Abstract
Objective
We compare rates of rapid HIV testing, test offer, and acceptance in an urban emergency department (ED) when conducted by dedicated HIV counselors versus current members of the ED staff.
Methods
The Universal Screening for HIV Infection in the Emergency Room [USHER] trial is a prospective randomized controlled trial that implemented an HIV screening program in the ED of an urban tertiary medical center. ED patients were screened and consented for trial enrollment by an USHER research assistant. Eligible subjects were randomized to rapid HIV testing (oral OraQuick) offered by a dedicated counselor (counselor arm) or by an ED provider (provider arm). In the counselor arm, counselors—without other clinical responsibilities—assumed nearly all testing-related activities (consent, counseling, delivery of test results). In the provider arm, trained ED emergency service assistants (nursing assistants) consented and tested the participant in the context of other ED-related responsibilities. In this arm, ED house officers, physician assistants, or attending physicians provided HIV test results to trial participants. Outcome measures were rates of HIV testing and test offer among individuals consenting for study participation. Among individuals offered the test, test acceptance was also measured.
Results
From February 2007 through July 2008, 8,187 eligible patients were approached in the ED, and 4,855 (59%) consented and were randomized to trial participation. The mean age was 37 years, 65% were women, and 42% were white. The overall testing rate favored the counselor arm (57% versus 27%; P < .001); 80% (1,959/2,446) of subjects in the counselor arm were offered an HIV test compared with 36% (861/2,409) in the provider arm (P < .001). HIV test acceptance was slightly higher in the provider arm (counselor arm 71% versus provider arm 75%; P = .025).
Conclusion
Routine rapid HIV testing in the ED was accomplished more frequently by dedicated HIV counselors than by ED staff in the course of routine clinical work. Without dedicated staff, HIV testing in this setting may not be truly routine.
INTRODUCTION
Background
Knowledge of HIV seropositivity is the first critical step in obtaining appropriate medical care; it allows individuals to receive timely prevention counseling and therapeutic interventions,1 improves clinical outcomes of HIV-infected patients, and potentially decreases rates of HIV transmission. However, opportunities for HIV counseling, testing, and referral are still missed in many medical care settings, including emergency departments (EDs). In EDs, routine HIV screening and appropriate referral to care have historically been the exception, rather than the rule. Emerging data suggest that HIV screening in the ED would identify numerous HIV-infected individuals who commonly use the ED as their sole source of medical care.2,3
Importance
In recognition of the expanding role of ED personnel in the provision of community preventive health care, recent literature has emphasized the critical role EDs could play as HIV testing sites.1 Although such data have motivated EDs nationwide to establish HIV testing programs,3–7 expansion of other public health efforts in this setting has stressed the already overworked staff and resources.8–11 The numerous HIV screening strategies in the ED setting previously published are ultimately incomparable because of differences in eligibility, data collection and reporting. Thus, the most effective mechanism to test patients for HIV infection in the ED setting remains unclear.
Goals of This Investigation
Our objective was to examine, in a randomized trial, whether ED providers can and will assume the rapid HIV testing role without the addition of extra personnel12 or whether the introduction of an HIV testing “team” (eg, counselors, social workers) is a more effective implementation strategy.
METHODS
Study Design
The National Institutes of Health–funded Universal Screening for HIV Infection in the Emergency Room (USHER) study is a single-center, randomized controlled trial of routine HIV screening. From February 7, 2007, to July 9, 2008, oral HIV testing was offered to eligible patients by either HIV counselors or emergency service assistants (existing members of ED personnel).13 All subjects provided separate written informed consent first for trial participation and again for rapid HIV testing. The study was approved by the Partners Human Research Committee and overseen by a data safety and monitoring board.
Setting
The study took place at Brigham and Women’s Hospital, a tertiary academic medical center in Boston, MA. The hospital’s ED treats more than 56,000 patients annually, with a faculty and staff of more than 300, including 148 nurses, 56 residents, and 35 attending physicians. The median patient age is 44 years, and approximately 60% are women. The patient population is racially and ethnically diverse, including 48% whites, 25% blacks, and 20% Hispanics. HIV-infected patients may receive their care at an on-site infectious disease/HIV clinic, and HIV testing in the ED was available only through participation in the USHER trial.
Selection of Participants
ED patients were offered USHER trial enrollment in a private area after being registered, triaged, and escorted to the patient care area to be evaluated for their chief complaint. USHER research assistants screened individuals to identify those meeting the following eligibility criteria: (1) aged 18 to 75 years; (2) clear mental status and an Emergency Severity Index score of 3, 4, or 5 on a scale of 1 (most severe) to 5 (least severe)14–16; (3) fluent in English or Spanish; (4) not engaged in prenatal care; (5) not known to be HIV infected; and (6) not enrolled in the USHER trial in the previous 3 months. Nearly all patients meeting criteria 1 through 3 above were approached for possible USHER trial participation. Day-of-week and time-of-day variation was captured by weekly changes in enrollment times, but enrollment always occurred greater than 60 hours per week and between 8 AM and midnight.
For those patients screened eligible and available, the USHER research assistant confirmed eligibility, described the study, and ascertained willingness to participate in a randomized trial on HIV counseling, testing, and referral. When an eligible patient who agreed to participate was identified, the research assistant explained that (1) participation was voluntary; (2) participation in the trial did not imply that an HIV test would be performed; (3) study procedures varied, depending on which arm of the trial the patient was randomized to; (4) an HIV knowledge questionnaire and brief risk factor assessment would be requested (by computer or with paper/pencil)17; and (5) a separate written informed consent would be requested and required for the rapid oral HIV test to be conducted. Interested subjects signed a written informed consent form (English or Spanish) for study participation. The process of informed consent for trial participation took approximately 15 minutes.
Participants who signed the trial informed consent were randomized to one of 2 arms: counselor-based HIV testing or ED provider-based HIV testing. We define “provider” as any member of the current ED care staff with previous predetermined responsibilities (ESA, nurse, resident or attending physician). Subjects, counselors, and providers were neither masked to the assigned arms nor incentivized to complete the testing process. Because it has been shown that HIV test acceptance is affected by sex and age,18 we randomized USHER study participants into 4 strata (ie, men <40 years, men ≥40 years, women <40 years, and women >40 years) and performed computer-generated block randomization (with blocks of variable size) within each stratum. Once a patient was consented for trial and randomized, the research assistant notified the appropriate USHER-trained personnel (counselor or ESA) of the name and location of the subject who had been consented for study. In addition to this direct communication, a color-coded sticker indicating the study arm, along with an envelope containing the blank consent for rapid HIV testing, was placed on the participant’s ED chart.
Trial participants were requested to complete a self-reported questionnaire (Appendix E1, available online at http://www.annemergmed.com). The 86-item survey included questions on patient demographics, testing history, sexual behavior and perceived HIV risk, and dependence on and use of tobacco, alcohol, and illicit drugs. The questionnaire, available by audio-computer assisted self-interview or by paper and pencil, took approximately 20 minutes to complete. Neither counselors nor providers had access to the results.
Interventions
HIV screening within the context of the USHER trial occurred only when USHER research assistants, counselors, and trained emergency service assistants were available. HIV counselors were required to complete the Massachusetts Department of Public Health HIV counselor certification process.19 USHER staff members who offered and conducted the test (counselors and emergency service assistants) attended the same 1-day OraQuick training program used by the Massachusetts Department of Public Health and successfully completed the accompanying competency test. Finally, a 90-minute training session (available by video on the hospital Intranet) was conducted by the principal investigator of the USHER trial (R.P.W.) and the HIV social workers and was required of all emergency service assistants; this program was also available (optional) for house officers, physician assistants, and attending physicians who were responsible for delivery of test results in the provider arm. The number of emergency service assistants trained and actively conducting HIV screening within the course of their clinical duties increased from 6 at the trial outset to 22 for the final 5 months of the trial. During the 17-month trial period, 9 counselors and 28 emergency service assistants were trained to conduct HIV testing.
The delineation of activities within each arm is described in Table 1 (further protocol details are available in Appendix E1, available online at http://www.annemergmed.com). Briefly, in the provider arm, emergency service assistants offered and consented (opt-in approach) participants for HIV testing, collected the specimen, and developed the test in an on-site laboratory. Nonreactive results were delivered by resident physicians or physician assistants. Reactive results were delivered by attending physicians, who then requested consent for confirmatory testing, including enzyme immunoassay (ADVIA Centaur, HIV 1/0/2; Bayer HealthCare LLC, Tarrytown, NY), serum Western blot (Genetic Systems HIV-1 WB; Bio-Rad, Redmond, WA), CD4 count, and plasma HIV-1 RNA. The ED care facilitator—a nurse employed to assist with transfers and the follow-up of the ED care plan— helped to arrange HIV clinic follow-up for patients with reactive test results. A positive HIV Western blot and plasma HIV-1 RNA result confirmed the diagnosis of chronic HIV infection at the HIV clinic follow-up.
Table 1.
HIV screening responsibilities within each trial arm.
| Counselor Arm | Provider Arm | |
|---|---|---|
| Trial enrollment and consent | Research assistant | Research assistant |
| HIV test consent and performance of test | Counselor | ESAs |
| Results delivery | ||
| Nonreactive | Counselor | Residents or PAs |
| Reactive | Counselor | Attending physicians |
| Linkage to HIV care for new HIV diagnoses | Counselor HIV social worker |
ED care facilitator |
| Confirmation of follow-up in HIV clinic | HIV social worker | HIV social worker |
ESA, Emergency service assistant; PA, physician assistant.
Participants randomized to the counselor arm had nearly all activities conducted by the HIV counselors hired for the trial, from test consent to delivery of reactive or nonreactive test results (Table 1). Individuals who received reactive test results in the counselor arm were requested by the counselor to consent for the same confirmatory blood testing as in the provider arm; the counselors were not permitted to write orders, nor were they trained in phlebotomy. All point-of-care HIV tests within the trial were performed with oral fluid sampling for the OraQuick Advance Rapid HIV-1/2 Antibody Test (OraSure Technologies, Inc., Bethlehem, PA).
Outcome Measures
We defined the overall testing rate as the number of participants tested among those randomized to each testing arm. The offer rate of the HIV test was defined as the proportion of enrolled study participants who were actually offered a test. Acceptance of the HIV test was defined as the proportion of study participants who received the HIV test among those offered the test.
Primary Data Analysis
All data were analyzed according to the arm in which participants were initially randomized. For baseline demographic information stratified by study arm, means and SDs are provided for continuous variables (age), whereas frequencies are presented for categorical variables (sex, race/ethnicity, primary language, and education). The rate difference and its 95% confidence interval (CI) were calculated. P values were calculated with the χ2 test of independence or Fisher’s exact test when appropriate. Subgroup analyses were performed for the “test offer” group for age, sex, race, Emergency Severity Index score, time of day, and month of study. We evaluated the interaction between study arm assignments and the factors listed above in a logistic regression model. We calculated the percentage and 95% CI of those offered testing, stratified by age, race/ethnicity, Emergency Severity Index score, time of day, and month of the study.
To examine whether providers (compared to counselors) were more likely to offer HIV testing to ED patients at higher risk for acquiring HIV infection, we grouped trial participants into 5 categories: (1) no self-reported sexual risk or drug-related risk, (2) high sexual-related risk alone, (3) high drug-related risk alone, (4) high sexual- and high drug-related risk, and (5) missing sexual- or drug-related risk. High sexual risk included any self-report of having more than 1 sexual partner in the last 3 months, men having sex with men, ever having sex with a person who was HIV positive or had AIDS, ever having been incarcerated, and ever having sex with someone who has been incarcerated. Drug-related high risk included any self-report of using 1 illicit drug at least occasionally or of confirming use (at least once) of 2 or more drugs. We then examined whether test offer or acceptance rates in each study arm varied according to risk, formally testing for interaction between study arm and risk behavior group. All analyses were performed with SAS statistical software (version 9.1; SAS Institute, Inc., Cary, NC).
RESULTS
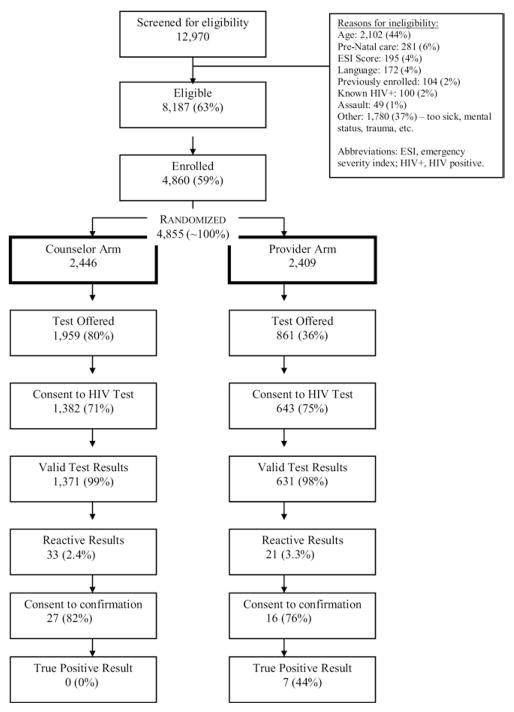
From February 7, 2007, through July 9, 2008, 12,970 ED visitors seeking health care were screened for USHER trial eligibility according to initial Emergency Severity Index score alone. The most frequently documented reason for ineligibility was age (n = 2,102; 44% of all ineligible). Among 8,187 eligible patients approached, 4,860 (59%) agreed to participate (Figure 1). The 3,327 eligible patients who refused trial enrollment were similar in sex and Emergency Severity Index score distribution to trial participants but were older (43 versus 37 years; P < .001).
Figure 1.
USHER trial enrollment schema. Percentages are calculated with the previous cell as the denominator. Results are reported as intention to treat.
More than 99% (4,855/4,860) of enrolled subjects were randomized: 2,446 to the counselor arm and 2,409 to the provider arm. Randomization achieved a balanced demographic distribution. The mean age of the study population was 37 years (SD 14), 65% of participants were women, 22% were black, and 29% were Hispanic (Table 2).
Table 2.
Demographic characteristics of patients randomized in the USHER trial.
| Characteristics | Counselor (N=2,446; 50.4%) | Provider (N=2,409; 49.6%) |
|---|---|---|
| Mean age, y (SD) (N=4,840) | 37.1 (14) | 37.1 (14) |
| Sex, No. (%) (N=4,820) | ||
| Male | 849 (35) | 832 (35) |
| Female | 1,585 (65) | 1,554 (65) |
| Race/ethnicity, No. (%) (N=4,800) | ||
| Non-Hispanic white | 938 (39) | 928 (39) |
| Non-Hispanic black | 526 (22) | 546 (23) |
| Hispanic | 707 (29) | 670 (28) |
| Asian/Asian-American | 66 (3) | 59 (2) |
| Native American/Alaskan Native | 12 (0.5) | 8 (0.3) |
| Multiracial/other | 176 (7) | 164 (7) |
| Primary language, No. (%) (N=4,817) | ||
| English | 1,788 (74) | 1,807 (76) |
| Spanish | 483 (20) | 448 (19) |
| Other | 154 (6) | 137 (6) |
| Education, No. (%) (N=4,814) | ||
| Less than high school | 310 (13) | 310 (13) |
| High school degree | 566 (23) | 575 (24) |
| Some college | 696 (29) | 713 (30) |
| College degree | 517 (21) | 438 (18) |
| Some postcollege/graduate degree | 342 (14) | 347 (15) |
Of the 2,002 study participants who had valid HIV test results, 1,929 (96.4%) met the Centers for Disease Control and Prevention (CDC) guidelines for screening because they were aged 64 years or younger. Seven new cases of HIV infection were identified, yielding a prevalence of new cases identification of 0.35% (95% CI 0.14% to 0.72%). Each of these cases was in the provider arm. The CD4 cell count of newly identified HIV-infected participants ranged from 74 to 1,276/μL; 3 of 7 had CD4 cell counts less than 250/μL. Of all participants tested, 1,405 (70%) completed the self-reported question about testing history; of these, 916 (65%) reported a history of testing. Aside from our previous report of a higher-than-expected rate of false-positive oral test results, there were no important trial-related adverse events.20
Rates of Overall Testing, Test Offer, and Acceptance
The overall testing rate—the proportion of subjects randomized to a given arm who completed the test—was 57% (1,382/2,446) in the counselor arm, more than twice as high as the overall testing rate in the provider arm (27%; 643/2,409; P < .001). Among participants randomized to the HIV counselor arm, 80% (1,959/2,446) were offered HIV testing. This test offer rate was higher than that of the HIV provider arm, 861 of 2,409 (36%; P < .001; Table 3). Among subjects offered HIV testing, the test acceptance rates were slightly higher in the provider arm (75% versus 71%; P = .025).
Table 3.
Summary of secondary endpoints by trial arm.
| Counselor, No. (%) (N=2,446) | Provider, No. (%) (N=2,409) | Difference, % (95% CI) | P Value* | |
|---|---|---|---|---|
| HIV test completed among patients randomized | 1,382 (57) | 643 (27) | 30 (27 to 32) | <.001 |
| HIV test offered | 1,959 (80) | 861 (36) | 44 (42 to 47) | <.001 |
| HIV test accepted among patients offered | 1,382 (71) | 643 (75) | −4 (−8 to −1) | .02 |
P value is from the χ2 test of independence.
The effect of sex (P for interaction = 0.92), race/ethnicity (P for interaction = 0.18), Emergency Severity Index score (P for interaction = 0.11), and time of day (P for interaction = .28) on offer rates did not vary by study arm. The effect of age on offer rate did vary by study arm (P for interaction = 0.02). The offer rate was similar across all ages in the counselor arm (79% to 83%), but the offer rate decreased with increasing age in the provider arm. For example, individuals older than 60 years were offered testing 25% of the time, whereas those aged 18 to 29 years were offered testing 39% of the time.
Figure 2 indicates HIV test offer rates, examined by trial arm, over calendar time. As the study progressed, the test offer rates remained steady in the counselor arm but decreased in the provider arm (P for interaction < .001).
Figure 2.
HIV test offer rates over calendar time, stratified by testing arm. For the first 3 months of the study (February through April 2007), HIV test offer rates were similar between trial arms (range counselor arm 82% to 90%, provider arm 61% to 76%). By May 2007, all test offer rates remained statistically significantly higher in the counselor arm. After September 2007, test offer rates in the provider arm never exceeded 40%; in the last 5 months of study, the peak offer rate in the provider arm was 30% (P for trend = .003).
Test Offer and Acceptance by Self-reported Risk
Among the 4,855 participants, 15% met criteria for high sexual risk, 8% met criteria for high drug-related risk, 15% met criteria for both, 26% self-reported no high-risk behavior, and 37% had missing data. In the counselor arm, there was no difference in rates of test offer by risk group (range 82% to 84% for individuals with identifiable risk group and 75% for those whose risk group was missing). In the provider arm, the offer rate among all risk groups was also similar (range 35% to 46% for individuals with identifiable risk group and 29% for those whose risk group was missing). The data did not provide evidence of targeted test offer or test acceptance in one arm compared with other; the P values corresponding to the formal test for interaction were 0.38 and 0.65, respectively.
LIMITATIONS
Results of this study should be interpreted within the context of its limitations. First, the USHER trial is a single-site study. Second, participants tested in the USHER trial were required to provide informed consent more than once for participation (one for trial, one for testing per Massachusetts state law, and one for confirmation of reactive results, if necessary). The lengthy consent process, though necessary to conduct a criterion standard randomized trial, may have affected participation and generalizability of the results. Although the intent was to offer routine HIV screening, only 80% of participants in the counselor arm were reached. Failure to offer testing in the counselor arm generally resulted from unexpectedly short ED visits, failure to anticipate the intensive ED care required, or inability to interrupt a clinical evaluation that was in process.
DISCUSSION
In a randomized controlled ED-based trial, we found that routine, voluntary HIV testing was completed more than twice as frequently when personnel were dedicated specifically to this task. Ultimately, more individuals were tested when the responsibility did not solely rely on the current ED staff.
In contrast to our results, data reported by the CDC on 3 ED HIV testing implementation projects (New York, NY, Los Angeles, CA, and Oakland, CA) suggest improved testing rates with a provider-based testing program.4 In the 2 sites in which counselors were used (New York, Los Angeles), 2% to 4% of presenting patients were tested. In the site in which a provider model was used (Oakland), approximately 10% of presenting patients were tested. In all of these sites, methods for patient screening, testing eligibility, hours of operation, and training differed, making them ultimately incomparable. Furthermore, only 10% of the target goal—to offer HIV testing to all presenting patients—was reached.21 To best identify the yield of testing according to each delivery model, both of which are now widely used, a head-to-head trial, with its inherent consent-process limitations, such as USHER is necessary.
ED providers have observed disincentives to conduct HIV testing, including insufficient time in patient encounters, inadequate training, and concerns about follow-up.22 Surveys of providers in the USHER trial identified similar concerns.23 However, these issues seemingly did not serve as obstacles at the study onset. Initial interest in testing in the provider arm, indicated by testing rates similar to those in the counselor arm, suggests that testing attrition over time may be due to waning enthusiasm for the program in the face of patient acuity and other clinical duties (Figure 2).
The USHER trial identified fewer cases of HIV infection than anticipated. Secondary analyses of self-reported data suggest that one reason for the low yield was a high rate (>60%) of previous testing. Nationally, incident HIV cases are less abundant than undiagnosed prevalent ones.24 If test acceptance in the ED setting is largely predicated on those with a testing history, fewer cases may be identified by routine screening efforts. With active scaling up of testing programs nationwide, the future challenge will be to build on these experiences to construct efforts that fundamentally improve on current standards in reaching patients with undiagnosed disease. In the meantime, although new case identification is the ultimate public health goal, the complementary value of informing patients they are HIV negative should not be underestimated.
Though the numbers are small, the fact that all newly diagnosed HIV cases occurred among patients randomized to the provider arm merits comment. Two explanations seem plausible: providers are better at targeting testing to patients with highest risk of infection, and HIV-infected patients preferentially accept testing offered by providers. Confined to the limitations of self-reported sexual and drug risk collected in the participant questionnaire, the data do not suggest that providers accurately target testing. Furthermore, because USHER is a study about HIV screening, research assistants and staff were discouraged from selectively offering trial eligibility and randomization according to physicians’ desire for HIV diagnostic testing on a particular patient. Although we cannot fully assess the second hypothesis with the data herein, self-reported data also suggest that high-risk patients are equally likely to accept testing regardless of who offers it.
In demonstrating that a counselor-based model will lead to the testing of more patients, we recognize that this model requires the addition of upfront resources. Certainly, resources to use dedicated counselors would not be merited if our findings about the yield of testing in the counselor arm were observed in other settings; this is not the case. Other ED studies corroborate our findings that testing rates are overall higher when provided by an HIV counselor. When routine screening coverage increases (compared with diagnostic testing), HIV prevalence decreases because of a lower pretest probability in individuals being tested; however, in contrast to our findings, yield in terms of absolute number of cases identified generally increases or is comparable.6,25
If enhanced resources are available, trained personnel might be further used to screen for HIV infection and also for other sexually transmitted diseases, hepatitis B and C. Although HIV counselor time certainly costs less than that of ED providers, the cost of additional trained personnel is not trivial. Further analyses should be conducted to evaluate the incremental cost-effectiveness of such an intervention compared with one in which less testing, but fewer supplemental resources, is required.
Unlike many HIV testing studies, which report on feasibility and overall experience, the strength of these results lies in the randomized controlled design. The single site may compose a limitation, but because Brigham and Women’s Hospital is an urban academic hospital with a busy ED, results of test offer and testing rates are likely generalizable to many other centers nationwide. Moreover, the findings from the USHER trial provide guidance where the CDC does not and where demonstration and feasibility projects are incomparable. Results of this study suggest that the availability of additional dedicated personnel to implement a routine, voluntary rapid HIV screening effort in an ED setting leads to more patients reached, with improved programmatic sustainability. Without such resources, rapid HIV testing in this setting is most likely diagnostic and not truly routine.
Acknowledgments
Funding and support: By Annals policy, all authors are required to disclose any and all commercial, financial, and other relationships in any way related to the subject of this article as per ICMJE conflict of interest guidelines (see www.icmje.org). This research was funded by the National Institute of Mental Health (R01 MH073445, R01 MH65869) and the Doris Duke Charitable Foundation, Clinical Scientist Development Award to Rochelle P. Walensky.
Publication of this article was supported by Centers for Disease Control and Prevention, Atlanta, GA.
Footnotes
No authors have conflicts of interest to disclose.
References
- 1.Rothman RE. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44:31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Missed opportunities for earlier diagnosis of HIV infection—South Carolina, 1997–2005. MMWR Morb Mortal Wkly Rep. 2006;55:1269–1272. [PubMed] [Google Scholar]
- 3.Haukoos JS, Hopkins E, Eliopoulos VT, et al. Development and implementation of a model to improve identification of patients infected with HIV using diagnostic rapid testing in the emergency department. Acad Emerg Med. 2007;14:1149–1157. doi: 10.1197/j.aem.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Rapid HIV testing in emergency departments—three US sites, January 2005–March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597–601. [PubMed] [Google Scholar]
- 5.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines: results from a high-prevalence area. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 6.Lyss SB, Branson BM, Kroc KA, et al. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J Acquir Immune Defic Syndr. 2007;44:435–442. doi: 10.1097/QAI.0b013e31802f83d0. [DOI] [PubMed] [Google Scholar]
- 7.Mehta SD, Hall J, Lyss SB, et al. Adult and pediatric emergency department sexually transmitted disease and HIV screening: programmatic overview and outcomes. Acad Emerg Med. 2007;14:250–258. doi: 10.1197/j.aem.2006.10.106. [DOI] [PubMed] [Google Scholar]
- 8.Davis RE, Harsh KE. Confronting barriers to universal screening for domestic violence. J Prof Nurs. 2001;17:313–320. doi: 10.1053/jpnu.2001.28181. [DOI] [PubMed] [Google Scholar]
- 9.Ellis JM. Barriers to effective screening for domestic violence by registered nurses in the emergency department. Crit Care Nurs Q. 1999;22:27–41. doi: 10.1097/00002727-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Larkin GL, Hyman KB, Mathias SR, et al. Universal screening for intimate partner violence in the emergency department: importance of patient and provider factors. Ann Emerg Med. 1999;33:669–675. doi: 10.1016/s0196-0644(99)70196-4. [DOI] [PubMed] [Google Scholar]
- 11.Nordqvist C, Johansson K, Lindqvist K, et al. Attitude changes among emergency department triage staff after conducting routine alcohol screening. Addict Behav. 2006;31:191–202. doi: 10.1016/j.addbeh.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, Division of HIV/AIDS Prevention. [Accessed November 8, 2010.];Advancing HIV prevention: interim technical guidance for selected interventions. Available at: http://www.cdc.gov/hiv/topics/prev_prog/ahp/resources/guidelines/Interim-Guidance.htm. Updated October 20, 2006.
- 13.National Institutes of Health. Two approaches to routine HIV testing in a hospital emergency department. [Accessed November 8, 2010.];National Institutes of Health Web site. Available at: http://clinicaltrials.gov/ct2/show/NCT00502944. Published July 16, 2007. Updated April 2, 2010.
- 14.Eitel DR, Travers DA, Rosenau AM, et al. The Emergency Severity Index triage algorithm version 2 is reliable and valid. Acad Emerg Med. 2003;10:1070–1080. doi: 10.1111/j.1553-2712.2003.tb00577.x. [DOI] [PubMed] [Google Scholar]
- 15.Wuerz RC, Milne LW, Eitel DR, et al. Reliability and validity of a new five-level triage instrument. Acad Emerg Med. 2000;7:236–242. doi: 10.1111/j.1553-2712.2000.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 16.Wuerz RC, Travers D, Gilboy N, et al. Implementation and refinement of the Emergency Severity Index. Acad Emerg Med. 2001;8:170–176. doi: 10.1111/j.1553-2712.2001.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 17.Reichmann WM, Losina E, Seage GR, et al. Does modality of survey administration impact data quality: audio computer assisted self interview (ACASI) versus self-administered pen and paper? PLoS One. 2010;5:e8728. doi: 10.1371/journal.pone.0008728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddicoat RV, Losina E, Kang M, et al. Refusing HIV testing in an urgent care setting: results from the “Think HIV” program. AIDS Patient Care STDS. 2006;20:84–92. doi: 10.1089/apc.2006.20.84. [DOI] [PubMed] [Google Scholar]
- 19.JRI Health HCoSM, Inc, AIDS Housing Corporation, AdCare Educational Institute. HIV/AIDS provider training calendar. September–December, 2008. [Google Scholar]
- 20.Walensky RP, Arbelaez C, Reichmann WM, et al. Revising expectations from rapid HIV tests in the emergency department. Ann Intern Med. 2008;149:153–160. doi: 10.7326/0003-4819-149-3-200808050-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55:1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 22.Fincher-Mergi M, Cartone KJ, Mischler J, et al. Assessment of emergency department health care professionals’ behaviors regarding HIV testing and referral for patients with STDs. AIDS Patient Care STDS. 2002;16:549–553. doi: 10.1089/108729102761041100. [DOI] [PubMed] [Google Scholar]
- 23.Arbelaez C, Wright EA, Losina E, et al. Emergency provider attitudes and barriers to universal HIV testing in the emergency department. J Emerg Med. 2009 Oct 13; doi: 10.1016/j.jemermed.2009.07.038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–529. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walensky RP, Losina E, Steger-Craven KA, et al. Identifying undiagnosed human immunodeficiency virus: the yield of routine, voluntary inpatient testing. Arch Intern Med. 2002;162:887–892. doi: 10.1001/archinte.162.8.887. [DOI] [PubMed] [Google Scholar]