Abstract
Mesolimbic dopamine (DA) is a critical component of the brain circuitry regulating behavioral activation and effort-related processes. Rats with impaired DA transmission reallocate their instrumental behavior away from food-reinforced tasks with high response requirements, and instead select less effortful food-seeking behaviors. Previous work showed that adenosine A2A antagonists can reverse the effects of DA D2 antagonists on effort-related choice. However, less is known about the effects of adenosine A1 antagonists. Despite anatomical data showing that A1 and D1 receptors are co-localized on the same striatal neurons, it is uncertain if A1 antagonists can reverse the effects DA D1 antagonists. The present work systematically compared the ability of adenosine A1 and A2A receptor antagonists to reverse the effects of DA D1 and D2 antagonists on a concurrent lever pressing/feeding choice task. With this procedure, rats can choose between responding on a fixed ratio 5 lever-pressing schedule for a highly preferred food (i.e., high carbohydrate pellets) vs. approaching and consuming a less preferred rodent chow. The D1 antagonist ecopipam (0.2 mg/kg IP) and the D2 antagonist eticlopride (0.08 mg/kg IP) altered choice behavior, reducing lever pressing and increasing lab chow intake. Co-administration of the adenosine A1 receptor antagonists DPCPX (0.375, 0.75, and 1.5 mg/kg IP), and CPT (3.0, 6.0, 12.0 mg/kg IP) failed to reverse the effects of either the D1 or D2 antagonist. In contrast, the adenosine A2A antagonist KW-6002 (0.125, 0.25 and 0.5 mg/kg IP) was able to produce a robust reversal of the effects of eticlopride, as well as a mild partial reversal of the effects of ecopipam. Adenosine A2A and DA D2 receptors interact to regulate effort-related choice behavior, which may have implications for the treatment of psychiatric symptoms such as psychomotor slowing, fatigue or anergia that can be observed in depression and other disorders.
Keywords: operant, reinforcement, motivation, behavioral economics, behavioral activation, reward, decision making
Activational aspects of motivated behavior (i.e. vigor, persistence, work output) are highly adaptive because they enable organisms to overcome obstacles or work related response costs that separate them from significant stimuli (Salamone 1991, 1992; Salamone et al. 1997, 2003, 2007; Salamone and Correa 2002; van de Bos et al. 2006). Several lines of evidence implicate dopamine (DA), particularly in nucleus accumbens, as a critical component of the brain circuitry regulating behavioral activation and effort-related processes (Salamone et al., 1991, 2003, 2005, 2007; Vezina et al., 2002; Zhang et al., 2003; Wakabayashi et al., 2004; Barbano and Cador, 2006, 2007; Cagniard et al., 2006; Phillips et al., 2007; Floresco et al., 2008; Salamone, 2010). The increased activity induced by periodic food presentation is accompanied by increases in accumbens DA release, and is reduced by DA antagonism and accumbens DA depletions (Salamone 1986, 1988; McCullough and Salamone 1992). Rats with accumbens DA depletions are very sensitive to ratio requirements in operant schedules (Sokolowski and Salamone 1998; Aberman and Salamone 1999; Correa et al. 2002; Ishiwari et al. 2004; Mingote et al. 2005), including progressive ratio schedules (Aberman et al. 1998; Hamill et al. 1999). Moreover, rats with impaired DA transmission show alterations in response allocation on tasks that measure effort-related choice behavior (Salamone et al. 1991, 1997, 2003, 2005, 2006, 2007). Studies of effort-related choice behavior typically offer animals alternative paths to obtain reinforcement, which involve cost/benefit trade-offs related to the work requirements for obtaining the reinforcer. Some investigations of effort-related choice have employed a T-maze barrier task (Salamone et al. 1994; Cousins et al. 1996; Walton et al. 2002, 2003; Denk et al. 2005; Floresco and Ghods-Sharifi 2007; Bardgett et al. 2009; Correa et al. 2009), and others have used effort discounting procedures (Floresco et al. 2008; Bardgett et al. 2009). Additional studies have employed a concurrent fixed ratio 5 (FR5)/chow feeding procedure (Salamone et al. 1991, 2002, 2003, 2007). With this task, rats can choose between responding on a FR5 lever-pressing schedule for a highly preferred food (i.e., high carbohydrate precision pellets) vs. approaching and consuming a freely available but less preferred food (rodent chow). Trained rats spend most of their time lever pressing for the preferred food, and eat very little of the concurrently available lab chow. Rats treated with relativity low doses of D1 or D2 family antagonists, or with intra-accumbens injections of D1 or D2 antagonists, show a suppression of food-reinforced lever pressing, but increased levels of chow intake (Salamone et al. 1991, 1996; 2002; Cousins et al. 1994; Koch et al. 2000; Nowend et al. 2001; Sink et al. 2008; Farrar et al. 2010). This task has been extensively studied, and considerable evidence indicates that the shift from lever pressing to chow intake that is induced by DA antagonism or accumbens DA depletions is not due to effects on appetite or food preference, and is not related to the kinds of forepaw motor control deficits that are seen after ventrolateral neostriatal DA depletions (Salamone et al. 1991, 1993, 2002, 2007, 2009; Cousins et al. 1993; Nowend et al. 2001; Sink et al. 2008).
Although mesolimbic DA is a critical component of the brain circuitry regulating work output and effort-related choice behavior (Salamone and Correa 2002; Salamone et al. 2003, 2005, 2006), other brain areas and transmitters also are involved (Walton et al. 2002, 2003; Floresco and Ghods-Sharifi 2007; Salamone et al. 2007; Farrar et al. 2008; Hauber and Sommer, 2009). Recent studies have implicated the purine nucleoside adenosine in this type of function (Salamone and Correa 2009). Striatal areas, including neostriatum as well as nucleus accumbens, have a high concentration of adenosine A2A receptors (Jarvis and Williams 1989; Schiffmann et al. 1991; DeMet and Chicz-DeMet 2002; Ferre et al. 2004). There is a functional interaction between DA D2 and adenosine A2A receptors, which are co-localized on enkephalin-containing medium spiny neurons (Fink et al. 1992; Ferré 1997; Ferré et al., 1997, 2008b; Hillion et al. 2002; Fuxe et al. 2003). Frequently, this interaction has been investigated in connection with neostriatal motor functions that are related to parkinsonism (Ferré et al. 1997, 2001; Hauber and Munkel 1997; Svenningsson et al. 1999; Hauber et. al 2001; Wardas et al. 2001; Morelli and Pinna 2002; Jenner 2003, 2005; Correa et al. 2004; Pinna et al. 2005; Ishiwari et al. 2007; Salamone et al. 2008a,b). More recently, researchers have identified additional functions of the A2A receptor related to cognition (Takahashi et al. 2008) and motivation (O’Neill and Brown 2006; Mingote et al. 2008; Font et al. 2008). It has been demonstrated that the adenosine A2A agonist CGS 21680, when microinjected into the nucleus accumbens, produced effects on instrumental behavior and effort-related choice that resembled those produced by accumbens DA depletions or antagonism (Font et al. 2008; Mingote et al. 2008).
Several studies have shown that adenosine A2A antagonists such as MSX-3 and KW-6002 are capable of reversing the effects of the DA D2 antagonists haloperidol and eticlopride, including experiments that used the concurrent FR5/feeding choice procedure (Farrar et al. 2007; Worden et al. 2009; Salamone et al. 2009), and the T-maze barrier choice task (Mott et al. 2009; Correa et al. 2009). These observations are consistent with anatomical data showing that adenosine A2A and DA D2 family receptors are co-localized in the same medium spiny neurons (Fink et al. 1992; Ferré 1997; Hillion et al. 2002; Fuxe et al. 2003). Yet despite the growing body of evidence demonstrating that the effort-related effects of D2 antagonists can be reversed by adenosine A2A antagonists (Salamone and Correa 2009), less is known about the general pattern of the interaction between antagonists that act upon specific subtypes of DA and adenosine receptors, and how this interaction regulates effort-related choice behavior. A few studies have shown that the adenosine A1 antagonist DPCPX could not reverse the effects of haloperidol on the concurrent FR5/feeding task (Salamone et al. 2009) or the T-maze barrier choice procedure (Mott et al. 2009). Nevertheless, the ability of adenosine antagonists with different patterns of receptor selectivity to reverse the effort-related effects of DA D1 and D2 family antagonists remains uncertain. Furthermore, in view of the fact that adenosine A1 and DA D1 receptors tend to be co-localized on the same striatal neurons (Ferré 1997, 2008; Ferré et al. 1997, 2005), it is particularly important to determine if A1 antagonists are capable of reversing the effects of DA D1 antagonists.
In view of these uncertainties about the overall pattern of interaction between selective adenosine and DA antagonists on effort-related choice, the current work was undertaken to examine the role of DA/adenosine receptor interactions using the concurrent lever pressing/chow feeding procedure. More specifically, the present experiments were conducted to determine if the ability of adenosine A1 or A2A antagonists to reverse the effects of DA antagonists is dependent upon the particular subtype of DA receptor that was being blocked. The adenosine A1 antagonists DPCPX (0.375–1.5 mg/kg IP) and CPT (3.0–12.0 mg/kg IP) were studied for their ability to reverse the effects of the D1 antagonist ecopipam (0.2 mg/kg IP) and the D2 antagonist eticlopride (0.08 mg/kg IP). Both of these adenosine A1 antagonists were investigated because evidence indicates that they may have different behavioral effects; although DPCPX generally fails to stimulate locomotion when administered on its own, CPT has been shown to induce locomotion in some studies (Martson et al. 1998), and also was reported to reverse some of the behavioral effects of haloperidol (Trevitt et al. 2009). In order to provide a direct and systematic comparison between the effects of adenosine A1 and A2A antagonism, the A2A selective drug KW-6002 (0.125–0.5mg/kg IP) also was assessed for its ability to reverse the behavioral effects of ecopipam and eticlopride.
EXPERIMENTAL PROCEDURES
Subjects
Adult male, drug- naïve, Sprague- Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) were housed in a colony maintained at 23°C with 12 hour light/dark cycles (lights on at 0:700 h). Rats (N = 48) weighed 290 – 340 g at the beginning of the study, and were initially food deprived to 85% of their free-feeding body weight for operant training. Rats were fed supplemental chow to maintain the food restriction throughout the study, with water available ad libitum in the home cages. Despite food restriction, rats were allowed modest weight gain throughout the experiment. All animal protocols were approved by the University of Connecticut institutional animal care and use committee, and followed NIH guidelines.
Pharmacological Agents and Selection of Doses
Eticlopride (S(−)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride) was obtained from Sigma Chemical Co. (St. Louis, MO) and SCH 39166 (ecopipam; (6aS-trans)-11-chloro-6,6a,7,8,9,13b-hexahydro-7-methyl-5H-benzo[d] naphtha[2,1-b]azepin-12-ol hydrobromide, obtained from Tocris, (Ellisville, MO) was dissolved in 0.9% saline. Ecopipam was used because it binds to D1 receptors with high affinity and selectivity, but has little affinity for 5HT2A and 5HT2C receptors (Alburges et al. 1992). Saline was also used as the vehicle control for the eticlopride and ecopipam injections. SCH 23390 ((R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride) also was obtained from Tocris, and dissolved in saline. The adenosine A2A antagonist used was KW-6002, which was generously donated by Lundbeck (Copenhagen, Denmark) and was dissolved in a DMSO/Tween-80/Saline solution. DPCPX (8-cyclopentyl-1,3-dipropylxanthine) was obtained from Tocris, and was dissolved in a 20% ethanol vehicle as in previous studies (Mott et al. 2009; Salamone et al. 2009). CPT (8-cyclopentyltheophylline; Sigma Chemical Co., St. Louis) was dissolved in 0.9% saline, which also was used as the vehicle control for CPT injections. All drug treatments were administered IP (see descriptions of individual experiments for drug injection schedule).
Doses of eticlopride and ecopipam used for the experiments were based upon previous research (Terry and Katz 1994; Clifton et al. 1995; Barrett et al. 2004; Sink et al., 2008; Worden et al., 2009) and on pilot studies. For this type of reversal study, picking a minimally significant dose of the DA antagonist does not ensure a large enough impairment in order to get a full dose/response curve for the reversal effect. The specific doses of each DA antagonist were selected in order to be high enough to produce a robust shift from lever pressing to chow intake (Sink et al. 2008), but low enough not to produce a general disruption of behavior. With eticlopride, the dose chosen (0.08 mg/kg) was slightly higher than the very lowest dose (0.05 mg/kg) that decreased lever pressing and increased chow intake in a previous study (Sink et al. 2008). With ecopipam (SCH 39166), the 0.2 mg/kg dose was also chosen based upon previous data showing that the 0.1 mg/kg dose produced a statistically significant but small decrease in lever pressing and increase in chow intake, but 0.2 mg/kg produced a more robust effect (Sink et al. 2008). Moreover, these doses of eticlopride and ecopipam were previously used in a reversal experiment (Worden et al. 2009). The dose range chosen for DPCPX and CPT was based upon doses listed in published behavioral studies involving IP administration in rats (Prediger et al. 2005; Aubel et al. 2007; Maione et al. 2007; Lobato et al. 2008; Karcz-Kubicha 2003; Marston et al, 1998; Salamone et al. 2009). Because some rats that received the combination of 12.0 mg/kg CPT plus eticlopride showed increased lever pressing compared to eticlopride alone, a second study was conducted to assess the effects of 24.0 mg/kg CPT and eticlopride. An additional study also was conducted to determine if DPCPX could reverse the effects of a D1 antagonist other than ecopipam; SCH 23390 was used for this study.
Behavioral Procedures
Behavioral sessions were conducted in operant conditioning chambers (28 cm × 23 cm × 23 cm; Med Associates). Rats were initially trained to lever press on a continuous reinforcement schedule (30-min sessions; 45-mg pellets, Bioserve, Frenchtown, NJ, were used for all operant behavior tests) and then were shifted to the FR5 schedule (30-min sessions, 5 days/week) and trained for several additional weeks. Rats were then trained on the concurrent FR5/chow-feeding procedure. With this task weighed amounts of lab chow (Lab Diet, 5P00 Prolab RMH 3000, Purina Mills, St. Louis, MO; typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the FR5 sessions. At the end of the session, rats were immediately removed from the chamber, and food intake was determined by weighing the remaining food (including spillage). Rats were trained until they attained stable levels of baseline lever pressing and chow intake (i.e., consistent responding over 1200 lever presses per 30 min), after which drug testing began. For most baseline days rats did not receive supplemental feeding, however, over weekends and after drug tests, rats usually received supplemental chow in the home cage. On baseline and drug treatment days, rats normally consumed all the operant pellets that were delivered from lever pressing during each session.
Experimental Procedures
Rats were trained on the concurrent FR5/chow-feeding procedure (as described above) before testing began, and each experiment employed different groups of rats. All six experiments used a within-groups design, with each rat receiving all combined IP drug treatments in their particular experiment in a randomly varied order (one treatment per week, with none of the treatment sequences repeated across different animals in the same experiment). Baseline (i.e. non-drug) sessions were conducted four additional days per week. The specific treatments and testing times for each experiment are listed below.
Ability of the A1 antagonists DPCPX and CPT to reverse the effects of the D1 antagonist ecopipam (SCH 39166)
On the test day, trained rats (n = 8) received the following treatments: 20 % ethanol vehicle (30 min before testing) plus saline vehicle IP (20 min before testing), 0.2 mg/kg ecopipam IP (20 min before testing) plus 20 % ethanol vehicle IP (30 min before testing), 0.2 mg/kg ecopipam IP (20 min) plus 0.375 mg/kg DPCPX IP (30 min), 0.2 mg/kg ecopipam IP (20 min) plus 0.75 mg/kg DPCPX IP (30 min), 0.2 mg/kg ecopipam IP (20 min) plus 1.5 mg/kg DPCPX IP (30 min). Because DPCPX was unable to reverse the effects of ecopipam, an additional experiment was conducted to determine if the same doses of DPCPX could reverse the effects of a different D1 antagonist (SCH 23390; 0.1 mg/kg IP; dissolved in saline, injected 60 min before testing). This experiment was conducted to determine if the inability of DPCPX to reverse the effects of ecopipam were unique to the particular D1 antagonist being used. For the CPT/ecopipam experiment, trained rats (n = 8) received the following treatments: saline vehicle (20 min before testing) plus saline vehicle IP (20 min before testing), 0.2 mg/kg ecopipam IP (20 min before testing) plus saline vehicle IP (20 min), 0.2 mg/kg ecopipam IP (20 min before testing) plus 3.0 mg/kg CPT IP (20 min), 0.2 mg/kg ecopipam IP (20 min) plus 6.0 mg/kg CPT IP (20 min), 0.2 mg/kg ecopipam IP (20 min) plus 12.0 mg/kg CPT IP (20 min).
Ability of the A1 antagonists DPCPX and CPT to reverse the effects of the D2 antagonist eticlopride
For the DPCPX/eticlopride experiment, trained rats (n = 8) received the folowing treatments: 20 % ethanol solution vehicle (30 min before testing) plus saline vehicle IP (30 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus 20 % ethanol solution vehicle IP (30 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus 0.375 mg/kg DPCPX IP (30 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus 0.75 mg/kg DPCPX IP (30 min before testing), 0.08 mg/kg eticlopride IP (30 min) plus 1.5 mg/kg DPCPX IP (30 min). For the CPT/eticlopride experiment, trained rats (n = 8) received the following treatments: saline vehicle (30 min before testing) plus saline vehicle IP (20 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus saline vehicle IP (20 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus 3.0 mg/kg CPT IP (20 min before testing), 0.08 mg/kg eticlopride IP (30 min) plus 6.0 mg/kg CPT IP (20 min), 0.08 mg/kg eticlopride IP (30 min) plus 12.0 mg/kg CPT IP (20 min). Because some animals that received the combination of 12.0 mg/kg CPT plus eticlopride showed increased lever pressing compared to eticlopride alone, a second experiment was conducted to assess the effects of a higher dose (24.0 mg/kg CPT) vs. eticlopride. On two successive weeks, rats (n = 8) received each of the following treatments in a randomly varied order: 0.08 mg/kg eticlopride IP (30 min before testing) plus saline vehicle IP (20 min before testing), and 0.08 mg/kg eticlopride IP (30 min) plus 24.0 mg/kg CPT IP (20 min).
Ability of the A2A antagonist KW-6002 to reverse the effects of the D1 antagonist ecopipam (SCH 39166) and the D2 antagonist eticlopride
Trained rats (n = 8) received the flowing treatments in the KW-6002/ecopipam experiment: DMSO/Tween-80/Saline vehicle (20 min before testing) plus saline vehicle IP (20 min before testing), 0.2 mg/kg ecopipam (20 min before testing) plus saline vehicle IP (20 min before testing), 0.2 mg/kg ecopipam IP (20 min before testing) plus 0.125 mg/kg KW-6002 IP (20 min before testing), 0.2 mg/kg ecopipam IP (20 min) plus 0.25 mg/kg KW-6002 IP (20 min), 0.2 mg/kg ecopipam IP (20 min) plus 0.5 mg/kg KW-6002 IP (20 min). In the KW-6002/eticlopride study, trained rats (n = 8) received the flowing treatments: DMSO/Tween-80/Saline vehicle (20 min before testing) plus saline vehicle IP (30 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus saline vehicle IP (20 min before testing), 0.08 mg/kg eticlopride IP (30 min before testing) plus 0.125 mg/kg KW-6002 IP (20 min), 0.08 mg/kg eticlopride IP (30 min) plus 0.25 mg/kg KW-6002 IP (20 min), 0.08 mg/kg eticlopride IP (30 min) plus 0.5 mg/kg KW-6002 IP (20 min).
Statistical Analyses
Total number of lever presses and gram quantity of chow intake from the 30 min sessions were analyzed with repeated measures of analysis of variance (ANOVA). When the overall ANOVA was significant, non-orthogonal planned comparisons using the overall error term were used to compare each treatment with the eticlopride or ecopipam vehicle control group (Keppel, 1991). For these comparisons, α level was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one. With this analysis, each condition that combined eticlopride or ecopicpam plus adenosine antagonist was compared with its respective eticlopride or ecopipam vehicle condition using the planned comparisons. Effect size calculations (R2 values; Keppel, 1991) were performed to assess the magnitude of the reversal effect; these analyses were conducted by removing the vehicle plus vehicle condition, and calculating the R2 value for the four treatments that included a DA antagonist injection. With this type of calculation, the magnitude of the treatment effect is independent of the number of animals, and is expressed as the proportion of total variance accounted for by treatment variance (for example R2 = 0.3 reflects 30% of the variance explained) across experiments and measures).
RESULTS
Ability of DPCPX and CPT to reverse the effects of D1 antagonism
DPCPX failed to attenuate the effects of ecopipam (SCH 39166) on the concurrent lever-pressing/chow feeding task (Fig. 1). There was a overall significant effect of drug treatment on lever pressing [F (4,28) = 20.539; p < 0.001]. Planned comparisons revealed that ecopipam significantly reduced lever pressing compared to vehicle control (p< 0.05), but co-administration of DPCPX with ecopipam did not produce a significant increase in lever pressing compared to ecopipam plus vehicle at any dose. There also was an overall significant effect of drug treatment on chow intake [F(4,28) =9.088, p < 0.001]. Planned comparisons revealed that ecopipam significantly increased chow increased compared to vehicle control (p< 0.05), but co-administration of DPCPX with ecopipam did not significantly decrease chow intake compared to ecopipam plus vehicle. An additional study assessed the ability of DPCPX to reverse the effects of another D1 antagonist, SCH 23390 (Table 1). There was a overall significant effect of drug treatment on lever pressing [F (4,28) = 27.52; p< .001]. Planned comparisons revealed that SCH 23390 produced a significant reduction in lever pressing compared to vehicle control (p< 0.05), but co-administration of DPCPX with SCH 23390 did not significantly increase lever pressing compared to SCH 23390 plus vehicle (Table 1). There also was a overall significant effect of drug treatment on chow consumption [F (4,28) = 6.99; p< 0.05]. SCH 23390 significantly increased chow intake compared to vehicle control (p< 0.05), but co-administration of DPCPX with SCH 23390 did not significantly increase lever pressing compared to SCH 23390 plus vehicle. For the CPT/ecopipam experiment (Fig. 2), there was a overall significant effect of drug treatment [F (4,28) = 19.469 p < .001]. Planned comparisons revealed that ecopipam significantly reduced lever pressing compared to vehicle control (p< 0.05). Co-administration of CPT with ecopipam did not significantly increase lever pressing compared to ecopipam plus vehicle. There also was a significant effect of drug treatment on chow intake [F(4,28) = 8.196, p < .001]. Planned comparisons revealed that ecopipam significantly increased chow intake compared to vehicle control (p< 0.05). Co-administration of CPT with ecopipam did not produce a significant decrease in chow consumption compared to ecopipam plus vehicle (p> 0.05).
Figure 1.
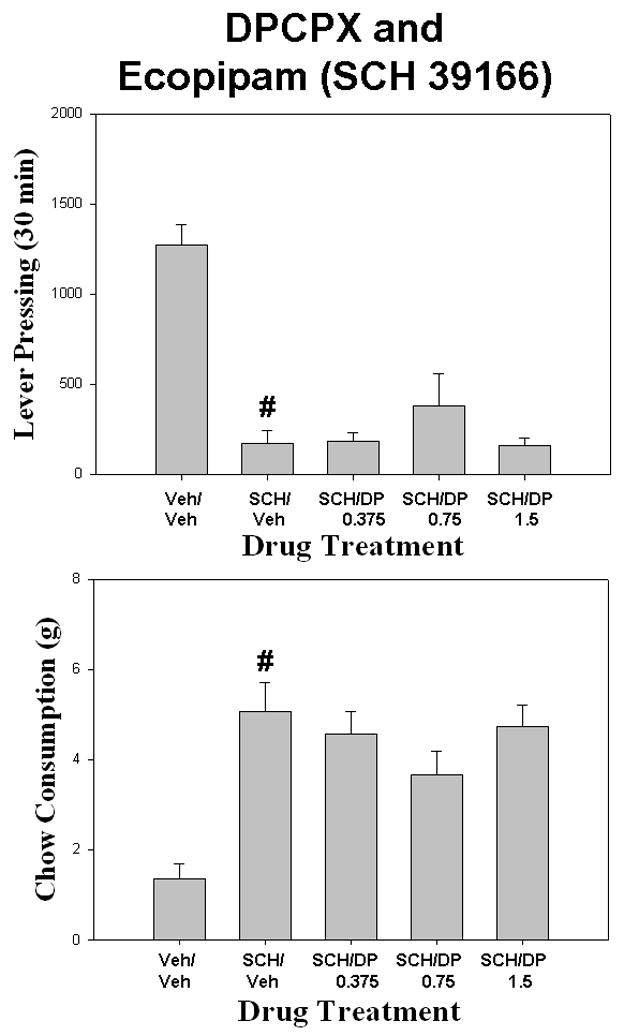
The effects of the adenosine A1 antagonist DPCPX on ecopipam-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.2 mg/kg ecopipam (SCH 39166) plus vehicle (SCH/Veh), and ecopipam (SCH 39166) plus 0.375, 0.75, or 1.5, mg/kg doses of DPCPX (SCH/DP). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Ecopipam significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05).
Table 1.
Effect of DPCPX in combination with the D1 antagonist SCH 23390 (0.1 mg/kg). Mean (± SEM) number of lever presses and amount of chow intake (in grams) after injection of vehicle, SCH 23390, and SCH 23390 plus various doses of DPCPX.
| Lever Presses | |
| vehicle plus vehicle | 1434.75 (±129.33) |
| SCH 23390 plus vehicle | 339.13 (±85.34)# |
| SCH 23390 plus 0.375 mg/kg DPCPX | 290.0 (±52.55) |
| SCH 23390 plus 0.75 mg/kg DPCPX | 404.0 (±160.89) |
| SCH 23390 plus 1.5 mg/kg DPCPX | 451.88 (±115.0) |
| Chow Intake | |
| vehicle plus vehicle | 1.5 (±0.43) |
| SCH 23390 plus vehicle | 4.75 (±0.65)# |
| SCH 23390 plus 0.375 mg/kg DPCPX | 4.19 (±0.45) |
| SCH 23390 plus 0.75 mg/kg DPCPX | 4.11 (±0.75) |
| SCH 23390 plus 1.5 mg/kg DPCPX | 3.53 (±0.61) |
different from vehicle plus vehicle, p < 0.05
Figure 2.
The effects of the adenosine A1 antagonist CPT on ecopipam-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.2 mg/kg ecopipam (SCH 39166) plus vehicle (SCH/Veh), and ecopipam plus 3.0, 6.0, or 12.0 mg/kg doses of CPT (SCH/CPT). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Ecopipam significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05).
Ability of DPCPX and CPT to reverse the effects of D2 antagonism
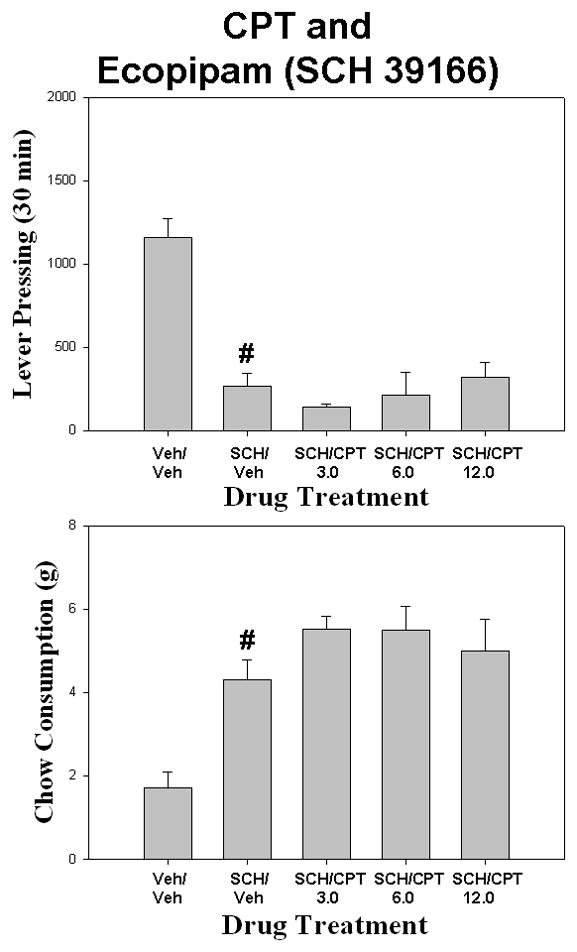
In the DPCPX/eticlopride study (Fig. 3), there was a significant overall effect of drug treatment [F (4,28) = 113.248 p< .001]. Planned comparisons revealed that eticlopride significantly lowered lever pressing compared to vehicle control (p< 0.05), but co-administration of DPCPX with eticlopride did not significantly increase lever pressing compared to eticlopride plus vehicle. There also was a significant effect of drug treatment on chow intake [F(4,28) = 8.449, p < .001]. Eticlopride significantly increased chow intake compared to vehicle control (planned comparisons, p< 0.05), but co-administration of DPCPX with eticlopride did not significantly affect chow intake compared to eticlopride plus vehicle. CPT also failed to significantly increase lever pressing and decrease chow intake in eticlopride-treated rats (Fig. 4). With lever pressing, there was a overall significant effect of drug treatment [F (4,28) = 17.349 p< 0.01]. Planned comparisons revealed that eticlopride significantly reduced lever pressing compared to vehicle control (p< 0.05), but co-administration of CPT with eticlopride did not significantly affect lever pressing compared to eticlopride plus vehicle. There also was an overall significant effect of drug treatment on chow intake [F(4,28) = 10.921 p < .001]. Planned comparisons showed that eticlopride produced a significant increase in chow consumption compared to vehicle control (p< 0.05), and co-administration of CPT with eticlopride did not significantly decrease chow consumption compared to eticlopride plus vehicle (p> 0.05). However, some of the rats that received 12.0 mg/kg CPT plus eticlopride did show increased lever pressing and decreased chow intake relative to eticlopride plus vehicle. For that reason, a second experiment was performed in which a higher dose of CPT was used. This study revealed that rats that received 24.0 mg/kg CPT plus eticlopride did not differ from eticlopride plus vehicle, either in terms of lever pressing (Mean (± SEM) number of lever presses: eticlopride plus vehicle, 7.0 (±2.62); eticlopride plus 24.0 mg/kg CPT, 69.5 (±36.10); t = −1.69, df = 7, n.s.) or chow intake (Mean (± SEM) amount of chow intake (grams): eticlopride plus vehicle, 4.1 (±0.91); eticlopride plus 24.0 mg/kg CPT, 3.7 (±0.89); t = 0.29, df = 7, n.s.).
Figure 3.
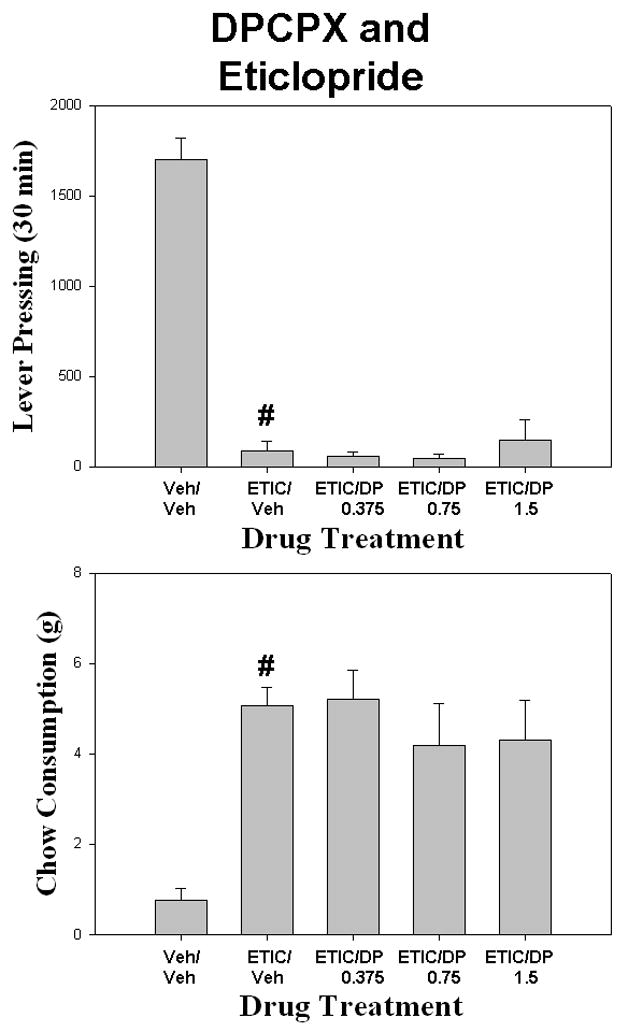
The effects of the adenosine A1 antagonist DPCPX on eticlopride-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.08 mg/kg eticlopride plus vehicle (ETIC/Veh), and eticlopride plus 0.375, 0.75, or 1.5, mg/kg doses of DPCPX (ETIC/DP). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Eticlopride significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05).
Figure 4.

The effects of the adenosine A1 antagonist CPT on eticlopride-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.08 mg/kg eticlopride plus vehicle (ETIC/Veh), and eticlopride plus 3.0, 6.0, or 12.0 mg/kg doses of CPT (ETIC/CPT). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Ecopipam significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05).
Ability of KW-6002 to reverse the effects of D1 or D2 antagonism
KW-6002 produced a partial reversal of the effects of ecopipam (Fig. 5). There was a overall significant effect of drug treatment on lever pressing [F (4,28) = 8.270 p< 0.01]. Planned comparisons showed that ecopipam produced a significant reduction in lever pressing compared to vehicle control (p< 0.05). Co-administration of KW-6002 with ecopipam significantly increased lever pressing compared to ecopipam plus vehicle, with the highest dose of KW–6002 (0.5mg/kg) increasing lever pressing relative to ecopipam plus vehicle (p< 0.05). There also was an overall significant effect of drug treatment on chow intake [F(4,28) = 5.280, p < .001]. Ecopipam produced a significant increase in chow consumption compared to vehicle control (p< 0.05). Administration of KW-6002 with ecopipam did not reverse the increase in chow intake produced by ecopipam plus vehicle. KW-6002 produced a robust and significant reversal of the effects of eticlopride on the concurrent lever pressing/chow-feeding task (Fig. 6). There was on overall effect of drug treatment on lever pressing [F(4,28) = 11.035, p < 0.001]. Eticlopride produced a significant reduction in lever pressing compared to vehicle control (planned comparison; p< 0.05). Co-administration of KW-6002 with eticlopride produced a significant increase in lever pressing at all doses (0.125, 0.25, and 0.5 mg/kg) compared to eticlopride plus vehicle (p< 0.05). There also was a significant overall effect of treatment on chow intake [F(4,28) = 10.029, p < 0.001]. Planned comparisons showed that eticlopride significantly increased chow intake compared to vehicle control (p< 0.05). Co-administration of KW-6002 with eticlopride produced a significant decrease in chow consumption at the two highest doses (0.25mg/kg and 0.5mg/kg) compared to eticlopride plus vehicle (p< 0.05).
Figure 5.
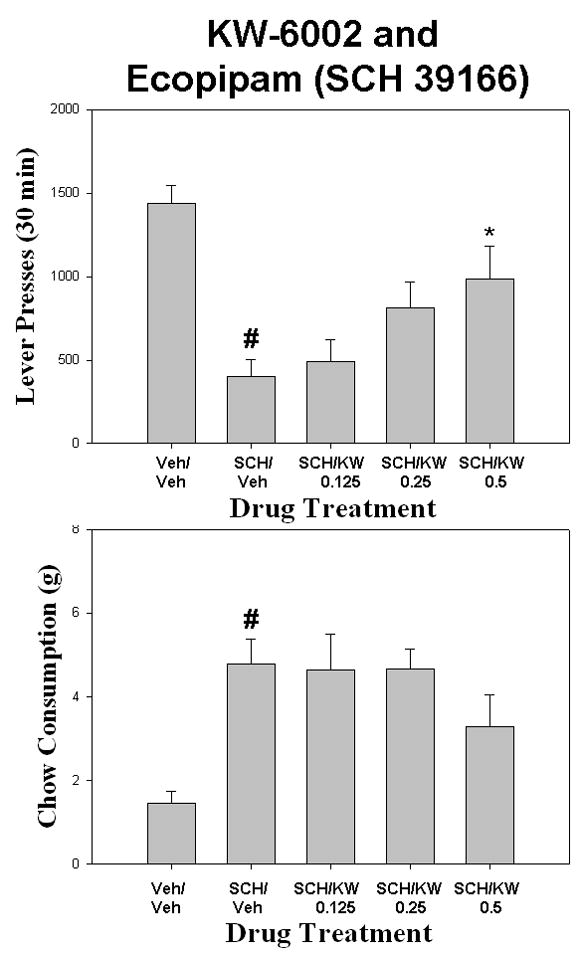
The effects of the adenosine A2A antagonist KW-6002 on ecopipam-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.2 mg/kg ecopipam (SCH 39166) plus vehicle (SCH/Veh), and ecopipam (SCH 39166) plus 0.125, 0.25, or 0.5, mg/kg doses of KW-6002 (SCH/KW). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Ecopipam significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05). KW-6002 administered to ecopipam-treated rats significantly increased lever pressing at the highest dose relative to treatment with ecopipam alone (* p < 0.05).
Figure 6.
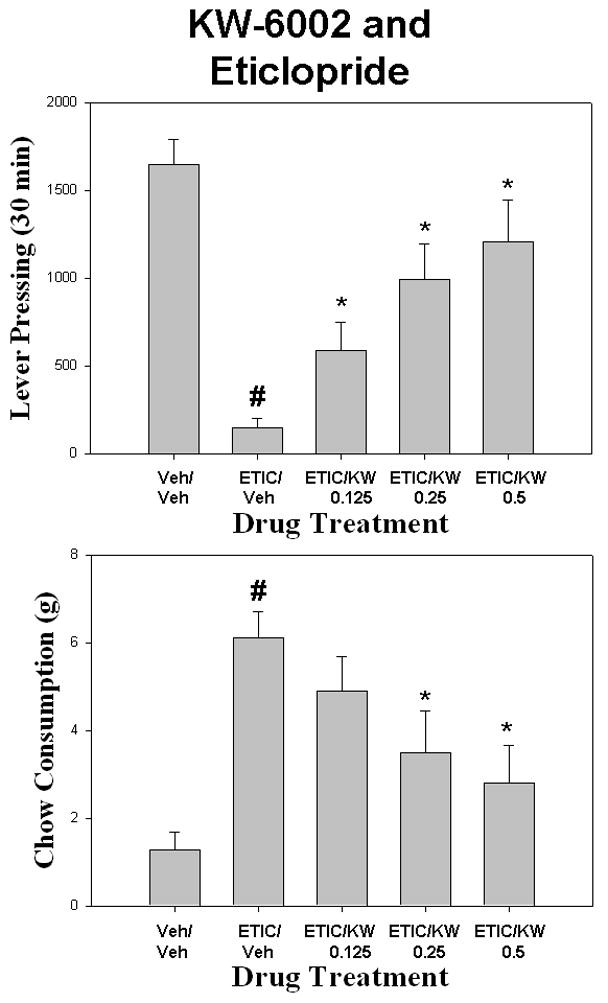
The effects of the adenosine A2A antagonist KW-6002 on eticlopride-induced changes in performance on the concurrent lever pressing/chow feeding procedure. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 0.08 mg/kg eticlopride plus vehicle (ETIC/Veh), and eticlopride plus 0.125, 0.25, or 0.5, mg/kg doses of KW-6002 (ETIC/KW). A. Mean (± SEM) number of lever presses (FR5 schedule) during the 30 min session. B. Mean (± SEM) gram quantity of chow intake. Eticlopride significantly decreased lever pressing and increased chow intake relative to vehicle (# p < 0.05). KW-6002 administered to eticlopride-treated rats significantly increased lever pressing and decreased chow intake relative to treatment with eticlopride alone (* p < 0.05).
Analysis of Effect Sizes
Table 2 shows the results of the effect size analyses for all the reversal effects in the DPCPX, CPT and KW-6002 experiments with ecopipam and eticlopride. By far, the largest effect size was shown for the ability of KW-6002 to reverse the effects of eticlopride.
Table 2.
Effect size calculations (R2 values) for the reversal effects.
| Experiment | Lever Pressing | Chow Intake |
|---|---|---|
| DPCPX vs. ecopipam | 0.11 | 0.12 |
| CPT vs. ecopipam | 0.07 | 0.10 |
| DPCPX vs. eticlopride | 0.06 | 0.05 |
| CPT vs. eticlopride | 0.10 | 0.07 |
| KW6002 vs. ecopipam | 0.26 | 0.10 |
| KW6002 vs. eticlopride | 0.43 | 0.27 |
DISCUSSION
Motivation is a complex and multifaceted process (Salamone 2010b), and a number of approaches have been used to study the impact of drugs on choice performance under conditions in which animals can select between multiple reinforcers that can be obtained via distinct instrumental behaviors (Routtenberg and Bulloch 1971; Salamone et al. 1994; Floresco et al. 2008; Bardgett et al. 2009). In the present studies, with rats responding on the concurrent FR5/chow feeding choice task, antagonism of either D1 or D2 receptors produced a substantial alteration in the relative allocation of behavior, decreasing lever pressing but increasing chow intake. The present results are consistent with previous studies showing that low-to-moderate doses of DA antagonists with varying degrees of selectivity, including cis-flupenthixol, SCH 23390, SCH 83566, haloperidol and raclopride, as well as ecopipam (SCH 39166) and eticlopride, all decrease lever pressing and increase chow intake in rats responding on this task (Salamone et al. 1991, 2002; Cousins et al. 1994; Sink et al. 2008). Because the present study was focused upon the interaction between DA and adenosine antagonists, only a single dose of each DA antagonist was used; nevertheless, the present doses were selected from a previous study that included a wider dose range for both ecopipam and eticlopride (Sink et al. 2008), and are the same doses as those used in a previous study of adenosine/DA interactions (Worden et al. 2009). Research with genetically altered mice also has shown that knockdown of the DA transporter, which elevates extracellular DA levels, can produce the opposite effect in animals tested on this task, i.e., increases in lever pressing and decreases in chow intake (Cagniard et al. 2006).
Several studies have been conducted to assess the validity of the concurrent lever pressing/chow intake choice task for assessing effort-related choice behavior. Attachment of higher ratio requirements (up to FR20) caused animals that were not drug treated to shift from lever pressing to chow intake (Salamone et al. 1997), indicating that this task is sensitive to lever pressing work requirements. Considerable evidence indicates that the shift from lever pressing to chow intake induced by interference with DA transmission is not due to a suppression of appetite for food or a change in food preference. Systemic or intra-accumbens injections of the DA antagonists haloperidol, SCH 23390 and sulpiride at doses that cause the shift from lever pressing to chow intake did not affect intake of either operant pellets or chow, and did not alter preference for the two types of food (Salamone et al. 1991; Koch et al., 2000). Future research should also study the effects of eticlopride and ecopipam on food preference. Additional studies have shown that chow intake is not generally affected by accumbens DA depletions (Koob et al., 1978; Salamone et al., 1993), or by injections of either D1 or D2 family antagonists into accumbens core or shell subregions (Baldo et al. 2002). Furthermore, the effects of DA antagonists on the concurrent choice procedure do not resemble those produced by prefeeding to reduce food motivation (Salamone et al. 1991), or appetite suppressant drugs such as amphetamine (Cousins et al. 1994), fenfluramine (Salamone et al. 2002) or CB1 antagonists (Sink et al., 2008); these appetite-related manipulations all fail to increase chow intake at doses that suppress food-reinforced lever pressing. In addition, several studies have demonstrated that the shift from lever pressing to chow intake that is induced by low doses of DA antagonists or accumbens DA depletions is not occurring because of gross impairments in the execution of motor acts that are necessary for lever pressing. DA depletions in ventrolateral neostriatum induce impairments in various markers of forepaw motor control, including grasping, forepaw usage during feeding, feeding rate, and lever press response duration (Salamone et al. 1993; Cousins and Salamone 1996), however, ventrolateral neostriatal DA depletions do not produce the shift from lever pressing to chow intake (Cousins et al. 1993). Instead, rats with ventrolateral striatal DA depletions show a suppression of both lever pressing and chow intake (Cousins et al. 1993). In contrast, conditions that decrease lever pressing and increase chow intake, such as low doses of DA antagonists or accumbens DA depletions, do not impair forepaw usage during feeding, food handling, or feeding rate (Salamone et al. 1990, 1993). Moreover, injections of DA D1 or D2 family antagonists into nucleus accumbens core and shell that produced the shift from lever pressing to chow intake did not increase lever press response duration (Nowend et al. 2001). These observations, coupled with results obtained from T-maze barrier choice tasks and discounting procedures, are generally interpreted to mean that low-to-moderate doses of DA antagonists and accumbens DA depletions or antagonism are not acting to suppress appetite or alter food preference, are not producing choice impairments that are dependent upon alterations in delay discounting, and are not producing gross impairments in response capacity, but instead are acting on behavioral arousal, activation, or effort-related processes (Salamone et al., 1991, 1997, 2002, 2003, 2005, 2007, 2009, 2010; Salamone and Correa 2002, 2009; Winstanley et al. 2005; Cagniard et al., 2006; Kelley et al., 2005; Baldo and Kelley 2007; Barbano and Cador, 2007; Niv et al., 2007; Phillips et al., 2007; Floresco et al., 2008; Sink et al., 2008; Bardgett et al., 2009; Salamone 2010a,b).
As described above, anatomical studies have demonstrated that adenosine A1 receptors are co-localized with DA D1 receptors in both ventral and dorsal striatal medium spiny neurons (Ferre 1997). Despite this post-synaptic co-localization pattern, the present studies demonstrated that injections of the A1 antagonists DPCPX and CPT failed to reverse the behavioral effects of the DA D1 antagonist ecopipam (SCH 39166). These results were not an artifact of using ecopipam as the D1 antagonist, because similar results were obtained when DPCPX was tested for its effects in rats treated with SCH 23390 (Table 1). In addition, the dose ranges for DPCPX and CPT that were used in the present experiments, though ineffective in terms of reversing the actions of ecopipam and eticlopride, were nevertheless effective in studies using other behaviors (Prediger et al. 2005; Aubel et al. 2007; Maione et al. 2007; Lobato et al. 2008; Karcz-Kubicha 2003; Marston et al, 1998). Of course, this is not to say that drugs that act on A1 and D1 receptors do not interact; there is behavioral evidence of A1/D1 receptor interactions from studies involving DA agonists. For example, CPT enhanced the locomotor stimulation produced by the D1 partial agonist SKF 38393, but not the D2 agonist quinpirole, in DA depleted animals (Popoli et al., 1996). Nevertheless, the present results indicate that systemic A1 antagonism does not reverse the effects of systemic D1 antagonism on operant behavior. There could be several reasons why CPT and DPCPX were unable to reverse the effects of ecopipam. Even though A1 and D1 receptors are co-localized postsynaptically on substance-P containing medium spiny neurons, form heteromeric complexes, and converge onto the same signal transduction pathways (Ferré et al. 1997, 2001, 2008b; Fuxe et al., 2007), it also is true that there are a considerable number of pre-synaptic A1 receptors (Ferre, 2008; Ferré et al. 2008a). Thus, it is possible that some of the presynaptic release-modulating effects of A1 antagonists work against the postsynaptic effects of A1 blockade. Furthermore, it should be emphasized that there is a widespread non-striatal distribution of A1 receptors (Svenningson et al., 1999; Ferré 2008), and that actions on these non-striatal sites could act to cancel out the effects of accumbens A1 receptor antagonism.
In addition to being ineffective at reversing the actions of the D1 antagonist ecopipam, DPCPX and CPT also did not significantly affect lever pressing or chow intake in eticlopride-treated rats. The results with DPCPX are consistent with previous reports showing that this drug failed to reverse the effects of haloperidol on effort-related choice behavior (Mott et al., 2009; Salamone et al., 2009). CPT injected in the dose range of 3.0–12.0 mg/kg did not significantly increase lever pressing or decrease chow intake in eticlopride-treated rats in the main study, and an additional experiment showed that 24.0 mg/kg CPT also was ineffective. However, some eticlopride-treated rats that received 12.0 mg/kg CPT did show increased lever pressing. A similar pattern was reported in a recent paper that studied the effects of CPT and DPCPX on the suppression of locomotor activity induced by eticlopride (Collins et al., submitted). This overall pattern suggests that CPT, unlike DPCPX, may have produced a mild partial reversal of the effects of eticlopride. CPT has lower A1 vs. A2A selectivity than DPCPX (Maemoto et al., 1997), and also can have different behavioral effects (Marston et al., 1998). Nevertheless, the magnitude of the reversal produced by CPT, as measured by the effect size (R2 = 0.10), was still very small.
The only drug that significantly increased lever pressing in rats treated with a DA antagonist was the adenosine A2A antagonist KW-6002. This drug produced a significant but small increase in lever pressing in rats treated with ecopipam, which is consistent with previous results obtained with the adenosine A2A antagonist MSX-3 (Worden et al., 2009). Thus, despite the anatomical data indicating that A2A and D1 receptors are not generally co-localized on the same medium spiny neurons, it appears that A2A antagonism can consistently produce a mild partial reversal of the effects of D1 antagonism. Since adenosine A2A receptors and D1 receptors are relatively segregated on different populations of cells, any interactions between them are likely to involve indirect effects on basal ganglia circuitry, rather than direct actions on the same neuron (Hauber et al., 2001; Pollack and Fink 1996; Worden et al., 2009; Collins et al., in press). The most robust reversal effect observed in the present paper was shown in the experiment with KW-6002 and eticlopride. KW-6002 produced substantial increases in lever pressing and decreases in chow intake in eticlopride-treated rats (Figures 5–6; Table 2). These results are consistent with a growing number of studies demonstrating that adenosine A2A antagonists can reverse the effects of DA D2 antagonists on effort-related choice behavior in studies using the concurrent choice task (Farrar et al., 2007, 2010; Worden et al., 2009; Salamone et al., 2009), and the T-maze barrier task (Mott et al., 2009; Correa et al. 2009). Furthermore, they are part of a much larger body of evidence demonstrating that A2A antagonism can generally reverse the effects of D2 antagonism across a wide range of behavioral contexts, including tasks that involve functions related to ventral and dorsal striatum (Correa et al., 2004; Ishiwari et al., 2007; Salamone et al., 2008a,b; Collins et al. in press). Adenosine A2A receptors are located on enkephalin-positive striatal neurons that also express DA D2 receptors (Schiffman et al., 1991; Fink et al., 1992; Ferré 1997; Svenningsson et al., 1999; Wang et al., 2000; Hettinger et al., 2001; Chen et al., 2001). A2A and D2 receptors are capable of forming hetromers, and also converge onto the same cAMP-related signal transduction pathways (Fink et al., 1992; Ferré 1997; Ferré et al., 1997, 2004, 2008b; Svenningsson 1999; Hillion et al., 2002; Fuxe et al., 2003). Recent evidence indicates that doses of adenosine A2A antagonists that reverse the effects of DA D2 antagonists on tremor and effort-related choice behavior also can reverse the D2-antagonist-induced enhancement of c-Fos expression in neostriatum (Betz et al., 2009) and nucleus accumbens core (Farrar et al., 2010), respectively. These studies provide a potential neural marker of the direct cellular interaction between D2 and adenosine A2A receptors.
CONCLUSION
In summary, the present experiments indicate that, despite the co-localization of adenosine A1 and D1 receptors in striatal medium spiny neurons, the adenosine A1 antagonists DPCPX and CPT failed to reverse the effects of the DA D1 receptor antagonist ecopipam on the concurrent lever pressing/feeding choice task. In general, the effects of the D1 antagonist ecopipam were harder to reverse with adenosine antagonists than the effects of the D2 antagonist eticlopride. Furthermore, the adenosine A2A antagonist KW-6002 produced the most robust effects in rats treated with DA antagonists, inducing a partial reversal of the effects of ecopipam and a nearly complete reversal of the effects of eticlopride. These findings can help to explicate the complex nature of the interactions between adenosine and DA receptor antagonists with different patterns of receptor selectivity, and shed light on the neural circuitry involved in behavioral activation and effort-related choice behavior. Future studies should use additional behavioral methods, including discounting tasks and other discrete-trial procedures, to assess the interaction between DA and adenosine (Bardgett et al. 2009; Floresco et al. 2008; Gan et al. 2010). Moreover, these studies also have potential clinical relevance. In humans, symptoms such as anergia, psychomotor slowing, and fatigue reflect pathologies in behavioral activation. These symptoms are fundamental aspects of depression and other psychiatric and neurological disorders (Tylee et al. 1999; Stahl 2002; Demyttenaere et al. 2005; Salamone et al. 2006, 2007; Yurgelun-Todd et al. 2007; Capuron et al. 2007; Majer et al. 2008). Thus, the present research may contribute to the development of novel treatments for effort-related disorders in humans, and clearly indicate that adenosine A2A antagonists could be useful for attenuating some of the motivational and other behavioral side effects of D2 antagonists (Correa et al. 2004; Salamone et al. 2008a,b; Varty et al., 2008).
Acknowledgments
This work was supported by a grant to J.S. from the National Institute of Mental Health (MH078023), and to Merce Correa from Conselleria de Empresa, Universitat i Ciència. Generalitat Valenciana. (BEST/2009/157).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberman JE, Salamone JD. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Alburges ME, Hunt ME, McQuade RD, Wamsley JK. D1-receptor antagonists: comparison of [3H] SCH39166 to [3H]SCH23390. J Chem Neuroanat. 1992;5:357–366. doi: 10.1016/0891-0618(92)90051-q. [DOI] [PubMed] [Google Scholar]
- Aubel B, Kayser V, Farré A, Hamon M, Bourgoin S. Evidence for adenosine and serotonin-mediated antihyperalgesic effects of cizolirtine in rats suffering from diabetic neuropathy. Neuropharmacology. 2007;52:487–496. doi: 10.1016/j.neuropharm.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Discrete neurochemical coding of distinguishable motivational processes: insights from nucleus accumbens control of feeding. Psychopharmacology. 2007;191:439–459. doi: 10.1007/s00213-007-0741-z. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Sadeghian K, Basso AM, Kelley AE. Effects of selective dopamine D1 or D2 receptor blockade within nucleus accumbens subregions on ingestive behavior and associated motor activity. Behav Brain Res. 2002;137:165–177. doi: 10.1016/s0166-4328(02)00293-0. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Differential regulation of the consummatory, motivational and anticipatory aspects of feeding behavior by dopaminergic and opioidergic drugs. Neuropsychopharmacology. 2006;31:1371–1381. doi: 10.1038/sj.npp.1300908. [DOI] [PubMed] [Google Scholar]
- Barbano MF, Cador M. Opioids for hedonic experience and dopamine to get ready for it. Psychopharmacology (Berl) 2007;191:497–506. doi: 10.1007/s00213-006-0521-1. [DOI] [PubMed] [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123:463–467. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett AC, Miller JR, Dohrmann JM, Caine SB. Effects of dopamine indirect agonists and selective D1-like and D2-like agonists and antagonists on cocaine self-administration and food maintained responding in rats. Neuropharmacology. 2004;47(Suppl 1):256–273. doi: 10.1016/j.neuropharm.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Betz AJ, Vontell R, Valenta J, Worden L, Sink KS, Font L, Correa M, Sager TN, Salamone JD. Effects of the adenosine A2A antagonist KW-6002 (istradefylline) on pimozide-induced oral tremor and striatal c-Fos expression: comparisons with the muscarinic antagonist tropicamide. Neuroscience. 2009;163:97–108. doi: 10.1016/j.neuroscience.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon -α therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Impagnatiello F, Grandy DK, Cuellar B, Rubinstein M, Beilstein MA, Hacket E, Fink JS, Low MJ, Ongini E, Schwarzschild MA. The role of the D2 dopamine receptor (D2R) in A2a adenenosine-receptor (A2aR) mediated behavioral and cellular responses as revealed by A2a and D2 receptor knockout mice. Proc Natl Acad Sci. 2001;98:1970–1975. doi: 10.1073/pnas.98.4.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PG. Effects of SCH39166 and domperidone on the meal patterning of male rats. Pharmacol Biochem Behav. 1995;52:265–270. doi: 10.1016/0091-3057(95)00094-d. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Collins P, Jones SK, Port RG, Paul NE, Hockemeyer J, Müller CE, Salamone JD. Interactions between adenosine and dopamine receptor antagonists with different selectivity profiles: Effects on locomotor activity. Behav Brain Res. 2010;211:148–155. doi: 10.1016/j.bbr.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Correa M, Carlson BB, Wisniecki A, Salamone JD. Nucleus accumbens dopamine and work requirements on interval schedules. Behav Brain Res. 2002;137:179–187. doi: 10.1016/s0166-4328(02)00292-9. [DOI] [PubMed] [Google Scholar]
- Correa M, Wisniecki A, Betz A, Dobson DR, O’Neill MF, O’Neill MJ, Salamone JD. The adenosine A2A antagonist KF17837 reverses the locomotor suppression and tremulous jaw movements induced by haloperidol in rats: possible relevance to parkinsonism. Behav Brain Res. 2004;148:47–54. doi: 10.1016/s0166-4328(03)00178-5. [DOI] [PubMed] [Google Scholar]
- Correa M, Pardo M, Hockemeyer J, Müller CE, Salamone JD. Neuroscience Meeting Planner Program Number 285.6. Chicago, IL: Society for Neuroscience; 2009. Dopamine D2 antagonism increases selection of less effortful food-seeking behavior in a T-maze procedure in mice: reversal of the motivational impairment with adenosine antagonists. Online. [Google Scholar]
- Cousins MS, Salamone JD. Nucleus accumbens dopamine depletions in rats affect relative response allocation in a novel cost/benefit procedure. Pharmacol Biochem Behav. 1994;49:85–91. doi: 10.1016/0091-3057(94)90460-x. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonist, cholinomimetic, sedative and stimulant drugs. Psychopharmacology. 1994;116:529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Atherton A, Turner L, Salamone JD. Nucleus accumbens dopamine depletions alter relative response allocation in a T-maze cost/benefit task. Behav Brain Res. 1996;74:189–197. doi: 10.1016/0166-4328(95)00151-4. [DOI] [PubMed] [Google Scholar]
- DeMet EM, Chicz-DeMet A. Localization of adenosine A2A-receptors in rat brain with [3H]ZM-241385. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:478–481. doi: 10.1007/s00210-002-0613-3. [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8:93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- Denk F, Walton ME, Jennings KA, Sharp T, Rushworth MF, Bannerman DM. Differential involvement of serotonin and dopamine systems in cost-benefit decisions about delay or effort. Psychopharmacology. 2005;179:587–596. doi: 10.1007/s00213-004-2059-4. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology. 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152:321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD. Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166:1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Popoli P, Gimenez-Llort L, Rimondini R, Muller CE, Stromberg I, Ogren SO, Fuxe K. Adenosine/dopamine interaction: implications for the treatment of Parkinson’s disease. Parkinsonism Relat Disord. 2001;7:235–241. doi: 10.1016/s1353-8020(00)00063-8. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Canals M, Marcellino D, Burgueno J, Casado V, Hillion J, Torvinen M, Fanelli F, Benedetti Pd P, Goldberg SR, Bouvier M, Fuxe K, Agnati LF, Lluis C, Franco R, Woods A. Adenosine A2A-dopamine D2 receptor-receptor heteromers. Targets for neuro-psychiatric disorders. Parkinsonism Relat Disord. 2004;10:265–271. doi: 10.1016/j.parkreldis.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Ferré S, Borycz J, Goldberg SR, Hope BT, Morales M, Lluis C, Franco R, Ciruela F, Cunha R. Role of adenosine in the control of homosynaptic plasticity in striatal excitatory synapses. J Integr Neurosci. 2005;4:445–464. doi: 10.1142/s0219635205000987. [DOI] [PubMed] [Google Scholar]
- Ferré S, Ciruela F, Borycz J, Solinas M, Quarta D, Antoniou K, Quiroz C, Justinova Z, Lluis C, Franco R, Goldberg SR. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front Biosci. 2008a;13:2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr Pharm Des. 2008b;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink JS, Weaver DR, Rivkees SA, Peterfreund RA, Pollack AE, Adler EM, Reppert SM. Molecular cloning of the rat A2 adenosine receptor: selective co-expression with D2 dopamine receptors in rat striatum. Brain Res Mol Brain Res. 1992;14:186–195. doi: 10.1016/0169-328x(92)90173-9. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17:251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, Port RG, Salamone JD. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl) 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati LF, Jacobsen K, Hillion J, Canals M, Torvinen M, Tinner-Staines B, Staines W, Rosin D, Terasmaa A, Popoli P, Leo G, Vergoni V, Lluis C, Ciruela F, Franco R, Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61:S19–23. doi: 10.1212/01.wnl.0000095206.44418.5c. [DOI] [PubMed] [Google Scholar]
- Gan JO, Walton ME, Phillips PE. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiology and Behavior. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Hamill S, Trevitt JT, Nowend KL, Carlson BB, Salamone JD. Nucleus accumbens dopamine depletions and time-constrained progressive ratio performance: effects of different ratio requirements. Pharmacol Biochem Behav. 1999;64:21–27. doi: 10.1016/s0091-3057(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Hauber W, Munkle M. Motor depressant effects mediated by dopamine D2 and adenosine A2A receptors in the nucleus accumbens and the caudate-putamen. Eur J Pharmacol. 1997;323:127–131. doi: 10.1016/s0014-2999(97)00040-x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Neuscheler P, Nagel J, Muller CE. Catalepsy induced by a blockade of dopamine D1 or D2 receptors was reversed by a concomitant blockade of adenosine A(2A) receptors in the caudate-putamen of rats. Eur J Neurosci. 2001;14:1287–1293. doi: 10.1046/j.0953-816x.2001.01759.x. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cort. 2009;19:2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- Hettinger BD, Lee A, Linden J, Rosin DL. Ultrastructural localization of adenosine A2A receptors suggests multiple cellular sites for modulation of GABAergic neurons in rat striatum. J Comp Neurol. 2001;431:331–346. doi: 10.1002/1096-9861(20010312)431:3<331::aid-cne1074>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hillion J, Canals M, Torvinen M, Casado V, Scott R, Terasmaa A, Hansson A, Watson S, Olah ME, Mallol J, Canela EI, Zoli M, Agnati LF, Ibanez CF, Lluis C, Franco R, Ferre S, Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002;277:18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Weber SM, Mingote S, Correa M, Salamone JD. Accumbens dopamine and the regulation of effort in food-seeking behavior: modulation of work output by different ratio or force requirements. Behav Brain Res. 2004;151:83–91. doi: 10.1016/j.bbr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Ishiwari K, Madson LJ, Farrar AM, Mingote SM, Valenta JP, DiGianvittorio MD, Frank LE, Correa M, Hockemeyer J, Muller C, Salamone JD. Injections of the selective adenosine A2A antagonist MSX-3 into the nucleus accumbens core attenuate the locomotor suppression induced by haloperidol in rats. Behav Brain Res. 2007;178:190–199. doi: 10.1016/j.bbr.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Williams M. Direct autoradiographic localization of adenosine A2 receptors in the rat brain using the A2-selective agonist, [3H]CGS 21680. Eur J Pharmacol. 1989;168:243–246. doi: 10.1016/0014-2999(89)90571-2. [DOI] [PubMed] [Google Scholar]
- Jenner P. Dopamine agonists, receptor selectivity and dyskinesia induction in Parkinson’s disease. Curr Opin Neurol. 2003;16(Suppl 1):S3–7. doi: 10.1097/00019052-200312001-00002. [DOI] [PubMed] [Google Scholar]
- Jenner P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin Investig Drugs. 2005;14:729–738. doi: 10.1517/13543784.14.6.729. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, Pezzola A, Reggio R, Müller CE, Fuxe K, Goldberg SR, Popoli P, Ferré S. Involvment of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–795. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: a researchers handbook. Englewood Cliffs, NJ: Prentice-Hall; 1991. [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens dopamine D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Lobato KR, Binfaré RW, Budni J, Rosa AO, Santos AR, Rodrigues AL. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:994–999. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Maione S, de Novellis V, Cappellacci L, Palazzo E, Vita D, Luongo L, Stella L, Franchetti P, Marabese I, Rossi F, Grifantini M. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–292. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Maemoto T, Finlayson K, Olverman HJ, Akahane A, Horton RW, Butcher SP. Species differences in brain adenosine A1 receptor pharmacology revealed by use of xanthine and pyrazolopyridine based antagonists. Br J Pharmacol. 1997;122:1202–1208. doi: 10.1038/sj.bjp.0701465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–880. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston HM, Finlayson K, Maemoto T, Olverman HJ, Akahane A, Sharkey J, Butcher SP. Pharmacological characterization of a simple behavioral response mediated selectively by central adenosine A1 receptors, using in vivo and in vitro techniques. J Pharmacol Exp Ther. 1998;285:1023–1030. [PubMed] [Google Scholar]
- McCullough LD, Salamone JD. Involvement of nucleus accumbens dopamine in the motor activity induced by periodic food presentation: a microdialysis and behavioral study. Brain Res. 1992;592:29–36. doi: 10.1016/0006-8993(92)91654-w. [DOI] [PubMed] [Google Scholar]
- Mingote S, Weber SM, Ishiwari K, Correa M, Salamone JD. Ratio and time requirements on operant schedules: effort-related effects of nucleus accumbens dopamine depletions. Eur J Neurosci. 2005;21:1749–1757. doi: 10.1111/j.1460-9568.2005.03972.x. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli M, Pinna A. Interaction between dopamine and adenosine A2A receptors as a basis for the treatment of Parkinson’s disease. Neurol Sci. 2002;22:71–72. doi: 10.1007/s100720170052. [DOI] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Muller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology. 2009;204:103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology. 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A2A antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology. 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Walton ME, Jhou TC. Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology. 2007;191:483–495. doi: 10.1007/s00213-006-0626-6. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Simola N, Morelli M. New therapies for the treatment of Parkinson’s disease: adenosine A2A receptor antagonists. Life Sci. 2005;77:3259–3267. doi: 10.1016/j.lfs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Pollack AE, Fink JS. Synergistic interaction between an adenosine antagonist and a D1 dopamine agonist on rotational behavior and striatal c-Fos induction in 6-hydroxydopamine-lesioned rats. Brain research. 1996;743:124–130. doi: 10.1016/s0006-8993(96)01036-0. [DOI] [PubMed] [Google Scholar]
- Popoli P, Giménez-Llort L, Pezzola A, Reggio R, Martínez E, Fuxe K, Ferré S. Adenosine A1 receptor blockade selectively potentiates the motor effects induced by dopamine D1 receptor stimulation in rodents. Neuroscience Letters. 1996;218:209. doi: 10.1016/s0304-3940(96)13143-8. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Fernandes D, Takahashi RN. Blockade of adenosine A2A receptors reverses short-term social memory impairments in spontaneously hypertensive rats. Behav Brain Res. 2005;159:197–205. doi: 10.1016/j.bbr.2004.10.017. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Bulloch GC. Self-starvation and rewarding brain stimulation: effects of chlorpromazine and pentobarbital. Learn Motiv. 1971;2:83–94. [Google Scholar]
- Salamone JD. Different effects of haloperidol and extinction on instrumental behaviours. Psychopharmacology (Berl) 1986;88:18–23. doi: 10.1007/BF00310507. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Dopaminergic involvement in activational aspects of motivation: effects of haloperidol on schedule induced activity, feeding and foraging in rats. Psychobiology. 1988;16:196–206. [Google Scholar]
- Salamone JD. Behavioral pharmacology of dopamine systems: A new synthesis. In: Willner P, Scheel-Kruger J, editors. The Mesolimbic Dopamine System: From Motivation to Action. Cambridge, England: Cambridge University Press; 1991. pp. 599–613. [Google Scholar]
- Salamone JD. Complex motor and sensorimotor functions of striatal and accumbens dopamine: involvement in instrumental behavior processes. Psychopharmacology. 1992;107:160–174. doi: 10.1007/BF02245133. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Involvement of nucleus accumbens dopamine in behavioral activation and effort-related functions. In: Iversen LL, Iversen SD, Dunnett SB, Bjorklund A, editors. Dopamine Handbook. Oxford: Oxford University Press; 2010a. pp. 286–300. [Google Scholar]
- Salamone JD. Motor function and motivation. In: Koob G, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 3. Oxford: Academic Press; 2010b. pp. 267–272. [Google Scholar]
- Salamone JD, Correa M. Motivational views of reinforcement: implications forunderstanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite. 2009;53:422–425. doi: 10.1016/j.appet.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience. 1990;39:17–24. doi: 10.1016/0306-4522(90)90218-s. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Mahan K, Rogers S. Ventrolateral striatal dopamine depletions impair feeding and food handling in rats. Pharmacol Biochem Behav. 1993;44:605–610. doi: 10.1016/0091-3057(93)90174-r. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Bucher S. Anhedonia or anergia? Effects of haloperidol and nucleus accumbens dopamine depletion on instrumental response selection in a T-maze cost/benefit procedure. Behav Brain Res. 1994;65:221–229. doi: 10.1016/0166-4328(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology (Berl) 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. Dopamine antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus Accumbens Dopamine and the forebrain circuitry involved in behavioral activation and effort- related decision making: implications for understanding anergia and psychomotor slowing in depression. Current Psychiatry Reviews. 2006;2:267–280. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Ishiwari K, Betz AJ, Farrar AM, Mingote SM, Font L, Hockemeyer J, Muller CE, Correa M. Dopamine/adenosine interactions related to locomotion and tremor in animal models: possible relevance to parkinsonism. Parkinsonism Relat Disord. 2008a;14(Suppl 2):S130–134. doi: 10.1016/j.parkreldis.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Betz AJ, Ishiwari K, Felsted J, Madson L, Mirante B, Clark K, Font L, Korbey S, Sager TN, Hockemeyer J, Muller CE. Tremorolytic effects of adenosine A2A antagonists: implications for parkinsonism. Front Biosci. 2008b;13:3594–3605. doi: 10.2741/2952. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, Collins LE, Sager TN. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of dopamine D2 antagonism. Behav Brain Res. 2009;201:216–222. doi: 10.1016/j.bbr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Pardo M. Dopamine, behavioral economics, and effort. Front Behav Neurosci. 2009;3:13. doi: 10.3389/neuro.08.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Collins LE. The role of dopamine/adenosine interactions in the brain circuitry regulating effort-related decision making: Insights into pathological aspects of motivation. Future Neurology. 2010 (in press) [Google Scholar]
- Schiffmann SN, Jacobs O, Vanderhaeghen JJ. Striatal restricted adenosine A2 receptor (RDC8) is expressed by enkephalin but not by substance P neurons: an in situ hybridization histochemistry study. J Neurochem. 1991;57:1062–1067. doi: 10.1111/j.1471-4159.1991.tb08257.x. [DOI] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196:565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Salamone JD. The role of accumbens dopamine in lever pressing and response allocation: effects of 6-OHDA injected into core and dorsomedial shell. Pharmacol Biochem Behav. 1998;59:557–566. doi: 10.1016/s0091-3057(97)00544-3. [DOI] [PubMed] [Google Scholar]
- Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiat. 2002;63:7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Le Moine C, Fisone G, Fredholm BB. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Takahashi RN, Pamplona FA, Prediger RD. Adenosine receptor antagonists for cognitive dysfunction: a review of animal studies. Front Biosci. 2008;13:2614–2632. doi: 10.2741/2870. [DOI] [PubMed] [Google Scholar]
- Terry P, Katz JL. A comparison of the effects of the D1 receptor antagonists SCH 23390 and SCH 39166 on suppression of feeding behavior by the D1 agonist SKF38393. Psychopharmacology. 1994;113:328–333. doi: 10.1007/BF02245205. [DOI] [PubMed] [Google Scholar]
- Trevitt J, Vallance C, Harris A, Goode T. Adenosine antagonists reverse the cataleptic effects of haloperidol: Implications for the treatment of Parkinson’s disease. Pharmacol Biochem Behav. 2009;92:521–527. doi: 10.1016/j.pbb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. Int Clin Psychopharmacol. 1999;14:139–151. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- van den Bos R, van der Harst J, Jonkman S, Schilders M, Spruijt B. Rats assess costs and benefits according to an internal standard. Behav Brain Res. 2006;171:350–354. doi: 10.1016/j.bbr.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hodgson RA, Pond AJ, Grzelak ME, Parker EM, Hunter JC. The effects of adenosine A2A receptor antagonists on haloperidol-induced movement disorders in primates. Psychpharmacology. 2008;200:393–401. doi: 10.1007/s00213-008-1214-8. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi KT, Fields HL, Nicola SM. Dissociation of the role of nucleus accumbens dopamine in responding to reward-predictive cues and waiting for reward. Behav Brain Res. 2004;154:19–30. doi: 10.1016/j.bbr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Rushworth MF. The role of rat medial frontal cortex in effort-based decision making. J Neurosci. 2002;22:10996–11003. doi: 10.1523/JNEUROSCI.22-24-10996.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MF. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WF, Ishiwata K, Nonaka H, Ishii S, Kiyosawa M, Shimada J, Suzuki F, Senda M. Carbon-11-labeled KF21213: a highly selective ligand for mapping CNS adenosine A(2A) receptors with positron emission tomography. Nucl Med Biol. 2000;27:541–546. doi: 10.1016/s0969-8051(00)00126-8. [DOI] [PubMed] [Google Scholar]
- Wardas J, Konieczny J, Lorenc-Koci E. SCH 58261, an A(2A) adenosine receptor antagonist, counteracts parkinsonian-like muscle rigidity in rats. Synapse. 2001;41:160–171. doi: 10.1002/syn.1070. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Muller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology (Berl) 2009;203:489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Sava S, Dahlgren MK. Mood disorders. Neuroimaging Clin N Am. 2007;17:511–521. doi: 10.1016/j.nic.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, GABaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]


