Abstract
OBJECTIVE
To estimate the independent effect of gestational impaired glucose tolerance, defined as a single abnormal oral glucose tolerance test (OGTT) value, on metabolic dysfunction at 3 years postpartum.
METHODS
We used multiple linear regression to measure associations between glucose testing during pregnancy and metabolic markers at 3 years postpartum in Project Viva, a prospective cohort study of maternal and infant health. We compared metabolic measures at 3 years postpartum among four groups: normal glucose challenge test (less than 140 mg/dL, n=461); abnormal glucose challenge test but normal glucose tolerance test (GTT) (n=39); impaired glucose tolerance (IGT) (a single abnormal GTT value, n=21); and gestational diabetes mellitus (GDM) (n=16).
RESULTS
Adjusting for age, race, parity, parental history of diabetes, and maternal BMI at 3 years postpartum, we found women with GDM had lower adiponectin (11.2 ng/mL vs. 20.7 ng/mL) and higher homeostatic model assessment – insulin resistance (3.1 vs. 1.3) and waist circumference (91.3 cm vs. 86.2 cm) compared with women with IGT or normal glucose tolerance. Women in both the IGT and GDM groups had lower high-density lipoprotein (GDM: 44.7 mg/dL; IGT: 45.4/dL vs normal glucose tolerance 55.8 mg/dL) and higher triglycerides (GDM: 136.1 mg/dL; IGT: 140.1 mg/dL, vs. normal glucose tolerance: 78.3), compared with women in the normal glucose tolerance group. We found the highest values for Hemoglobin A1c (GDM: 5.1%; IGT 5.3%, normal glucose tolerance 5.1%) and high-sensitivity c reactive protein (GDM 1.4 mg/dL IGT: 2.2 mg/dL; NGT 1.0 mg/dL) among women with IGT.
CONCLUSION
GDM and IGT during pregnancy are associated with persistent metabolic dysfunction at 3 years postpartum, independent of other clinical risk factors.
Introduction
Metabolic dysfunction causes substantial morbidity and mortality among women. Among women, pregnancy complications predict metabolic disease risk. Women with gestational diabetes (GDM) have an 17 to 63% risk of developing type 2 diabetes within 5 to 16 years of the index pregnancy(1), and recent studies have linked a history of gestational diabetes with cardiovascular risk(2, 3).
The diagnosis of GDM presumes a threshold value above which women are at increased risk of pregnancy complications; however, recent work shows that adverse pregnancy outcomes rise continuously with increasing fasting glucose values(4). Maternal metabolic risk may similarly rise with glucose values. Indeed, recent studies have linked gestational impaired glucose tolerance with subsequent diabetes and cardiovascular disease risk(3, 5–9). However, it is not known whether impaired glucose tolerance during pregnancy predicts metabolic dysfunction independent of clinical risk factors such as body mass index and family history.
The aim of our study was to determine whether a history of GDM or gestational impaired glucose tolerance (IGT) is predictive of maternal metabolic dysfunction, independent of recognized clinical risk factors. We hypothesized that we would find a monotonic relationship between degree of gestational glucose tolerance and metabolic dysfunction at 3 years postpartum. To test this hypothesis, we compared measures of metabolic dysfunction at 3 years postpartum among women with normal glucose tolerance (NGT), abnormal glucose loading test results but normal GTT values, gestational impaired glucose tolerance (IGT), or GDM, in Project Viva, a prospective cohort study of maternal and infant health.
Materials and Methods
We performed an unplanned secondary analysis of participants in Project Viva, a longitudinal cohort study of maternal and child health(10). Women were recruited for Project Viva at their first prenatal visit at one of eight obstetrical offices of a multispecialty group practice in Eastern Massachusetts from 1999 to 2002. To be eligible for the study, potential participants were required to be fluent in English, <22 weeks gestation at study entry, and have a singleton pregnancy All participants provided written informed consent, and the Institutional Review Board of Harvard Pilgrim Health Care approved all procedures.
Assessment of gestational glucose tolerance
Obstetrical care providers assessed gestational glucose tolerance among women in our cohort according to the following guidelines: At 26 to 28 weeks’ gestation, all mothers underwent a non-fasting 50g oral glucose challenge test (GCT). Women with a result of 140 mg/dL or higher underwent a 100g oral glucose tolerance test (OGTT), administered the morning after an overnight fast. Normal results were defined by Carpenter-Coustan criteria: fasting <95 mg/dL, 1 hour <180 mg/dL, 2 hour < 155 mg/dL, 3 hour < 140 mg/dL. Gestational glucose tolerance was categorized as normal (GLT < 140 mg/dL), Abnormal GLT, normal GTT (GLT >=140 mg/dL, GTT with no abnormal results), gestational impaired glucose tolerance (IGT) (GLT >=140 mg/dL, GTT with only 1 abnormal result) (8, 11), or gestational diabetes (GLT >=140 and GTT with 2 or more abnormal results).
Assessment of metabolic parameters at 3 years postpartum
Women returned at 3 years postpartum for a physical examination that included anthropometric measurements and a blood sample. Methodology for anthropometric measures has been previously described elsewhere(12). We tested all blood samples (N=537) for HbA1c, sex hormone binding globulin (SHBG), C-reactive protein (CRP), and the adipokines leptin(13) and adiponectin(14). We identified as fasting those participants who did not eat or drink anything other than water for 8 hours before blood samples were obtained (N=166). We tested fasting blood samples for insulin, glucose, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, IL 6, ghrelin(15 ) and PYY(16). When we compared women who provided fasting samples with those who provided non-fasting samples, we found no differences in age, race, parity, family history of DM, gestational glucose tolerance, or in any outcome variables measured in both fasting and non-fasting participants. Laboratory methods for assessment of metabolic markers in this study have been previously described (12, 17).
Study covariates
Participants reported sociodemographic variables including parity, race/ethnicity, and personal history of type 1 or type 2 diabetes at the initial study visit during prenatal care. They reported parental history of type 2 diabetes at three-year follow-up visit. Women missing data on study covariates were excluded.
Data Analysis
We used analysis of variance and chi square tests to measure bivariate associations between sociodemographic characteristics and gestational glucose tolerance. We used multiple linear regression to model the relation between gestational glucose tolerance category and metabolic markers at three-years. Because BMI may have a non-linear association with metabolic markers, we used linear, quadratic and 3-knot cubic spline models(18) to adjust for maternal BMI 3 years postpartum, retaining the more complex model if the log likelihood ratio test p value was < 0.05. Because inclusion of quadratic and 3-knot quadratic spline terms did not improve model fit, we modeled BMI as a linear variable. We further adjusted for maternal age, race, parity, and parental history of type 2 diabetes to ascertain the predictive role of gestational glucose tolerance independent of clinical risk factors for metabolic disease. Adjustment for breastfeeding duration and weight change from pre-pregnancy to the three-year visit did not materially change our results, and they were therefore excluded from our model.
The Sharpiro-Wilk test and visual inspection of regression residuals suggested that normality should not be assumed in several cases. Log transformation of HOMA-IR, insulin, sex hormone binding globulin, triglycerides, C-reactive protein and IL6 improved normality of regression residuals. We present p values for the partial F test to assess the joint null hypothesis of equality across all of the glucose tolerance categories(19).
To facilitate interpretation of both the magnitude and clinical significance of differences among gestational glucose tolerance groups, we present results as predicted means and 95% confidence intervals for the mean. We present both unadjusted mean values and adjusted predicted mean values for participants of average postpartum BMI (26.2) who were white, age 35 to <40, had two children, and had no parental history of type 2 diabetes.
Data analyses were performed using SAS 9.2. Two-tailed P values < 0.05 were considered statistically significant.
Results
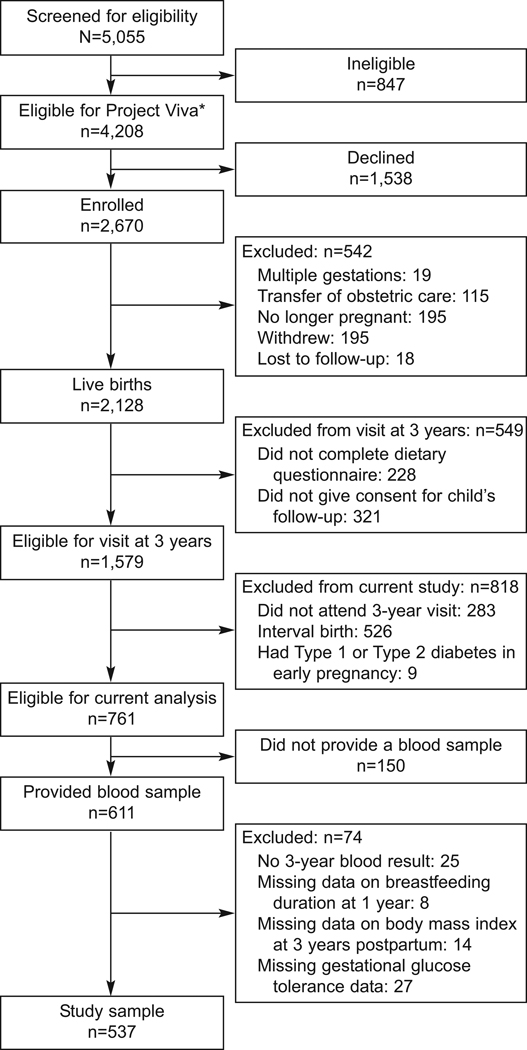
Of 5055 women screened for Project Viva, 4208 were eligible, and 2670 enrolled (Figure 1). Among the 2128 Project Viva participants who gave birth, 1579 met criteria for a three-year follow-up examination with their children by virtue of completing a pregnancy dietary questionnaire and contenting for child follow-up. Of these women, 611 met criteria for the current analysis because they attended the three-year visit, had not delivered another child since the birth of the index child three-years previously, denied a diagnosis of type 1 or type 2 diabetes early in the index pregnancy and provided a blood sample. We excluded women with missing data for three-year lab results (n=25), breastfeeding duration at 1 year (n=8), BMI at 3 years postpartum (n=14), or gestational glucose tolerance (n=27), leaving 537 women for analysis. At this three-year visit, 166 women provided a fasting blood sample.
Figure 1.
Flow of patients through the current study, which is a secondary analysis of Project Viva, a longitudinal study of maternal and child health. *Eligibility requirements included singleton gestation, ability to answer questions in English, plans to remain in the area through delivery, and gestational age less than 22 weeks at initial prenatal clinical appointment.
Glucose challenge test results were normal for 85.9% (N=461, 95%CI 82.9–88.8%) of women in our cohort. Among the 76 women with a GCT >=140, 39 had all normal values on the 100g GTT, 21 had one abnormal value (IGT), and 16 met the diagnostic criteria for GDM. Maternal age, parity, body mass index at 3 years postpartum, and race were similar among the glucose tolerance groups. Women with a parental history of type 2 diabetes were more likely to have had GDM (Table 1).
Table 1.
Baseline characteristics of study population, by pregnancy glucose tolerance category. Data from 578 participants in Project Viva who presented for follow-up at three years postpartum without an intervening birth.
| Normal | Abnormal GCT, normal GTT |
Impaired glucose tolerance during pregnancy |
Gestational diabetes |
Missing gestational glucose category2 |
p1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 461 | 39 | 21 | 16 | 28 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| BMI at 3 year pp visit (kg/m2) | 25 | (5) | 26 | (5) | 25 | (4) | 27 | (6) | 27 | (6) | 0.15 |
| Age at 3 year pp visit | 37 | (5) | 39 | (5) | 38 | (4) | 38 | (5) | 38 | (6) | 0.51 |
| N | % | N | % | N | % | N | % | ||||
| Race | 0.45 | ||||||||||
| Asian | 18 | (82) | 0 | (0) | 1 | (5) | 2 | (9) | 1 | (5) | |
| Black | 68 | (83) | 5 | (6) | 4 | (5) | 3 | (4) | 2 | (2) | |
| Hispanic | 29 | (74) | 5 | (13) | 2 | (5) | 0 | (0) | 3 | (8) | |
| Other | 22 | (85) | 0 | (0) | 0 | (0) | 1 | (4) | 3 | (12) | |
| White | 333 | (81) | 32 | (8) | 15 | (4) | 10 | (2) | 19 | (5) | |
| Parity | 0.78 | ||||||||||
| 1 | 131 | (79) | 11 | (7) | 7 | (4) | 7 | (4) | 10 | (6) | |
| 2 | 227 | (83) | 17 | (6) | 10 | (4) | 6 | (2) | 12 | (4) | |
| 3+ | 112 | (80) | 14 | (10) | 5 | (4) | 3 | (2) | 6 | (4) | |
| Parental History DM | 0.02 | ||||||||||
| Yes | 65 | (74) | 6 | (7) | 4 | (5) | 7 | (8) | 6 | (7) | |
| No | 405 | (83) | 36 | (7) | 18 | (4) | 9 | (2) | 22 | (4) | |
| Missing BMI at 3 years2 | 0.12 | ||||||||||
| Yes | 9 | (60) | 3 | (20) | 1 | (7) | 0 | (0) | 2 | (13) | |
| No | 461 | (82) | 39 | (7) | 21 | (4) | 16 | (3) | 26 | (5) | |
ANOVA p value for continuous variables, chi square p value for categorical variables.
Participants missing pregnancy glucose tolerance (N=27) or BMI at 3 years (N=14) were excluded from the analysis.
Most women in our cohort gained weight from prior to the index pregnancy to the three-year visit: the median weight gain was 2.2 kg (Interquartile range -0.4 to 5.3 kg), and 26.5% (95%CI 22.8–30.4%) of women had gained 5 kg or more during this interval. Most participants had normal glucose tolerance at 3 years postpartum by ADA criteria(20). Among 535 women for whom HbA1C was obtained, 14 were at increased risk of diabetes (A1C 5.7–6.4%) and one, with an A1C of 6.58%, met criteria for ADA diabetes (A1C ≥6.5%). In addition, one participant self-reported a diagnosis of type 2 diabetes. Among 164 women for whom fasting glucose was obtained, 2 had impaired fasting glucose (FPG 100–125 mg/dl) and none met criteria for diabetes (FPG > 126 mg/dl).
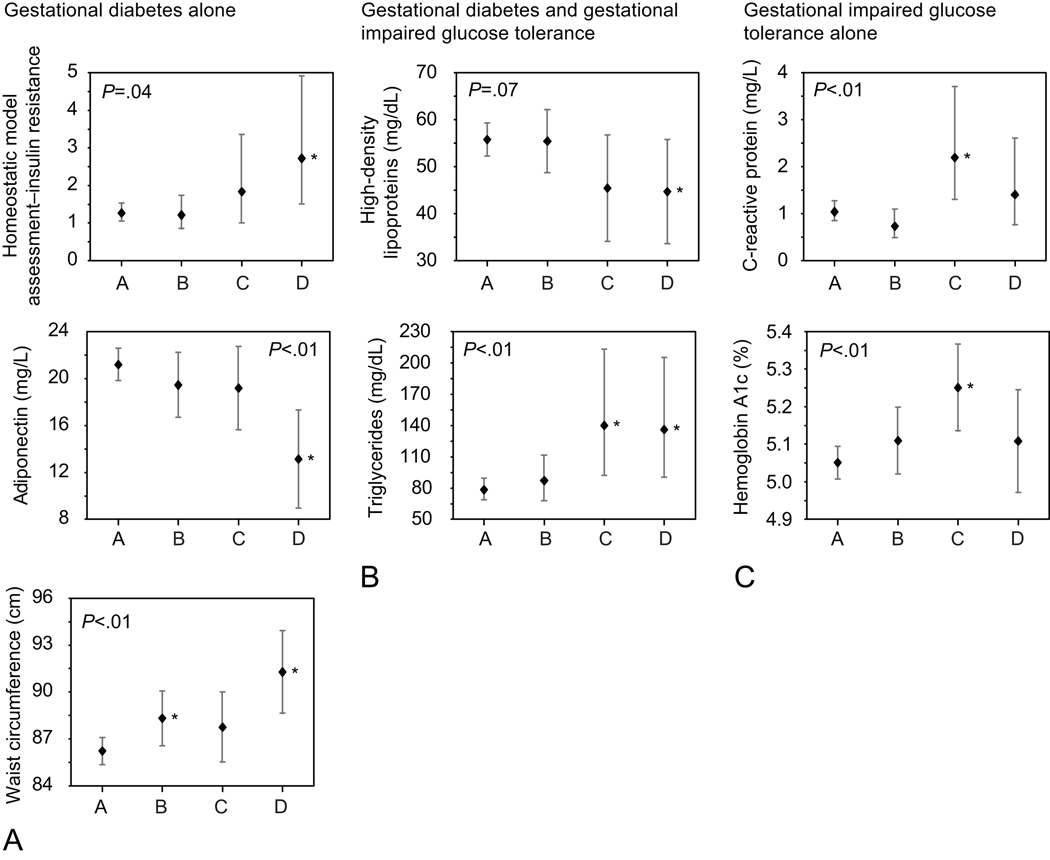
As we hypothesized, women with both IGT and GDM had more adverse metabolic profiles than women with normal GCT results or with abnormal GCT but normal values on the GTT. These patterns were similar in unadjusted models and in models adjusted for BMI at 3 years postpartum, parity, age, self-reported race and parental history of diabetes (Tables 2, 3 and 4). Women with GDM had lower adiponectin and higher HOMA-IR and waist circumference compared with women with IGT or normal glucose tolerance (Figure 2a, HOMA-IR GDM: 2.7 vs. NGT:1.3; adiponectin GDM 13.1 ng/mLvs. NGT 21.2 ng/mL; waist circumference GDM 91.3 cm vs. NGT 86.2 cm; partial F test p < 0.05 for all models). Women in both the IGT and GDM groups had lower HDL and higher triglycerides, compared with women in the NGT group (Figure 2b: HDL GDM: 44.7 mg/dL; IGT: 45.4 mg/dL vs NGT 55.8 mg/dL, partial F test p=0.07; triglycerides GDM: 136.1 mg/dL; IGT: 140.1 mg/dL, vs. NGT: 78.3 mg/dL; partial F test p<0.01). We had hypothesized that we would find a monotonic association between metabolic dysfunction and degree of glucose intolerance; however, we found the highest values for Hemoglobin A1c and CRP among women with IGT (Figure 2c: Hemoglobin A1c GDM: 5.1%; IGT 5.3%, NGT 5.1%, partial F test p<0.01; hsCRP GDM 1.4 mg/dL IGT: 2.2 mg/dL; normal 1.0 mg/dL, partial F test p<0.01). We found no pattern of association between gestational glucose tolerance category and sex hormone binding globulin, total cholesterol, fasting IL6, leptin, ghrelin or PYY (Tables 2 and 3).
Table 2.
Markers of glucose and lipid homeostasis at three-years postpartum, by gestational glucose tolerance category. Predicted values1 from multiple linear regression models. Data from 537 participants in Project Viva who presented for follow-up at three years postpartum without an intervening birth.
| N | Normal | Abnormal GCT, normal GTT |
Gestational impaired glucose tolerance |
Gestational diabetes |
P3 | |
|---|---|---|---|---|---|---|
| Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
|||
| Glucose metabolism | ||||||
| Hemoglobin A1c%, N | 460 | 38 | 20 | 15 | ||
| Unadjusted | 533 | 5.10 | 5.18 | 5.324 | 5.24 | <.001 |
| (5.07–5.12) | (5.09–5.26) | (5.20–5.44) | (5.10–5.37) | |||
| MV-Adjusted | 533 | 5.05 | 5.11 | 5.254 | 5.11 | <.01 |
| (5.01–5.09) | (5.02–5.20) | (5.14–5.37) | (4.97–5.24) | |||
| HOMA IR2, N | 142 | 12 | 4 | 5 | ||
| Unadjusted | 163 | 1.3 | 1.3 | 1.6 | 3.14 | 0.04 |
| (1.2–1.5) | (0.9–1.8) | (0.9–3.1) | (1.7–5.5) | |||
| MV-Adjusted | 163 | 1.3 | 1.2 | 1.8 | 2.74 | 0.04 |
| (1.1–1.5) | (0.8–1.7) | (1.0–3.4) | (1.5–4.9) | |||
| Insulin, u/mL2 | ||||||
| Unadjusted | 163 | 7.5 | 6.9 | 8.7 | 15.34 | 0.09 |
| (6.8–8.4) | (4.8–9.9) | (4.7–16.2) | (8.8–26.7) | |||
| MV-Adjusted | 163 | 6.8 | 6.3 | 8.8 | 12.14 | 0.12 |
| (5.8–8.1) | (4.5–8.7) | (5.1–15.3) | (7.1–20.8) | |||
| Glucose, mg/dL | ||||||
| Unadjusted | 163 | 72.6 | 76.1 | 77.5 | 82.8 | 0.3 |
| (70.3–74.8) | (68.3–83.8) | (64.1–90.9) | (70.8–94.8) | |||
| MV-Adjusted | 163 | 76.5 | 79.0 | 82.9 | 89.04 | 0.19 |
| (72.3–80.6) | (71.0–87.0) | (69.4–96.4) | (75.8 –102.3) | |||
| Sex hormone binding globulin2, N | 459 | 39 | 20 | 16 | ||
| Unadjusted | 534 | 48.7 | 44.9 | 45.1 | 50.8 | 0.86 |
| (45.7–51.8) | (36.1–55.7) | (33.4–61.1) | (36.2–71.2) | |||
| MV-Adjusted | 534 | 50.0 | 48.8 | 50.3 | 59.2 | 0.77 |
| (44.8–55.8) | (39.1–61.0) | (37.5–67.4) | (42.3–82.9) | |||
| Lipid metabolism | ||||||
| Total cholesterol, mg/dL, N | 142 | 12 | 4 | 5 | ||
| Unadjusted | 163 | 176.8 | 185.1 | 195.5 | 164.0 | 0.34 |
| (171.9 –181.6) | (168.3 –201.8) | (166.5 –224.5) | (138.1 –189.9) | |||
| MV-Adjusted | 163 | 175.8 | 180.5 | 194.7 | 165.9 | 0.48 |
| (166.6 –185.0) | (162.9 –198.0) | (165.1 –224.4) | (136.8 –194.9) | |||
| LDL cholesterol, mg/dL | ||||||
| Unadjusted | 163 | 106.2 | 113.4 | 126.0 | 90.9 | 0.22 |
| (101.7 –110.7) | (98.0 –128.8) | (99.3 –152.6) | (67.1 –114.8) | |||
| MV-Adjusted | 163 | 102.7 | 106.4 | 126.1 | 92.8 | 0.26 |
| (94.4 –111.0) | (90.6 –122.3) | (99.3 –152.8) | (66.6 –119.0) | |||
| HDL cholesterol, mg/dL | ||||||
| Unadjusted | 163 | 54.5 | 54.0 | 47.3 | 41.84 | 0.09 |
| (52.5–56.4) | (47.2–60.7) | (35.5–59.0) | (31.3–52.3) | |||
| MV-Adjusted | 163 | 55.8 | 55.4 | 45.4 | 44.74 | 0.07 |
| (52.2–59.3) | (48.7–62.2) | (34.1–56.7) | (33.6–55.8) | |||
| Triglycerides, mg/dL2 | ||||||
| Unadjusted | 163 | 73.6 | 85.1 | 128.84 | 133.34 | 0.001 |
| (68.6–78.9) | (67.0 –108.1) | (85.1 –195.0) | (92.0 –193.2) | |||
| MV-Adjusted | 163 | 78.3 | 87.1 | 140.14 | 136.14 | <.01 |
| (68.7–89.2) | (68.0 –111.7) | (92.1 –213.0) | (90.3 –205.2) |
Data presented are mean predicted values. MV-adjusted results are predicted means for a white participant age 35–<40 with a BMI of 26.2, the study population mean, who has two children and no parental history of diabetes.
Results are geometric means.
Partial F test p among categories
Effect estimate p<.05 vs. NGT group
Table 3.
Inflammatory markers and adipokines at three-years postpartum, by gestational glucose tolerance category. Predicted values1 from multiple linear regression models. Data from 537 participants in Project Viva who presented for follow-up at three years postpartum without an intervening birth.
| N | Normal | Abnormal GCT, normal GTT |
Gestational impaired glucose tolerance |
Gestational diabetes |
P3 | |
|---|---|---|---|---|---|---|
| Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
|||
| Inflammatory markers | ||||||
| hsCRP, mg/dL2 | 460 | 39 | 21 | 16 | ||
| Unadjusted | 536 | 0.8 | 0.6 | 1.94 | 1.2 | <.01 |
| (0.7–0.9) | (0.4–0.9) | (1.1–3.4) | (0.6–2.4) | |||
| MV-Adjusted | 536 | 1.0 | 0.7 | 2.24 | 1.4 | <.01 |
| (0.8–1.3) | (0.5–1.1) | (1.3–3.7) | (0.8–2.6) | |||
| IL6, pg/mL 2, N | 142 | 12 | 4 | 5 | ||
| Unadjusted | 163 | 1.1 | 1.6 | 1.0 | 1.5 | 0.44 |
| (0.9–1.2) | (0.9–2.6) | (0.4–2.5) | (0.7–3.3) | |||
| MV-Adjusted | 163 | 0.9 | 1.2 | 1.1 | 1.3 | 0.38 |
| (0.7–1.1) | (0.7–2.0) | (0.4–2.6) | (0.6–3.1) | |||
| Adipokines | ||||||
| Leptin ng/mL, N | 456 | 39 | 20 | 16 | ||
| Unadjusted | 531 | 8.6 | 10.0 | 10.7 | 9.5 | 0.25 |
| (8.0–9.1) | (8.1 – 11.9) | (8.0 – 13.3) | (6.5 – 12.4) | |||
| MV-Adjusted | 531 | 8.3 | 8.6 | 9.4 | 8.1 | 0.56 |
| (7.7–8.9) | (7.4–9.9) | (7.7 – 11.0) | (6.2–9.9) | |||
| Ghrelin pg/mL, N | 144 | 11 | 5 | 5 | ||
| Unadjusted | 165 | 769.1 | 699.8 | 693.8 | 459.54 | 0.16 |
| (717.8–820.4) | (514.2–885.4) | (418.5–969.0) | (184.2–734.8) | |||
| MV-Adjusted | 165 | 819.8 | 762.4 | 663.8 | 562.54 | 0.11 |
| (739.8–899.7) | (603.0–921.8) | (430.8–896.7) | (306.6–818.5) | |||
| Adiponectin ng/mL, N | 457 | 39 | 21 | 16 | ||
| Unadjusted | 533 | 20.7 | 19.1 | 18.0 | 11.24 | <.001 |
| (19.9–21.5) | (16.4–21.7) | (14.4–21.7) | (7.1–15.4) | |||
| MV-Adjusted | 533 | 21.2 | 19.4 | 19.2 | 13.14 | <.001 |
| (19.8–22.6) | (16.7–22.2) | (15.6–22.7) | (9.0–17.3) | |||
| PYY2 pg/mL, N | 143 | 12 | 5 | 5 | ||
| Unadjusted | 165 | 58.5 | 55.2 | 51.8 | 71.3 | 0.42 |
| (55.4–61.7) | (45.8–66.5) | (38.8–69.1) | (53.5–95.1) | |||
| MV-Adjusted | 165 | 59.9 | 55.8 | 52.0 | 71.8 | 0.38 |
| (54.3–66.1) | (46.1–67.5) | (39.1–69.2) | (52.5–98.4) |
Data presented are mean predicted values. MV-adjusted results are predicted means for a white participant age 35–<40 with a BMI of 26.2, the study population mean, who has two children and no parental history of diabetes.
Results are geometric means.
Partial F test p among categories
Effect estimate p<.05 vs. NGT group
Table 4.
Anthropometry at three-years postpartum, by gestational glucose tolerance category. Predicted values1 from multiple linear regression models. Data from 537 participants in Project Viva who presented for follow-up at three years postpartum without an intervening birth.
| N | Normal | Abnormal GCT, normal GTT |
Gestational impaired glucose tolerance |
Gestational diabetes |
P3 | |
|---|---|---|---|---|---|---|
| Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
Mean (95%CI) |
|||
| Anthropometry | ||||||
| 3-year postpartum weight retention, kg, N | 460 | 39 | 21 | 16 | ||
| Unadjusted | 536 | 2.3 | 3.1 | 4.9 | −0.1 | 0.16 |
| (1.7–3.0) | (0.9–5.2) | (1.9–7.8) | (−3.5–3.3) | |||
| MV-Adjusted | 536 | 1.6 | 1.9 | 3.5 | −1.6 | 0.12 |
| (0.5–2.7) | (−0.3–4.1) | (0.7–6.4) | (−4.9–1.8) | |||
| 1-year postpartum weight retention, kg, N | 399 | 32 | 17 | 16 | ||
| Unadjusted | 464 | 0.9 | 0.5 | 2.4 | −0.2 | 0.56 |
| (0.3–1.4) | (−1.4–2.4) | (−0.2–5.0) | (−2.9–2.5) | |||
| MV-Adjusted | 464 | −0.2 | −0.5 | 1.0 | −1.3 | 0.65 |
| (−1.2–0.8) | (−2.5–1.5) | (−1.7–3.6) | (−4.2–1.5) | |||
| Subscapular:Triceps skin fold ratio ,N | 460 | 39 | 21 | 14 | ||
| Unadjusted | 534 | 0.8 | 0.8 | 0.8 | 0.94 | 0.08 |
| (0.7–0.8) | (0.7–0.9) | (0.7–0.9) | (0.8–1.0) | |||
| MV-Adjusted | 534 | 0.7 | 0.8 | 0.8 | 0.94 | 0.07 |
| (0.7–0.8) | (0.7–0.9) | (0.7–0.9) | (0.8–1.0) | |||
| Waist circumference, cm, N | 461 | 39 | 21 | 16 | ||
| Unadjusted | 537 | 86.0 | 90.64 | 89.6 | 93.34 | 0.01 |
| (84.9–87.1) | (86.8–94.5) | (84.3–94.9) | (87.3–99.4) | |||
| MV-Adjusted | 537 | 86.2 | 88.34 | 87.8 | 91.34 | <.001 |
| (85.4–87.1) | (86.6–90.1) | (85.5–90.0) | (88.6–93.9) |
Data presented are mean predicted values. MV-adjusted results are predicted means for a white participant age 35–<40 with a BMI of 26.2, the study population mean, who has two children and no parental history of diabetes.
Results are geometric means.
Partial F test p among categories
Effect estimate p<.05 vs. NGT group
Figure 2.
Mean predicted values for a white participant between 35 and less than 40 years of age with a body mass index (BMI) of 26.2 (the study population mean), who has two children and no parental history of diabetes. P-values on graph are for partial F test. The figure shows adverse metabolic markers that differ with gestational diabetes mellitus (GDM) alone (column A), both gestational impaired glucose tolerance and GDM (column B), or gestational impaired glucose tolerance alone (column C). In each graph’s x-axis, A, B, C, and D represent the following groups: Group A, 50-g screen less than 140 mg/dL; B, 50-g screen 140 mg/dL or more with normal oral glucose tolerance test (OGTT); C, 50-g 140 mg/dL or more with one abnormal OGTT value; D, gestational diabetes mellitus (GDM: two or more abnormal OGTT values). Predicted mean, 95% confidence limit of the mean, adjusted for BMI, age, race, parity, and parental history of diabetes mellitus. *P<.05 compared with normal glucose tolerance group.
Discussion
In this community-based prospective cohort study, we found that both gestational diabetes and gestational impaired glucose tolerance were associated with an adverse metabolic profile at 3 years postpartum, independent of body mass index and parental history of diabetes.
Strengths of our study include its prospective assessment of gestational glucose tolerance and standardized assessment of three-year outcomes. Nevertheless, our results must be interpreted within the context of the study design. Our population was healthy, resulting in low rates of gestational diabetes and impaired glucose tolerance. Among the 91 women ages 30–39 for whom we had data on waist circumference, blood pressure, serum lipids and glucose, only 5 (5.5%, 95% CI 1.8–12.4%) met criteria for the metabolic syndrome, compared with 15% of women in this age range in the general US population(21). In addition, the number of participants with fasting blood samples limited power to detect subtle differences among glucose tolerance groups, and we were not able to define metabolic syndrome in the full cohort. Further studies in larger populations will be needed to validate our findings. Nevertheless, our study size is comparable to several other studies that have assessed metabolic markers among postpartum women with a history of GDM(2, 22). We did not measure post-glucose load insulin or glucose in our population, and therefore we were unable to compare indices of glycemia, insulin sensitivity and beta-cell function. Nevertheless, our study included postpartum measures of adiponectin, which is highly correlated with beta cell dysfunction during pregnancy(23) and with 2-h post OGTT in the postpartum period(24).
Our results confirm and extend earlier work linking gestational glucose tolerance with an adverse maternal metabolic profile in later life. Several authors have reported an increased risk of impaired glucose tolerance and type 2 diabetes among women with abnormal glucose screening results in pregnancy in the setting of both normal OGTT(7) and one abnormal GTT result (5, 6, 9, 25). Moreover, both IGT and GDM have been associated with the metabolic syndrome at 3 months postpartum(8). Other authors have reported associations between GDM and markers of metabolic dysfunction after pregnancy. At a mean of 2 years postpartum, Costacou et al reported adverse associations between history of GDM (N=22) and waist circumference, hemoglobin A1c, and HOMA-IR, compared with women without a history of pregnancy complications (N=29)(22). Heitritter et al similarly compared women with a GDM history (N=23) with normal controls (N=23) at a mean of 4 years postpartum. Women in the GDM group had higher diastolic blood pressure, mean arterial pressure, heart rate, fasting glucose, HOMA, triglycerides, CRP, IL-6, and PAI-1 and lower adiponectin than women in the control group.
No studies to our knowledge have measured associations between IGT and LDL, inflammatory markers or adipokines, or with other metabolic markers beyond 3 months postpartum. We found that women with impaired glucose tolerance during pregnancy had elevations of triglycerides, hemoglobin A1c and CRP, as well as lower HDL, after adjustment for current body mass index and parental history of diabetes. Women with a history of GDM had triglyceride and HDL levels that were similar to those with IGT, but they had higher HOMA-IR and waist circumference, as well as lower adiponectin levels.
These adverse profiles of intermediate markers among women with pregnancy dysglycemia imply increased risk for cardiovascular disease, which is consistent with findings in a recent population-based cohort study (3). In that study, compared with women who did not undergo glucose tolerance testing during pregnancy and therefore were presumed to have had normal glucose screening test results, women with both IGT and GDM were more likely to experience cardiovascular events (IGT OR 1.19, 95% CI 1.02–1.39; GDM OR 1.66, 95% CI 1.30–2.13).
Compared with women with normal glucose testing during pregnancy, we found that women with a history of gestational glucose intolerance had unfavorable markers of glucose and lipid homeostasis and inflammation. These findings persisted with adjustment for current body mass index, suggesting that normal or overweight women with a history of IGT may be at risk for metabolic dysfunction at 3 years postpartum. These women may therefore benefit from dietary changes, physical activity, and/or screening for metabolic syndrome. Current guidelines recommend screening women with a history of GDM for type 2 diabetes(26, 27).
In conclusion, in a prospective study of maternal and infant health, we found that maternal gestational glucose intolerance and gestational diabetes were both associated with adverse metabolic profile at 3 years postpartum, independent of other clinical risk factors.
Acknowledgments
Funded by National Institutes of Health grants R21 DK053537, R01’s HD 034568, HL 064925, HL 075504.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary results were presented at the Society for Gynecologic Investigation, Glasgow, Scotland, Abstract #577, March 20, 2009.
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10):1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 2.Heitritter SM, Solomon CG, Mitchell GF, Skali-Ounis N, Seely EW. Subclinical inflammation and vascular dysfunction in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2005 Jul;90(7):3983–3988. doi: 10.1210/jc.2004-2494. [DOI] [PubMed] [Google Scholar]
- 3.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009 Sep 15;181(6–7):371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Hapo Study Cooperative Research Group. Hyperglycemia and Adverse Pregnancy Outcomes. The New England journal of medicine. 2008 May 8;358(19):1991–2002. doi: 10.1056/NEJMoa0707943. 2008. [DOI] [PubMed] [Google Scholar]
- 5.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. Glucose intolerance in pregnancy and future risk of pre-diabetes or diabetes. Diabetes Care. 2008 Oct;31(10):2026–2031. doi: 10.2337/dc08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJG. Isolated Hyperglycemia at 1-Hour on Oral Glucose Tolerance Test in Pregnancy Resembles Gestational Diabetes in Predicting Postpartum Metabolic Dysfunction. Diabetes Care. 2008 March 20;2008 doi: 10.2337/dc08-0126. dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJ, Zinman B. An abnormal screening glucose challenge test in pregnancy predicts postpartum metabolic dysfunction, even when the antepartum oral glucose tolerance test is normal. Clin Endocrinol (Oxf) 2009 Aug;71(2):208–214. doi: 10.1111/j.1365-2265.2008.03460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinman B, Hanley AJ. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010 Feb;95(2):670–677. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr DB, Newton KM, Utzschneider KM, Tong J, Gerchman F, Kahn SE, et al. Modestly elevated glucose levels during pregnancy are associated with a higher risk of future diabetes among women without gestational diabetes mellitus. Diabetes Care. 2008 May;31(5):1037–1039. doi: 10.2337/dc07-1957. [DOI] [PubMed] [Google Scholar]
- 10.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004 Feb;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 11.Herring SJ, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Kleinman KP, et al. Weight gain in pregnancy and risk of maternal hyperglycemia. American journal of obstetrics and gynecology. 2009 Jul;201(1):61. doi: 10.1016/j.ajog.2009.01.039. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stuebe AM, Kleinman K, Gillman MW, Rifas-Shiman SL, Gunderson EP, Rich-Edwards J. Duration of lactation and maternal metabolism at 3 years postpartum. J Womens Health (Larchmt) 2010 May;19(5):941–950. doi: 10.1089/jwh.2009.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz MW, Niswender KD. Adiposity Signaling and Biological Defense Against Weight Gain: Absence of Protection or Central Hormone Resistance? J Clin Endocrinol Metab. 2004 December 1;89(12):5889–5897. doi: 10.1210/jc.2004-0906. 2004. [DOI] [PubMed] [Google Scholar]
- 14.Antuna-Puente B, Feve B, Fellahi S, Bastard JP. Adipokines: the missing link between insulin resistance and obesity. Diabetes Metab. 2008 Feb;34(1):2–11. doi: 10.1016/j.diabet.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003 Oct;52(10):2546–2553. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 16.Karra E, Chandarana K, Batterham RL. The role of peptide YY in appetite regulation and obesity. J Physiol. 2009 Jan 15;587(Pt 1):19–25. doi: 10.1113/jphysiol.2008.164269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Gunderson EP, et al. Duration of Lactation and Maternal Adipokines At 3 Years Postpartum. Diabetes. 2011 Feb 24;60(4):1277–1285. doi: 10.2337/db10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology (Cambridge Mass. 1995 Jul;6(4):356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Neter J, Kutner M, Nachtsheim C, Wasserman W. Applied Linear Statistical Models. 4th ed. Boston: WCB/McGraw-Hill; 1996. [Google Scholar]
- 20.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010 Jan;33 Suppl 1:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ford ES, Giles WH, Dietz WH. Prevalence of the Metabolic Syndrome Among US Adults: Findings From the Third National Health and Nutrition Examination Survey. JAMA. 2002 January 16;287(3):356–359. doi: 10.1001/jama.287.3.356. 2002. [DOI] [PubMed] [Google Scholar]
- 22.Costacou T, Bosnyak Z, Harger GF, Markovic N, Silvers N, Orchard TJ. Postpartum adiponectin concentration, insulin resistance and metabolic abnormalities among women with pregnancy-induced disturbances. Prev Cardiol. 2008 Spring;11(2):106–115. doi: 10.1111/j.1751-7141.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Hanley AJ, Raif N, Hirning CR, Connelly PW, Sermer M, et al. Adiponectin and beta cell dysfunction in gestational diabetes: pathophysiological implications. Diabetologia. 2005 May;48(5):993–1001. doi: 10.1007/s00125-005-1710-x. [DOI] [PubMed] [Google Scholar]
- 24.Choi SH, Kwak SH, Youn BS, Lim S, Park YJ, Lee H, et al. High plasma retinol binding protein-4 and low plasma adiponectin concentrations are associated with severity of glucose intolerance in women with previous gestational diabetes mellitus. J Clin Endocrinol Metab. 2008 Aug;93(8):3142–3148. doi: 10.1210/jc.2007-1755. [DOI] [PubMed] [Google Scholar]
- 25.Vambergue A, Dognin C, Boulogne A, Rejou MC, Biausque S, Fontaine P. Increasing incidence of abnormal glucose tolerance in women with prior abnormal glucose tolerance during pregnancy: DIAGEST 2 study. Diabet Med. 2008 Jan;25(1):58–64. doi: 10.1111/j.1464-5491.2007.02306.x. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Gestational Diabetes Mellitus. Diabetes Care. 2004 January 1;27(90001):88S–90. 2004. [Google Scholar]
- 27.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No 435: Postpartum Screening for Abnormal Glucose Tolerance in Women Who Had Gestational Diabetes Mellitus. Obstetrics and gynecology. 2009;113(6):1419–1421. doi: 10.1097/AOG.0b013e3181ac06b6. [DOI] [PubMed] [Google Scholar]