Abstract
There is a growing appreciation for the role for B cells in autoimmune disorders in which inflammation is driven by T cells, in addition to the well-established role for B cells in autoimmune disorders characterized by pathogenic auto-antibodies. Current information on tolerance checkpoints in B cells, B cell depletion, BAFF blockade, regulatory B cells and clonal ignorance mediated by the SIAE/Siglec pathway will be reviewed.
The mechanisms of immune mediated injury have long provided a basis for categorizing most autoimmune disorders into two broad sets of diseases – one set of disorders in which inflammation is driven by T cells and another group of conditions in which auto-antibodies play a key role, either by binding to tissue antigens or by forming immune complexes. This approach to segregating autoimmune disorders into T cell mediated and so-called B cell mediated diseases has eroded quite dramatically in recent years.
It is now recognized that T cell help for B cells during adaptive immune responses is reciprocated by a critical role for B cell help during CD4+T cell activation, as discussed below. While auto-antibody linked diseases such as systemic lupus erythematosus, myasthenia gravis, and Goodpasture’s syndrome, among many others, likely involve a break in tolerance in antigen-specific B cells, an important role for T cells in this category of diseases has also long been recognized. Most disease related auto-antibodies are IgGs that are somatically mutated suggesting that helper T cells drive the autoimmune B cell response [1]. More recently it has also been recognized that B cells play important roles in inflammatory conditions such as rheumatoid arthritis, multiple sclerosis, and type I diabetes, disorders that have long been considered to be mediated primarily by T cells. It is clear that in most autoimmune disorders cells of both lymphocyte lineages cooperate closely in disease pathogenesis.
Although studies on the role of B cells in autoimmunity have focused primarily on the mechanisms of B cell tolerance and how tolerance may be abrogated in auto-antibody mediated diseases, it is possible that a loss of B cell tolerance might occur in almost all autoimmune disorders. In diseases in which specific autoimmune T cell clones drive the process of inflammation, auto-antibody production may represent a marker for the expansion of auto-antigen specific B cells that capture and present self-antigen peptides to inflammatory T cells. In other disorders, T cell help appears to be a crucial component in driving self-reactive B cells to make pathogenic auto-antibodies.
In this review we will first attempt to provide an overview of what is known regarding B cell tolerance checkpoints during development and in germinal centers, both in rodents and humans, and discuss the postulated role of B regulatory cells in tolerance and autoimmunity. We will then consider why B cell depletion facilitates the remission of some autoimmune disorders; in this context we will examine the role of B cells in antigen presentation and the induction of CD4+ T cell memory and/or effectors in the context of autoimmunity. We will finally consider the phenomenon of clonal ignorance in B cells mediated by the Siglec/SIAE inhibitory pathway.
Tolerance checkpoints during B cell development – lessons from rodents
B cell tolerance has been studied extensively using BCR transgenic and knockin mice as well as mice in which critical recognition or signaling molecules of developmental relevance have been mutated.
A major tolerance checkpoint in the bone marrow occurs just after small pre-B cells (surface IgM negative cells that are actively rearranging at the κ light chain locus) transition into the immature B cell stage. Immature B cells are the first cells to express the BCR which at this stage is made up the of μ heavy chain (and not the δ chain), a newly rearranged and synthesized κ light chain (not λ, which is rearranged later) and an Igα/Igβ heterodimer. For multivalent antigens such as membrane proteins, membrane glycolipids and nucleic acids, this checkpoint is mediated primarily by the process of receptor editing wherein immature B cells that recognize self-antigens with high avidity are apparently induced to revert to a pre-B cell like phenotype, express Rag genes and induce additional κ light chain gene rearrangements [2–7]. Novel Vκ-Jκ rearrangements, involving upstream V segments and downstream J segments, delete the original Vκ-Jκ rearrangement that had produced a self-reactive light chain. The new rearrangement may be out-of- frame, or could be in-frame but perhaps still produce a κ light chain that is self-reactive. Continuing BCR signaling because of self-reactivity at this stage presumably results in ongoing receptor editing on both κ light chain chromosomes, followed by rearrangements at the λ light chain gene locus, accompanied by deletion of the κ loci. The presence of a heptamer (CACAGTG) containing RS (recombining sequence) element downstream of Cκ [8] permits the deletion of the κ locus during receptor editing and appears to be crucial for the induction of λ light chain gene rearrangement [9]. RS element knockout mice have both tolerance defects and defective Igλ gene rearrangement [9]. In humans the analogous sequence similar to the RS element is called the κ deleting element or KDE [10]. An assay for receptor editing has been developed that identifies the frequency of RS/KDE recombination events in B cells [11]. This assay has revealed defects in receptor editing in human subjects with lupus and type I diabetes.
While multivalent antigens appear to primarily mediate receptor editing, soluble antigens mainly induce anergy [12,13]. Some deletion of self- reactive B cells also probably occurs in immature and transitional B cells in vivo. Evidence for deletion and anergy was originally obtained from pioneering and elegant studies [12,14–16] employing conventional BCR transgenic models. More “physiological” BCR knockin models however provide strong evidence supporting the view that receptor editing rather than deletion is the dominant mechanism of tolerance induction by multivalent antigens in developing B cells [4–7]. In BCR knockin models rearranged Ig heavy and κ light chain genes are inserted respectively into the endogenous heavy chain locus on mouse chromosome 12 and the κ light chain locus on mouse chromosome 6, and not into random sites on other chromosomes. It is therefore possible for receptor editing at the κ locus to delete the original self-reactive Vκ-Jκ exon that had been inserted in the correct position in the κ locus. In contrast, in conventional BCR transgenic mice, the “offending” self-reactive light chain is expressed by a transgene inserted in some random chromosomal location and this cannot be deleted by a new Vκ-Jκ rearrangement on chromosome 6. In such situations the self-reactive light chain persists even if receptor editing does occur, and continuing signaling might result in cell death. Indeed while the original conventional BCR transgenic studies using membrane bound hen egg lysozyme and MHC class I as self antigens revealed an important role for deletion, knockin BCR experiments with the same antigens revealed that tolerance was mediated primarily by receptor editing [5,7].
Clonal deletion may occur at some frequency in B cells that recognize multivalent self antigens even though receptor editing may well be the dominant mechanism. Deletion may be crucial when all editing options are exhausted, and may be the major mechanism that drives pre-BCR censoring (see below) when most of the self-recognition is mediated by the heavy chain alone. It could be argued that multivalent antigens that do not have access to the bone marrow but which can encounter transitional B cells in the spleen might mediate deletional tolerance since receptor editing is no longer an option. There is no evidence at present to support this speculation. The relevance of deletion as a central tolerance mechanism receives support from studies in which transgenic expression of Bcl-2 preserves self-reactive B cells beyond those tolerized by receptor editing [9].
The earliest tolerance related checkpoint during B cell development may occur at the pro-B to pre-B cell transition and is a phenomenon known as pre-BCR censoring [17]. Assembly of the pre-BCR results in positive selection of pre-B cells in which heavy chain gene rearrangement has been productive and signals are generated in a ligand independent fashion [18]. In contrast, during pre-BCR censoring, it is possible that specific self-ligands do interact with some pre-BCRs. In surrogate light chain deficient mice, some autoreactive Ig heavy chains are found in increased frequency in the periphery and auto-antibodies are generated [17]. Some pre-B cells that express potentially self-reactive Ig heavy chains are culled by a poorly understood mechanism, possibly involving self-antigen recognition by the pre-BCR coupled to deletion. It is difficult to assess exactly how important pre-BCR censoring is in terms of tolerance. Indeed it has been shown that pre-BCR signaling resembles the analogous process initiated by auto-reactive BCRs: both categories of receptors initiate light chain gene rearrangement, primary light chain gene rearrangement in the case of the pre-BCR and secondary rearrangements, as discussed above, when signals are initiated by autoreactive BCRs [19].
Immature B cells migrate from the bone marrow to the spleen as part of the maturation process although some maturation can continue in the bone marrow as well [20,21]. Splenic cells with a phenotype that resembles that of immature B cells (IgM+IgD− CD23− CD21loCD93hi CD24hi) are recent emigrants from the bone marrow that are assumed to have not yet entered the follicular niche and are called T1 cells. Splenic B cells that retain markers of recent generation (CD93hiCD24hi B cells) and which are CD23+ begin to express IgD (they can be IgD− or IgD+), are called T-2 transitional B cells and represent recent emigrants that have entered the follicular niche and have acquired the ability to recirculate. T-1 and T-2 B cells that encounter self-antigen that is multivalent may be clonally deleted. Immature, T-1, and T-2 B cells that are repeatedly stimulated by high affinity self antigen (likely monovalent protein antigens for the most part) may be anergized and acquire an IgDhiIgMloCD93+ T-3 B cell phenotype. Most transitional cells however are not as strongly self-reactive as cells that will be deleted or anergized and these less obviously self-reactive B cells differentiate either into long-lived follicular B cells or into MZ B cells. Long-lived recirculating follicular B cells can be divided into IgDhiIgMhiCD93− CD21int FO-II cells that do not require self- antigen and Btk during ontogeny and IgDhiIgMloCD93− FO-I B cells that require Btk for their development [22,23]. The possible role of BAFF in regulating anergy and deletion will be discussed in a subsequent section.
In rodents splenic B cells that are IgMhiIgDhiCD21hiCD1dhi are categorized as marginal zone precursor B cells or MZP cells. Mutations that block MZ B cell development almost always result in the loss of MZP cells [24]. Although MZP B cells have sometimes been called T2-MZP cells (and we are responsible in part for this older terminology, [25]), it is more appropriate to define T2 cells as a distinct population as described above. In in vitro stimulation studies MZP cells are capable of making high levels of IL-10, have been called B regulatory cells, and in certain models these cells can suppress the disease process when introduced into recipient mice. B regulatory cells will also be discussed separately below.
B cell tolerance in the context of the germinal center reaction
Epitopes on the surface of microbial protein antigens are recognized by specific B cells in the follicle and the B cell activation that results typically does not suffice to induce cell cycle progression but can contribute to endocytosis via the BCR and initiate the expression of CCR7 on these B cells that can now begin to migrate out of the follicle towards the T cell zone. The same protein antigen when initially internalized and presented by activated dendritic cells results in specific CD4+ T cell activation and expansion, induction of CXCR5 expression on these T cells and their migration towards the B cell zone. CD4+ T cell mediated activation of specific B cells via CD40L –CD40 interactions induces B cell activation and expansion initially at extra-follicular sites. Activated B cells in turn can trigger activated CD4+ T cells, in part via ICOSL-ICOS interactions to differentiate into T follicular helper (TFH) cells (reviewed in [26,27]). Interactions between activated B cells and activated CD4+ T cells in the inter-follicular zone can be prominently observed using multi-photon microscopy and B-T interactions are important beyond TFH cell differentiation for many CD4+ T cell responses as will be discussed below.
TFH cells express high levels of CXCR5, migrate into the follicle and help set up the environment for germinal center formation [26,28,29]. Activated B cells undergo rapid proliferation and somatic mutation in the dark zone of the germinal center [30,31] and in the light zone antigen bound to FDCs may be captured by high affinity B cells which may then be able to present peptides to TFH cells, receive help and survive [26,27,32].
IL-21 secreted by TFH cells may contribute to the high levels of Fas and the low levels of Bim expressed by GC B cells which might in turn render these cells prone to apoptotic death by both the mitochondrial pathway and death receptor signaling. It is possible that high affinity B cells may be induced to express c-FLIP, an antagonist of caspase-8, either though the BCR or because they more readily capture and present antigen in order to receive T cell help. These cells may thus not be killed by FasL expressed on TFH cells resulting in the predominance of CD40 mediated survival, proliferation and differentiation signals. Some regulation of B cells in the germinal center may be mediated by a subset of regulatory T cells that resemble TFH cells and express CXCR5 [33]. Whether these T follicular regulatory cells contribute to self-tolerance in the germinal center reaction remains to be determined. There are two broad issues to be considered in the context of B cell tolerance in the germinal center reaction.
Firstly, in responses to a microbial/foreign antigen, somatic hypermutation may cause a B cell clone that was specific for a foreign epitope to mutate towards specificity for a self antigen. Such a self antigen, being ubiquitous, could potentially select a self-reactive B cell in the light zone and contribute to its expansion. How are such self-reactive B cells tolerized? It could be argued that if TFH derived signals are essential for B cell selection and survival in the light zone, most often self-antigens will not yield peptides that can be recognized by TFH cells and help will not be provided to autoimmune clones. It is possible that on occasion the very rare self-reactive B cell generated by somatic hypermutation is specific for a self –antigen that can complex with a foreign antigen and thus break tolerance. How exactly such a scenario may be avoided is unclear. Single cell cloning studies have revealed a tolerance checkpoint between the germinal center B cell and plasma cell stages [34] that is regulated in part by FcγRIIb. In the absence of FcγRIIb an accumulation of self-reactive GC B cells synthesizing somatically mutated IgGs was observed suggesting that this inhibitory receptor contributes to the elimination of strongly self-reactive GC B cells [34]. It is possible that IgG immune complexes containing low affinity self antigens produce relatively weak BCR signals that are overpowered by negative signals from FcγRIIb, leading to deletion of self-reactive B cells. Alternatively perhaps inhibitory signaling through FcγRIIb that is induced by IgG immune complexes must be overcome by positive signals received from a TFH cell in order for a GC B cell to survive in the light zone; self-reactive B cells presumably fail to receive the requisite T cell help to survive.
The second issue relates to weakly self-reactive B cells that are not of sufficient affinity for self to be tolerized by receptor editing, deletion, or anergy. Such clones may sometimes be activated by self-antigen complexed with a microbial protein. Such a scenario could result in T-B collaboration and somatic mutation. How tolerance may be regulated in such a scenario will be discussed in a later section on clonal ignorance in B cells.
B cell tolerance checkpoints in humans
Studies on tolerance checkpoints in humans have typically depended on an approach originally developed in the Nussenzweig laboratory that involves single cell cloning of B cells at different stages of development or activation, expression of matched Ig heavy and light chain genes in non-lymphoid cells and assays for self-reactivity or poly-reactivity of the expressed antibodies [35–37]. Using such an approach it has been shown that “Early Immature B cells” – phenotypically surface IgM negative pre-B cells in the bone marrow that are actively rearranging their κ light chain genes – contain rearranged μ heavy chain and κ light chain genes that are about 75% self-reactive, many encoding anti-nuclear antibodies. These early immature B cells also have an enhanced frequency of polyreactive B cells, containing immunoglobulins that can bind to structurally diverse antigen such as DNA, LPS and some protein antigens including insulin. There is a significant drop in self-reactive and polyreactive B cells at the immature B cell stage. This checkpoint is sometimes referred to as the “central” checkpoint and most likely reflects ongoing receptor editing.
A second “peripheral” checkpoint has been described at which a loss of autoreactive B cells is observed when the frequencies of self-reactive B cells at the transitional B cell stage and in mature B cells are compared. Based on analogies with tolerance mechanisms in mice it would be reasonable to speculate that this second checkpoint reflects B cell deletion at the transitional B cell stage or anergy, or both. Individuals lacking MHC class II (the bare lymphocyte syndrome) or with CD40L defects causing the hyper IgM syndrome have a defect at this checkpoint. It has been suggested that naïve B cells present self-antigen to T cells, likely T regulatory cells, and that these Tregs specifically suppress the antigen presenting self-reactive B cells [38]. Defects in the central checkpoint as well as in the peripheral checkpoint have been observed in subjects with rheumatoid arthritis and systemic lupus erythematosus [39–42].
A third potential checkpoint has been observed in human subjects. A significant decrease in autoreactive B cells is seen between naïve B cells and IgM+CD27+ B cells – also known as IgM memory B cells or marginal zone B cells. This population of cells lacks self-reactive or polyreactive B cell receptors [43]. The mechanisms that regulate this third checkpoint are not understood.
In human subjects the IgG+CD27+ presumed class switched memory B cell population exhibits both some auto-reactivity as well as some polyreactivity even in healthy subjects, suggesting that a break in tolerance occurs during the germinal center reaction [44]. Since this polyreactivity is observed in healthy subjects it is likely that these self-reactive IgGs are not pathogenic.
Regulatory B cells
An inflammatory colitis seen in TCRα knockout mice was shown to be exacerbated in the absence of B cells and ameliorated by the introduction of wild type B cells in transfer experiments [45]. IL-10 producing CD1dhi B cells were found to accumulate in the intestine and to suppress inflammation suggesting that a CD1dhi subset of B cells may represent regulatory B cells [46]. A similar regulatory role for IL-10 producing B cells was described in a collagen induced arthritis model [47] and in EAE models [48–50].
The in vivo source of regulatory B cells is unclear and the existence of a single defined population in an in vivo setting remains to be established. Many B cell populations, including B-1 B cells [51] and MZ B cells [52,53] have been suggested to possess regulatory properties, but two independently described but likely overlapping populations have been most thoroughly examined. IgMhiIgDhiCD21hiCD1dhi splenic T2-MZP B cells and CD5hiCD1dhi splenic B cells are the best studied putative regulatory B cell populations [54,55]. Both these CD1dhi populations secrete IL-10 when stimulated. Transfer of putative regulatory B cells (Bregs) with an MZP B cell phenotype (that are capable of secreting IL-10 upon activation in vitro) into mice with collagen arthritis and other inflammatory models have been shown to reduce inflammation [54]. In the arthritis model, transfer of in vitro activated MZP/Breg cells that are capable of secreting IL-10 results in the induction of FoxP3 expressing Tregs and a reduction in Th1 and Th17 CD4+ T cells [56]. Suppression of inflammation by Bregs may however also be observed in mice that lack Tregs, and the exact mechanism of Breg action remains unclear. Studies involving B lineage specific deletion of MyD88 indicate that Bregs may be activated via TLR ligands in order to suppress other immune cells that are activated via TLRs [57].
There is some indirect evidence that Bregs might be relevant in human disease. Exacerbation of ulcerative colitis has been observed in subjects in whom B cells have been depleted with Rituxumab and colitis has been induced in subjects receiving Rituximab for lymphoma and Graves disease [58–61]. These and other case reports suggest that Bregs that dominantly inhibit intestinal inflammation might have been depleted resulting in an ulcerative colitis like presentation. Human CD19+CD10+CD38+ B cells, that correspond to a transitional B cell population [62,63] can be induced in vitro to make IL-10 and to suppress T cell responses [64]. Cells with the same phenotype from lupus subjects were less efficient at suppression arguing that a defect in Breg function might be relevant in the pathogenesis of this disease [64]. A CD24hiCD27+ human B cell population that can be induced to secrete IL-10 in vitro has also been identified as a putative Breg population [65]. The relationship between these two putative human Breg populations remains to be determined.
Significant gaps remain in our appreciation of the in vivo role of Bregs. Bregs, as commonly defined, overlap with cells that are precursors of marginal zone B cells. The absence of MZP B cells is always correlated with an absence of MZ B cells. Mice with engineered genetic mutations in which MZP and MZ B cells are absent do not present with florid autoimmunity - certainly any comparison of relevance in a functional sense with Tregs would be premature. Detailed studies of Breg function in genetic mutants that cannot make MZP and MZ B cells need to undertaken.
While mice lacking B cells (μMT mice) have been reconstituted with mixtures of IL-10−/− and μMT bone marrow to study the role of IL-10 secreting B cells in vivo [56], conditional deletion of IL-10 in B cells, and eventually, when the technology becomes available, in defined subsets of B cells should be undertaken to further understand the in vivo relevance of IL-10 secretion by B cells. Indeed while evidence for cytokine secretion by B cells activated in vitro is strong [66], in vivo evidence for the relevance of B cell mediated cytokine secretion must be ascertained by B cell specific conditional knockout studies, and this is still lacking.
B cell help for CD4+ effector and memory T cell generation
As mentioned earlier, in the first day or two after immunization with a protein antigen, multiphoton microscopy reveals a large influx of activated helper T cells into the inter-follicular area [67]. A number of studies have established that B cells are required for the generation of CD4+ memory cells in most antigenic contexts with some increasingly rare exceptions [68–70]. It appears therefore that although dendritic cells are the key antigen presenting cells for the initiation of CD4+ T cell activation, B cells are required for subsequent activation of the CD4+ T cells [71], particularly for memory generation. Although evidence for an antigen presentation function for B cells was not observed in earlier studies, it is possible that B cells that have expanded in response to T cell help via CD40 activation, present antigen to T cells during CD4+ memory generation. CD4+ effector and memory cells that drive the pathological changes in specific autoimmune disorders may therefore require B cells for their generation.
Lessons from Rituximab and Bemilumab: revisiting BAFF dependent tolerance checkpoints
CD20 is expressed on mature B cells and memory cells of the B lineage but not on long-lived plasma cells. Clinical trials have established that B cell depletion using Rituximab (anti-CD20) significantly ameliorates the disease process in a large number of autoimmune and chronic inflammatory diseases [72–74]. Its efficacy in diseases in which autoantibodies are not though to play a key role (multiple sclerosis and type I diabetes for instance) may reflect the importance of B cells as APCs and their role in sustaining CD4+ effector and memory responses. It has been shown that remission in certain diseases can be linked to a striking reduction in B cells so it is perhaps less likely, as it has been sometimes argued, that an increase in regulatory B cells seen during the early phases of bone marrow recovery contributes to a suppression of disease symptoms [62]. In subjects with systemic lupus erythematosus Rituximab has been of variable efficacy. One possible explanation may be linked to the role of BCR – TLR collaboration during the process of lupus B cell differentiation [75]. In some autoimmune states autoantibodies arise from short-lived plasma cells in extra-follicular foci perhaps because auto-antigens generally lack the ability to provide conventional costimulatory stimuli [76]. Since lupus pathogenesis is driven by chromatin derived autoantigens that can both engage the BCR and activate endosomal TLRs, auto-antigen specific B cell differentiation into long-lived plasma cells may occur more readily in this disease than in many other auto-immune disorders. Since Rituximab cannot typically deplete these long-lived cells, the secretion of pathogenic auto-antibodies might not be attenuated by this biological therapy.
T cell responses have been monitored in Rituximab-treated patients with pemphigus vulgaris (PV). Although B cell depletion had no effect on the total T cell pool in these subjects, the frequency of desmoglein-specific autoreactive T cells in peripheral blood that produced interferon-γ (IFNγ) or interleukin-4 (IL-4) declined significantly following B cell depletion. In addition a reduction in desmoglein specific autoantibodies also accompanied the decrease in desmoglein-reactive T cells [77]. Similar results were obtained in patients with immune thrombocytopenic purpura (ITP); although autoantibodies contribute largely to the pathogenesis of ITP, disease subjects also present with predominantly monoclonal or pauciclonal T cell repertoires that are skewed towards the production of TH1 type cytokines like IFNγ [78]. These T cells from ITP patients express higher levels of Bcl-2 and lower levels of BAX, suggesting the development of a memory or memory-like phenotype. These T cell changes were reversed in ITP subjects who responded positively to B cell depletion therapy.
Rituximab treatment has also been reported to promote the expansion of Treg populations [79,80]. These results have been reproduced in autoimmune mouse models. When B cells were depleted before the onset of disease in NOD mice expressing a human CD20-transgene the numbers of Foxp3+ T cells increased, and CD4+ T cells from these animals suppressed diabetes in adoptive transfer experiments [81].
BAFF/BLyS is a TNF family protein that functions as a growth factor for B cells after the T-1 stage of differentiation, essentially in the follicular milieu. Its receptor on developing B cells is BAFF-R [82]. BAFF and a related protein, APRIL, can interact with two other receptors, TACI and BCMA, not considered further in this review. BAFF is also required for the differentiation in the spleen of transitional and FO-II cells into MZP B cells and MZ B cells. High level overexpression of BAFF in transgenic mice results in a lupus like disorder, and it has been shown in transgenic BCR models that engineered high levels of BAFF can prevent the death of cells that would be otherwise anergized or deleted by self-antigen encounter [83,84]. This has led to the view that high levels of BAFF can contribute to autoimmune disease pathogenesis by preventing the tolerization of self-reactive B cells thus allowing them to enter B follicles and survive [85]. Subjects with lupus and Sjogren’s syndrome have elevated levels of BAFF but these levels are considerably lower in relative terms than the BAFF levels in BAFF transgenic mice. The elevated levels of BAFF appear to be secondary to inflammation and activation of myeloid cells in autoimmune subjects.
Blockade of BAFF using antibodies or TACI-Ig in mice has ameliorated disease in animal models of autoimmunity [86], but Bemilumab (an anti-BAFF monoclonal antibody) therapy in human subjects with SLE has been disappointing, although it does reduce peripheral B cell numbers without reducing numbers of memory B cells or long-lived plasma cells [87,88]. BAFF levels may not be high enough to break tolerance in lupus subjects. Even if, at best, BAFF plays a secondary role in pathogenesis and contributes to a break in tolerance, Bemilumab would be unlikely to eliminate the cells that are the source of the pathogenic auto-antibodies in lupus subjects. Bemilumab would be expected to perhaps cause an increase in transitional and presumed Breg cells but such increases, if they do occur, do not appear to be effective in controlling disease at least in subjects with SLE.
Given the fact that Bemilumab only targets BAFF, while Atacicept (TACI-Ig) blocks both BAFF and APRIL, it might be assumed that the latter reagent could possibly phenocopy Rituxan when used in subjects with autoimmunity, given the requirement for BAFF and/or APRIL for B cell survival. BAFF and APRIL, however, are required for naïve follicular B cell and marginal zone B cell survival but may not be essential for memory B cells. While Rituxan can lead to the elimination of both naïve and memory B cells, Atacicept does not contribute to a significant reduction in the memory B cell pool. As a result, in disorders such as rheumatoid arthritis and multiple sclerosis, while Rituxan has proven to be therapeutically effective, Atacicept therapy has not proven to be useful (89–91). In subjects with multiple sclerosis Atacicept led to an unexpected increase in inflammation, and trials in subjects with this disease were suspended (91). Although with Rituxan, naïve and memory B cells are depleted, it is possible that with Atacicept the memory B cell pool is preserved while perhaps regulatory B cells may actually be lost (along with naïve B cells), thereby perhaps leading to disease progression. The phenotype of bona fide human regulatory B cells remain to be unequivocally established, their requirement for BAFF and APRIL are unknown, and the relevance of BAFF blockade in autoimmune therapy remains questionable.
B cell immunodeficiencies and autoimmunity
Immunodeficiency states can result in autoimmunity for a number of reasons. Robust BCR signaling is required for receptor editing, clonal deletion and anergy, and defective BCR signaling could therefore contribute to autoimmunity. In milder cases of X-linked agammaglobulinemia, attenuated BCR signaling because of defective Btk molecules results in a breakthrough of self-reactive B cells at the bone marrow and early peripheral B cell tolerance checkpoints [92].
Hypomorphic Rag mutations causing the Omenn syndrome for instance could contribute to defective receptor editing, and an increase in BAFF levels that occurs when B cell numbers are depleted, and these phenomena (as well as T cell defects) may explain the autoimmune phenomena observed in this syndrome [93].
Examination of individuals with the bare lymphocyte syndrome and with the X-linked hyper-IgM syndrome has revealed a requirement for T cells in peripheral B cell tolerance as discussed earlier [38]. In subjects with mutations in activation induced cytidine deaminase (AID), the hyper-IgM syndrome may also be accompanied by autoimmune manifestations. Somatic mutation and isotype switching both depend on AID, and the possibility has been raised that estrogen receptor mediated regulation of AID transcription might help explain the gender bias in autoimmunity [94]. AID has been shown to be expressed not only in activated B cells but also in ES cells and in developing B cells in the bone marrow. In patients with defective AID autoimmune B cells accumulate both at the late pre-B checkpoint bone marrow checkpoint as well as at the peripheral naïve B cell checkpoint [95]. The exact function of AID in terms of tolerance in developing B cells is unclear. Since AID can contribute to DNA demethylation by deaminating methylated cytidines, it might possibly contribute to the regulation of V(D)J recombination and receptor editing at the bone marrow checkpoint. AID could potentially contribute to the induction of DNA lesions and contribute to the process of apoptosis during clonal deletion in the peripheral B cell checkpoint. AID knockout mice exhibit a defect in central tolerance which may be linked to defective apoptosis (96).
Some interesting information on the pathogenic role of TLR signaling in autoimmunity has been obtained from studying human subjects with genetic defects in IRAK-4, MyD88 and UNC93B. Signaling downstream of all TLRs except TLR3 makes use of the MyD88 adaptor and the IRAK-4 kinase. UNC93B is a polytopic membrane protein that is required for localization and signaling mediated by nucleic acid specific endosomal TLRs. Although all subjects with these disorders accumulated auto-reactive B cells at the bone marrow and peripheral B cell tolerance checkpoints, the patients did not exhibit the presence of auto-antibodies or develop any other features of autoimmunity [97]. TLRs are required for tolerance in part perhaps because TLRs on B cells contribute to tolerance induction, but TLRs are also required for auto-immune B cell activation and disease progression.
Clonal Ignorance in B cells – the SIAE/Siglec pathway
Weakly self-reactive B cells that fail to induce receptor editing, deletion, or anergy, mature and differentiate into follicular or marginal zone B cells. While such weakly self-reactive cells were once considered to be innocuous it is clear that these cells have the potential to be dangerous if they are permitted to receive T cell help and undergo somatic mutation.
It appears to be important for weakly self-reactive B cells to be controlled by inhibitory signaling and maintained in a state of clonal ignorance in order to protect against the development of autoimmunity. If a weakly self-reactive B cell were to be engaged by a self-antigen that is physically complexed to a foreign antigen, activation of such B cells could potentially facilitate the endocytic process, induce the expression of CCR7 and drive the migration of such a B cell towards the T cell zone where it could present foreign peptides to previously activated CD4+T cells. This could result in T-B collaboration between a T cell specific for a foreign peptide and a B cell specific for a self epitope. Somatic mutation at either extra-follicular sites or in the germinal center could result in the generation of strongly self-reactive B cells and auto-antibodies.
Although defects in BCR signaling would be expected to lead to a failure of receptor editing, or deletion or anergy, enhanced BCR signaling is observed in mutations of certain negative regulators of the BCR which can contribute to the development of autoimmunity. CD22/Siglec-2 is a negative regulator of the BCR which binds to α2, 6 linked sialic acid containing N-glycans and is then phosphorylated on cytoplasmic tail ITIM tyrosines by the Lyn tyrosine kinase, resulting in the recruitment of the SH2 domain containing tyrosine phosphatase SHP-1. Activated SHP-1 contributes to the attenuation of BCR signaling. Mice with a mutation in Lyn develop autoimmunity as do mice in which SHP-1 is conditionally deleted in B cells [98–101]. CD22 knockout mice develop anti-DNA antibodies as they age [102]. Some sialic acid containing glycoconjugates are modified in the Golgi by the addition of an acetyl moiety at the 9-OH position. This acetyl group can be removed by an enzyme known as sialic acid acetyl esterase or SIAE. CD22 cannot bind to 9-O-acetylated sialic and siae knockout mice present with enhanced BCR signaling and an autoimmune phenotype [103]. SIAE may regulate more than one Siglec on B cells and CD22−/−/Siglec-G−/− mice present with an autoimmune phenotype although single mutant mice do not develop autoimmunity [104]. Defective rare variants of SIAE are enriched in human subjects with autoimmunity suggesting that the SIAE/Siglec pathway protects against autoimmunity in both mice and men [105].
CD22 and SIAE are expressed primarily in mature B cells and the Lyn knockout autoimmune phenotype is recreated when Lyn in knocked out only in the periphery [106]. The overall evidence suggests that the SIAE/Siglec pathway maintains peripheral tolerance by setting a threshold for B cell activation, and presumably prevents weak self-antigens from drawing cognate B cells into T-B collaboration and the risk of somatic mutation [107]. SIAE, Siglecs, Lyn and SHP-1 may function primarily to maintain B cells in a state of clonal ignorance.
Conclusions
Considerable progress has been made in understanding the role of B cells in autoimmunity from studies in mice as well as from therapeutic interventions, especially antibody mediated B cell depletion studies in human subjects. These analyses have revealed unexpected roles for B cells in large numbers of human inflammatory and autoimmune disorders previously thought to be primarily T cell mediated diseases. Novel tools have been developed in the field to identify tolerance checkpoints in human subjects that probably correspond to more functionally defined checkpoints in mice. Emerging roles for regulatory B cells and inhibitory mechanisms that mediate B cell clonal ignorance will likely contribute to and be integrated with the explosive growth in knowledge regarding human autoimmunity that is certain to result from numerous ongoing studies on rare human genetic alterations that make individuals more susceptible to autoimmune disorders.
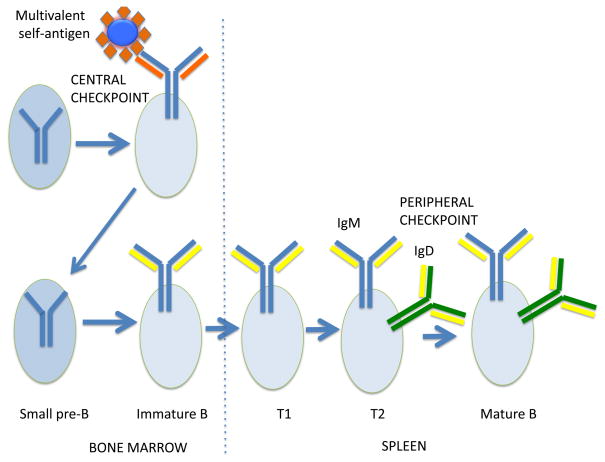
Figure 1. Receptor editing is a major mechanism of central tolerance in B cells.
Immature B cells in the bone marrow that encounter multivalent self antigens revert to the small pre-B stage, continue to rearrange κ and if necessary λ light chain genes and generate newly generated B cells that have a novel light chain that is no longer self-reactive. Immature B cells with novel light chains that are no longer part of a self-reactive B cell receptor, then migrate to the periphery as T1 B cells where they mature into newly generated IgM and IgD expressing recirculating T2 B cells and then into mature recirculating B cells.
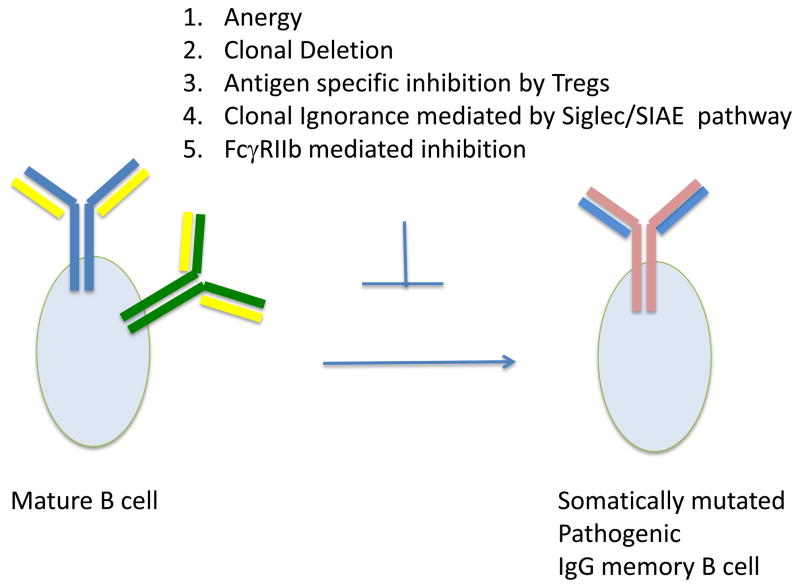
Figure 2. A number of tolerance mechanisms prevent mature peripheral B cells from developing into pathogenic class switched and somatically mutated auto-antibody producing B cells.
These mechanisms include anergy, clonal deletion, specific inhibition of naïve B cells by Tregs in an MHC class II and CD40L dependent manner, clonal inhibition by the Siglec/SIAE pathway, and inhibition by FcγRIIb.
Highlights.
B cells contribute to both auto-antibody linked and helper T cell mediated inflammatory disorders
Tolerance checkpoints in murine and human B lymphocytes are discussed
Lessons from Rituximab mediated B cell depletion and Bemilumab mediated BAFF antagonism in human autoimmune subjects are considered
Current views on Regulatory B cells has been described
The contribution of the SIAE/Siglec pathway and clonal ignorance in autoimmunity have been discussed
Acknowledgments
This work was supported by grants AI076505, AR058481, and AI064930 from the NIH and a grant from the Alliance for Lupus Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shlomchik MJ, Marshak-Rothstein A, Wolfowicz CB, Rothstein TL, Weigert MG. The role of clonal selection and somatic mutation in autoimmunity. Nature. 1987;328:805–811. doi: 10.1038/328805a0. [DOI] [PubMed] [Google Scholar]
- 2.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. The Journal of experimental medicine. 1993;177:1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. The Journal of experimental medicine. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelanda R, Schwers S, Sonoda E, Torres RM, Nemazee D, Rajewsky K. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–775. doi: 10.1016/s1074-7613(00)80395-7. This was the first study using BCR knockin mice to explore the phenomenon of receptor editing, established in conventional BCR transgenic mice in the studies in references 2 and 3. [DOI] [PubMed] [Google Scholar]
- 5.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nature immunology. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 6.Casellas R, Shih TA, Kleinewietfeld M, Rakonjac J, Nemazee D, Rajewsky K, Nussenzweig MC. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–1544. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 7.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. Journal of immunology. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 8.Durdik J, Moore MW, Selsing E. Novel kappa light-chain gene rearrangements in mouse lambda light chain-producing B lymphocytes. Nature. 1984;307:749–752. doi: 10.1038/307749a0. [DOI] [PubMed] [Google Scholar]
- 9.Vela JL, Ait-Azzouzene D, Duong BH, Ota T, Nemazee D. Rearrangement of mouse immunoglobulin kappa deleting element recombining sequence promotes immune tolerance and lambda B cell production. Immunity. 2008;28:161–170. doi: 10.1016/j.immuni.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siminovitch KA, Bakhshi A, Goldman P, Korsmeyer SJ. A uniform deleting element mediates the loss of kappa genes in human B cells. Nature. 1985;316:260–262. doi: 10.1038/316260a0. [DOI] [PubMed] [Google Scholar]
- 11.Panigrahi AK, Goodman NG, Eisenberg RA, Rickels MR, Naji A, Luning Prak ET. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. The Journal of experimental medicine. 2008;205:2985–2994. doi: 10.1084/jem.20082053. This report describes a method for assessing receptor editing that can be applied to subjects with autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 13.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. Journal of immunology. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 14.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 15.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 16.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 17.Keenan RA, De Riva A, Corleis B, Hepburn L, Licence S, Winkler TH, Martensson IL. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–699. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 18.Aoki Y, Isselbacher KJ, Cherayil BJ, Pillai S. Tyrosine phosphorylation of Blk and Fyn Src homology 2 domain-binding proteins occurs in response to antigen-receptor ligation in B cells and constitutively in pre-B cells. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:4204–4208. doi: 10.1073/pnas.91.10.4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kohler F, Hug E, Eschbach C, Meixlsperger S, Hobeika E, Kofer J, Wardemann H, Jumaa H. Autoreactive B cell receptors mimic autonomous pre-B cell receptor signaling and induce proliferation of early B cells. Immunity. 2008;29:912–921. doi: 10.1016/j.immuni.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- 21.Lindsley RC, Thomas M, Srivastava B, Allman D. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–2528. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 22.Cariappa A, Boboila C, Moran ST, Liu H, Shi HN, Pillai S. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. Journal of immunology. 2007;179:2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 23.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nature reviews Immunology. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 24.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annual review of immunology. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 25.Pillai S, Cariappa A, Moran ST. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunological reviews. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 26.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annual review of immunology. 2008;26:741–766. doi: 10.1146/annurev.immunol.26.021607.090344. [DOI] [PubMed] [Google Scholar]
- 27.Crotty S. Follicular helper CD4 T cells (TFH) Annual review of immunology. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 28.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. The Journal of experimental medicine. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacLennan IC. Somatic mutation. From the dark zone to the light Current biology: CB. 1994;4:70–72. doi: 10.1016/s0960-9822(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 32.Szakal AK, Kosco MH, Tew JG. A novel in vivo follicular dendritic cell-dependent iccosome-mediated mechanism for delivery of antigen to antigen-processing cells. Journal of immunology. 1988;140:341–353. [PubMed] [Google Scholar]
- 33.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nature medicine. 2011 doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, Oden F, Mertes MM, Ehlers M, Wardemann H. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice. The Journal of experimental medicine. 2010;207:2767–2778. doi: 10.1084/jem.20100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. This report establishes an approach to identify B cell tolerance checkpoints in humans. [DOI] [PubMed] [Google Scholar]
- 36.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Advances in immunology. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 37.Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity. Current opinion in immunology. 2008;20:632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Herve M, Isnardi I, Ng YS, Bussel JB, Ochs HD, Cunningham-Rundles C, Meffre E. CD40 ligand and MHC class II expression are essential for human peripheral B cell tolerance. The Journal of experimental medicine. 2007;204:1583–1593. doi: 10.1084/jem.20062287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. The Journal of experimental medicine. 2005;201:1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. The Journal of experimental medicine. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, Nussenzweig MC. Persistent expression of autoantibodies in SLE patients in remission. The Journal of experimental medicine. 2006;203:2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Menard L, Samuels J, Ng YS, Meffre E. Inflammation-independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis and rheumatism. 2011;63:1237–1245. doi: 10.1002/art.30164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. The Journal of experimental medicine. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizoguchi A, Mizoguchi E, Smith RN, Preffer FI, Bhan AK. Suppressive role of B cells in chronic colitis of T cell receptor alpha mutant mice. The Journal of experimental medicine. 1997;186:1749–1756. doi: 10.1084/jem.186.10.1749. This is the first report to indicate the presence of regulatory B cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 47.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. The Journal of experimental medicine. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 49.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. Journal of immunology. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 50.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. The Journal of clinical investigation. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimomura Y, Mizoguchi E, Sugimoto K, Kibe R, Benno Y, Mizoguchi A, Bhan AK. Regulatory role of B-1 B cells in chronic colitis. International immunology. 2008;20:729–737. doi: 10.1093/intimm/dxn031. [DOI] [PubMed] [Google Scholar]
- 52.Gray M, Miles K, Salter D, Gray D, Savill J. Apoptotic cells protect mice from autoimmune inflammation by the induction of regulatory B cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:14080–14085. doi: 10.1073/pnas.0700326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. Journal of clinical immunology. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 54.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. Journal of immunology. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 55.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 56.Carter NA, Vasconcellos R, Rosser EC, Tulone C, Munoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. Journal of immunology. 2011;186:5569–5579. doi: 10.4049/jimmunol.1100284. [DOI] [PubMed] [Google Scholar]
- 57.Lampropoulou V, Hoehlig K, Roch T, Neves P, Calderon Gomez E, Sweenie CH, Hao Y, Freitas AA, Steinhoff U, Anderton SM, et al. TLR-activated B cells suppress T cell-mediated autoimmunity. Journal of immunology. 2008;180:4763–4773. doi: 10.4049/jimmunol.180.7.4763. [DOI] [PubMed] [Google Scholar]
- 58.Goetz M, Atreya R, Ghalibafian M, Galle PR, Neurath MF. Exacerbation of ulcerative colitis after rituximab salvage therapy. Inflammatory bowel diseases. 2007;13:1365–1368. doi: 10.1002/ibd.20215. [DOI] [PubMed] [Google Scholar]
- 59.El Fassi D, Nielsen CH, Kjeldsen J, Clemmensen O, Hegedus L. Ulcerative colitis following B lymphocyte depletion with rituximab in a patient with Graves’ disease. Gut. 2008;57:714–715. doi: 10.1136/gut.2007.138305. [DOI] [PubMed] [Google Scholar]
- 60.Ardelean DS, Gonska T, Wires S, Cutz E, Griffiths A, Harvey E, Tse SM, Benseler SM. Severe ulcerative colitis after rituximab therapy. Pediatrics. 2010;126:e243–246. doi: 10.1542/peds.2009-3395. [DOI] [PubMed] [Google Scholar]
- 61.Blombery P, Prince HM, Levinson M, Pianko S, Maxwell E, Bhathal P. Rituximab-induced immunodysregulatory ileocolitis in a patient with follicular lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2011;29:e110–112. doi: 10.1200/JCO.2010.31.8899. [DOI] [PubMed] [Google Scholar]
- 62.Palanichamy A, Barnard J, Zheng B, Owen T, Quach T, Wei C, Looney RJ, Sanz I, Anolik JH. Novel human transitional B cell populations revealed by B cell depletion therapy. Journal of immunology. 2009;182:5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sims GP, Ettinger R, Shirota Y, Yarboro CH, Illei GG, Lipsky PE. Identification and characterization of circulating human transitional B cells. Blood. 2005;105:4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 65.Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM, Szabolcs PM, Bernstein SH, Magro CM, Williams AD, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nature immunology. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 67.Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH, Haberman AM. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity. 2011;34:947–960. doi: 10.1016/j.immuni.2011.03.024. This report describes the visualization of a large scale migration of activated B and T cells into the interfollicular zone after immune activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Essen D, Dullforce P, Brocker T, Gray D. Cellular interactions involved in Th cell memory. Journal of immunology. 2000;165:3640–3646. doi: 10.4049/jimmunol.165.7.3640. [DOI] [PubMed] [Google Scholar]
- 69.Christensen JP, Kauffmann SO, Thomsen AR. Deficient CD4+ T cell priming and regression of CD8+ T cell functionality in virus-infected mice lacking a normal B cell compartment. Journal of immunology. 2003;171:4733–4741. doi: 10.4049/jimmunol.171.9.4733. [DOI] [PubMed] [Google Scholar]
- 70.Whitmire JK, Asano MS, Kaech SM, Sarkar S, Hannum LG, Shlomchik MJ, Ahmed R. Requirement of B cells for generating CD4+ T cell memory. Journal of immunology. 2009;182:1868–1876. doi: 10.4049/jimmunol.0802501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. The Journal of experimental medicine. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunological reviews. 2010;237:264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 73.Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kotter I, Haas J, Unger L, Lovric S, Haubitz M, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID) Arthritis research & therapy. 2011;13:R75. doi: 10.1186/ar3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. The New England journal of medicine. 2009;361:2143–2152. doi: 10.1056/NEJMoa0904452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 76.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 77.Eming R, Nagel A, Wolff-Franke S, Podstawa E, Debus D, Hertl M. Rituximab exerts a dual effect in pemphigus vulgaris. The Journal of investigative dermatology. 2008;128:2850–2858. doi: 10.1038/jid.2008.172. [DOI] [PubMed] [Google Scholar]
- 78.Stasi R, Del Poeta G, Stipa E, Evangelista ML, Trawinska MM, Cooper N, Amadori S. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–2930. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 79.Saadoun D, Rosenzwajg M, Landau D, Piette JC, Klatzmann D, Cacoub P. Restoration of peripheral immune homeostasis after rituximab in mixed cryoglobulinemia vasculitis. Blood. 2008;111:5334–5341. doi: 10.1182/blood-2007-11-122713. [DOI] [PubMed] [Google Scholar]
- 80.Sfikakis PP, Souliotis VL, Fragiadaki KG, Moutsopoulos HM, Boletis JN, Theofilopoulos AN. Increased expression of the FoxP3 functional marker of regulatory T cells following B cell depletion with rituximab in patients with lupus nephritis. Clinical immunology. 2007;123:66–73. doi: 10.1016/j.clim.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 81.Hu CY, Rodriguez-Pinto D, Du W, Ahuja A, Henegariu O, Wong FS, Shlomchik MJ, Wen L. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. The Journal of clinical investigation. 2007;117:3857–3867. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mackay F, Schneider P. Cracking the BAFF code. Nature reviews Immunology. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 83.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 84.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Current opinion in immunology. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 86.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. The Journal of clinical investigation. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wallace DJ, Stohl W, Furie RA, Lisse JR, McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis and rheumatism. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis and rheumatism. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van Vollenhoven RF, Kinnman N, Vincent E, Wax S, Bathon J. Atacicept in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase II, randomized, placebo-controlled trial. Arthritis and rheumatism. 2011;63:1782–92. doi: 10.1002/art.30372. [DOI] [PubMed] [Google Scholar]
- 90.Genovese MC, Kinnman N, de La Bourdonnaye G, Pena Rossi C, Tak PP. Atacicept in patients with rheumatoid arthritis and an inadequate response to tumor necrosis factor antagonist therapy: results of a phase II, randomized, placebo-controlled, dose-finding trial. Arthritis and rheumatism. 2011;63:1793–803. doi: 10.1002/art.30373. [DOI] [PubMed] [Google Scholar]
- 91.Hartung HP, Kieseier BC. Atacicept: targeting B cells in multiple sclerosis. Therapeutic advances in neurological disorders. 2010;3:205–16. doi: 10.1177/1756285610371146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ng YS, Wardemann H, Chelnis J, Cunningham-Rundles C, Meffre E. Bruton’s tyrosine kinase is essential for human B cell tolerance. The Journal of experimental medicine. 2004;200:927–934. doi: 10.1084/jem.20040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walter JE, Rucci F, Patrizi L, Recher M, Regenass S, Paganini T, Keszei M, Pessach I, Lang PA, Poliani PL, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. The Journal of experimental medicine. 2010;207:1541–1554. doi: 10.1084/jem.20091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pauklin S, Sernandez IV, Bachmann G, Ramiro AR, Petersen-Mahrt SK. Estrogen directly activates AID transcription and function. The Journal of experimental medicine. 2009;206:99–111. doi: 10.1084/jem.20080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meyers G, Ng YS, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, et al. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11554–11559. doi: 10.1073/pnas.1102600108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuraoka M, Holl TM, Liao D, Womble M, Cain DW, Reynolds AE, Kelsoe G. Activation induced cytidine deaminase mediates central tolerance in B cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11560–11565. doi: 10.1073/pnas.1102571108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Isnardi I, Ng YS, Srdanovic I, Motaghedi R, Rudchenko S, von Bernuth H, Zhang SY, Puel A, Jouanguy E, Picard C, et al. IRAK-4- and MyD88-dependent pathways are essential for the removal of developing autoreactive B cells in humans. Immunity. 2008;29:746–757. doi: 10.1016/j.immuni.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 99.Nishizumi H, Taniuchi I, Yamanashi Y, Kitamura D, Ilic D, Mori S, Watanabe T, Yamamoto T. Impaired proliferation of peripheral B cells and indication of autoimmune disease in lyn-deficient mice. Immunity. 1995;3:549–560. doi: 10.1016/1074-7613(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 100.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 101.Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 102.O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. The Journal of experimental medicine. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cariappa A, Takematsu H, Liu H, Diaz S, Haider K, Boboila C, Kalloo G, Connole M, Shi HN, Varki N, et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. The Journal of experimental medicine. 2009;206:125–138. doi: 10.1084/jem.20081399. This study describes a role for sailic acid acetyl esterase in Siglec regulation and the prevention of autoimmunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. The Journal of experimental medicine. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Surolia I, Pirnie SP, Chellappa V, Taylor KN, Cariappa A, Moya J, Liu H, Bell DW, Driscoll DR, Diederichs S, et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. Journal of immunology. 2009;182:5382–5392. doi: 10.4049/jimmunol.0803941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends in immunology. 2009;30:488–493. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]