Abstract
Socially withdrawn individuals display solitary behavior across wide contexts with both unfamiliar and familiar peers. This tendency to withdraw may be driven by either past or anticipated negative social encounters. In addition, socially withdrawn individuals often exhibit right frontal electroencephalogram (EEG) asymmetry at baseline and when under stress. In the current study we examined shifts in frontal EEG activity in young adults (N=41) at baseline, as they viewed either an anxiety-provoking or a benign speech video, and as they subsequently prepared for their own speech. Results indicated that right frontal EEG activity increased, relative to the left, only for socially withdrawn participants exposed to the anxious video. These results suggest that contextual affective cues may prime an individual’s response to stress, particularly if they illustrate or substantiate an anticipated negative event.
Keywords: Social Withdrawal, EEG, Frontal Asymmetry, Presentation Task, Affective Cue
1. Introduction
The term “social withdrawal” has often been used interchangeably with terms such as shyness, inhibition, and social isolation (Rubin & Coplan, 2004). Each term refers to a similar set of behaviors exhibited by children and adults when faced with novel or unfamiliar situations. Specifically, social withdrawal characterizes children or adults who display solitary behavior across different contexts when exposed to unfamiliar and familiar peers (Rubin & Burgess, 2001). Individuals who are socially withdrawn are rejected by peers, and tend to attribute this rejection to their own personal deficiencies (Rubin, Copeland, & Bowker, 2009). Feelings of deficiency can then lead to further withdrawal from social contact, creating a “feedback loop” that strengthens withdrawal behavior (Rubin & Burgess, 2001).
Social withdrawal is thought to be highly stable and evident across time and context (Caspi et al., 2003; Newman, Caspi, Moffitt, Silva, 1997), with several precursors of adult social withdrawal identifiable in infancy and early childhood. One such precursor that has gained empirical support is the temperamental trait of behavioral inhibition (Rubin & Burgess, 2001). Children and infants characterized as behaviorally inhibited in childhood have several distinct behavioral and physiological characteristics. These children are shy, fearful, and tend to retreat from novel situations (Kagan, Reznick, Clarke, Snidman, & Garcia-Coll, 1984). In addition these children are characterized as having a specific psychophysiological profile marked by faster heart rates (Andersson, Bohlin & Hagekull, 1999; Kagan, Reznick, & Snidman, 1987) and a high vagal tone (Calkins & Fox, 1992; Fox, 1989) both at rest and when under stress. These peripheral markers are often coupled with a pattern of right frontal electroencephalogram (EEG) asymmetry (Davidson & Fox, 1989; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001).
Researchers have suggested that both anxiety and social withdrawal are driven by over activation of the amygdala, especially to novelty (Kagan, Snidman, & Arcus, 1993). Based on the large number of connections between the amygdala and the prefrontal cortex (PFC), researchers have also suggested that the PFC is in part responsible for regulating the activity of the amygdala (Davidson, 2004). In animal models, stimulation of the left PFC decreases responsiveness of neurons in the central amygdala (Quirk et al., 2003). It is posited, therefore, that greater left activation of the prefrontal cortex indicates greater amygdala inhibition while greater right activation of the PFC indicates less inhibition of the amygdala (Davidson, 2004).
To examine the relationship between the prefrontal cortex and anxiety, researchers often measure variations in EEG patterns. Patterns of EEG asymmetry are captured by calculating a difference score from the respective levels of activation between the left and right side of the scalp (Harmon-Jones, Gable & Peterson, 2010; Silva, Pizzagalli, Larson, Jackson, & Davidson, 2002). While this calculation can be carried out across all scalp electrodes, much of the literature examining variations in affect and social behavior has focused on asymmetries across right and left frontal electrodes.
Individuals with greater activation of the right as compared to the left frontal cortical areas are considered to display right frontal asymmetry, while those displaying greater activation of the left as compared to the right frontal cortical areas are considered as displaying left frontal EEG asymmetry (Silva, et al., 2002). The literature suggests that frontal EEG asymmetry represents an underlying disposition to approach or withdraw from challenging or novel situations (Harmon-Jones et al., 2010), reflecting the PFC- amygdala relation noted above. As such, behaviorally inhibited, anxious, or depressed children and adults have all been shown to exhibit right EEG asymmetry (i.e., greater activity over the right hemisphere versus the left) at baseline (Baving, Laucht, Schmidt, 2002; Buss et al., 2003; Davidson & Fox, 1989; Diego, Field, & Hernandez-Reif, 2001; Fox et al., 1995; Henriques & Davidson, 1991; Thibodeaua, Jorgensena & Kima, 2006).
Frontal EEG asymmetry may also be induced or exacerbated through experimental manipulation of affect (Schmidt, Fox, Schulkin, & Gold, 1999), often achieved by presenting negative stimuli (e.g., sad movies), (Davidson & Fox, 1982). In an example of such research, Harmon-Jones and Sigelman (2001) measured resting frontal EEG in undergraduate participants. Participants were then asked to write an essay on one of several topics. After finishing writing their essay participants were either given positive or negative feedback. Negative essay feedback was designed to provoke anger in the participants, thereby activating the approach systems of the individual. As mentioned above, left frontal EEG activation is posited to be related to an individual’s underlying approach tendencies. As expected, participants given negative feedback on their essay displayed increases from baseline in left frontal EEG activity while those who received positive feedback displayed no changes in EEG activity. In addition, increases in left frontal EEG activity relative to baseline in the negative feedback group paralleled self-report measures of anger and aggression. In a similar study, Field and colleagues (1998) played pleasing music to clinically depressed adolescents. At baseline, participants displayed greater right frontal EEG activity. The participants’ asymmetry values shifted during, and immediately following the music session, moving significantly closer to a more symmetric activation pattern.
In a study of infant EEG, Fox and Davidson (1987) placed infants in four different situations; one in which the infants’ mother approached them, one in which the infants’ mother was separated from them, one in which a stranger approached the infant while the mother was in the room, and one in which a stranger approached the infant with the mother absent from the room. Frontal EEG was recorded during each of these four situation periods. Results indicated that EEG asymmetry was sensitive to both the task context and the infant’s affective style. For example, an increase in right frontal EEG activity was observed during mother separation for children who displayed a negative affective style (cried) while a slight decrease in right frontal activation relative to the left was observed for children with a more positive affective style (non-crier). These studies illustrate that changes in frontal EEG asymmetry can both be induced through manipulation of task condition, but are also linked to individual differences in socio-emotional characteristics from infancy through young adulthood.
Similar to depressed and anxious individuals, socially withdrawn individuals also show a pattern of greater right frontal EEG activity. For example, Schmidt (1999) identified four groups of undergraduates who characterized themselves as high or low on self-report measures of shyness and sociability. Participants identified by Schmidt (1999) as being high shy (regardless of sociability level) had greater right frontal EEG activity at baseline than those identified as low shy. Additionally, participants identified as high shy/low sociability (i.e. the most socially withdrawn participants) exhibited the largest right frontal EEG activity values.
Although numerous studies have examined the psychobiological profile of adults with social withdrawal or anxiety at baseline (Schmidt, 1999), there is relatively less information concerning how these individuals respond to emotionally charged social situations. There is some indication that socially withdrawn or anxious individuals are particularly sensitive to social scenarios that place them at perceived risk or in a position to challenge social conventions (Giesen & Rollison, 1980). In a study focusing specifically on socially anxious individuals, Davidson and colleagues (Davidson, Marshall, Tomarken & Henriques, 2000) examined socially phobic and healthy adults during various stages of a speech presentation task. Participants were told they would be giving a speech in front of 24 graduate students who would note the strengths and weaknesses of their presentation. EEG, heart-rate and blood pressure were recorded during baseline, in anticipation of the speech, while planning for the speech, and immediately after finishing their speech. Results indicated a pattern of increased right sided activation in anterior temporal and lateral prefrontal brain regions of socially phobic participants as compared to non-phobic participants. Increased right sided activation was specifically seen during the anticipation and planning stages of the speech, but was not seen immediately following speech completion. The authors (Davidson et al, 2000) argued that activation in the right hemisphere was associated with anxiety relating to the performed task.
An additional study focusing on the link between EEG activity and social difficulties was recently conducted by Miskovic and colleagues (Miskovic, et al., 2010). Here, the authors examined delta-beta EEG power coupling in adults high and low in social anxiety during baseline and in anticipation of a speech presentation task. Delta-beta coupling is thought to represent communication between cortical and sub-cortical brain areas, and may reflect the motivational state of the individual. Indeed, responses associated with stress (such as increases in the hormone cortisol) tend to strengthen delta-beta coupling (van Peer, Roelofs, & Spinhoven, 2008). While Miskovic et al. (2010) found no differences in delta-beta coupling at baseline, socially anxious participants exhibited significant delta-beta coupling at the right frontal electrode site during the speech anticipation phase, as compared to non-anxious participants.
These studies suggest that the pattern of brain activity in socially and non-socially anxious adults may be similar under non-stressful circumstances, and that it is only when these groups are specifically exposed to affective stresses that differences in electrical brain responses emerge. This reflects the broader literature linking social withdrawal to greater sensitivity to environmental conditions (Kashdan & Herbert, 2001).
With this in mind, the current study examined the impact of task specific affective cues and task-related stress on frontal EEG asymmetry patterns in individuals characterized as either high or low in social withdrawal. We had three main questions of interest. First, would individuals characterized as either low or high in social withdrawal show EEG asymmetry differences at baseline? Second, would EEG asymmetry values change with the introduction of an increasingly stressful task? Third, would prior exposure to an affective cue moderate EEG asymmetry patterns across task conditions?
In the current study, EEG measures were recorded at baseline and as the participants silently prepared for a standard speech presentation task (Hoffman, Moscovitch & Kim, 2006; Schmidt at al., 1999; Miskovic, et al., 2010). A speech presentation task was chosen specifically because studies have indicated that such procedures produce feelings of social threat marked by anxiety, nervousness, and stress (Schmidt at al., 1999; Westenburg et al., 2009). Physiological responses such as increases in heart rate, skin conductance, and cortisol response have also been noted during this task (Westenburg et al., 2009). Based on prior work (Schmidt, Fox, Shulkin, & Gold, 1999, Perez-Edgar & Fox, 2005), the participants were specifically asked to recount their “most embarrassing moment.”
In order to examine the potential effect of an affective prime, participants were shown a video of a “previous participant” performing the speech task before they were instructed to prepare their own speech. For one-half of the participants (counterbalanced across social withdrawal status), the confederate displayed clear signs of distress and anxiety while recounting the event. For the other half of the participants, the same confederate was at ease and laughing as she recounted the incident. This video was designed to serve as a task specific affective prime, either heightening or alleviating the participants’ anxiety in anticipation of their own speech. As such, the video served as an example against which the participants could compare their own performance and also potentially serve as a model for their social fears (Mineka & Zinbarg, 2006). EEG asymmetry pattern was noted at three points during the study: At baseline, during video viewing, and while preparing for the speech.
This study therefore addressed two parallel issues. First, the study examined potential shifts in frontal EEG asymmetry patterns across dynamic contexts of st ress as a function of individual differences in social withdrawal. Second, the study examined the impact of affective social cues on subsequent patterns of frontal EEG asymmetry.
We hypothesized that participants characterized as highly socially withdrawn would have greater right frontal EEG activity at baseline and when under stress compared to subjects identified as low in social withdrawal. Additionally, we hypothesized increases in right frontal EEG activity for individuals in the high social withdrawal group during speech preparation relative to baseline. Finally, we hypothesized that video type (anxious or non-anxious) would differentially effect changes in EEG asymmetry. Specifically, participants who saw the anxious video before the speech presentation would have greater increases in right fontal EEG activity relative to baseline. Again, this would be moderated by social withdrawal.
2. Methods
2.1. Participants
Participants were 59 undergraduate students (M = 21.3 years, SD = 5.6; 23 male) at George Mason University, Fairfax VA. Participants were primarily Caucasian, Non-Hispanic (66.1%) with the remaining participants self-identifying as Asian/Pacific Islander (13.6%), African-American (11.95%), or Hispanic (8.5%). Students enrolled voluntarily and received compensation in the form of research participation credits. This study was approved by the University Human Subjects Review Board.
Eight subjects were excluded for failing to complete the social withdrawal self-report measure. An additional 10 subjects either declined to participate in the EEG portion of the study, or did not provide viable EEG data. Therefore the final sample consisted of 41 participants (M = 21.2 years, SD = 5.6, 17 male). Participants were primarily Caucasian, Non-Hispanic (75.61%) with the remaining participants self-identifying as Asian/Pacific Islander (9.54%), African-American (7.31%), or Hispanic (7.31%).
2.2. Procedure
Prior to data collection the participants were briefed concerning the task and consent was obtained. Participants were asked to complete the Cheek and Buss Shyness and Sociability scale (CBSS; Cheek & Buss, 1981) and were then prepared for EEG recording. EEG was recorded during baseline, video viewing, and speech preparation. Participants rated both the observed speech and their own speech for nervousness, embarrassment, and levels of emotional distress. EEG was not recorded while participants completed the task questionnaires or during their own speech due to artifact.
2.2.1. Self Report Measure
The CBSS (Cheek and Buss, 1981) consists of 18 items. 13 items focus on shyness including questions such as “I find it hard to talk to strangers”. The sociability subscale is comprised of five questions, with items such as “I prefer working with others than alone”. Items on both of these scales are rated from 0 to 4, with 0 being extremely uncharacteristic and 4 being extremely characteristic.
Initial review found that the separate shyness and sociability scores were highly correlated, r(41) = −0.476, p = 0.002. As such, they were standardized and combined (after reverse scoring sociability) to create a single measure of social withdrawal. Subjects were then median split into high (N=21 [9 males, 12 females], M = 1.45, SD = 0.90) and low (N=20 [7 males, 13 females], M = −1.54, SD = 1.01) social withdrawal groups, t(38) = −9.87, p < 0.001, d = 3.20.
2.2.2. Electrophysiology Recording
Across all conditions electroencephalogram measures were recorded from 64 EEG and EOG channels, using the Lycra NeuroScan Quick-cap system (NeuroScan, Texas, USA). EEG channels were referenced to an electrode 2 cm posterior to Cz. Vertical eye movements (VEOG) were collected through electrodes placed above and below the left eye, while horizontal eye movements (HEOG) were collected through electrodes placed on the external canthi of each eye. Researchers attempted to keep all electrode impedances below 10 K ohms. The data from each channel were digitized at a 500 Hz sampling rate (High pass 0.10 Hz; Low pass 40 Hz).
The digitized EEG data were manually inspected and channels with unreliable EEG signals were removed. The data were then re-referenced via the software to give an average reference configuration. Portions of the EEG data contaminated with eye movement or motor artifact were automatically removed from all channels using predetermined parameters (signal ±100 μV). The re-referenced, artifact-free EEG data were submitted to a discrete Fourier transform using a 1-s Hanning window with 50% overlap between consecutive windows.
2.2.2.1. Baseline
Participants sat at rest for a total of four minutes with their eyes alternately open and closed for one minute periods.
2.2.2.2. Speech Video
After completing the baseline procedure, participants were told that they would have to give a videotaped speech describing a past embarrassing moment, which would be shown to future research participants. Prior to giving their speech, participants watched one of two two-minute videos of a research confederate giving a speech about an event consisting of spilling food in a cafeteria. In one video the female confederate appeared embarrassed and anxious over the past event (anxious condition; N=10 low social withdrawal, 10 high social withdrawal). In the other video (non-anxious condition; N=10 low social withdrawal, 11 high social withdrawal) the same confederate displayed positive affect when recounting the same event and treated the incident jokingly. Participants were then asked to rate the emotional quality and overall presentation style of the video.
2.2.2.3. Speech Preparation
After viewing the video participants were given two minutes to silently prepare for their own presentation. After the two-minute period, participants recounted their embarrassing moment while being video recorded. Following the presentation participants were asked to rate their own speech performance using an identical rating sheet to the one used with the confederate speech.
2.2.3. Speech Ratings
We asked participants to rate the videos and their own speeches with a brief questionnaire in order to verify our anxious video manipulation and assess how participants subjectively viewed their own performance. The questionnaire consisted of four items: (1) “How well did the individual give his or her speech?”, (2) “How nervous did the individual appear?”, (3) “How emotionally upset did the individual appear?”, and (4) “How embarrassing was the speech?” The questions were re-worded to refer to the participant for ratings after the laboratory speech.
Question 1 was assessed using a 5-point Likert scale ranging from 1=Poor to 5=Excellent. Questions 2 through 4 used a parallel scale with 1=Extremely to 5=Not at all. Scores were then summed across the four questions (maximum score = 20) with high scores indicating little to no anxiety or discomfort and better overall performance.
2.2.4. EEG Asymmetry Calculation
Data analysis focused on the F3 and F4 frontal electrodes (Silva et al., 2002). For each electrode site, alpha power was computed as the natural logarithm of power in the 8-13 Hz frequency band during the baseline (eyes-open), during video watching, and during speech preparation conditions. Asymmetry scores were then calculated by subtracting the natural log of alpha power from the left electrode (F3) from the corresponding electrode over the right hemisphere (F4).
For alpha asymmetry, a positive score reflects greater relative right-sided power (or increased left-sided activity), whereas a negative score reflects greater relative left-sided power (or increased right-sided activity). This is because alpha power is thought to be inversely related to brain activity (Davidson, 2004). Each participant had three EEG asymmetry scores available from the baseline (eyes open), the speech watching, and the speech preparing conditions.
In order to better understand the pattern of EEG activity that may be underlying any significant findings with EEG asymmetry scores (Schmidt, 1999), power values from the right (F4) and left (F3) hemisphere electrodes were used in follow-up ANOVAs. In this way we were able to gauge if findings were driven primarily by changes in one hemisphere in response to the task manipulations.
Most studies of this nature have focused on frontal EEG asymmetry. Indeed, the literature suggests that asymmetry relations are either specific to or at the very least, strongest for the frontal lobe (Harmon-Jones et al, 2010). However, asymmetries derived from other brain regions also reflect underlying differences in emotional or motivational systems. Right parietal alpha asymmetry has been noted in children and adults viewing emotional stimuli (Davidson & Fox, 1982; Davidson & Henriques, 2000; Davidson, Schwartz, Saron, Bennett, & Goleman, 1979) and show differences at rest as a function of shyness and sociability (Schmidt & Fox, 1994). Heller (Heller, Nitschke, Etienne, & Miller, 1997) also found that stressful events can produce greater right parietal activation, particularly in anxious individuals. In order to test the specificity of our hypotheses, we therefore repeated the central analyses of our study using asymmetry scores derived from parietal electrodes (P3 and P4).
3. Statistical Analysis
3.1 Speech Ratings and Social Withdrawal
The initial analysis assessed whether individual ratings of the video and laboratory speeches varied as a function of social withdrawal and the observed speech (descriptive measures of the ratings are presented in Table 1). As such we relied on a 2 (speech performance rating: self performance, viewed performance) X 2 (video type: anxious, non-anxious) X 2 (gender: male, female) X 2 (social withdrawal: high, low) mixed model ANOVA. Here we found a significant two-way interaction between speech rating and video type viewed, F(1,41) = 13.92, p < 0.001, d = 1.17, indicating that we had successfully manipulated the emotional tone of the two videos.
Table 1.
Descriptive measures (means and standard deviations) for the video ratings and EEG measures collected. Values are presented separately for the high and low social withdrawal groups.
| Low Social Withdrawal |
High Social Withdrawal |
|
|---|---|---|
| Anxious Video | 12.00 | 12.30 |
| -Self Rating | (2.35) | (2.50) |
| Anxious Video | 10.50+ | 9.23+ |
| -Presenter Rating | (2.03) | (1.29) |
| Non-Anxious Video | 11.82 | 12.85 |
| -Self Rating | (2.48) | (2.30) |
| Non-Anxious Video | 13.36 | 14.54 |
| -Presenter Rating | (1.63) | (1.98) |
| EEG Asymmetry | 0.077 | -0.018 |
| -Baseline | (0.241) | (0.188) |
| EEG Asymmetry | 0.189** | -0.161** |
| -Viewing | (0.210) | (0.290) |
| EEG Asymmetry | 0.184+ | -0.082+ |
| -Preparing | (0.670) | (0.210) |
p<0.01;
p<0.10
An independent samples t-test found that participants rated the anxious video performance (M = 9.76) significantly worse than the non-anxious performance (M = 14.05), t(39) = −7.04, p <0.001, d = 2.25. Participants did not differ in their evaluation of their own performance, regardless of which video they had viewed, t(39) = −0.96, p = 0.35, d = 0.31.
Additionally, paired samples t-tests indicated participants watching the anxious video rated their own performance better (M = 11.63) than that of the video presenter (M = 9.76), t(18) = 3.09, p = 0.006, d = 1.46, while participants watching the non-anxious video rated themselves worse (M = 12.32) than the video presenter (M = 14.05), t(21) = −2.12, p = 0.046, d = 0.92 (Figure 1).
Figure 1.
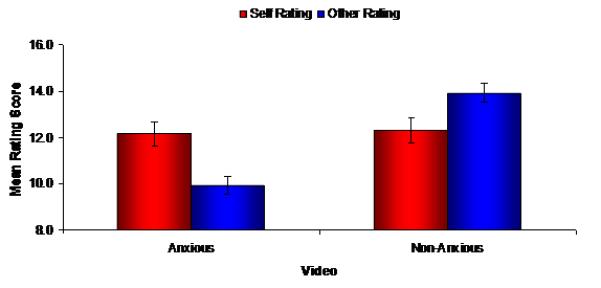
Rating scores for the viewed video speech (other) and the participant’s own speech (self) Mean scores are presented separately for the groups exposed to the anxious and non-anxious videos
Analyses indicated no significant interaction between social withdrawal and the performance ratings, F(1,41) = 0.23, p = 0.64, d = 0.15. There were no relations between frontal EEG asymmetry scores and performance ratings across conditions, p’s > 0.16.
3.2 Shifts in EEG Asymmetry across Conditions: Social Withdrawal and Video Type
Our central analysis examined potential shifts in frontal EEG asymmetry patterns across the task conditions presented. In addition, we looked to see if the emotional tone of the confederate video shifted this pattern, either overall or as a function of social withdrawal. An omnibus 3 (EEG asymmetry: baseline, viewing, preparing) X 2 (video type: anxious, non- anxious) X 2 (gender: male, female) X 2 (social withdrawal: high, low) mixed model ANOVA was therefore used.
Overall, there was a main effect of social withdrawal, such that individuals high in social withdrawal exhibited negative asymmetry scores (M=−0.104), while participants with low social withdrawal had positive scores (M =0.148), F(1,29)=7.58, p = 0.01, d = 1.02 (see Table 1). Follow-up analyses with the power values from each electrode (F3, F4) found a significant hemisphere by social withdrawal interaction, F(1,29)=8.40, p = 0.007, d = 1.08, indicating significant differences in power levels across hemisphere (F4 > F3) for the high social withdrawal group (p = 0.02), that were in the opposite direction and approaching significance (p = 0.06) for the low social withdrawal group.
The initial omnibus ANOVA also found a significant three-way interaction between task condition, video type and social withdrawal, F(1,29) = 4.79, p = 0.037, d = 0.81. Follow-up analyses indicated that social withdrawal moderated shifts in EEG asymmetry (from baseline to viewing to preparing) only among participants who were exposed to the anxious video, F(2,32) = 4.30, p = 0.022, f = 0.52 (Figure 2). In particular, socially withdrawn individuals showed an increase in right frontal EEG activity relative to baseline while viewing the anxious video (baseline vs. viewing: 0.025 vs. −0.190); the non-withdrawn group did not (0.026 vs. 0.195). While not as extreme, withdrawn individuals continued to show greater right frontal EEG activity scores during the stressful speech preparation period (−0.090); again, non-withdrawn individuals did not show this effect (0.130).
Figure 2.
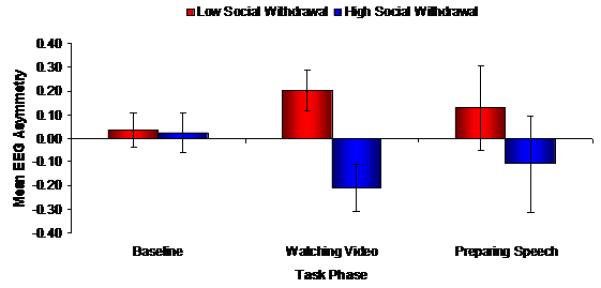
EEG asymmetry values across the three testing conditions (baseline, video watching, and speech preparing) for participants who viewed the anxious video as a function of social withdrawal
The parallel analysis for individuals observing the non-anxious video was not significant, F(2,34) = 0.64, p = 0.54, f = 0.19.
When examining electrode patterns for this analysis, power in the F3 and F4 electrodes did not modify the three-way interaction noted above, indicating that the effect may be driven by the relative balance in EEG activity, as opposed to significant shifts in activity in one location.
In order to assess the specificity of our initial findings, analyses were then replicated using parietal asymmetries. The main effect of social withdrawal noted above was no longer significant, F(1,29) = 0.31, p = 0.58, d = 0.21. The initial task condition by video type by social withdrawal interaction was also non-significant, F(1,29) = 2.80, p = 0.11, d = 0.62. Rather, there was a two-way interaction between task condition and video type, F(1,29) = 5.94, p = 0.021, d = 0.91. The data indicate that asymmetry scores fluctuated across conditions for individuals who viewed the anxious video (−0.032 vs. −0.098 vs. 0.062). However, the EEG asymmetry scores shifted toward zero across conditions (0.278 vs. 0.125 vs. 0.018) for participants who viewed the non-anxious video.
As a final analysis of the strength of the relationship between social withdrawal and frontal EEG asymmetry, we examined the correlations between EEG asymmetry (baseline, viewing, preparing) and social withdrawal scores for the anxious and non-anxious video groups. For participants exposed to the anxious video, there was no significant correlation between social withdrawal and EEG asymmetry at baseline, r (19) = −0.02, p = 0.94, (Figure 3). However, a significant correlation emerged while viewing the anxious video, r (17) = −0.49, p = 0.044, that continued during speech preparation, r (17) = −0.68, p =0.003. Among the participants who watched the non-anxious video, the null EEG asymmetry-social withdrawal finding was evident across all three conditions: baseline, r (21) = −0.14, p=0.31, viewing, r (19) = −0.23, p=0.53, and preparation, r (19) = −0.31, p=0.19. These data indicate that individual differences in social withdrawal may emerge to shape manifest patterns of approach and withdrawal biases (as marked by shifts in EEG asymmetry scores) for individuals primed by a task-specific anxious stimulus,.
Figure 3.
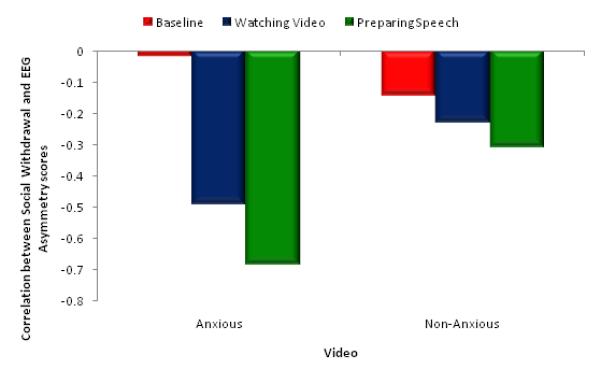
Correlations between social withdrawal values and EEG asymmetry scores for the participants exposed to the anxious and non-anxious videos
4. Discussion
Past studies indicate that socially withdrawn individuals display greater right frontal EEG activity at baseline (Schmidt, 1999), and that this frontal EEG asymmetry increases in step with individual levels of stress or negative affect (Davidson, et al., 2000). The current study aimed to build upon this work by examining specific changes in EEG asymmetry magnitude across a task reflecting many of the psychological and social concerns seen in withdrawn individuals.
Counter to our hypothesis, but in line with recent work (e.g., Miskovic et al., 2010), group differences in frontal EEG asymmetry were not evident specifically at baseline. Rather, frontal asymmetry differences emerged when assessed across all conditions. This would indicate that differential patterns of asymmetry at rest are most evident when comparing extreme trait or clinical differences across groups (Harmon-Jones et al., 2010). Group differences in our healthy sample were most striking when socially withdrawn participants were exposed to a stressful video exemplifying and illustrating potentially anticipated negative outcomes for the upcoming speech. Indeed, our correlational analysis suggests that this prime strengthened the relation between frontal EEG asymmetry and social withdrawal across the sample. Further analysis indicated that changes in frontal asymmetry across task condition were the result of overall changes in the balance of asymmetry, rather than a dramatic shift of activity in either the left or right frontal location.
Counter to previous work (Davidson et. al., 1979; Heller et. al., 1997; Schmidt & Fox, 1994), analysis of parietal sites (P3, P4) did not indicate significant differences in EEG asymmetry as a function of social withdrawal. Instead, we noted a two-way interaction such that parietal asymmetry was sensitive to task conditions (peaking during viewing) for the anxious video condition. Parietal asymmetry drifted toward zero for the participants viewing the non-anxious video, perhaps reflecting their gradual acclimation to the laboratory and task conditions. These findings may indicate that while EEG-affective context links are not exclusive to EEG measured from frontal recording sites, these locations may be more sensitive to individual differences in social withdrawal than EEG recorded from parietal sites.
These findings reinforce our current understanding of the physiological and motivational correlates of social withdrawal and anxiety. In particular, avoidance of social settings is often driven by a fear of future interactions and the anticipation that they may produce negative evaluations or affect (Kashdan & Herbet, 2001). Specifically, many socially withdrawn individuals feel that they are in constant danger of acting in an inept or inappropriate manner during social interactions, and they believe that these actions will lead to loss of status, worth, and ultimately, rejection by peers (Clark & Wells, 1995). Withdrawn individuals may therefore remove themselves from these situations rather than risk these anticipated negative consequences.
In addition, socially anxious individuals tend to use audience cues to provide them with indications of the quality of their performance, and place great emphasis on pleasing others (Kashdan & Herbert, 2001). Indications of negative performance increases anxiety level in this group which in turn negatively impacts social performance; thus making it more likely that they will indeed be rejected by their peers (Kashdan & Herbert, 2001). Socially withdrawn or anxious individuals also tend to compare themselves more negatively to others. They often perceive themselves as being less competent, less attractive and less socially accepted than others (Cunha, Soares, & Pinto-Gouveia, 2008). When withdrawn individuals engage in this type of negative self comparison they will seek to avoid other individuals as a way of reducing anxiety and stress thereby perpetuating and strengthening their maladaptive behavior pattern over time (Trower & Gilbert, 1989).
When negative interactions do occur, anxious and socially withdrawn individuals tend to overestimate the intensity or severity of the interaction (Teachman & Allen, 2007), will often ruminate on the interaction (Mellings & Alden, 2000), and will regularly point to intrinsic, rather than contextual reasons for the poor outcome (Rubin & Burgess, 2001; Rubin et al., 2009). These interpretive biases, coupled with a coping mechanism marked by avoidance, may in turn create a self-reinforcing cycle that results in individuals’ becoming less-and-less adept at social exchanges and more prone to social rejection and/or self-imposed social isolation (Rubin & Burgess, 2001; Rubin et al., 2009).
Another explanation of the findings in this study may be found in social learning theory. Mineka and Zinbarg (2006) suggest that social anxiety may arise through vicarious conditioning. The mere observation of another person being embarrassed or humiliated may be enough to induce fear or phobia of similar situations in the observer. In this model, people with pre-existing vulnerabilities to stress may be more affected by negative vicarious experiences. Socially withdrawn individuals may be more sensitive to vicarious learning experiences modeled by the negative affective video, thus triggering an immediate aversion to the same situation. This would then be reflected in EEG patterns as they prepare to engage in the same activity.
This model would explain why only participants high in social withdrawal who were exposed to the negative video produced an acute stress response. Participants high in social withdrawal that were exposed to the positive affective video would have a positive vicarious learning experience. In addition, those participants low in social withdrawal are, according to this model, less sensitive to the effects of vicarious learning and therefore would not be as affected by the negative or positive presentation as compared to those participants with high levels of social withdrawal. Further examination of the underlying mechanisms leading to the observed physiological stress reaction in the socially withdrawn participants is warranted.
In the current study, the use of an affectively-charged anxious video exemplar, coupled with having participants actively rate the videos, may have substantiated the underlying concerns of the socially withdrawn participants. This substantiation of underlying concerns is evidenced through the changes in frontal EEG asymmetry observed across task conditions. Stress levels in socially anxious individuals are often influenced by the individual’s hypothesized reception by others, and through a negative self-comparison of the individual’s abilities to others. The negative affective cue (the video in our task) potentially resulted in an increased stress response by the socially withdrawn individuals by heightening their expectation of the likelihood of negative evaluation and criticism of their performance.
Non-socially withdrawn individuals are less negative in their expectation of their performance reception, and place less emphasis on audience reaction than socially withdrawn individuals (Rapee & Heimberg, 1997), therefore the negative cue should have disproportionately affected the stress level of the socially withdrawn individuals as compared to the non-socially anxious individuals. Indeed in our study, frontal EEG asymmetry values only shifted in participants who displayed social withdrawal and were exposed to the negative video. This illustrates the disproportionate sensitivity of this group to cues regarding both positive and negative task performance and the perceived stress of the task. The increase in right frontal EEG activity relative to the left observed as task stress increased indicates that frontal EEG asymmetry may not only act as a neural correlate of trait levels of anxiety or depression, but also reflects acute levels of distress a person may be experiencing.
The results of the current study should be examined in light of several limitations. First, our study examined a non-clinical sample of socially withdrawn subjects. It is therefore not clear how our results would generalize to individuals with more extreme social withdrawal or clinical social anxiety. Second, social withdrawal groups were created using a self-report measure. Future studies incorporating behavioral observations or clinical screening may help clarify the reach of our findings. Lastly, we collected EEG measures during a single te st session. Therefore, it was not possible from our results to determine whether the observed relations between frontal EEG asymmetry and social withdrawal are stable over long periods of time.
The current study was specifically designed to provoke concerns central to persons prone to social withdrawal. The study demonstrates that the affective context of a task affects the relationship between EEG asymmetry responses to stress in individuals with social withdrawal. A better understanding of this relation may provide important insights into the development and response of socially withdrawn individuals to stress, contributing to potential diagnostic or treatment procedures.
Acknowledgments
Role of the Funding Source Support for study preparation was provided by National Institute of Mental Health grants MH073569 and MH094633 to Koraly Perez-Edgar. The National Institute of Mental Health did not play a role in creating the study design, in the collection of study data, or in the analysis and interpretation of study data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson K, Bohlin G, Hagekull B. Early temperament and stranger wariness as predictors of social inhibition in 2-year-olds. The British Psychological Society. 1999;17:421–434. doi: 10.1348/026151099165375. [Google Scholar]
- Baving L, Laucht M, Schmidt M. Frontal brain activation in anxious school children. Journal of Child Psychology and Psychiatry. 2002;43:265–274. doi: 10.1111/1469-7610.00019. doi: 10.1111/1469-7610.00019. [DOI] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker D, Rosenbaum J, Hérot C, Friedman D, Snidman N, Kagan J, Faraone S. Further evidence of association between behavioral inhibition and social anxiety in children. American Journal of Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. Retrieved from http://ajp.psychiatryonline.org/index.dtl. [DOI] [PubMed] [Google Scholar]
- Buss A, Schumacher J, Dolski I, Kalin N, Goldsmith H, Davidson R. Right frontal brain activity, cortisol, and withdrawal behavior in 6-month-old infants. Neuroscience. 2003;117:11–20. doi: 10.1037//0735-7044.117.1.11. doi: 10.1037/0735-7044.117.1.11. [DOI] [PubMed] [Google Scholar]
- Calkins S, Fox NA. The relations among infant temperament, security of attachment, and behavioral inhibition at twenty-four months. Child Development. 1992;63:1456–1472. doi:10.1111/j.1467-8624.1992.tb01707.x. [PubMed] [Google Scholar]
- Calkins S, Fox NA. Self regulatory processes in early personality development: A multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology. 2002;14:477–498. doi: 10.1017/s095457940200305x. doi: 10.1017/S095457940200305X. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell J, Theodore R, Moffitt T. Children’s behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality. 2003;71:495–513. doi: 10.1111/1467-6494.7104001. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Cheek J, Buss A. Shyness and sociability. Journal of Personality and Social Psychology. 1981;41:330–339. doi:10.1037/0022-3514.41.2.330. [Google Scholar]
- Chronis-Tuscano A, Degnan K, Pine D, Perez-Edgar K, Henderson H, Diaz Y, Raggi V, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Wells A. A cognitive model of social phobia. In: Heimberg R, Liebowitz M, Hope D, Schneier F, editors. Social Phobia, Diagnosis, Assessment and Treatment. The Guilford Press; New York: 1995. pp. 69–93. [Google Scholar]
- Cunha M, Soares I, Pinto-Gouveia J. The role of individual temperament, family and peers in social anxiety disorder: A controlled study. International Journal of Clinical and Health Psychology. 2008;8:631–633. Retrieved from http://www.aepc.es/ijchp/index.php?coid=English. [Google Scholar]
- Davidson R. Affective style, psychopathology, and resilience: brain mechanisms and plasticity. American Psychologist. 2000;55:1196–1214. doi: 10.1037//0003-066x.55.11.1196. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11280935. [DOI] [PubMed] [Google Scholar]
- Davidson R. What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biological Psychology. 2004;67:219–233. doi: 10.1016/j.biopsycho.2004.03.008. doi: 10.1016/j.biopsycho.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Davidson R, Fox NA. Asymmetrical brain activity discriminates between positive and negative affective stimuli in human infants. Science. 1982;218:1235–1237. doi: 10.1126/science.7146906. doi: 10.1126/science.7146906. [DOI] [PubMed] [Google Scholar]
- Davidson R, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. Journal of Abnormal Psychology. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. Retrieved from http://www.apa.org/pubs/journals/abn/ [DOI] [PubMed] [Google Scholar]
- Davidson R, Henriques J. Regional brain function in sadness and depression. In: Borod J, editor. The Neuropsychology of Emotion. Oxford University Press; New York: 2000. pp. 269–297. [Google Scholar]
- Davidson R, Marshall J, Tomarken A, Henriques J. While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry. 2000;47:85–95. doi: 10.1016/s0006-3223(99)00222-x. doi: 10.1016/s0006-3223(99)00222-X. [DOI] [PubMed] [Google Scholar]
- Davidson R, Schwartz G, Saron C, Bennett J, Goleman D. Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology. 1979;16:202–203. Retrieved from http://www.blackwellpublishing.com/journal.asp?ref=0048-5772. [Google Scholar]
- Diego M, Field T, Hernandez-Reif M. Bis/Bas scores are correlated with frontal EEG asymmetry in intrusive and withdrawn depressed mothers. Infant Mental Health Journal. 2001;22:665–675. doi: 10.1002/imhj.1025. [Google Scholar]
- Field T, Martinez A, Nawrocki T, Pickens J, Fox N, Schanberg S. Music shifts frontal EEG in depressed adolescents. Adolescence. 1998;129:109–116. [PubMed] [Google Scholar]
- Fox NA. Psychophysiological correlates of emotional reactivity during the first year of life. Developmental Psychology. 1989;25:364–372. Retrieved from http://www.apa.org/pubs/journals/dev/ [Google Scholar]
- Fox NA, Copelan R, Rubin K, Porges S, Calkins S, Long J, Marshall T, Stewart S. Frontal activation asymmetry and social competence at four years of age. Child Development. 1995;66:1770–1784. doi: 10.1111/j.1467-8624.1995.tb00964.x. [PubMed] [Google Scholar]
- Fox NA, Davidson R. Electroencephalogram asymmetry in response to the approach of a stranger and maternal separation in 10-month old infants. Developmental Psychology. 1987;23:233–240. Retrieved from http://www.apa.org/pubs/journals/dev/ [Google Scholar]
- Fox NA, Henderson H, Rubin K, Calkins S, Schmidt L. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72:1–21. doi: 10.1111/1467-8624.00262. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Giesen M, Rollison M. Guilt knowledge versus innocent associations: Effects of trait anxiety and stimulus context on skin conductance. Journal of Research in Personality. 1980;14:1–11. doi: 10.1016/0092-6566(80)90035-5. [Google Scholar]
- Harmon-Jones E, Gable P, Peterson C. The role of frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology. 2010;84:451–462. doi: 10.1016/j.biopsycho.2009.08.010. doi: 10.1016/j.biopsycho.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Sigelman J. State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology. 2001;80:797–803. doi: 10.1037//0022-3514.80.5.797. [PubMed] [Google Scholar]
- Heller W, Nitschke J, Etienne M, Miller G. Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology. 1997;106:376–385. doi: 10.1037//0021-843x.106.3.376. doi: 10.1037/0021-843X.106.3.376. [DOI] [PubMed] [Google Scholar]
- Henriques J, Davidson R. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. Retrieved from http://www.apa.org/pubs/journals/abn/ [DOI] [PubMed] [Google Scholar]
- Hoffman S, Moscovitch D, Kim H. Autonomic correlates of social anxiety and embarrassment in shy and non-shy individuals. International journal of Psychophysiology. 2006;61:134–142. doi: 10.1016/j.ijpsycho.2005.09.003. doi: 10.1016/j.ijpsycho.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick S, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55:2212–2225. Retrieved from http://www.wiley.com/bw/journal.asp?ref=0009-3920. [Google Scholar]
- Kagan J, Reznick S, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. Retrieved from http://www.wiley.com/bw/journal.asp?ref=0009-3920. [PubMed] [Google Scholar]
- Kashdan T, Herbert J. Social anxiety disorder in childhood and adolescence: Current status and future directions. Clinical Child and Family Psychology Review. 2001;4:37–61. doi: 10.1023/a:1009576610507. doi: 10.1023/A:1009576610507. [DOI] [PubMed] [Google Scholar]
- Mellings T, Alden L. Cognitive processes in social anxiety: The effects of self-focus, rumination, and anticipatory processing. Behaviour Research and Therapy. 2000;38:243–257. doi: 10.1016/s0005-7967(99)00040-6. doi: 10.1016/s0005-7967(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on anxiety disorders: It’s not what you thought it was. American Psychologist. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Ashbaugh A, Santesso D, McCabe R, Antony M, Schmidt L. Frontal brain oscillations and social anxiety: A cross-frequency spectral analysis during baseline and speech anticipation. Biological Psychology. 2010;83:125–132. doi: 10.1016/j.biopsycho.2009.11.010. doi: 10.1016/j.biopsycho.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Newman D, Caspi A, Moffitt T, Silva P. Antecedents of adult interpersonal functioning: Effects of individual differences in Age 3 temperament. Developmental Psychology. 1997;33:206–217. doi: 10.1037//0012-1649.33.2.206. Retrieved from http://www.apa.org/pubs/journals/dev/ [DOI] [PubMed] [Google Scholar]
- Perez-Edgar K, Fox NA. A behavioral and electrophysiological study of children’s selective attention under neutral and affective conditions. Journal of Cognition and Development. 2005;6:89–118. Retrieved from http://www.cogdevsoc.org/jcd/jcd-home.php. [Google Scholar]
- Quirk G, Likhtik E, Pelletier J, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. Retrieved from http://www.jneurosci.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin K, Asendorpf J, editors. Social withdrawal inhibition, and shyness in childhood. Lawrence Erlbaum Associates; Hillsdale, NJ: 1993. [Google Scholar]
- Rubin K, Burgess K. Social withdrawal. In: Vasey MW, Dadds MR, editors. The developmental psychopathology of anxiety. Oxford University Press; Oxford, UK: 2001. pp. 407–434. [Google Scholar]
- Rubin K, Coplan R. Paying attention to and not neglecting social withdrawal and social isolation. Merrill-Palmer Quarterly. 2004;50:506–534. [Google Scholar]
- Rubin K, Coplan R, Bowker J. Social withdrawal in childhood. Annual Review of Psychology. 2009;60:141–171. doi: 10.1146/annurev.psych.60.110707.163642. doi: 10.1353/mpq.2004.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt L. Frontal brain electrical activity in shyness and sociability. Psychological Science. 1999;10:316–320. doi: 10.1111/1467-9280.00161. [Google Scholar]
- Schmidt L, Buss A. Understanding shyness: Four questions and four decades of research. In: Rubin K, Coplan R, editors. The Development of Shyness and Social Withdrawal. The Guilford Press; New York, NY: 2010. pp. 23–41. [Google Scholar]
- Schmidt L, Fox N. Patterns of cortical electrophysiology and autonomic activity in adults’ shyness and sociability. Biological Psychology. 1994;38:183–198. doi: 10.1016/0301-0511(94)90038-8. doi: 10.1016/0301-0511(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schmidt L, Fox N, Shulkin J, Gold P. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Developmental Psychobiology. 1999;35:119–135. doi: 10.1002/(SICI)1098-2302(199909) [PubMed] [Google Scholar]
- Schwartz C, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Silva J, Pizzagalli D, Larson C, Jackson D, Davidson R. Frontal brain asymmetry in restrained eaters. Journal of Abnormal Psychology. 2002;111:676–681. doi: 10.1037//0021-843x.111.4.676. doi: 10.1037//0021-843X.111.4.676. [DOI] [PubMed] [Google Scholar]
- Teachman B, Allen J. Development of social anxiety: Social interaction predictors of implicit and explicit fear of negative evaluation. Journal of Abnormal Child Psychology. 2007;35:63–78. doi: 10.1007/s10802-006-9084-1. doi: 10.1007/s10802-006-9084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeaua R, Jorgensena R, Kima S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. doi:10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]
- Trower P, Gilbert P. New theoretical conceptions of social anxiety and social phobia. Clinical Psychology Review. 1989;9:19–35. doi: 10.1016/0272-7358(89)90044-5. [Google Scholar]
- van Peer J, Roelofs K, Spinhoven P. Cortisol administration enhances the coupling of midfrontal delta and beta oscillations. International Journal of Psychophysiology. 2008;67:144–150. doi: 10.1016/j.ijpsycho.2007.11.001. doi:10.1016/j.ijpsycho.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Westenberg M, Bokhorst C, Miers A, Sumter S, Kallen V, van Pelt J, Blöte A. A prepared speech in front of a pre-recorded audience: Subjective, physiological, and neuroendocrine responses to the Leiden Public Speaking Task. Biological Psychology. 2009;82:116–124. doi: 10.1016/j.biopsycho.2009.06.005. doi:10.1016/j.biopsycho.2009.06.005. [DOI] [PubMed] [Google Scholar]


