, et al. In vivo sensitivity monitoring of mefloquine monotherapy and artesunate-mefloquine combinations for the treatment of uncomplicated falciparum malaria in Thailand in 2003. Trop Med Int Health. 2006;11:211–219. doi: 10.1111/j.1365-3156.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- 16.Denis MB, Tsuyuoka R, Poravuth Y, et al. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health. 2006;11:1360–1366. doi: 10.1111/j.1365-3156.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 17.Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 18•.Noedl H, Se Y, Sriwichai S, et al. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in Southeast Asia. Clin Infect Dis. 2010;51:e82–e89. doi: 10.1086/657120. This article provides additional details and data about the earlier report by Noedl et al. [17] that identified patients with artesunate-resistant malaria along the Thai–Cambodian border. [DOI] [PubMed] [Google Scholar]
- 19.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Htut ZW. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:1807–1808. author reply 1808. [PubMed] [Google Scholar]
- 21••.Dondorp AM, Yeung S, White L, et al. Artemisinin resistance: current status and scenarios for containment. Nat Rev Microbiol. 2010;8:272–280. doi: 10.1038/nrmicro2331. A comprehensive review of ART resistance and proposed ways in which it might be contained. [DOI] [PubMed] [Google Scholar]
- 22.Newton PN, Fernandez FM, Plancon A, et al. A collaborative epidemiological investigation into the criminal fake artesunate trade in South East Asia. PLoS Med. 2008;5:e32. doi: 10.1371/journal.pmed.0050032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rathod PK, McErlean T, Lee PC. Variations in frequencies of drug resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 1997;94:9389–9393. doi: 10.1073/pnas.94.17.9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Anderson TJ, Nair S, Nkhoma S, et al. High heritability of malaria parasite clearance rate indicates a genetic basis for artemisinin resistance in western Cambodia. J Infect Dis. 2010;201:1326–1330. doi: 10.1086/651562. Genotypic analysis of South-east Asian parasites that provides evidence of a heritable component of parasite clearance rates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White NJ, Pongtavornpinyo W. The de novo selection of drug-resistant malaria parasites. Proc Biol Sci. 2003;270:545–554. doi: 10.1098/rspb.2002.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Enserink M. Malaria’s drug miracle in danger. Science. 2010;328:844–846. doi: 10.1126/science.328.5980.844. This article discusses the evidence for emerging resistance to ARTs in South-east Asia and the initiatives being taken to suppress its spread. [DOI] [PubMed] [Google Scholar]
- 27.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 28.Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 29.Wootton JC, Feng X, Ferdig MT, et al. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 30.Roper C, Pearce R, Nair S, et al. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 31••.Cui L, Yan G, Sattabongkot J, et al. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta Trop. (in press). This article provides an outstanding summary of malaria epidemiology in countries throughout the GMS and the evidence for emerging ART resistance. Also, it discusses the different Anopheles vectors, the status of insecticide resistance in this region, malaria elimination campaigns, and the issue of counterfeit drugs. [Google Scholar]
- 32.Carrara VI, Zwang J, Ashley EA, et al. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thanh NV, Toan TQ, Cowman AF, et al. Monitoring for Plasmodium falciparum drug resistance to artemisinin and artesunate in Binh Phuoc Province, Vietnam: 1998–2009. Malar J. 2010;9:181. doi: 10.1186/1475-2875-9-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Sibley CH, Guerin PJ, Ringwald P. Monitoring antimalarial resistance: launching a cooperative effort. Trends Parasitol. 2010;26:221–224. doi: 10.1016/j.pt.2010.02.008. This article summarizes the collaborative effort between the WHO and the WorldWide Antimalarial Resistance Network, whose objective is to create a global, comprehensive network of information on antimalarial drug resistance. [DOI] [PubMed] [Google Scholar]
- 35.Kachur SP, MacArthur JR, Slutsker L. A call to action: addressing the challenge of artemisinin-resistant malaria. Expert Rev Anti Infect Ther. 2010;8:365–366. doi: 10.1586/eri.10.23. [DOI] [PubMed] [Google Scholar]
- 36.Picot S, Olliaro P, de Monbrison F, et al. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Lim P, Wongsrichanalai C, Chim P, et al. Decreased in vitro susceptibility of Plasmodium falciparum isolates to artesunate, mefloquine, chloroquine, and quinine in Cambodia from 2001 to 2007. Antimicrob Agents Chemother. 2010;54:2135–2142. doi: 10.1128/AAC.01304-09. This article reported drug profile data on approximately 1600 Cambodian isolates, revealing a positive association between IC50 values of artesunate, mefloquine, chloroquine, and quinine. Genometric mean IC50 values from patients who failed artesunate–mefloquine therapy were significantly higher than the values from patients who were cured. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huttinger F, Satimai W, Wernsdorfer G, et al. Sensitivity to artemisinin, mefloquine and quinine of Plasmodium falciparum in northwestern Thailand. Wien Klin Wochenschr. 2010;122 (Suppl 3):52–56. doi: 10.1007/s00508-010-1438-6. [DOI] [PubMed] [Google Scholar]
- 39•.Ding XC, Beck HP, Raso G. Plasmodium sensitivity to artemisinins: magic • bullets hit elusive targets. Trends Parasitol. 2010;27:73–81. doi: 10.1016/j.pt.2010.11.006. This article reviews the different models proposed for ART action and the evidence for associations between resistance and candidate genes. [DOI] [PubMed] [Google Scholar]
- 40•.O’Neill PM, Barton VE, Ward SA. The molecular mechanism of action of artemisinin: the debate continues. Molecules. 2010;15:1705–1721. doi: 10.3390/molecules15031705. This article discusses the potential mechanisms of bioactivation of ARTs and related endoperoxides and proposed cellular modes of action. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krishna S, Pulcini S, Fatih F, Staines H. Artemisinins and the biological basis for the PfATP6/SERCA hypothesis. Trends Parasitol. 2011;26:517–523. doi: 10.1016/j.pt.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Ecker A, Lehane A, Fidock DA. Molecular markers of Plasmodium resistance to antimalarials. In: Staines HM, Krishna S, editors. Treatment and prevention of malaria: antimalarial drug chemistry, action and use. Basel, Switzerland: Birkhauser Verlag; (in press) [Google Scholar]
- 43.Raj DK, Mu J, Jiang H, et al. Disruption of a Plasmodium falciparum multidrug resistance-associated protein (PfMRP) alters its fitness and transport of antimalarial drugs and glutathione. J Biol Chem. 2009;284:7687–7696. doi: 10.1074/jbc.M806944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunt P, Martinelli A, Modrzynska K, et al. Experimental evolution, genetic analysis and genome re-sequencing reveal the mutation conferring artemisinin resistance in an isogenic lineage of malaria parasites. BMC Genomics. 2010;11:499. doi: 10.1186/1471-2164-11-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valderramos SG, Fidock DA. Transporters involved in resistance to antimalarial drugs. Trends Pharmacol Sci. 2006;27:594–601. doi: 10.1016/j.tips.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46••.Klonis N, Crespo-Ortiz MP, Bottova I, et al. Artemisinin activity against Plasmodium falciparum requires hemoglobin uptake and digestion. Proc Natl Acad Sci U S A. 2011;108:11405–11410. doi: 10.1073/pnas.1104063108. This article presents evidence that hemoglobin uptake and proteolysis is required for ART activity and supports prior observations that ART triggers oxidative stress via the production of free radicals in parasite membranes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Witkowski B, Lelievre J, Barragan MJ, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Anti-microb Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. The first report of P. falciparum parasites selected for resistance to an ART in vitro. The survival of parasites exposed to extremely high ART concentrations was attributed to a subpopulation of ring-stage parasites that entered into a state of developmental quiescence. These parasites resumed normal growth within a day of removing drug pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Teuscher F, Gatton ML, Chen N, et al. Artemisinin-induced dormancy in Plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis. 2010;202:1362–1368. doi: 10.1086/656476. This article documents the ability of ring-stage parasites to enter dormancy following short-term exposure to an ART. This phenomenon may help explain the high rates of P. falciparum recrudescence following treatment with ART derivatives. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Mok S, Imwong M, Mackinnon MJ, et al. Artemisinin resistance in Plasmodium falciparum is associated with an altered temporal pattern of transcription. BMC Genomics. 2011;12:391. doi: 10.1186/1471-2164-12-391. A genome-wide transcriptional analysis of P. falciparum isolates from South-east Asia, showing differential expression of multiple genes and gene sets in western Cambodian isolates showing delayed parasite clearance times. This altered profile was associated with slower growth and maturation of early stages of intra-erythrocytic development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–291. doi: 10.1007/3-540-29088-5_11. [DOI] [PubMed] [Google Scholar]
- 51.Hartwig CL, Rosenthal AS, D’Angelo J, et al. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem Pharmacol. 2009;77:322–336. doi: 10.1016/j.bcp.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui L, Su XZ. Discovery, mechanisms of action and combination therapy of artemisinin. Expert Rev Anti Infect Ther. 2009;7:999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valderramos SG, Scanfeld D, Uhlemann AC, et al. Investigations into the role of the Plasmodium falciparum SERCA (PfATP6) L263E mutation in artemisinin action and resistance. Antimicrob Agents Chemother. 2010;54:3842–3852. doi: 10.1128/AAC.00121-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Price RN, Uhlemann AC, Brockman A, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sidhu AB, Uhlemann AC, Valderramos SG, et al. Decreasing pfmdr1 copy number in Plasmodium falciparum malaria heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine, and artemisinin. J Infect Dis. 2006;194:528–535. doi: 10.1086/507115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavchich M, Gerena L, Peters J, et al. Role of pfmdr1 amplification and expression in induction of resistance to artemisinin derivatives in Plasmodium falciparum. Antimicrob Agents Chemother. 2010;54:2455–2464. doi: 10.1128/AAC.00947-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen N, Chavchich M, Peters JM, et al. Deamplification of pfmdr1-containing amplicon on chromosome 5 in Plasmodium falciparum is associated with reduced resistance to artelinic acid in vitro. Antimicrob Agents Chemother. 2010;54:3395–3401. doi: 10.1128/AAC.01421-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borges S, Cravo P, Creasey A, et al. Genome-wide scan reveals amplification of mdr1 as a common denominator of resistance to mefloquine, lumefantrine and artemisinin in P. chabaudi malaria parasites. Antimicrob Agents Chemother. 2011;55:4858–4865. doi: 10.1128/AAC.01748-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veiga MI, Ferreira PE, Jornhagen L, et al. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One. 2011;6:e20212. doi: 10.1371/journal.pone.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunt P, Afonso A, Creasey A, et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61•.Imwong M, Dondorp AM, Nosten F, et al. Exploring the contribution of candidate genes to artemisinin resistance in Plasmodium falciparum. Anti-microb Agents Chemother. 2010;54:2886–2892. doi: 10.1128/AAC.00032-10. A molecular analysis of patient isolates from Cambodia and Thailand, showing that delayed parasite clearance times are not associated with changes in the proposed candidate genes pfmdr1, pfatp6, or ubp-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beez D, Sanchez CP, Stein WD, Lanzer M. Genetic predisposition favors the acquisition of stable artemisinin resistance in malaria parasites. Antimicrob Agents Chemother. 2011;55:50–55. doi: 10.1128/AAC.00916-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63••.Yuan J, Cheng KC, Johnson RL, et al. Chemical genomic profiling for antimalarial therapies, response signatures, and molecular targets. Science. 2011;333:724–729. doi: 10.1126/science.1205216. This article describes a powerful combination of high-throughput chemical screening and genome-wide association analysis to define highly active compounds that are differentially active against distinct strains of P. falciparum. The analysis identified multiple genes associated with drug susceptibility, including 15 genes associated with artesunate and mefloquine response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Hu G, Cabrera A, Kono M, et al. Transcriptional profiling of growth perturbations of the human malaria parasite Plasmodium falciparum. Nat Biotechnol. 2010;28:91–98. doi: 10.1038/nbt.1597. Microarray analysis was applied to define changes in gene expression associated with chemical perturbation of parasite growth, leading the authors to construct a gene interaction network. These studies also documented downregulation of many parasite genes following exposure to ART. [DOI] [PubMed] [Google Scholar]
- 65.Codd A, Teuscher F, Kyle DE, et al. Artemisinin-induced parasite dormancy: a plausible mechanism for treatment failure. Malar J. 2011;10:56. doi: 10.1186/1475-2875-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66•.Saralamba S, Pan-Ngum W, Maude RJ, et al. Intrahost modeling of artemisinin resistance in Plasmodium falciparum. Proc Natl Acad Sci U S A. 2010;108:397–402. doi: 10.1073/pnas.1006113108. This article describes a mathematical model of intra-host parasite stage-specific pharmacokinetic–pharmacodynamic relationships, using data obtained from Pailin, Cambodia and Wang Pha, Thailand. The model predicted that artesunate efficacy against ring-stage parasites was significantly reduced in Pailin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.The malERA Consultative Group on Drugs. A research agenda for malaria eradication: drugs. PLoS Med. 2011;8:e1000402. doi: 10.1371/journal.pmed.1000402. This article defines an agenda for drug research and development aimed at achieving malaria eradication. Topics include the need for drugs that are prophylactic and curative and that could be administered in a single encounter and result in radical cure of all life cycle stages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.The malERA Consultative Group on Diagnoses and Diagnostics. A research agenda for malaria eradication: diagnoses and diagnostics. PLoS Med. 2011;8:e1000396. doi: 10.1371/journal.pmed.1000396. This article summarizes the existing tools and needs for accurate malaria diagnosis and diagnostics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69•.Tshefu AK, Gaye O, Kayentao K, et al. Efficacy and safety of a fixed-dose oral combination of pyronaridine-artesunate compared with artemether-lumefantrine in children and adults with uncomplicated Plasmodium falciparum malaria: a randomised noninferiority trial. Lancet. 2010;375:1457–1467. doi: 10.1016/S0140-6736(10)60322-4. A clinical noninferiority trial of pyronaridine-artesunate compared with ATM–lumefantrine for the treatment of uncomplicated P. falciparum malaria. The study observed equivalent treatment efficacy and reduced rates of reinfection with pyronaridine–artesunate presumably as a result of the long half-life of the pyronaridine partner drug. These features along with the once-daily dosing for 3 days lead the authors to propose this combination for inclusion in malaria treatment programs. [DOI] [PubMed] [Google Scholar]
- 70.Song J, Socheat D, Tan B, et al. Randomized trials of artemisinin-piperaquine, dihydroartemisinin-piperaquine phosphate and artemether-lumefantrine for the treatment of multidrug resistant falciparum malaria in Cambodia-Thailand border area. Malar J. 2011;10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71••.Trape JF, Tall A, Diagne N, et al. Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis. doi: 10.1016/S1473-3099(11)70194-3. (in press). A longitudinal study from Senegal showing significant decreases in malaria incidence following the introduction of ACTs and long-lasting insecticide-treated bednets. The report also documents worrying signs of the recent, rapid increase in Anopheles resistance to pyrethroids associated with a sudden rebound in malaria attacks. [DOI] [PubMed] [Google Scholar]
- 72.Laxminarayan R, Over M, Smith DL. Will a global subsidy of new antimalarials delay the emergence of resistance and save lives? Health Aff (Millwood) 2006;25:325–336. doi: 10.1377/hlthaff.25.2.325. [DOI] [PubMed] [Google Scholar]
- 73•.Adeyi O, Atun R. Universal access to malaria medicines: innovation in financing and delivery. Lancet. 2010;376:1869–1871. doi: 10.1016/S0140-6736(10)61189-0. This article describes a financing mechanism, termed AMFm, designed to expand access to ACTs through the public and private sectors and nongovernmental organizations and displace oral ART monotherapies from the market place. [DOI] [PubMed] [Google Scholar]