Abstract
Objective
The functioning of neural systems supporting emotion processing and regulation in bipolar disorder-not otherwise specified (BP-NOS) youth remains poorly understood. We sought to examine patterns of activity and connectivity in BP-NOS youth relative to youth with BP-I and healthy controls (HC).
Method
Participants (18 BP-I youth, 16 BP-NOS youth, and 18 HC) underwent functional magnetic resonance imaging while performing two emotional-face gender labeling tasks (happy/neutral, fearful/neutral). Analyses focused on a priori neural regions supporting emotion processing (amygdala) and emotion regulation (ventromedial prefrontal cortex (VMPFC), dorsolateral prefrontal cortex (DLPFC). Connectivity analyses used VMPFC as a seed region.
Results
During the happy-face task, BP-I youth had greater amygdala, VMPFC, and DLPFC activity to happy faces whereas BP-NOS youth had reduced VMPFC and DLPFC activity to neutral faces relative to HC, and reduced amygdala, VMPFC, and DLPFC activity to neutral faces versus BP-I. During the fearful-face task, BP-I youth had reduced DLPFC activity to fearful faces whereas BP-NOS youth had reduced DLPFC activity to neutral faces relative to HC. BP-NOS youth showed greater VMPFC-DLPFC connectivity to happy faces relative to HC and BP-I youth. BP-I youth showed reduced VMPFC-amygdala connectivity to fearful faces relative to HC and BP-NOS youth.
Conclusions
This is the first study to document differential patterns of abnormal neural activity in, and connectivity between, neural regions supporting emotion processing and regulation in BP-NOS versus BP-I youth. Findings suggest that despite similarities in symptom presentation, there are differential patterns of abnormal neural functioning in BP-NOS and BP-I relative to HC, which might reflect an “intermediate state” in the course of BP-I illness. Future longitudinal studies are needed to relate these findings with future conversion to BP-I/II.
Introduction
Bipolar disorder (BP) is a devastating psychiatric illness that affects 2–5% of the adult population and remains a leading cause of morbidity, functional impairment, and completed suicide.1 Abnormal emotion processing and regulation, marked by swings from depression to mania, are key clinical features of the illness.2 Accurate diagnosis of BP has critical implications for treatment, yet identifying BP in youth can be challenging. Many youth present with manic symptoms but fail to meet strict DSM-IV criteria for BP-I/II. Those with subthreshold symptoms of BP, often receive the diagnosis of BP Not Otherwise Specified (BP-NOS). BP-NOS youth are particularly vulnerable because they are at very high risk for developing BP-I/II. One way to help identify which BP-NOS youth are likely to develop BP-I/II in the future is to elucidate objective markers that may index pathophysiologic mechanisms of BP, which may not be observable at the behavioral level.3, 4 Elucidating such markers could impact early intervention strategies.
Neuroimaging studies in BP-I youth indicate functional abnormalities in neural systems supporting emotion processing and regulation.5 One study reported abnormally elevated amygdala activity to neutral faces in BP-I youth, particularly in those who perceived these faces as threatening.6 Other findings indicated elevated amygdala and striatal activity,7, 8 and reduced lateral prefrontal cortical activity7 during passive viewing of happy faces in euthymic BP-I versus controls, and elevated amygdala activity during gender labeling of happy and fearful faces in BP-I youth in manic, euthymic, and depressed mood.9 Additional findings show greater striatal activity to positive emotional scenes in BP-I versus healthy youth,10 and greater amygdala activity to negative emotional words in euthymic BP-I versus controls.11
In contrast to studies focused on regional changes in brain activity, connectivity analyses examine the integrity of distributed neural systems by correlating activity in different regions over time. One study documented significantly reduced VLPFC regulation of the amygdala response during an emotion labeling task in manic BP-I adults.12 Another study reported significantly reduced connectivity between the amygdala and cortical association regions in BP-I youth versus controls while processing neutral faces perceived as threatening.13 Taken together, these findings suggest that functional abnormalities in neural systems supporting emotion processing and regulation may underlie the pathophysiology of BP in youth.14, 15
This study aimed at performing a first-stage, cross-sectional examination of the extent to which BP-I and BP-NOS youth share a common neuropathophysiology, by examining neural systems supporting emotion processing and regulation in both youth groups relative to healthy youth. We employed an emotional face gender labeling task16 known to reliably activate subcortical regions (i.e., amygdala) implicated in emotion processing17 and ventromedial, ventrolateral and dorsolateral prefrontal cortices (VMPFC, VLPFC, DLPFC, respectively) implicated in emotion regulation,5 reported to be functionally impaired in BP-I youth.15 Given the rich connections between the amygdala and the VMPFC and the role of the VMPFC in regulating activity in the amygdala, particularly during implicit or automatic emotion regulation tasks such as the task employed in the present study, our connectivity analyses included the VMPFC as a seed region.
We hypothesized that, relative to healthy controls (HC), both BP-I and BP-NOS youth would show significantly elevated amygdala and reduced VMPFC/VLPFC and DLPFC activity to emotional faces but not to neutral faces. Given that only a fraction of BP-NOS youth might convert to BP-I/II, we hypothesized that the magnitude of elevated amygdala and reduced VMPFC/VLPFC and DLPFC would be attenuated relative to that shown by BP-I youth (versus HC). The paucity of extant connectivity findings in BP prevented us to make specific hypotheses regarding connectivity abnormalities in BP-I or BP-NOS youth. Nevertheless, because of the role of VMPFC in implicit emotion regulation and evidence of altered functioning of VMPFC in BP-I youth (see 5), we focused our analyses on VMPFC connectivity while processing emotional and neutral faces and hypothesized reduced VMPFC-amygdala connectivity to emotional faces in both BP-I and BP-NOS youth (versus HC).
Method
Participants
Useable fMRI data were acquired from 52 youth (8–17 years old) with BP-I (n=18), BP-NOS (n=16), as well as age- and sex-matched healthy control youth (HC) (n=18) (Table 1). The study was approved by the University of Pittsburgh Institutional Review Board. Parents signed consent forms; youth signed assent forms. BP-I and BP-NOS youth were recruited from the following sources1: 1) Course and Outcome of Bipolar Youth multicenter study (Pittsburgh site); 2) Longitudinal Assessment of Manic Symptoms multicenter study (Pittsburgh site); 3) Child and Adolescent Bipolar Services outpatient program at University of Pittsburgh Medical Center (UPMC). Participants recruited from these sources were interviewed in their respective studies by the same child psychiatrists (B. B., D. A.) or experienced clinicians using the same semi-structured interviews for children and parents. HC were recruited from the community control group in the Bipolar Offspring Study.18 They had neither lifetime psychiatric diagnoses nor 1st-degree relative with a history of recurrent unipolar depression, mania, hypomania or psychosis, nor 2nd-degree relative with a history of mania, hypomania or psychosis.
Table 1.
Demographic and Clinical Characteristics of Bipolar Disorder type I (BP-I), Bipolar Disorder Not Otherwise Specified (BP-NOS), and Healthy Controls (HC)
| Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| BP-I (N = 18) | BP-NOS (N = 16) | HC (N = 18) | Statistic | P value | ||||
| Characteristic | Mean | SD | Mean | SD | Mean | SD | ||
| Age at Scan (years) | 14.57 | 1.98 | 12.59 | 2.27 | 13.67 | 2.55 | F2,51=3.2 | .07 |
| Socio-economic Statusa | 44 | 12.90 | 42 | 12.62 | 46 | 10.32 | F2,40=.45 | .80 |
| Age of illness onset (years)b | 7.95 | 2.5 | 9.36 | 2.9 | --- | --- | t18= −1.15 | .27 |
| Number of Comorbid | 1.47 | 1.06 | 1.53 | 0.99 | --- | --- | t28= −.18 | .86 |
| Diagnoses Number of Medications | 2.4 | 1.46 | 1.94 | .99 | --- | --- | t32= 1.17 | .25 |
| K-MRSc | 17.17 | 10.93 | 15.50 | 12.04 | --- | --- | t32=.42 | .68 |
| K-DRSc | 13.17 | 10.04 | 14.11 | 8.99 | --- | --- | t31=−.28 | .78 |
| MFQ – parent versionc | 14.0e | 12.9 | 18.9e | 13.1 | 1.3f | 1.2 | F2, 51=12.7 | <.001 |
| MFQ – child version | 11.1 | 11.8 | 11.4 | 11.6 | 5.9 | 5.8 | F2, 51=1.63 | .21 |
| CALSc | 25.7e | 19.4 | 34.6e | 22.5 | 2.4f | 2.2 | F2, 50=16.6 | <.001 |
| SCARED – parent versionc | 18.4e | 12.9 | 22.4e | 14.9 | 3.4f | 3.0 | F2, 50=13.2 | <.001 |
| SCARED – child versionc | 18.5e | 15.9 | 19.4e | 14.1 | 8.4f | 5.1 | F2, 51=4.1 | <.05 |
| N | N | N | |||
|---|---|---|---|---|---|
| Male | 11 | 11 | 7 | χ2,52=3.3 | .18 |
| Comorbid Disorders (current/lifetime) | |||||
| Anxiety disorder | 8/11 | 5/10 | --- | ||
| ADHD | 8/9 | 10/10 | --- | ||
| Oppositional defiant disorder or conduct disorder | 2/16 | 6/6 | --- | ||
| Medication | |||||
| No medication | 2 | 0 | 18 | ||
| Lithium | 6 | 1 | --- | ||
| Atypical Antipsychotic | 14 | 13 | --- | ||
| Antiepileptic | 6 | 2 | --- | ||
| Antidepressant | 6 | 4 | --- | ||
| Stimulants | 6 | 8 | --- | ||
| Otherd | 10 | 7 | --- | ||
Note: CALS = Child Affect Lability Scale (range, 0–80); K-DRS, 12-item depression questionnaire from the Schedule for Affective Disorders and Schizophrenia for School-Age Children (KSADS) 22; K-MRS = KSADS Mania Rating Scale21; MFQ = Mood and Feelings Questionnaire (range, 0–68); SCARED = Screen for Childhood Anxiety and Related Disorders (range, 0–82).
Measured using the Hollingshead Four-Factor Index26
Because the validity of DSM-IV diagnostic criteria for preschool-aged children has not been established, the minimum age of onset for BP-I and BP-NOS was set at 4 years.
Significant main effect of group.
Medications used to treat medical condition (e.g., allergies, diabetes).
means with different letters in their superscript differ at the p<.05 level, with Bonferroni correction.
All youth were assessed using the Schedule for Affective Disorders and Schizophrenia for School Aged Children–Present and Lifetime Version (K-SADS-PL19). Inter-rater reliability was excellent (k≥.90 for all diagnoses, including differentiating BP-I and BP-NOS). BP-I youth met DSM-IV diagnostic criteria for BP-I. BP-NOS was defined as clinically relevant BP symptoms that did not fulfill DSM-IVcriteria for BP-I or BP-II. Subjects were also required to have a minimum of elated mood plus two associated DSM symptoms or irritable mood plus three DSM associated symptoms and change in functioning. These symptoms were required to last a minimum of 4 hours within a 24-hour period, and at least 4 cumulative lifetime days meeting the criteria. Most of the BP-NOS youth had both elated and irritable mood associated with manic symptoms; one had a history of elated mood only and one had a history of irritable mood only. Age of onset for a BP-I or BP-NOS diagnosis was considered to be when youth first met DSM-IV criteria for a major mood episode or COBY study criteria for BP-NOS. Given that the validity of DSM-IV diagnostic criteria for preschool-aged children has not been established, the minimum age of onset for BP-I or BP-NOS was set at 4 years.20
To determine mood state on the day of the scan, clinicians administered the KSADS-PL Mania Rating Scale (K-MRS)21 and the KSADS-PL Depression Rating Scale (K-DRS)22 immediately before the scan. Parents completed the following questionnaires about their children: the Mood and Feelings Questionnaire (MFQ)23, to assess depression symptoms; the Child Affect Lability Scale (CALS)24, to assess mood lability, and the Screen for Childhood Anxiety and Related Disorders (SCARED) 25 to assess anxiety symptoms. Youth completed the child self-report version of the MFQ and SCARED. Socio-economic status (SES) was measured with the Hollingshead Four-Factor Index.26 Handedness was determined using the Edinburgh Handedness Inventory.27
At the time of scanning, 33% (n=6) of BP-I and 37.5% (n=6) of BP-NOS youth were euthymic (K-MRS<12 and K-DRS<10); 22% (n=4) of BP-I and 0.6% (n=1)of BP-NOS youth had clinically significant manic symptoms20 with no or minimal depression symptoms (K-MRS>12 and K-DRS<10); 0% of BP-I and 0.6% (n=1) of BP-NOS youth had clinically significant depression symptoms with few manic symptoms (K-MRS<12 and K-DRS>10); and 44% (n=8) of BP-I and 50% (n=8) of BP-NOS youth were in mixed mood state (K-MRS>12 and K-DRS>10).
Exclusion criteria included: IQ<70, history of head trauma, neurological disorder, substance abuse/dependence, developmental delay, hand-eye coordination problem, and mood disorders secondary to substance abuse, medical conditions, pregnancy, presence of metal in the body. All medication usage over the past 48 hours was recorded. Youth medicated for BP and ADHD were not excluded from the study.
fMRI Paradigm
An emotional face gender labeling event-related fMRI paradigm was used.28 It comprised two, well-validated 6-minute fast event-related neuroimaging tasks examining neural activity to happy versus neutral (happy face task) and fearful versus neutral (fearful face task) emotional facial expressions.29 The happy face task comprised happy and neutral facial expressions whereas the fearful face task comprised fearful face task comprised fearful and neutral facial expressions; each task was presented as a separate run. In both tasks, stimuli were gray-scale digitized photographs from that were of fixed size (15×10.5 cm), cropped, and morphed using software to depict emotional expressions ranging from neutral (0%) to mild (50%) to prototypical (100%) intensity of each emotion. Each stimulus was presented for 2 sec. with a mean inter-stimulus interval of 4.9 sec. during which a fixation cross was displayed. In each task, subjects viewed 20 neutral, 20 mild, and 20 prototypical faces. The order of which task would be presented first was counterbalanced across subjects. Subjects were asked to respond with their index finger or the middle finger to indicate whether the actor in the picture was a woman or a man. They were also asked to try to respond as quickly but also as accurately as possible. Because of the complexity of the design, in the current study we focused analyses on the prototypical (100%) and neutral faces in each task.
fMRI Data Acquisition
A 3.0T Siemens Allegra MRI scanner was used to acquire 3D Sagittal MPRAGE images (TE:2.48 ms, TR:1630 ms, IT:800ms, flip angle:8°, field of view:200 mm, slice thickness:0.8 mm, image matrix:256 × 256, 208 slices) and mean blood-oxygenation-level-dependent (BOLD) images. We used a reverse gradient-echo EPI sequence (34 axial slices, 3mm thick, 0mm gap; TR/TE=2000/25msec, FOV=205 mm, matrix=64×64), parallel to the AC-PC line and encompassing the entire cerebrum and the majority of the cerebellum. Participants were placed in a simulator to habituate to the scanning environment and minimize head movement.
Behavioral Data
Mean percent accuracy scores and correct-trial reaction times were computed for each condition for each participant. Data were analyzed using mixed ANCOVA models in SPSS, with group as between-subject and emotion condition as within-subject variables, with age and sex as covariates.
fMRI Data Analysis
Preprocessing
Data preprocessing was performed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm). Functional data for each participant were first corrected for differences in acquisition time between slices, realigned using the first slice as a reference, and unwarped to correct for static inhomogeneity of the magnetic field and movement by inhomogeneity interactions. Each volume was co-registered by aligning the first scan from each volume to the first scan of the first volume with regard to the subject’s MPRAGE image and segmented. Data were normalized to Montreal Neurological Institute standardized template and spatially smoothed with a Gaussian kernel of 6-mm full-width at half-maximum.
Individual and Group-level Analyses
Individual, or first-level, analyses, model specification and estimation were performed using a General Linear Model (GLM) within SPM5. For each task (happy and fearful) separately, the first-level fixed-effect model was defined by entering emotion condition (emotional face: happy or fearful, neutral) as separate conditions in an event-related design matrix with fixation cross as baseline. Uncorrelated low frequency noise was removed by using high-pass filter (cut-off 128sec). Motion-related regressors were included as covariates of no interest in the GLM to control signal change related to motion.
Contrast maps from first-level analyses (i.e., happy face task: happy minus fixation, neutral face minus fixation; fearful face task: fearful face minus fixation, neutral face minus fixation) were then entered into the random-effects group- or second-level analyses using a full factorial model. Separate models were computed for happy and fearful face tasks. Age, sex, and accuracy rates were included as covariates in each of the models. We performed a 3 × 2 ANCOVA including the three groups (BP-I, BP-NOS, HC) and two emotion conditions (emotional face: happy, or fearful, neutral) for each task (happy or fearful) separately. This provided an omnibus test of our hypotheses regarding group x emotion condition interactions for each of the happy and fearful face tasks. Because we had strong region-based hypotheses of group differences, region-of-interest (ROI) analyses were conducted to examine between-group differences in neural activity in a priori ROIs, which were defined by Wake Forest University Pick Atlas.30 Specifically, three predefined anatomical masks were used: 1) bilateral amygdala, 2) bilateral VMPFC, which also incorporated part of the ventrolateral cortex (BA 11/25/47) and 3) bilateral DLPFC (BA 9/46).
To control for multiple voxelwise tests we used AlphaSim.31 In all analyses, images were spatially thresholded based on the functional data within the area of the hypothesized ROI and cluster-level significance was thresholded for multiple comparisons [p<.05] based on 104 Monte Carlo simulations from voxels across the individual ROIs. The number of contiguous voxels needed to maintain this false positive detection rate in each ROI was computed separately for each statistical model (happy, fearful).
Post hoc analyses to identify between group differences in activity underlying any observed significant group x emotion condition interactions in each ROI were performed in SPM5. Here, significant group x emotion condition interaction statistical maps in ROIs were used as masks and independent t-tests examined between-group differences in activity in these maps for each emotion condition (happy/neutral; fearful/neutral), with AlphaSim correction.
We also conducted exploratory whole-brain analyses to examine the extent to which group × emotion condition interactions occurred throughout the brain. A random effects GLM was used to examine activity patterns associated with the same contrasts as in the ROI analyses, using a threshold of p<.05, uncorrected, kE = 50 voxels, with small volume correction for clusters of interest.
fMRI Connectivity Analysis
Psychophysiological Interaction (PPI) is a valuable method of evaluating connectivity between the physiological response of a source region-of-interest and a psychological task of interest.32 Specifically, PPI analysis reflects changes in a regression slope associated with the differential BOLD response from one neural region under the influence of particular experimental contexts.32 Thus, PPI provides information about the modulatory effects of an experimental condition on the functional coupling between two neural regions. PPI analysis consists of a design matrix with three regressors: (1) “psychological regressor” (e.g., happy-fixation), (2) “physiological regressor”, determined by the neural response in VMPFC, and (3) interaction term of (1) and (2), which is referred to as the “PPI regressor”. The PPI regressor refers to brain areas that show a greater functional connectivity with VMPFC during one of the emotional face conditions.
At the first-level, a PPI regressor was created for each participant by computing an interaction term between the emotion condition effect (i.e., happy face task: happy minus fixation, neutral face minus fixation; fearful face task: fearful face minus fixation, neutral face minus fixation) and the mean seed region (VMPFC) time series. The seed region was extracted from a volume-of-interest (radius=2mm) centered on the significance peak of the condition-of-interest in left and right VMPFC, defined based on findings from previous studies pertaining to the role of the VMPFC in implicit emotion regulation.5, 33 The time series was then convolved with the paradigm (i.e., emotional face: happy or fearful minus fixation, neutral minus fixation) for each task (happy, fearful) separately and resultant interaction term was weighted −1 (indicating negative modulation), given that our main hypotheses focused on group differences in VMPFC-amygdala coupling and evidence of negative associations between these two regions. The resulting voxel-specific r-coefficients were converted to Z-scores images and entered into second-level random effects analyses testing for group differences and group x emotion condition interactions. A GLM was implemented to examine neural regions that showed significant increases in functional connectivity with seed activity (right VMPFC, left VMPFC) during task performance in BP-I youth, BP-NOS youth, and HC. We performed two separate 3 × 2 × 2 ANCOVA models, including group (BP-I, BP-NOS, HC), emotion condition (emotional face: happy or fearful, neutral) and hemisphere (left, right) for each task (happy, fearful) separately. Age, sex, and accuracy rates were included as covariates in both models. In contrast to usual fMRI analyses, our model included not only the experimental condition (emotional face: happy, or fearful, neutral) as the predictor variable but also activity level in the seed region (VMPFC) and interaction between the task and seed activity variables (PPI). Second-level analyses were restricted to any DLPFC and amygdala target ROIs, that showed connectivity with right and left VMPFC seed regions, and were thresholded at p<0.05. We used AlphaSim correction [p<.05], as described above, to correct for multiple voxelwise tests in clusters resulting from the interaction or main effects analyses as well as post hoc analyses.
Exploratory Analyses Examining Relationships with Clinical Variables
Mean eigenvalues from clusters that survived AlphaSim correction in the above statistical analyses for activity and connectivity analyses were extracted for each participant and condition. Pearson correlational analyses were performed to examine relationships between neural activity and connectivity indices with scores on the clinical measures (i.e., K-MRS, K-DRS, MFQ, CALS, SCARED) in BP-I and BP-NOS youth as well as maximum scores from the K-MRS associated with manic symptoms (i.e., elation, irritability) in BP-NOS youth. Furthermore, the influence of comorbid diagnoses (i.e., ADHD, Anxiety Disorders) and medication on neural activity and connectivity was examined by stratifying each BP-I and BP-NOS group as a function of presence/absence of comorbid diagnoses (e.g., BP-I_withADHD/Anx, BP-I_withoutADHD/Anx) and taking/not taking psychotropic medications in Table 1 (e.g., BP-I_taking stimulants, BP-NOS_not taking stimulants). Separate t-tests were conducted comparing each subgroup within BP-I and BP-NOS groups. One set of analyses was conducted for the presence of ADHD and another for the presence of Anxiety Disorders (i.e., GAD, separation anxiety, social phobia); few participants had only one comorbid condition. Because of the low number of BP-I and BP-NOS youth who were unmedicated, we could not perform between-group comparisons of neural activity and connectivity measures between HC and unmedicated BP-I and HC and unmedicated BP-NOS. The statistical threshold was set at p<0.05 because of the exploratory nature of these analyses.
Results
Demographic and Clinical Characteristics
Groups did not significantly differ in age, sex, and, socio-economic status. There was a significant main effect of group for scores on parent MFQ, parent CALS, parent SCARED, and child SCARED, but not child MFQ, indicating that both BP-I and BP-NOS youth had significantly higher scores than HC on these measures. There were no significant differences between BP-I and BP-NOS youth in K-MRS and K-DRS scores, age of illness onset, number of comorbid diagnoses, or number of medications (Table 1).
Behavioral Data
Accuracy
There were no significant group effects or group x emotion condition interactions [ps>.1].
Reaction times
For the happy-face task, there were no significant group effects or group x emotion condition interactions [ps>.1]. For the fearful-face task, there was no significant group x emotion condition interaction [ps>.1]. However, there was a significant group effect [F(2,44)=4.03, p<.05]. Post hoc comparisons indicated that BP-I youth were significantly slower than HC on this task [p<.05]; there were no significant differences between BP-I and BP-NOS youth, or BP-NOS youth and HC [ps>.1] (see Table S1, available online).
fMRI Data
In the following section, we present the fMRI and PPI results examining differences in the patterns of activity in, and connectivity between, neural regions implicated in emotion processing and regulation. To correct for multiple tests within the search volume of each of the ROIs, we used AlphaSim, which served as a family-wise error (FWE) correction [p<0.05] using a spatial extent threshold. The number of contiguous voxels needed to maintain this false positive detection rate in each ROI was as follows: bilateral amygdala (happy: kE=16; fearful: kE=26), bilateral VMPFC (happy: kE=61; fearful: kE=61), bilateral DLPFC (happy: kE=57; fearful: kE=55).
Region-of-interest Analyses
Happy-Face Task
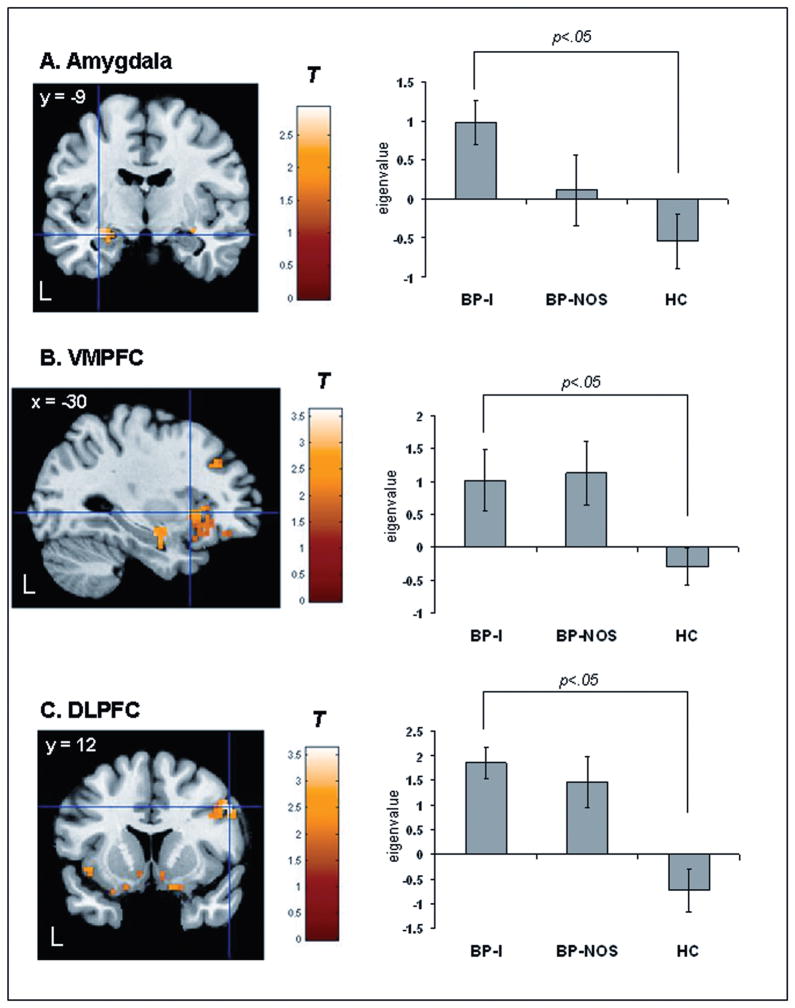
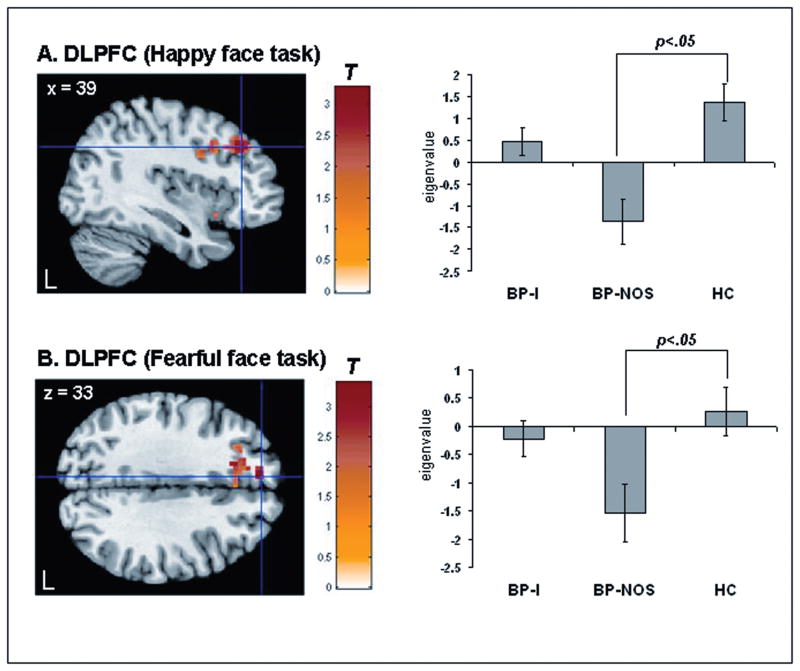
There was a significant group x emotion condition interaction in bilateral amygdala, bilateral VMPFC, and bilateral DLFPC [p<.05, corrected] (Table 2). Post hoc between-group comparisons for happy faces revealed that relative to HC, BP-I youth had significantly greater activity in left amygdala, left VMPFC, and right DLPFC [p<.05, corrected] (Table 2; Figure 1A, 1B, 1C, respectively). There was a trend suggesting that BP-NOS also had greater activity in left VMPFC compared to HC but this contrast did not reach the corrected statistical threshold [t(95)=2.45, p=.008, p<.10, corrected] (Table 2; Figure 1B). Post hoc analyses for neutral faces revealed that relative to HC, BP-NOS, but not BP-I youth, had significantly reduced activity in left VMPFC, and right DLPFC (Table 2, Figure 2A). BP-NOS had reduced activity to neutral faces versus BP-I youth in left amygdala, left VMPFC, and bilateral DLPFC (Table 2).
Table 2.
Regions Showing Greater and Reduced Activation in Response to Happy and Neutral Faces, Bipolar Disorder Type I (BP-I), Bipolar Disorder Not Otherwise Specified (BP-NOS), Healthy Controls (HC)
| Talairach Coordinatesa | |||||||
|---|---|---|---|---|---|---|---|
| Region | kE | Side | x | y | z | Statistic | Uncorrected P value |
| Happy Face Taskb | |||||||
|
| |||||||
| Group × Condition | |||||||
| Amygdalac | 36 | R | 33 | −4 | −17 | F=6.35 | .003 |
| 36 | L | −21 | −9 | −12 | F=5.69 | .005 | |
| VMPFCc | 1064 | L | −21 | 37 | −12 | F=14.92 | <.001 |
| 93 | R | 6 | 61 | −11 | F=13.87 | <.001 | |
| DLPFCc | 1095 | R | 48 | 35 | 1 | F=10.12 | <.001 |
| 67 | L | −56 | 4 | 27 | F=6.52 | .002 | |
| Group Comparisonsd | |||||||
| Happy BP-I>HC | |||||||
| Amygdalac | 29 | L | −30 | −9 | −12 | T=2.93 | .002 |
| VMPFCc | 138 | L | −30 | 15 | −1 | T=2.52 | .007 |
| DLPFCc | 70 | R | 53 | 13 | 32 | T=3.63 | <.001 |
| Happy BP-NOS>HC | |||||||
| VMPFC | 51 | L | −38 | 11 | −5 | T=2.45 | .008 |
| Neutral BP-NOS<HC | |||||||
| VMPFCc | 292 | L | −42 | 37 | −9 | T=3.27 | .001 |
| 234 | L | −6 | 20 | −6 | T=3.23 | .001 | |
| DLPFCc | 543 | R | 39 | 34 | 31 | T=3.24 | .001 |
| Neutral BP-NOS<BP-I | |||||||
| Amygdalac | 17 | L | −30 | −7 | −17 | T=2.76 | .003 |
| VMPFCc | 644 | L | −3 | 0 | −5 | T=4.49 | <.001 |
| DLPFCc | 320 | L | −18 | 25 | 32 | T=3.23 | .001 |
| 78 | R | 36 | 13 | 35 | T=3.00 | .002 | |
|
| |||||||
| Fearful Face Taske | |||||||
| Group × Condition | |||||||
| DLPFCc | 111 | L | −15 | 42 | 31 | F=9.03 | <.001 |
| Group Comparisonsd | |||||||
| Fear BP-I<HC | |||||||
| DLPFCc | 55 | L | −24 | 42 | 31 | T=3.76 | <.001 |
| Neutral BP-NOS<HC | |||||||
| DLPFCc | 73 | L | −6 | 48 | 28 | T=3.40 | .001 |
Note: DLPFC = dorsolateral prefrontal cortex; kE = cluster size in voxels; L = left side; R = right side; VMPFC = ventromedial prefrontal cortex.
Each line in the table represents the voxel of peak activity difference within the specified region.
BP-I (n=18), BP-NOS (n=16), HC (n=18).
Survived familywise error correction using AlphaSim, [p<.05]. The following number of contiguous voxels were used, [p<.05]: bilateral amygdala (happy: kE=16; fearful: kE=26), bilateral VMPFC (happy: kE=61; fearful: kE=61), bilateral DLPFC (happy: kE=57; fearful: kE=55).
Post hoc group comparisons: group contrasts that were masked with the significant group x condition interaction map for each of the regions of interest (ROI) analyses.
BP-I (n=17), BP-NOS (n=15), HC (n=18).
Figure 1.
Statistical parametric map displaying between-group contrasts for happy faces masked with the significant group x emotion condition interaction in the happy face task (left). Note: Histogram displaying mean eigenvalues extracted from the peak voxel of the cluster that reached statistical threshold (right). A. Bipolar Disorder Type I (BP-I) > healthy controls (HC) (left amygdala)[t(95)=2.93, p=.002, p<.05, corrected]. B. BP-I > HC (left ventromedial prefrontal cortex (VMPFC)) [t(95)=2.52, p=.007, p<.05 corrected]. There was a trend for Bipolar Disorder Not Otherwise Specified (BP-NOS)>HC (left VMPFC) [t(95)=2.45, p=.008, p<.10, corrected]. C. BP-I > HC (right dorsolateral prefrontal cortex (DLPFC)) [t(95)=3.63, p=<.001, p<.05, corrected]. Statistics and coordinates are given in Table 3. Color bars ranging from red to yellow represent T statistics. For display purposes, cluster threshold was set at 55 voxels. L = left.
Figure 2.
Statistical parametric map displaying between-group contrasts for neutral faces masked with the corresponding significant group x emotion condition interaction statistical map (happy or fearful face task) (left). Note: Histogram displaying mean eigenvalues extracted from the peak voxel of the cluster that reached statistical threshold (right). A. Bipolar Disorder Not Otherwise Specified (BP-NOS)< healthy controls (HC) to neutral faces dorsolateral prefrontal cortex (DLPFC) [t(91)==3.24, p<.001, p<.05, corrected] masked with the significant group x emotion condition interaction statistical map in the happy face task. B. BP-NOS < HC to neutral faces (DLPFC) [t(91)=3.40, p<.001, p<.05, corrected] masked with the significant group x condition interaction statistical map in the fearful face task. Statistics and coordinates are given in Table 3. Color bars ranging from red to yellow represent T statistics. For display purposes, cluster threshold was set at 55 voxels. L = left.
Fearful-Face Task
There was a significant group x emotion condition interaction in left DLFPC [p<.05, corrected]. Post hoc between-group comparisons for fearful faces revealed that relative to HC, BP-I, but not BP-NOS youth, had reduced activity in left DLPFC (Table 2). Post hoc analyses for neutral faces revealed that relative to HC, BP-NOS, but not BP-I youth, had reduced activity in left DLPFC (Table 2, Figure 2B). There were no significant differences in activity, however, between BP-I and BP-NOS youth for fearful or neutral faces.
Whole-brain Analyses
Voxel-wise whole-brain analyses also revealed group x emotion condition interactions for the happy-face task in the superior frontal gyrus, superior temporal gyrus, and cerebellum and for the fearful-face task in the medial frontal gyrus, superior frontal gyrus, cerebellum, and visual areas (see Table S2, available online). Post hoc analyses that decomposed the significant group x emotion condition interactions generally supported ROI analyses of reduced prefrontal cortical activation to neutral faces in BP-NOS youth and to fearful faces in BP-I youth relative to healthy controls (see Table S2, available online).
fMRI Connectivity Analysis
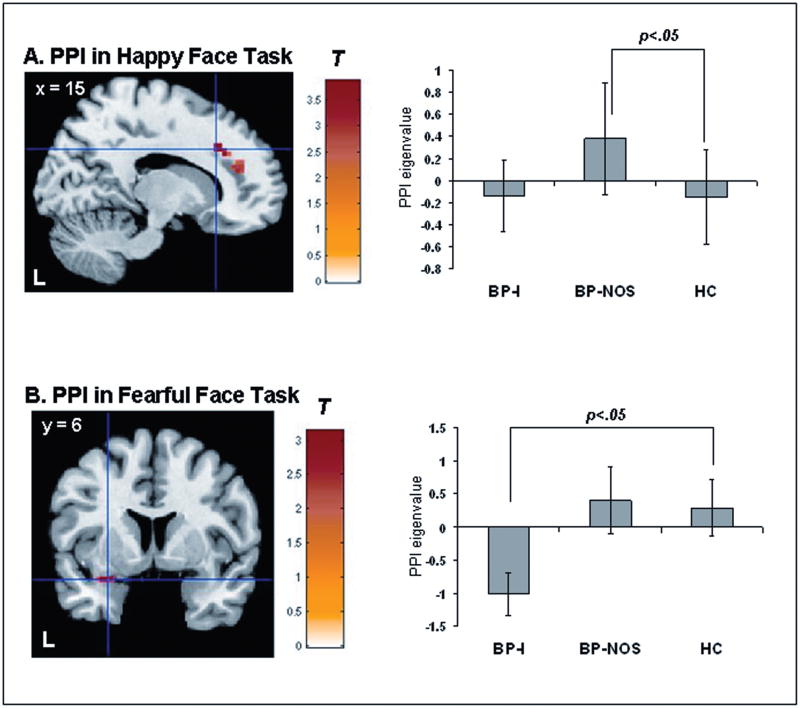
For the happy-face task, PPI analysis showed a significant group effect for happy faces (vs. fixation) [F(2,95)=8.04, p<.05, corrected, kE=93]. Post hoc group contrasts masked with the group effect statistical map revealed that relative to HC, BP-NOS, but not BP-I youth, showed greater functional coupling between bilateral VMPFC and right DLPFC to happy faces [t(95)=3.87, p<.05, corrected, kE=93] (Figure 3A). BP-NOS youth also showed greater coupling between these regions than BP-I youth [t(95)=3.66, p<.05, corrected, kE=70] (Figure 3A).
Figure 3.
A. Neural connectivity between bilateral ventromedial prefrontal cortex (VMPFC) and right dorsolateral prefrontal cortex (DLPFC) to happy faces. Note: Statistical parametric map (SPM-T) displaying a significant between-group contrast that was masked with the significant main effect of group statistical map (SPM-F), [F(2,95)=8.04, p<.001; p<.05, corrected, kE=93] (left). Relative to healthy controls (HC) (n=18), bipolar disorder not otherwise specified (BP-NOS) youth (n=16) showed significantly greater connectivity between ventromedial prefrontal cortex (VMPFC) and right dorsolateral prefrontal cortex (DLPFC) (Talairach x, y, z: 15, 25, 29) to happy faces, [t(95)=3.87, p<.001, p<.05, corrected], kE=93). B. Neural connectivity between bilateral VMPFC and left amygdala to fearful faces. SPM-T displaying a significant between-group contrast that was masked with the significant main effect of SPM-F, [F(2,91)=12.11, p<.001, p<.05, corrected, kE=19] (left). Relative to HC (n=18), BP-I youth (n=17) showed significantly reduced connectivity between VMPFC and left amygdala (Talairach x, y, z: −30, 2, −13) to fearful faces, [t(91)=3.13, p<.001, p<.05, corrected, kE=17]. For A and B, histograms on the right display mean eigenvalues extracted from the peak voxel of the cluster that reached statistical threshold (right). Color bars ranging from red to yellow represent T statistics. For display purposes, cluster threshold was set at 55 voxels. L = left; PPI = Psychophysiological Interaction.
For the fearful-face task, PPI analysis showed a significant group effect for fearful faces (vs. fixation)[F(2,95)=12.11, p<.05, corrected, kE=19]. Post hoc group contrasts masked with the group effect statistical map revealed that relative to HC, BP-I, but not BP-NOS youth, showed less coupling between bilateral VMPFC and left amygdala to fearful faces [t(91)=3.13, p<0.05, corrected, kE=17] (Figure 3B). BP-I also showed less coupling between these regions than BP-NOS youth [t(95)=4.91, p<.05, corrected, kE=19] (Figure 3B).
Exploratory Analyses Examining Relationships with Clinical Variables
Correlational analyses revealed a significant positive correlation for BP-NOS youth between mean K-DRS depression scores and PPI measures of VMPFC-DLPFC connectivity to happy faces (r=.73, p<.05). There were no other significant correlations in either BP-I or BP-NOS between neural activity or connectivity measures and clinical measures [ps>.1]. Within-group comparisons of neural activity and connectivity measures in BP-I and BP-NOS youth based on the presence/absence of a comorbid conditions (ADHD and/or Anxiety disorders) and being on/off medications did not yield any significant group differences [ps>.1].
Because BP-I youth had significantly slower reaction times on the fearful-face task, we examined correlations between reaction times and neural activity and connectivity measures extracted from the significant group contrasts in the fearful-face task for each of the groups. There were no significant correlations for either BP-I, BP-NOS, or HC youth [p>.1].
Discussion
The role of amygdala-prefrontal cortical circuitry in processing and regulating emotional information has been documented, with specific patterns of abnormal functioning reported mainly in BP-I.6, 10, 34 To our knowledge, this is the first study to examine whether BP-I and BP-NOS youth show similar patterns of abnormal activity in, and connectivity between, neural regions supporting emotion processing and regulation. We hypothesized that both BP-I and BP-NOS, relative to HC youth, would show significantly elevated amygdala and reduced VMPFC/VLPFC and DLPFC activity to emotional faces but that this pattern of neural activity would be attenuated in BP-NOS relative to that shown by BP-I youth(versus HC). Furthermore, we hypothesized reduced VMPFC-amygdala connectivity to emotional faces in both BP-I and BP-NOS youth (versus HC).
Our findings partially support our hypotheses. That is, BP-I youth versus HC did show elevated activity in key emotion processing and regulation regions to happy faces and reduced DLPFC activity to fearful faces. This pattern was not observed, however, in BP-NOS versus HC. Rather they showed reduced prefrontal cortical activity to neutral faces versus HC. Moreover, BP-I versus HC showed reduced VMPFC-amygdala coupling to fearful faces, which was not observed in BP-NOS. Rather, BP-NOS exhibited greater VMPFC-DLPFC coupling to happy faces versus HC and BP-I youth. Together, these findings suggest that, relative to healthy controls, BP-I and BP-NOS youth exhibit different patterns of neural activation and connectivity when processing emotional facial expressions.
In order to better understand these differential patterns of abnormal activation and connectivity in BP-NOS youth relative to BP-I youth, we first discuss findings in BP-I youth in relation to the extant neuroimaging literature in BP-I youth. Our findings of elevated amygdala and VMPFC activity to happy faces and reduced DLPFC to fearful faces in BP-I versus HC youth are consistent with results from previous studies using the same gender labeling task in BP-I adults.28, 35 Elevated amygdala activity to happy faces may reflect greater attentional bias and associated appraisal while elevated VMPFC activity may index greater implicit regulation of emotional responses toward these positive emotional stimuli in BP-I youth.5, 16 Although we did not replicate previous findings of elevated amygdala activity to fearful faces in BP-I youth versus HC,6, 9 we did replicate previous findings of reduced DLPFC to fearful faces reported in adult bipolar patients.35 Such reduction in DLPFC activity to fearful faces in BP-I youth may reflect reduced orienting to these negative emotional stimuli in bipolar individuals, as there is evidence to suggest that, during emotion processing, the orienting of attention toward emotional stimuli places demands on DLPFC.36 The pattern of elevated, rather than reduced, DLPFC activity to happy faces in BP-I youth in the present study may therefore reflect greater recruitment of orienting-related attentional and effortful regulatory resources in BP-I youth versus HC. Our findings in BP-I youth are also largely consistent with findings from the few extant studies in BP-I youth that reported abnormally elevated amygdala and prefrontal cortical activity to happy or positive emotional stimuli, and abnormally reduced DLPFC activity to threat-related stimuli. In particular, our findings of elevated amygdala activity to happy faces are consistent with recent findings of elevated amygdala activity during gender labeling of happy faces in BP-I youth versus HC9 and passive viewing of happy and angry faces in euthymic BP-I youth versus HC.7
With regard to connectivity, BP-I youth displayed reduced connectivity versus HC between VMPFC and left amygdala to fearful faces. Few studies have examined amygdala-prefrontal connectivity in pediatric bipolar disorder. The only study, to our knowledge, reported reduced connectivity between left amygdala and cortical association regions in BP-I youth versus HC to neutral faces subjectively rated as threatening.13 Given the role of VMPFC in modulating amygdala activity, our findings of reduced VMPFC-amygdala connectivity to fearful faces may be associated with altered functional maturation of the VMPFC in BP-I youth.37
The major findings in BP-NOS youth from the current study were unexpected. The neural activation findings suggest that BP-NOS youth exhibit reduced prefrontal cortical activation compared with HC to neutral faces when these faces are presented in the context of emotional faces (i.e. happy and fearful faces). There was, however, a trend effect suggesting that BP-NOS youth did exhibit, similar to BP-I youth versus HC, greater VMPFC activity to happy faces than HC. With regard to findings associated with neutral faces, there is evidence to suggest that emotional context can affect perception of, and neural activity to, neutral faces36. Reductions in DLPFC activity to neutral faces in each of the two experiments (and in VMPFC to neutral faces in the happy experiment) in BP-NOS relative to HC, and reduced amygdala, VMPFC and DLPFC activity to neutral faces (in the happy experiment) in BP-NOS relative to BP-I, may therefore suggest that BP-NOS youth perceived neutral faces as less salient in the context of emotional faces such as happy or fearful facial expressions, than did HC or BP-I, but this requires further study. Taken together, neuroimaging findings to happy faces in BP-NOS relative to BP-I and HC indicate that BP-NOS may represent an “intermediate” state between being healthy and having BP-I. The reduced activity in prefrontal cortical regions, and prefrontal cortical regions and amygdala, to neutral faces presented in the happy experiment, in BP-NOS versus HC and BP-I, respectively, could represent a potential biological mechanism that prevents BP-NOS youth from developing more severe symptoms of bipolar disorder. Given the paucity of neuroimaging research in BP-NOS youth, however, these interpretations remain speculative.
With regard to functional connectivity, BP-NOS youth compared with HC exhibited greater VMPFC-DLPFC coupling while viewing happy faces. According to animal models and recent neuroimaging studies, the DLPFC plays an indirect role in modulating the amygdala through connections via the VMPFC.5, 38 Thus, it is possible that greater VMPFC-DLPFC coupling in BP-NOS youth may explain why there was a trend for elevated VMPFC and DLPFC activity, but reduced amygdala activity, to happy faces in BP-NOS versus HC. Greater VMPFC-DLPFC coupling to happy faces in BP-NOS youth may therefore reflect enhanced recruitment of cognitive control resources during modulation of attention to perform the gender labeling task with happy faces, and is consistent with findings from a recent study demonstrating greater DLPFC activation during incidental versus direct processing of happy faces in euthymic BP-I youth versus HC.34 Our exploratory correlational analyses indicated that VMPFC-DLPFC coupling to happy faces was greater in BP-NOS youth with more severe depressive symptoms at the time of the scan. Such findings may be interpreted as suggesting that depressed BP-NOS youth may need to recruit more attentional resources when processing mood incongruent positive stimuli such as happy faces, but this should be the focus of future studies.
The fact that the BP-NOS youth did not exhibit reduced VMPFC-amygdala connectivity to fearful faces compared with HC suggests that, unlike BP-I youth, functioning of these regulatory systems may be less affected in BP-NOS youth. It is possible that, like BP-I youth, BP-NOS youth may tend toward having elevated neural activity to emotional stimuli but that such activity is dampened by compensatory patterns of activity such as greater coupling in regulatory systems, as shown with happy faces. Such compensatory patterns in BP-NOS youth may not be observed in contexts that do not elicit elevated neural responses in BP-NOS youth, for example, fearful faces. Previous findings in adult BP-I do, for example, indicate elevated amygdala and striatal activity to happy faces more than to fearful faces.16, 35 As such, there may be less need for recruitment of DLPFC regulatory circuitry in either youth patient group in the present study to fearful relative to happy faces. Our findings would then suggest that while BP-I youth adopted an inefficient strategy (given the presence of elevated amygdala activity to happy faces in BP-I youth) of activating DLPFC to happy faces, BP-NOS youth adopted a preferable strategy (given the absence of elevated amygdala activity in BP-NOS youth to these faces) of recruiting VMPFC-DLPFC connectivity. These differential patterns of connectivity in BP-I and BP-NOS youth relative to HC youth suggest that these groups do not share the same profile of alterations in the functioning of neural systems implicated in emotion processing and regulation. Further research is needed to better understand associations between such differential patterns and clinical presentation of bipolar disorder in youth.
Exploratory whole-brain analyses supported some of the ROI findings, particularly with regard to prefrontal cortical regions, as well as findings from emotional face processing neuroimaging studies.17 In particular, the group by emotion condition interaction for both the happy and fearful face tasks showed VMPFC activation. Other regions implicated in this interaction were the cerebellum, temporal cortex, and visual cortical regions. Although there were no significant findings in the amygdala in any of the whole-brain interactions, findings from these analyses did indicate recruitment of temporal regions, which are implicated in emotional information processing.17 Further, results from the post hoc analyses following the significant group by condition interactions generally supported ROI analyses of reduced prefrontal cortical activation to neutral faces in BP-NOS youth and to fearful faces in BP-I youth relative to healthy controls In contrast to our ROI findings yielding mostly bilateral findings, however, whole-brain analyses for the group by emotion condition interaction suggested a certain degree of laterality with mostly right hemispheric activation for the happy face task and left hemispheric activation for the fearful face condition. Findings from meta-analyses suggest that emotional face processing typically recruits bilateral prefrontal-subcortical regions.39 There is, however, evidence of valence-specific lateralization of brain response during negative emotion processing, particularly in left amygdala.39 Given the exploratory nature of our whole-brain analyses, these findings pertaining to laterality associated with emotional face processing in BP-NOS should be considered preliminary, and would require further study.
Certain limitations merit consideration. First, most BP-I and BP-NOS youths were medicated, which prevented us from comparing unmedicated BP-I and BP-NOS youth to HC. However, prior work suggests that medications may reduce group differences in activation.40 Second, there was no emotion labeling task to account for the possible deficits in emotion labeling in BP-I youth, given recent findings suggesting emotional face processing deficits in BP-I youth.41, 42 However, gender-labeling accuracy scores, which were included as covariates in the analyses, suggest that all youth attended to the stimuli equally well. Nevertheless, future studies should include an emotion labeling task either inside or outside of the scanner to investigate this issue further. Third, our sample size precluded the testing of a full group by emotion condition by hemisphere interaction. As such, PPI analyses were performed for each emotional face versus fixation separately. The valence specific group differences argue against a general face processing explanation of the functional connectivity analyses. Fourth, although differences in mood state between BP-I and BP-NOS could have impacted results, we did not find any significant group differences on mania or depression scores from interviews conducted at the time of the scan. However, high depression scores were positively correlated with VMPFC-DLPFC coupling in BP-NOS only suggesting that mood may have a differential role on emotion processing and regulation in this group and is an issue that requires further examinations.
This is the first study to demonstrate differential patterns of abnormal activity in, and connectivity between, amygdala-prefrontal cortical regions supporting emotion processing and regulation in youth with BP-I and BP-NOS versus HC. Our findings indicate that BP-I youth exhibit greater activation in corticolimbic regions to happy faces, reduced DLPFC activation to fearful faces, and prefrontal-amygdala disconnectivity to fearful faces, versus HC. BP-NOS youth, however, did not differ from HC when processing happy or fearful faces. Rather, they exhibited, compared to HC, reduced prefrontal activation to neutral faces along with greater VMPFC-DLPFC coupling to happy faces, which was positively associated with depressive symptoms. These differential patterns in BP-I and BP-NOS youth suggest that BP-NOS youth do not exhibit elevated reactivity to positive emotional stimuli versus HC perhaps because of greater prefrontal connectivity enabling them to modulate attention in such contexts. Future longitudinal studies are warranted to examine whether these differential patterns of abnormal activity and connectivity predict future onset of BP-I/II in BP-NOS youth.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the children and their families for participating in this study as well as Jacqueline Rosenstern and Claire Dempsey, of the University of Pittsburgh Medical Center, for their assistance in research procedures, and Richard White, of Wayne State University, for his assistance in data analyses procedures. They also thank Dr. KJ Jung, Scott Kurdilla and Debbie Vizslay, of the University of Pittsburgh and Carnegie Mellon’s Brain Imaging Research Center for their help acquiring the neuroimaging data.
This study was supported in part by a Klingenstein Third Generation Foundation Fellowship and an APIRE/Lilly Psychiatric Research Fellowship (TF), the National Institute of Mental Health grants (DAA; R01 MH 059929), (CDL; K01 MH083001), and (BB; R01 MH60952), and the National Alliance for Research on Schizophrenia and Depression Blowitz-Ridgeway Young Investigator Award (CDL) and Nellie Blumenthal Independent Investigator Award to (MLP).
Footnotes
Supplemental material cited in this article is available online.
Disclosure: Dr. Birmaher receives research support from the National Institute of Mental Health (NIMH). He has served as a consultant for Schering Plough. He has or will receive royalties from Random House and Lippincott Williams and Wilkins. Dr. Phillips receives research support from NIMH. Dr. Diwadkar receives research support from NIMH and funding from the Children’s Research Center of Michigan and the National Alliance for Research on Schizophrenia and Depression. Drs. Ladouceur, Farchione, and Axelson, and Mr. Pruitt and Ms. Radwan report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dr. Cecile D. Ladouceur, University of Pittsburgh School of Medicine, Pittsburgh, PA
Dr. Tiffany Farchione, University of Pittsburgh School of Medicine, Pittsburgh, PA
Dr. Vaibhav Diwadkar, University of Pittsburgh School of Medicine, Pittsburgh, PA. Wayne State University School of Medicine, Detroit, MI
Mr. Patrick Pruitt, Wayne State University School of Medicine, Detroit, MI
Ms. Jacqueline Radwan, Wayne State University School of Medicine, Detroit, MI
Dr. David A. Axelson, University of Pittsburgh School of Medicine, Pittsburgh, PA
Dr. Boris Birmaher, University of Pittsburgh School of Medicine, Pittsburgh, PA
Dr. Mary L. Phillips, University of Pittsburgh School of Medicine, Pittsburgh, PA. Cardiff University School of Medicine, Cardiff, UK
References
- 1.Merikangas KR, Akiskal H, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 3.Charney DS, Babich KS. Foundation for the NIMH strategic plan for mood disorders research. Biol Psychiatry. 2002;52(6):455–456. doi: 10.1016/s0006-3223(02)01543-3. [DOI] [PubMed] [Google Scholar]
- 4.Phillips ML, Frank E. Redefining Bipolar Disorder: Toward DSM-V. Am J Psychiatry. 2006;163:1135–1136. doi: 10.1176/ajp.2006.163.7.1135. [DOI] [PubMed] [Google Scholar]
- 5.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13(9):829, 833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rich B, Vinton D, Roberson-Nay R, et al. Limbic hyperactivation during processing of neutral facial expressions in children with bipolar disorder. Proc Natl Acad Sci U S A. 2006;103:8900–8905. doi: 10.1073/pnas.0603246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry. 2007;62(2):158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein DP, Rich BA, Roberson-Nay R, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9(7):679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalmar JH, Wang F, Chepenik LG, et al. Relation Between Amygdala Structure and Function in Adolescents With Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(6):636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang K, Adleman NE, Dienes K, Simeonava D, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: A functional magnetic resonance imaging investigation. Arch Gen Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- 11.Pavuluri MN, O’Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficient modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatric Research. 2008;162(1):27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich B, Fromm S, Berghorst L, et al. Neural connectivity in children with bipolar disorder: impairment in the face of emotion processing circuit. Journal of Child Psychology and Psychiatry. 2008;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leibenluft E, Charney DS, Pine DS. Researching the pathophysiology of pediatric bipolar disorder. Biol Psychiatry. 2003;53:1009–1020. doi: 10.1016/s0006-3223(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein DP, Leibenluft E. Emotion regulation in children and adolescents: Boundaries between normalcy and bipolar disorder. Dev Psychopathol. 2006;18:1105–1131. doi: 10.1017/S0954579406060536. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence N, Williams A, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Sabatinelli D, Fortune EE, Qingyang L, et al. Emotional perception: Meta-analyses of face and natural scene processing. Neuroimage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Birmaher B, Axelson DA, Monk K, et al. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder. Arch Gen Psychiatry. 2009;66:287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63(10):1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 21.Axelson D, Birmaher BJ, Brent D, et al. A Preliminary Study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children Mania Rating Scale for Children and Adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463–470. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 22.Chambers W, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-age Children, Present Episode Version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 23.Angold A, Costello E, Messer S, Pickles A, Winder F, Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research. 1995;5:237–249. [Google Scholar]
- 24.Gerson A, Gerring JP, Freund L, et al. The children’s affective lability scale: A psychometric evaluation of reliability. Psychiatr Res Rep Am Psychiatr Assoc. 1996;65:189–198. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- 25.Birmaher B, Brent D, Chiapetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the screen for child anxiety related emotional disorders scale (SCARED): A replication study. J Am Acad Child Adolesc Psychiatry. 1999;38:1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 26.Hollingshead AB. Four Factor Index of Social Status. New Haven, CT: Yale University Department of Sociology; 1975. [Google Scholar]
- 27.Oldfield RC. The assessment and analysis of handedness: the Edingurgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence NS, Williams AM, Surguladze S, et al. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004 Mar 15;55(6):578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 29.Surguladze SA, Brammer MJ, Young AW, et al. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003 Aug;19(4):1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- 30.Maldjian J, Laurienti P, Kraft R, Burdette J. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 31.Ward D. [Accessed June, 2009];Simultaneous Inference for fMRI Data. http://afni.nimh.nih.gov./pub/dist/doc/manual/AlphaSim.pdf.
- 32.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997 Oct;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 33.Almeida JR, Versace A, Mechelli A, et al. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biol Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009 Mar;48(3):308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hassel S, Almeida J, Kerr N, et al. Elevated striatal but not amygdala activity to positive emotional stimuli in bipolar disorder: comorbid anxiety, illness duration and medication effects. Bipolar Disorders. 2008;10(8):916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halgren E, Marinkovic K. Neurophysiological networks integrating human emotions. In: MG, editor. Cognitive Neurosciences. The Cambridge: MIT Press; 1995. pp. 1137–1151. [Google Scholar]
- 37.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 38.Price JL, Carmichael ST, Drevets WC. Networks related to the orbital and medial prefrontal cortex; a substrate for emotional behavior? In: Holstege G, Bandler R, Saper CB, editors. Progress in Brain Research. Vol. 107. Amsterdam: Elsevier Science B.V; 1996. pp. 523–536. [DOI] [PubMed] [Google Scholar]
- 39.Fusar-Poli P, Placentino A, Carletti F, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neurosci Lett. 2009;452:262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- 40.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 42.Guyer AE, McClure EB, Adler AD, et al. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry. 2007;48(9):863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.