Abstract
Gadd45 genes have been implicated in stress signaling in response to physiological or environmental stressors, which results in either cell cycle arrest, DNA repair, cell survival and senescence, or apoptosis. Evidence accumulated implies that Gadd45 proteins function as stress sensors is mediated by a complex interplay of physical interactions with other cellular proteins that are implicated in cell cycle regulation and the response of cells to stress. These include PCNA, p21, cdc2/cyclinB1, and the p38 and JNK stress response kinases. Recently we have taken advantage of gadd45a and gadd45b deficient mice to determine the role gadd45a and gadd45b play in the response of bone marrow (BM) cells to genotoxic stress. Myeloid enriched BM cells from gadd45a and gadd45b deficient mice were observed to be more sensitive to ultraviolet radiation (UVC), VP-16 and daunorubicin (DNR) induced apoptosis compared to wild-type (wt) cells. The increased apoptosis in gadd45a and gadd45b deficient cells was evident also by enhanced activation of caspase-3 and PARP cleavage and decreased expression of cIAP-1, Bcl-2, Bcl-xL compared to wt cells. Reintroduction of gadd45 into gadd45 deficient BM cells restored the wt apoptotic phenotype. Both gadd45a and gadd45b deficient BM cells also displayed defective G2/M arrest following exposure to UVC and VP-16, but not to DNR, indicating the existence of different G2/M checkpoints that are either dependent or independent of gadd45. Additional work conducted in this laboratory has shown that in hematopoietic cells exposed to UV radiation gaddd45a and gadd45b cooperate to promote cell survival via two distinct signaling pathways involving activation of the Gadd45a-p38-NF-kB mediated survival pathway and Gadd45b mediated inhibition of the stress response MKK4-JNK pathway (59). These data reveal novel mechanisms that mediate the pro-survival functions of gadd45a and gadd45b in hematopoietic cells following UV irradiation. Taken together, these findings identify gadd45a and gadd45b as anti-apoptotic genes that increase the survival of hematopoietic cells following exposure to UV radiation and certain anticancer drugs. This knowledge should contribute to a greater understanding of the genetic events involved in the pathogenesis of different leukaemias and response of normal and malignant hematopoietic cells to chemo and radiation therapy. These observations set the stage to evaluate, in clinically relevant settings, the impact that the status of gadd45a and gadd45b might have on the efficacy of DNR or VP-16 in killing leukemic cells.
Overview
Gadd45 Functions
The gadd45 family of genes [originally termed gadd45, MyD118, & CR6 and now referred to as Gadd45a, Gadd45b, & Gadd45g, respectively (1-2)] encodes for small (18kd), evolutionarily conserved proteins that are highly homologous to each other (55%-57% overall identity at the amino acid level), are highly acidic, and are primarily, but not exclusively, localized within the cell nucleus. (1-3).
Gadd45 family members are rapidly induced by genotoxic agents (5-7) as well as by terminal differentiation and hematopoietic cytokines (3, 8-11). In recent years evidence has emerged that the proteins encoded by these genes play pivotal roles as stress sensors, either dependent or independent of p53 (3, 12-16a).
Data accumulated suggests that Gadd45 proteins serve similar, but not identical, functions along different apoptotic and growth inhibitory pathways. We have observed that only Gadd45b is induced upon TGF-b1 mediated apoptosis (17); in contrast, only gadd45a is a p53 target gene (18-20). All three genes are induced with different expression kinetics during terminal hematopoietic differentiation 3). Distinct expression patterns for these genes were observed also in a variety of murine tissues (3,9 8). Importantly, individual members of the Gadd45 family are differentially induced by a variety of genotoxic and environmental stress agents, indicating that each gene is induced by a distinct subset of environmental and physiological stresses. To what extent the function of each of Gadd45 proteins is unique or overlaps with the functions of the other proteins, is still unclear.
Gadd45 proteins have been implicated in cycle arrest (3,5,21-22), DNA repair (11,23-24), cell survival (24,25-29) and apoptosis (1,30-34)
Cell cycle arrest
In human endothelial & fibroblast cells inhibiting endogenous Gadd45 expression by antisense gadd45 impaired the G2/M checkpoint following exposure to either UV radiation or MMS (2). In addition, microinjecting a gadd45a expression vector into primary human fibroblasts arrested the cells at the G2/M boundary of the cell cycle (21). Given that gadd45a is a transcriptional target to p53 activation, these observations implicate Gadd45a in p53 mediated G2/M cell cycle arrest in response to certain genotoxic stress agents (21). The genomic instability observed in gadd45a null mice (22) may reflect perturbations in G2/M cell cycle progression &/or impaired DNA repair. In another study (35), ectopic deregulated expression of CR6 (gadd45g) in HeLa cells had little effect on HeLa cell growth under normal culture conditions; however, following serum withdrawal the G2/M transition was blocked and resulted in endo-reduplication. In contrast, under normal culture conditions, ectopic expression of any one of the Gadd45 proteins in USO2 cells resulted in blocking either G1/S or G2/M transitions (35). In this laboratory it was observed that IPTG-induced ectopic expression of any of the gadd45 genes in p53 null H1299 retarded cell growth and increased accumulation of cells in the G1 phase of the cell cycle (36).
Apoptosis
Several observations are consistent with a role for Gadd45 proteins in programmed cell death. For example, blocking gadd45b expression, by either antisense or using a KO mouse model, abolished TGF-b induced cell death, thereby implicating gadd45b as a positive modulator of TGF-b induced apoptosis (17,34). IPTG-inducible ectopic expression of gadd45b accelerated TGF-b induced apoptosis in M1 cells (9), and significantly enhanced apoptosis in H1299 lung carcinoma cells (36). Also, BRCA-1 induced gadd45a has been implicated in apoptosis of breast cancer cells (32), whereas transient ectopic expression of all three Gadd45 proteins has been implicated in apoptosis of HeLa cells. Furthermore, gadd45a has been reported to induce cell cycle arrest and apoptosis in uv-irradiated keratinocytes (30).
Survival
Interestingly, in apparent contradiction to the role Gadd45 proteins play in apoptosis, other observations suggest that Gadd45 proteins also function in cell survival, protecting cells from genotoxic stress (27). Using gadd45 KO MEFs (24) and RKO cells expressing antisense gadd45 RNA in clonogenic survival assays, has shown that deficiency of gadd45 increases the sensitivity of cells to killing by UV irradiation or cisplatin (25). Recently, Gadd45b has been suggested to play a role in TNFa-NF-kB mediated cell survival (26), and to mediate the protective effects of CD40 co-stimulation against Fas-induced apoptosis (37). The role Gadd45 proteins play in DNA repair (11,23-24) is compatible with a survival function.
Gadd45 Mode of Action: Interaction with partner proteins
Evidence has been obtained that Gadd45 proteins display a complex array of physical interactions with other cellular proteins, and these interactions are implicated in cell cycle regulation and the response of cells to stress.
PCNA
Work conducted in our laboratory and in the laboratory of Dr. Albert Fornace (Georgetown University) has shown that all three Gadd45 proteins interact with PCNA (11,23,33), a nuclear protein that plays a central role in DNA repair and replication (38-39 & therein). It was suggested that interaction of Gadd45a with PCNA is involved in nucleotide excision repair (NER) of DNA (24). Whether the ability of either Gadd45b or GAdd45g to interact with PCNA serves a role in DNA repair remains to be explored. Recently, we have obtained data suggesting that Gadd45a and Gadd45b co-operate in DNA repair (Azam et al., unpublished. & 11).
Cdc2/cyclinB1
Gadd45a also interacts with cdc2 (Cdk1), inhibiting of the kinase activity of the cdc2/cyclinB1 complex (2,40-42) that is known to play a key role in transition of cells from the G2 to the M phase of the cell cycle (43-44). This interaction has been implicated in Gadd45a mediated activation of the G2/M checkpoint in response to certain genotoxic stress agents (21). Recently, we have shown that both Gadd45b and Gadd45g also interact with the cdc2/cyclinB1 complex and inhibit cdc2 kinase activity following exposure of cell to genotoxic stress (2).
p21
We have shown that all three Gadd45 proteins interact with the cyclin dependent kinase inhibitor p21 (11,33), a p53 target gene (45) implicated in G1/S (46-49) and G2/M (50) cell cycle arrest. The role of Gadd45 protein interactions with p21 is still unclear.
MEKK4/p38/JNK
Gadd45 proteins interact with and activate the stress-responsive MEKK4/MTK1 kinase, which is an upstream activator of the p38/JNK MAP kinase pathways implicated in environmental stress induced responses (9). Evidence has been obtained that Gadd45 activate p38/JNK signaling and cytokine production in effector T cells (51-52), whereas Gadd45a activation of p38/JNK in keratinocytes has been implicated in apoptosis (30). The role of the interactions of Gadd45 proteins with MEKK4 in activation of JNK/p38 stress response pathways needs to be established (53-54). We have data indicating that all three Gadd45 proteins also directly bind to and activate p38 kinase (p38a) (55). The role of the interactions of Gadd45 proteins with p38 in stress signaling is not clear; it is possible that it plays an ancillary function to Gadd45 interactions with MEKK4.
Gadd45 homo- and hetero-interactions
Gadd45 proteins are also capable of homo- & hetero-oligomerization (56), reminiscent of p53 oligomerization. The role played by Gadd45 homo- and hetero-oligomers in interaction with partner proteins and in mediation of gadd45 functions has not been established, and is the subject of current investigations in my and Dr. Albert Fornace's laboratories.
Gadd45 null mice
Understanding how gadd45 genes function and interact in normal cells in response to stress can be accomplished using gene targeting, to first generate mouse strains that are null for each of these genes, which then can be used to generate strains of mice that are null for two, and eventually for all 3, family members. Mice that are deficient for gadd45b have been generated in this laboratory (29), whereas gadd45a null mice have been generated in the laboratory of Dr. Albert Fornace at the NCI, NIH. (22). Mice null for gadd45g have been generated recently in collaboration with the Fornace group. Mice null for each gadd45 gene have been used to generated mice null for any two gadd45 genes. These mice are viable but have multiple defects in several neuronal and hematopoietic compartments (unpublished). Mice that are null for all three gadd45 genes are very sick and colonies are difficult to maintain. Thus, efforts are being made to generate a mouse strain in which deletion of one of the three Gadd45 genes (gadd45a) can be temporally controlled, using the Cre-loxP recombination system.
Hematopoietic Cells from Gadd45a and Gadd45b Deficient Mice are Sensitized to Genotoxic-Stress Induced Apoptosis
Recently we have taken advantage of gadd45a and gadd45b deficient mice to determine the role gadd45a and gadd45b play in the response of bone marrow (BM) cells to genotoxic stress. Myeloid enriched BM cells from gadd45a and gadd45b deficient mice were observed to be more sensitive to ultraviolet radiation (UVC), VP-16 and daunorubicin (DNR) induced apoptosis compared to wild-type (wt) cells (27).
The increased apoptosis in gadd45a and gadd45b deficient cells was evident also by enhanced activation of caspase-3 and PARP cleavage and decreased expression of cIAP-1, Bcl-2, Bcl-xL compared to wt cells (27).
Reintroduction of gadd45 into gadd45 deficient BM cells restored the wt apoptotic phenotype (27).
Both gadd45a and gadd45b deficient BM cells also displayed defective G2/M arrest following exposure to UVC and VP-16, but not to DNR, indicating the existence of different G2/M checkpoints that are either dependent or independent of gadd45 (27).
Taken together, these findings identify gadd45a and gadd45b as anti-apoptotic genes that increase the survival of hematopoietic cells following exposure to UV radiation and certain anticancer drugs.
Our findings are surprising in light of previous data that have identified gadd45 genes as pro-apoptotic. It was shown that ectopic expression of gadd45a or gadd45b sensitizes H1299 human lung carcinoma cells to apoptosis induced by genotoxic stress (36). Recently, by taking advantage on the gadd45b-/- mice, it has been documented that gadd45b-/- hepatocytes are resistant to TGF-b mediated apoptosis (34), which was consistent with our previous observations (17). Also, using the gadd45a-/-mice, it has been reported that gadd45a promotes UV induced apoptosis in skin keratinocytes (57). Additional evidence for a pro-apoptotic function of gadd45a and gadd45g has been documented using either over-expression of these proteins in prostate and breast cancer cell lines or siRNA-mediated knockdown that was observed to block IkB mediated apoptosis in these cell lines (58). On the other hand, our results are consistent with an earlier report where it was shown that p53 null MEFS that lack gadd45a are sensitized to UV and cisplatin induced cell death (24). Also, a recent study on B cells showed that gadd45b mediates the protective effects of CD40 co-stimulation against Fas-induced apoptosis (37). Thus, it is possible that the stress stimulus encountered, the cell type, and interaction with other proteins that modulate gadd45 function, ultimately determine whether the outcome will be DNA repair and cell survival, or apoptotic cell death. This notion is supported by our observation that the gadd45b-/- BM cells, though sensitized to genotoxic stress induced apoptosis, are largely resistant to TGF-b induced apoptosis compared to wt BM cells.
Generation of gadd45a and gadd45b null mice allowed us to address the role of gadd45 in apoptosis. We showed that induction of an important anti-apoptotic gene, cIAP-1 along with bcl-2 and bcl-xL are reduced in both gadd45 null mice. cIAP-1 inhibits the active forms of caspase-3 which are responsible for PARP cleavage. This might explain the increased sensitivity of gadd45 null BM cells compared to wt cells to genotoxic stress induced apoptosis. Ongoing studies are targeted at identifying the signaling pathways which gadd45a and gadd45b utilize to protect hematopoietic cells from UV, VP16 or DNR inflicted genotoxic stress.
Several earlier studies have demonstrated that gadd45a and gadd45b mediate a G2/M cell cycle checkpoint in both human and murine cells (21,40). In the present study, cell cycle analysis of the gadd45a and gadd45b BM null cells in response to various DNA damaging agents indicated that these cells are deficient in both UV and VP-16 induced G2/M arrest. This data is consistent with an earlier report showing that anti-sense gadd45a expressing human colon carcinoma cells are defective in the UV induced G2/M checkpoint (2). In contrast, when either gadd45a or gadd45b deficient BM cells were treated with DNR they still retained a functional G2/M checkpoint. Thus, the DNR-mediated G2/M checkpoint pathway differs from the UV and VP-16 mediated pathways. Wang et al, reported that gadd45a deficient cells still retain a functional G2/M checkpoint after IR yet they are deficient in UV and MMS induced G2/M (2 21,40). These observations are consistent with the existence of multiple G2/M checkpoints that are stimulus specific, and are either gadd45 dependent or gadd45 independent. Our data suggests that both gadd45a and gadd45b are required in hematopoietic cells for a normal G2/M checkpoint in response to certain types of DNA damage. Further studies are needed to decipher the underlying mechanisms. Nevertheless, it is plausible that the increased apoptotic response triggered by UV or VP-16 in gadd45a and gadd45b deficient BM cells also reflects the failure of these cells to arrest in G2/M to allow repair of damaged DNA.
Overall, these data identify both gadd45a and gadd45b as pro-survival gene(s), that protect hematopoietic cells against certain DNA damage agents, and suggest that cells disabled for either gene may display higher susceptibility to killing by UV and certain anticancer drugs. Together, these findings demonstrate that loss of either gadd45a or gadd45b function may have a profound impact on the apoptotic efficacy of certain anti cancer agents. This knowledge should contribute to a greater understanding of the genetic events involved in the pathogenesis of different leukaemias and response of normal and malignant hematopoietic cells to chemo and radiation therapy.
Gadd45a and Gadd45b Protect Hematopoietic Cells from UV Induced Apoptosis via Distinct Signaling Pathways Including P38 Activation and JNK Inhibition
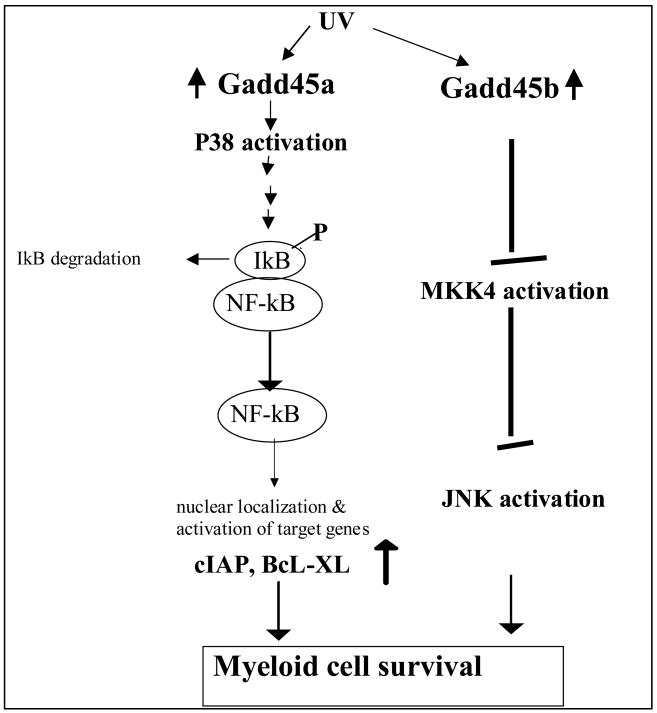
Additional work conducted in this laboratory has shown that in hematopoietic cells exposed to UV radiation gaddd45a and gadd45b cooperate to promote cell survival via two distinct signaling pathways involving activation of the Gadd45a-p38-NF-kB mediated survival pathway and Gadd45b mediated inhibition of the stress response MKK4-JNK pathway (59) (Figure 1). These data reveal novel mechanisms that mediate the pro-survival functions of gadd45a and gadd45b in hematopoietic cells following UV irradiation.
Figure 1.
Effect of ultraviolet (UV) light on Gadd45a and Gadd45b (see text for futher explanation).
Previous evidence has shown that gadd45a mediates activation of p38 by TGFb to promote hepatocyte cell death (34). Furthermore Gadd45a mediated activation of p38 has been linked to apoptosis induced by UV in keratinocytes. Our data provides first evidence for crosstalk between Gadd45a mediated activation of p38 and the NFkb pathway, which is known to play a major role in cell survival (60-61). Following UV irradiation Gadd45a is essential for p38 activation. In addition, Gadd45a mediated activation of p38 results in phosphorylation and degradation of IkB, which in turn allows nuclear localization of NF-kB and activation of its target genes. This Gadd45a-p38-NF-kB crosstalk pathway differs from another recently documented crosstalk between p38 and NF-kB, which had shown that p38 modulates the transcriptional activity of NF-kB via phosphorylation of RelA (62). Our data regarding a Gadd45a-p38-NF-kB survival pathway that protects hematopoietic cells from UV induced apoptosis differ sharply from previous work showing that p38 activation is linked to cell death in endothelial and epithelial cells (30,32). These data are, however, consistent with other studies showing that activation of p38 is linked to survival in hematopoietic cells (64-65).
Our data indicate that Gadd45b mediated inhibition of UV induced JNK activity cooperates with p38 activation in promoting hematopoietic cell survival. Previously, Gadd45b induction by NF-kB and subsequent inhibition of JNK activity has been implicated in MEF survival in response to TNFa (26). In contrast, our studies using Gadd45b-/- MEFs have shown that Gadd45b deficiency does not prolong JNK activity in response to TNFa (29). The reason for this discrepancy is not clear. In this work, using BM cells deficient for Gadd45b, we did obtain data indicating that UV induced Gadd45b blunts JNK activity and, thereby, promotes hematopoietic cell survival. Our data show that in myeloid cells gadd45b targets MKK4 rather than MKK7 (59) as the upstream regulator of JNK activity. Whereas prolonged JNK activation has been linked to cell death, it has been suggested that transient activation of JNK plays a role in cell survival (66). Whether this may be the case in hematopoietic cells remains to be determined.
In conclusion, we have shown that in hematopoietic cells Gadd45a and Gadd45b co-operate via utilizing two distinct signaling paths, namely p38 activation and JNK inhibition, to protect hematopoietic cells from genotoxic stress-induced cell death. Disrupting either pathway increases the apoptotic response of hematopoietic cells to UV treatment. Pretreatment of gadd45b-/- BM cells with the p38 inhibitor did not alter the apoptotic response to UV, although similar treatment of WT BM significantly increased the apoptotic response. This data suggests that although each pathway is necessary, the protective effects of the two pathways are not additive. Using mice deficient for both gadd45a and gadd45b, currently being bred, would further clarify this issue.
Taken together, this data and previous observations where Gadd45 mediated activation of p38 and JNK has been implicated in cell death, indicate that the nature of the stress stimulus encountered, its magnitude, and gadd45 interaction with proteins that modulate its function, as well as the specific cell type, ultimately determine whether the outcome will be cell cycle arrest, DNA repair and cell survival, or apoptotic cell death. In this context it will be of interest to determine the mechanisms involved in protecting hematopoietic cells from VP16 and DNR induced apoptosis on the one hand, and on the other the mechanisms that promote TGFb induced apoptosis.
Conclusions & Future Prospects
Gadd45 genes have been implicated in stress signaling in response to physiological or environmental stressors, which results in either cell cycle arrest, DNA repair, cell survival and senescence, or apoptosis. Evidence accumulated implies that Gadd45 proteins function as stress sensors is mediated by a complex interplay of physical interactions with other cellular proteins that are implicated in cell cycle regulation and the response of cells to stress. These include PCNA, p21, cdc2/cyclinB1, and the p38 and JNK stress response kinases. What deterministic factors dictate whether Gadd45 and partner proteins function in either cell survival or apoptosis remines to be determined. An attractive working model to consider is that the extent of cellular/DNA damage, in a given cell type, dictates the association of different Gadd45 proteins with particular partner proteins, which determines the outcome. ongoig research is aimed at testing this conjecture.
Acknowledgments
This paper is based on a presentation at the Seventh International Workshop on Molecular Aspects of Myeloid Stem Cell Development and Leukemia in Annapolis, Maryland May 13-16, 2007, sponsored by The Leukemia & Lymphoma Society
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Takekawa M, Saito H. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4/MAPKKK. Cell. 1998;95:521–30. doi: 10.1016/s0092-8674(00)81619-0. [DOI] [PubMed] [Google Scholar]
- 2.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. GADD45b and GADD45g are cdc2/cyclinB1 kinase inhibitors with a role in S and G2/M cell cycle checkpoints induced by genotoxic stress. J Cell Physiol. 2002;192:327–38. doi: 10.1002/jcp.10140. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Bae I, Krishnaraju K, Azam N, Fan W, Smith K, Hoffman B, Liebermann DA. CR6: A third member in the MyD118 & Gadd 45 gene family which functions in negative growth control. Oncogene. 1999;18:4899–4907. doi: 10.1038/sj.onc.1202885. [DOI] [PubMed] [Google Scholar]
- 4.Carrier F, Smith ML, Bae I, Kilpatrick KE, Lansing TJ, Chen CY, Engelstein M, Friend SH, Henner D, Gilmer TM, Kastan MB, Fornace AJ., Jr Characterization of human Gadd45, a p53-regulated protein. J Biol Chem. 1995;269:32672–7. [PubMed] [Google Scholar]
- 5.Beadling C, Johnson KW, Smith KA. Isolation of interleukin 2-induced immediate-early genes. Proc Natl Acad Sci USA. 1993;90:2719–23. doi: 10.1073/pnas.90.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papathanasiou MA, Kerr NC, Robbins JH, McBride OW, Alamo I, Jr, Barrett SF, Hickson ID, Fornace AJ., Jr Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989;10:4196–203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornace AJ, Jr, Nebert DW, Hollander MC, Luethy JD, Papathanasiou M, Fargnoli J, Holbrook NJ. Induction by ionizing radiation of the gadd45 gene in cultured human cells: lack of mediation by protein kinase C. Mol Cell Biol. 1991;11:1009–16. doi: 10.1128/mcb.11.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan Q, Lord KA, Alamo I, Jr, Hollander MC, Carrier F, Ron D, Kohn KW, Hoffman B, Liebermann DA, Furnace AJ., Jr The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol Cell Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W. Thesis. Temple University; Jul, 2000. The MyD118/Gadd4/CR6 gene gamily in negative growth control. [Google Scholar]
- 10.Abdollahi A, Hoffman-Liebermann B, Liebermann D. Sequence and expression of a cDNA encoding MyD118: A novel myeloid differentiation primary response gene induced by multiple cytokines. Oncogene. 1990;6:165–167. [PubMed] [Google Scholar]
- 11.Vairapandi M, Balliet AG, Hoffman B, Liebermann DA. The differentiation primary response gene MyD118, related to GADD45, encodes for a nuclear protein which interacts with PCNA and p21WAF1/CIP1. Oncogene. 1996;12:2579–2594. [PubMed] [Google Scholar]
- 12.Liebermann DA, Hoffman B. MyD genes in negative growth control. Oncogene Reviews. 1998;17:3319–30. doi: 10.1038/sj.onc.1202574. [DOI] [PubMed] [Google Scholar]
- 13.Liebermann DA, Hoffman B, Steinmann RA. Molecular controls of growth arrest and apoptosis:p53-dependent and independent pathways. Oncogene. 1995;11:199–210. [PubMed] [Google Scholar]
- 14.Fornace AJ, Jackman J, Hollander MC, Hoffman-Liebermann B, Liebermann DA. Genotoxic-stress-response genes and growth-arrest genes: gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann of the New York Acad of Sci. 1992;663:139–154. doi: 10.1111/j.1749-6632.1992.tb38657.x. [DOI] [PubMed] [Google Scholar]
- 15.Fornace AJ., Jr Mammalian genes induced by radiation; activation of genes associated with growth control. Annu Rev Genet. 1992;26:507–526. doi: 10.1146/annurev.ge.26.120192.002451. [DOI] [PubMed] [Google Scholar]
- 16.Zhan Q, Fan S, Smith ML, Bae I, Yu K, Alamo I, Jr, O'Connor PM, Fornace AJ., Jr Abrogation of p53 function affects gadd gene responses to DNA base-damaging agents and starvation. DNA Cell Biol. 1996;15:805–15. doi: 10.1089/dna.1996.15.805. [DOI] [PubMed] [Google Scholar]
- 16a.Liebermann DA, Hoffman Barbara. MyD/GADD genes in terminal differentiation growth arrest and apoptosis. In: Waxman S, editor. Differentiation Therapy. Vol. 10. 1995. pp. 107–116. (Ares-Serono Symposia-Series Challange of Modern Medicine). [Google Scholar]
- 17.Selvakumaran M, Lin HK, Tjin Tham Sjin R, Reed J, Liebermann D, Hoffman B. The novel primary response gene MyD118 and the proto-oncogenes myb, myc and bcl-2 modulate transforming growth factor b1-induced apoptosis of myeloid leukemia cells. Mol Cell Biol. 1994;14:2352–2360. doi: 10.1128/mcb.14.4.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvakumaran M, Lin HK, Miyashita T, Wang HG, Krajewski S, Reed JC, Hoffman B, Liebermann D. Immediate early up-regulation of bax expression by p53 but not TGFb1: A paradigm for distinct apoptotic pathways. Oncogene. 1994;9:1791–1798. [PubMed] [Google Scholar]
- 19.Guillouf C, Grana X, Selvakumaran M, Hoffman B, Giordano A, Liebermann DA. Dissection of the genetic programs of p53 G1 growth arrest and apoptosis: Blocking p53-induced apoptosis unmasks G1 arrest. Blood. 1995;85:2691–98. [PubMed] [Google Scholar]
- 20.Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B, Fornace AJ, Jr A mammalian cell cycle checkpoint utilizing p53 and gadd45 is defective in Ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, Hollander MC, O'Connor PM, Fornace AJ, Jr, Harris CC. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci U S A. 1999;96:3706–11. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hollander MC, Sheikh MS, Bulavin DV, Lundgren K, Augeri-Henmueller L, Shehee R, Molinaro TA, Kim KE, Tolosa E, Ashwell JD, Rosenberg MP, Zhan Q, Fernandez-Salguero PM, Morgan WF, Deng CX, Fornace AJ., Jr Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23:176–84. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 23.Smith ML, Chen IT, Zhan Q, Bae I, Chen CY, Gilmer TM, Kastan MB, O'Connor PM, Fornace AJ., Jr Protein interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994;266:1376–80. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- 24.Smith ML, Ford JM, Hollander MC, Bortnick RA, Amundson SA, Seo YR, Deng CX, Hanawalt PC, Fornace AJ., Jr p53-mediated DNA repair responses to UV radiation: studies of mouse cells lacking p53, p21, and/or gadd45 genes. MCB. 2000;20:3705–14. doi: 10.1128/mcb.20.10.3705-3714.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith ML, Kontny HU, Zhan Q, Sreenath A, O'Connor PM, Fornace AJ., Jr Antisense GADD45 expression results in decreased DNA repair and sensitizes cells to u.v.-irradiation or cisplatin. Oncogene. 1996;13:2255–63. [PubMed] [Google Scholar]
- 26.De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001 Nov 15;414(6861):308–13. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 27.Gupta M, Gupta S, Balliet AG, Hollander MC, Fornace AJ, Hoffman B, Liebermann DA. Hematopoietic cells from gadd45a and gadd45b deficient mice are sensitized to genotoxic-stress induced apoptosis. Oncogene. 2005;24:7170–9. doi: 10.1038/sj.onc.1208847. [DOI] [PubMed] [Google Scholar]
- 28.Gupta SK, Gupta M, Hoffman B, Liebermann DA. Hematopoietic cells from gadd45a-deficient and gadd45b-deficient mice exhibit impaired stress responses to acute stimulation with cytokines, myeloablation and inflammation. Oncogene. 2006;25:5539–46. doi: 10.1038/sj.onc.1209555. [DOI] [PubMed] [Google Scholar]
- 29.Amanullah A, Azam N, Balliet A, Hollander C, Hoffman B, Fornace A, Liebermann D. Cell signalling: cell survival and a Gadd45-factor deficiency. Nature. 2003;424:741–42. doi: 10.1038/424741b. [DOI] [PubMed] [Google Scholar]
- 30.Hildesheim J, Bulavin DV, Anver MR, Alvord WG, Hollander MC, Vardanian L, Fornace AJ., Jr Gadd45a protects against UV irradiation-induced skin tumors, and promotes apoptosis and stress signaling via MAPK and p53. Cancer Res. 2002;62:7305–15. [PubMed] [Google Scholar]
- 31.Vairapandi M, Azam N, Balliet AG, Hoffman B, Liebermann DA. Characterization of MyD118, Gadd45, and PCNA interacting domains: PCNA impedes MyD/Gadd Mediated Negative Growth Control. J Biol Chem. 2000;275:16810–16819. doi: 10.1074/jbc.275.22.16810. [DOI] [PubMed] [Google Scholar]
- 32.Harkin DP, Bean JM, Miklos D, Song YH, Truong VB, Englert C, Christians FC, Ellisen LW, Maheswaran S, Oliner JD, Haber DA. Induction of GADD45 and JNK/SAPK-dependent apoptosis following inducible expression of BRCA1. Cell. 1999;97:575–86. doi: 10.1016/s0092-8674(00)80769-2. [DOI] [PubMed] [Google Scholar]
- 33.Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA, Azam N, Vairapandi M, Zhang W, Hoffman B, Liebermann DA. Interaction of CR6 (GADD45gamma) with proliferating cell nuclear antigen impedes negative growth control. J Biol Chem. 2001;276:2766–74. doi: 10.1074/jbc.M005626200. [DOI] [PubMed] [Google Scholar]
- 34.Yoo J, Ghiassi M, Jirmanova L, Balliet AG, Hoffman B, Fornace AJ, Jr, Liebermann DA, Bottinger EP, Roberts AB. TGF-beta-induced apoptosis is mediated by Smad-dependent expression of GADD45B through p38 activation. JBC. 2003 Aug 21; doi: 10.1074/jbc.M307869200. [DOI] [PubMed] [Google Scholar]
- 35.Fan W, Richter G, Cereseto A, Beadling C, Smith KA. Cytokine response gene 6 induces p21 and regulates both cell growth and arrest. Oncogene. 1999;18:6573–82. doi: 10.1038/sj.onc.1203054. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W, Hoffman B, Liebermann DA. Ectopic expression of MyD118/Gadd45/CR6 (Gadd45beta/alpha/gamma) sensitizes neoplastic cells to genotoxic stress-induced apoptosis. Int J Oncol. 2001;18:749–57. [PubMed] [Google Scholar]
- 37.Zazzeroni F, Papa S, Algeciras-Schimnich A, Alvarez K, Melis T, Bubici C, Majewski N, Hay N, De Smaele E, Peter ME, Franzoso G. Gadd45 beta mediates the protective effects of CD40 costimulation against Fas-induced apoptosis. Blood. 2003;102(9):3270–9. doi: 10.1182/blood-2003-03-0689. [DOI] [PubMed] [Google Scholar]
- 38.Sancar A. Mechanisms of DNA excision repair. Science. 1994;266:1954–1956. doi: 10.1126/science.7801120. [DOI] [PubMed] [Google Scholar]
- 38a.Jonsson ZO, Hubsche U. Proliferating cell nuclear antigen: more than a clamp for DNA polymerases. Bio Essays. 1997;19:967–975. doi: 10.1002/bies.950191106. [DOI] [PubMed] [Google Scholar]
- 39.Kelman Z, Hurwitz J. Protein-PCNA interactions: a DNA-scanning mechanism? Trends Biochem Sci. 1998;23:236–238. doi: 10.1016/s0968-0004(98)01223-7. [DOI] [PubMed] [Google Scholar]
- 40.Zhan Q, Antinore MJ, Wang XW, Carrier F, Smith ML, Harris CC, Fornace AJ., Jr Association with Cdc2 and inhibition of Cdc2/Cyclin B1 kinase activity by the p53-regulated protein Gadd45. Oncogene. 1999;18:2892–900. doi: 10.1038/sj.onc.1202667. [DOI] [PubMed] [Google Scholar]
- 41.Jin S, Antinore MJ, Lung FD, Dong X, Zhao H, Fan F, Colchagie AB, Blanck P, Roller PP, Fornace AJ, Jr, Zhan Q. The GADD45 inhibition of Cdc2 kinase correlates with GADD45-mediated growth suppression. J Biol Chem. 2000;275:16602–8. doi: 10.1074/jbc.M000284200. [DOI] [PubMed] [Google Scholar]
- 42.Yang Q, Manicone A, Coursen JD, Linke SP, Nagashima M, Forgues M, Wang XW. Identification of a functional domain in a Gadd45-mediated G2/M checkpoint. J Biol Chem. 2000 Sep 5; doi: 10.1074/jbc.M005319200. [DOI] [PubMed] [Google Scholar]
- 43.O'Connor PM. Mammalian G1 and G2 phase checkpoints. Cancer Surv. 1997;29:151–82. [PubMed] [Google Scholar]
- 44.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–72. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 45.El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, Lin D, Mercer WE, Kinzler KW, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–25. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Xiong Y, Beach D. Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Molecular Biology of the Cell. 1993;4:897–906. doi: 10.1091/mbc.4.9.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 48.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1. Cell. 1993;75(4):805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 49.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 50.Chan TA, Hwang PM, Hermeking H, Kinzler KW, Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000;14(13):1584–8. [PMC free article] [PubMed] [Google Scholar]
- 51.Lu B, Yu H, Chow C, Li B, Zheng W, Davis RJ, Flavell RA. GADD45gamma mediates the activation of the p38 and JNK MAP kinase pathways and cytokine production in effector TH1 cells. Immunity. 2001;14:583–90. doi: 10.1016/s1074-7613(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 52.Yang J, Zhu H, Murphy TL, Ouyang W, Murphy KM. IL-18-stimulated GADD45 beta required in cytokine-induced, but not TCR-induced, IFN-gamma production. Nat Immunol. 2001;2:157–64. doi: 10.1038/84264. [DOI] [PubMed] [Google Scholar]
- 53.Shaulian E, Karin M. Stress-induced JNK activation is independent of Gadd45 induction. J Biol Chem. 1999;274:29595–8. doi: 10.1074/jbc.274.42.29595. [DOI] [PubMed] [Google Scholar]
- 54.Holbrook NJ, Liu Y, Fornace AJ., Jr Signaling events controlling the molecular response to genotoxic stress. EXS. 1996;77:273–88. doi: 10.1007/978-3-0348-9088-5_18. [DOI] [PubMed] [Google Scholar]
- 55.Bulavin DV, Kovalsky O, Hollander MC, Fornace AJ., Jr Loss of oncogenic H-ras-induced cell cycle arrest and p38 mitogen-activated protein kinase activation by disruption of Gadd45a. Mol Cell Biol. 2003;23:3859–71. doi: 10.1128/MCB.23.11.3859-3871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kovalsky O, Lung FD, Roller PP, Fornace AJ., Jr Oligomerization of human Gadd45a protein. J Biol Chem. 2001;276(42):39330–9. doi: 10.1074/jbc.M105115200. [DOI] [PubMed] [Google Scholar]
- 57.Maeda T, Hanna AN, Sim AB, Chua PP, Chong MT, Tron VA. J Invest Dermatol. 2002;119:22–6. doi: 10.1046/j.1523-1747.2002.01781.x. [DOI] [PubMed] [Google Scholar]
- 58.Zerbini LF, Wang Y, Czibere A, Correa RG, Cho JY, Ijiri K, Wei W, Joseph M, Gu X, Grall F, Goldring MB, Zhou JR, Libermann TA. Proc Natl Acad Sci U S A. 2004;101:13618–23. doi: 10.1073/pnas.0402069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta M, Gupta SK, Hoffman B, Liebermann DA. Gadd45a and gadd45b protect hematopoietic cells from UV induced apoptosis via distinct signaling pathways including p38 activation and JNK inhibition. J Biol Chem. 2006;281:17552–8. doi: 10.1074/jbc.M600950200. [DOI] [PubMed] [Google Scholar]
- 60.Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221–7. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 61.Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-kappaB: a key to survival. J Cell Sci. 2004;117:5197–208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- 62.Jijon H, Allard B, Jobin C. NF-kappaB inducing kinase activates NF-kappaB transcriptional activity independently of IkappaB kinase gamma through a p38 MAPK-dependent RelA phosphorylation pathway. Cell Signal. 2004;16:1023–3. doi: 10.1016/j.cellsig.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 63.Park JM, Greten FR, Li ZW, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–51. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- 64.Platanias LC. Map kinase signaling pathways and hematologic malignancies. Blood. 2003;101:4667–79. doi: 10.1182/blood-2002-12-3647. [DOI] [PubMed] [Google Scholar]
- 65.Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–53. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]