Abstract
Carvedilol is one of the most effective beta-blockers for preventing ventricular tachyarrhythmias (VTs) in heart failure (HF), but the mechanisms underlying its favorable anti-arrhythmic benefits remain unclear. Spontaneous Ca2+ waves, also termed store-overload-induced Ca2+ release (SOICR), are known to evoke VTs in patients with HF. Here we show that carvedilol is the only beta-blocker that effectively suppresses SOICR by directly reducing the open duration of the cardiac ryanodine receptor (RyR2). This unique anti-SOICR activity of carvedilol combined with its beta-blocking activity likely contributes to its favorable anti-arrhythmic effect. To allow individual and optimal titration of these beneficial activities, we developed a novel SOICR-inhibiting, minimally-beta-blocking carvedilol analogue VK-II-86. We found that VK-II-86 alone prevented stress-induced VTs in RyR2 mutant mice, and was more effective when combined with a selective beta-blocker metoprolol or bisoprolol. Thus, SOICR inhibition combined with optimal beta-blockade presents a new, promising and potentially patient-tailorable anti-arrhythmic approach.
INTRODUCTION
Ventricular tachyarrhythmias (VTs) are a leading cause of sudden death. Over the past 40 years, a variety of pharmacological therapies for VTs have been developed. Unfortunately, large clinical trials have recently demonstrated that most anti-arrhythmic drugs currently used have little or no survival benefit and, indeed, may even be harmful1-3. There is one exception. Beta-blockers generally reduce VTs and prevent sudden death. Particularly, carvedilol, a non-selective beta-blocker with anti-oxidant and alpha-blocking activities, is one of the most effective beta-blockers in reducing VTs and mortality in individuals with heart failure (HF)4-7. However, the mechanism underlying carvedilol’s favorable anti-arrhythmic effect remains unclear8, 9. The anti-oxidant and alpha-blocking activities of carvedilol have been suggested to contribute to its beneficial effects, but the benefits of anti-oxidants and alpha-blockers have not been corroborated by clinical studies10, 11. Thus, some other unknown carvedilol-specific action must be involved. Identifying this unknown action could have significant implications for the understanding and treatment of cardiac arrhythmias and the development of novel anti-arrhythmic therapies.
It is well known that excessive beta-adrenergic receptor (AR) stimulation can result in sarcoplasmic recticulum (SR) Ca2+ overload that can trigger spontaneous Ca2+ waves12-14, also known as store-overload-induced Ca2+ release (SOICR), mediated by the cardiac ryanodine receptor (RyR2)15, 16. This SOICR can evoke delayed afterdepolarizations (DADs) that can lead to triggered arrhythmias and sudden death17-23. Indeed, SOICR-evoked DADs are the cause of catecholaminergic polymorphic ventricular tachycardia (CPVT) associated with naturally occurring RyR2 mutations15, 16, 24-29. SOICR-evoked DADs are also a major cause of VTs and sudden death in HF12, 13, 30, 31. Beta-blockers suppress beta-AR mediated, stress-induced SR Ca2+ overload and its arrhythmogenicity. However, beta-blocker effectiveness is limited by drug intolerance and the existence of beta-AR independent causes of overload (e.g. Na+-dependent Ca2+ overload)2, 32, 33.
We propose that carvedilol, in addition to its beta-blocking action, directly suppresses SOICR itself. We tested this in two well-established SOICR cell models, HEK293 cells and cardiomyocytes expressing a SOICR-promoting, CPVT-causing RyR2 mutation (R4496C). Our data reveal that carvedilol is the only beta-blocker that effectively suppresses SOICR by directly altering the function of RyR2, independent of its beta- or alpha-blocking, or anti-oxidant activities. Our results also suggest that SOICR-inhibition combined with optimal beta-blockade is an effective and potentially patient-tailorable means to treat Ca2+-mediated VTs.
RESULTS
Carvedilol is the only beta-blocker that suppresses SOICR
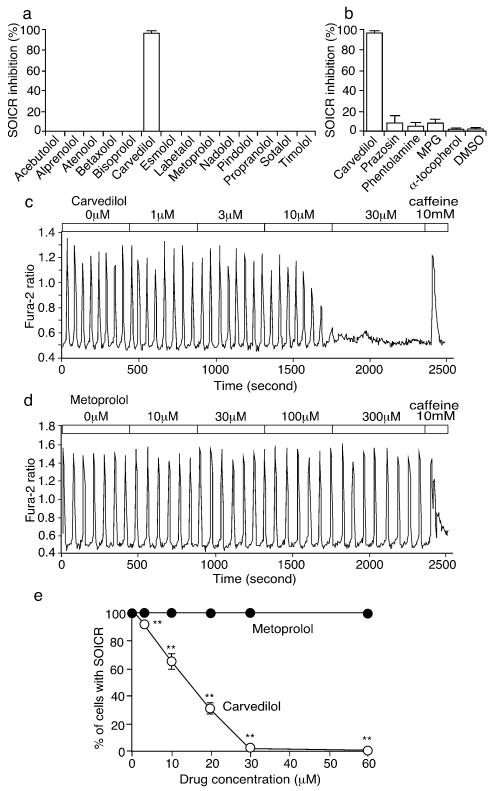
Of 14 beta-blockers tested, carvedilol was the only one that effectively inhibited SOICR (>95%). All the other beta-blockers had no impact on SOICR even at relatively high concentrations (30μM) (Fig.1).
Fig. 1. Carvedilol inhibits SOICR in HEK293 cells.
(a) HEK293 cells expressing RyR2- R4496C, treated with various beta-blockers (30μM) or (b) with prazosin (30μM), phentolamine (30μM), N-(2-mercaptopropionyl)-glycine (MPG, 1mM), α-tocopherol (600μM), or DMSO (control). SOICR Inhibition (%) denotes the percentage of HEK293 cells (120-370) in which SOICR were completely inhibited (n=3-7). Fura-2 ratios of representative HEK293 cells treated with carvedilol (c) or metoprolol (d). (e) The % cells with SOICR. All data shown are mean ± SEM (*P < 0.05; ** P < 0.01; vs control).
To further assess whether the alpha-blocking and anti-oxidant activities of carvedilol contribute to its unique action, we tested the impact of alpha-blockers (prazosin and phentolamine) and anti-oxidants ((N-(2-mercaptopropionyl)-glycine (MPG) and α-tocopherol) on SOICR. None of these agents significantly altered SOICR (Fig.1b). Thus, carvedilol is the only beta-blocker that effectively suppresses SOICR, and this action is independent of its beta- and alpha-blocking and anti-oxidant activities.
Carvedilol directly modifies the function of the RyR2 channel
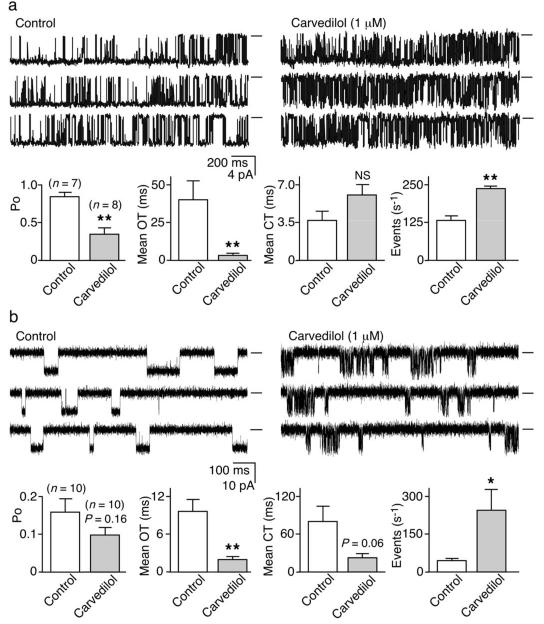
To test the possibility that carvedilol suppresses SOICR by directly altering the function of RyR2, we assessed the impact of carvedilol on single native RyR2 channels from SR microsomes fused into lipid bilayers. Carvedilol (1 μM) significantly reduced RyR2 mean open time (OT) (P < 0.01) and open probability (Po) (P < 0.01), and increased the event frequency (P < 0.01). The mean closed time (CT) was not statistically altered by carvedilol (P= 0.10) (Fig.2a).
Fig. 2. Carvedilol modifies the gating of single RyR2 channels.
(a) native RyR2s from rat SR microsomes or (b) purified recombinant RyR2-R4496C channels in the absence (control) or the presence of carvedilol. Openings downward and baselines indicated (short bars). Open probability (Po), mean open time (OT), meant closed time (CT), and event frequency (s−1) (*P < 0.05; ** P < 0.01; vs control).
We also assessed the impact of carvedilol on single purified recombinant RyR2 channels. Carvedilol (1 μM) significantly reduced their mean OT (P < 0.01) and increased their event frequency (P< 0.05) (Fig.2b). Carvedilol also reduced intra-burst event duration, while increasing intra-burst event frequency (P<0.05) (Fig.S1). Thus, carvedilol consistently reduces the duration and increases the frequency of single RyR2 openings.
Carvedilol suppresses SOICR in cardiomyocytes
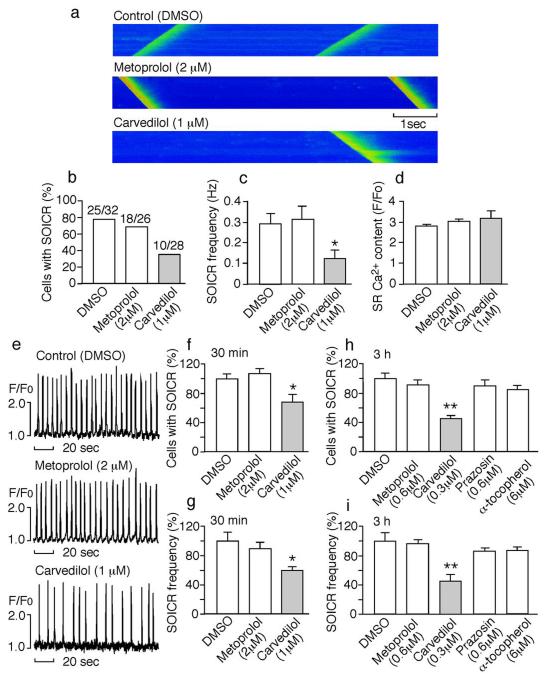
We next determined whether carvedilol is able to suppress SOICR in cardiomyocytes from mice harboring the SOICR-promoting, CPVT-causing RyR2 mutation, R4496C (Fig.S2). Line-scan confocal Ca2+ imaging showed that carvedilol (1 μM) significantly reduced the occurrence and frequency of SOICR (P < 0.05), whereas metoprolol (2 μM) and the vehicle DMSO had no significant effect (Fig.3a–c). The SR Ca2+ contents in myocytes treated with carvedilol, metoprolol, or DMSO were not significantly different (Fig.3d). We also employed epifluorescence single cell Ca2+ imaging to determine the impact of carvedilol on SOICR in a large number of myocytes. Consistently, carvedilol (1 μM) significantly decreased the occurrence and frequency of SOICR (P < 0.05). In contrast, metoprolol (2 μM) and the vehicle DMSO had no significant effect (Fig. 3e–g).
Fig. 3. Carvedilol suppresses SOICR in mouse ventricular myocytes.
(a) Line-scan confocal imaging of R4496C+/− ventricular myocytes treated with drugs for 30 min in the presence of 6 mM external Ca2+. The % cells with SOICR (b), frequency of SOICR (c), and SR Ca2+ content (d). (e) Single cell epifluorescence imaging of SOICR in the presence of DMSO, metoprolol, or carvedilol. (f) The % cells with SOICR and (g) SOICR frequency in cells (187-202) treated with drugs for 30 min (n=6). The % cells with SOICR (h) and SOICR frequency (i) in cells (147-225) treated with drugs for 3 hours (n=4-6) (*P < 0.05; ** P < 0.01; vs DMSO).
We also determined the impact of low concentrations of carvedilol on SOICR. Myocytes were incubated with 0.3 μM (or 122 ng/ml) carvedilol, which is similar to the peak plasma concentration of carvedilol (~100 ng/ml)34, 35, for 3 hours before the induction of SOICR. Myocytes treated with 0.3 μM carvedilol had a significantly reduced occurrence and frequency of SOICR compared to DMSO-treated cells (P < 0.01). The frequency and occurrence of SOICR in myocytes treated with 0.6μM metoprolol, 0.6 μM prazosin, or 6 μM α-tocopherol did not differ significantly from those in DMSO-treated cells (Fig.3h,i). Thus, carvedilol at clinically relevant concentrations suppresses SOICR in cardiomyocytes, independent of its beta- and alpha-blocking, and anti-oxidant activities.
Generation of novel SOICR-inhibiting carvedilol analogues with minimal beta-blockade
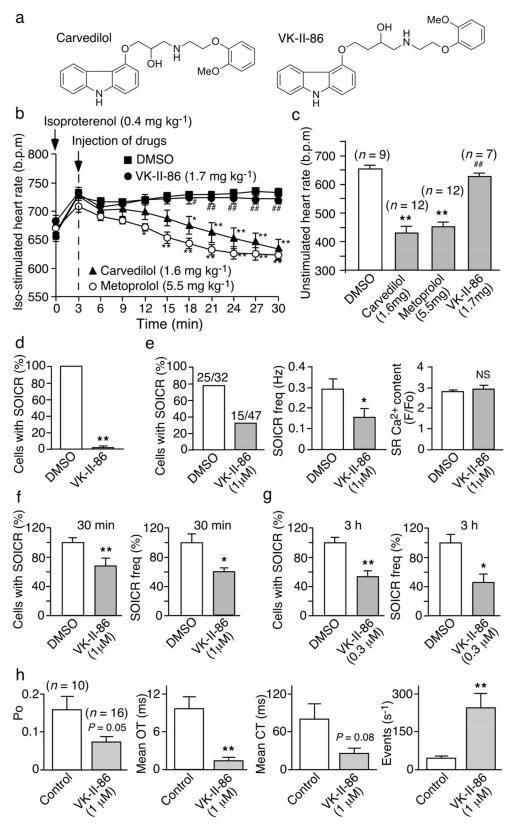
To be able to individually and optimally titrate SOICR inhibition and beta-blockade, we set out to separate the anti-SOICR activity of carvedilol from its beta-blocking effect by generating SOICR-inhibiting carvedilol analogues with minimal beta-blockade. To this end, we have made a number of structural modifications to carvedilol (Fig.S3). Carvedilol and metoprolol markedly suppressed the enhanced heart rate in WT mice induced by isoproterenol (Iso), but one of the analogues VK-II-86 (like the vehicle DMSO) did not (Fig.4a,b). Treating the R4496C+/− mice with carvedilol or metoprolol for 5 days decreased the heart rate (P<0.01), but a 5-day VK-II-86 treatment did not (P=0.18) (Fig.4c).
Fig. 4. Effect of VK-II-86 on heart rate, SOICR, and single RyR2 channels.
(a) Chemical structures of carvedilol and VK-II-86. (b) Iso-stimulated heart rate and (c) unstimulated heart rate in R4496C+/− mice treated with drugs (mg kg−1 or mg kg−1 d−1 for 5 days). (d) The % HEK293-R4496C cells with SOICR, treated with VK-II-86 (30μM, 515 cells) (n=10). (e) Line-scan confocal imaging of SOICR in R4496C+/− ventricular myocytes treated with VK-II-86 for 30 min. Single cell epifluorescence imaging of SOICR in R4496C+/− myocytes (177-208) treated with VK-II-86 for 30 min (n=6) (f) or for 3 hours (n=6) (g). (h) Po, mean OT, meant CT, and event frequency (s−1) of single purified RyR2-R4496C channels pre-incubated (1 h) with VK-II-86 (1μM) (*P < 0.05; ** P < 0.01; vs DMSO or control: # P < 0.05; ## P < 0.01 vs carvedilol).
Carvedilol, but not VK-II-86, abolished the Iso-induced PKA phosphorylation of RyR2 (Fig.S4). Radioligand binding studies also revealed that VK-II-86 exhibited >2,000-fold lower beta-AR binding affinity than carvedilol (Fig.S5, Supplementary Table 1). Thus, VK-II-86 lacks the normal beta-blocking activity of carvedilol and has little impact on heart rate.
Action of VK-II-86 on SOICR and single RyR2 channels
We next assessed the impact of VK-II-86 on SOICR and single RyR2 channels. Like carvedilol, VK-II-86 completely abolished SOICR in HEK293 cells (P<0.01) (Fig.4d, Fig.S4). VK-II-86 (1 μM) pre-treatment of cardiomyocytes for 30 min significantly reduced the occurrence and frequency of SOICR in both confocal (Fig.4e) and epifluorescence (Fig.4f, Fig.S4) imaged cells (P<0.01). Furthermore, pre-treatment with a lower concentration of VK-II-86 (0.3μM) for a longer period of time (3 hours) also significantly inhibited the occurrence (P<0.01) and frequency (P<0.05) of SOICR (Fig.4g).
Importantly, VK-II-86 also modified the function of both single native and purified recombinant RyR2 channels. Like carvedilol, it reduced the duration and increased the frequency of channel openings (Figs.4h, Fig.S6; P<0.01). It also reduced intra-burst event duration and increased intra-burst event frequency (Fig.S1). Thus, VK-II-86 is a minimally beta-blocking carvedilol analogue with anti-SOICR activity.
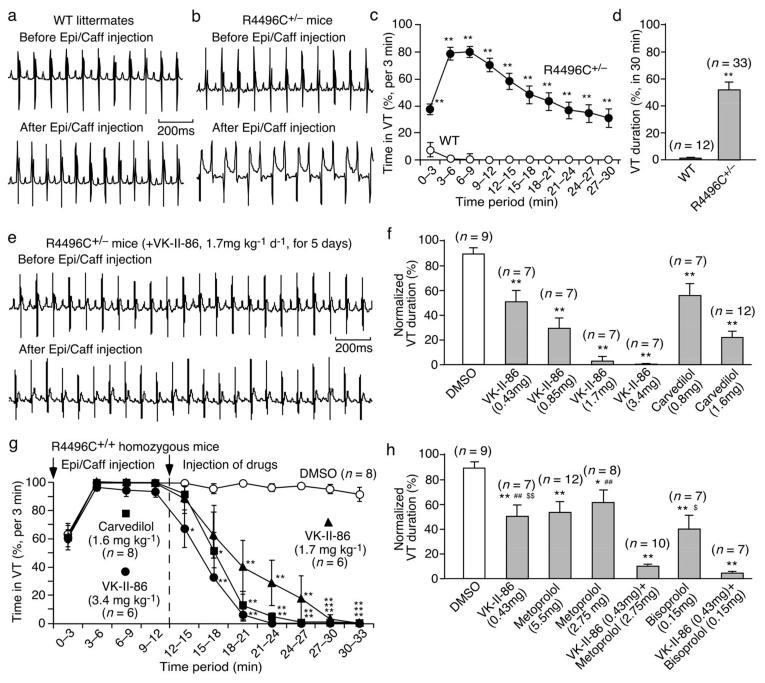
Effect of SOICR-inhibiting, minimally-beta-blocking carvedilol analogues on CPVT
We determined whether the SOICR-inhibiting carvedilol analogues with minimal beta-blockade are effective in suppressing CPVT. We used the RyR2 R4496C+/− mice that are known to develop SOICR-driven CPVT24-26. In these mice (unlike their WT littermates), long-lasting VTs, including bidirectional VTs, can be evoked pharmacologically using caffeine and epinephrine (Fig.5a–d). Importantly, VK-II-86 suppressed CPVT in RyR2-R4496C+/− mice in a dose dependent manner (Fig.5e,f, Fig.S7). At 3.4 mg kg−1 day−1, VK-II-86 reduced the VT duration by 99.9% (P<0.01) without adversely lowering the heart rate (8%, P=0.08) (Figs.4c,5f), whereas carvedilol (1.6 mg kg−1 day−1) reduced the VT duration by 79% (P<0.01) and the heart rate by 36% (P<0.05)(Figs.4c,5f).
Fig. 5. Effect of VK-II-86 on CPVT in R4496C+/− mice.
Representative ECG recordings of WT (a) and R4496C+/− mutant (b) mice before and after injection (i.p.) of epinephrine (1.6 mg/kg) and caffeine (120 mg/kg). VT duration (%) in WT or in R4496C+/− mice per 3-min period (c) or per 30-min period (d) of ECG recordings (**P < 0.01 vs WT). (e) Representative ECG recordings of R4496C+/− mice treated with VK-II-86 before and after triggers. (f) VT duration (%) in R4496C+/− mice treated with drugs (mg kg−1 d−1for 5 days). (g) VT duration (%) per 3-min period in RyR2 R4496C+/+ homozygous mice post-treated with drugs (*P < 0.05; ** P < 0.01 vs DMSO). (h) VT duration (%) in R4496C+/− mice treated with drugs (mg kg−1 d−1 for 5 days) (*P < 0.05; ** P < 0.01 vs DMSO; ## P<0.01 vs VK-II-86/metoprolol combined; $ P<0.05; $$ P<0.01 vs VK-II-86/bisoprolol combined).
We also determined the acute effect (post treatment) of VK-II-86 on VT in RyR2 R4496C+/+ homozygous mice (Fig. 5g). R4496C+/+ mice injected with the vehicle DMSO displayed VT for >30 min. VK-II-86 (3.4 mg kg−1) completely stopped the VT. Carvedilol (1.6 mg kg−1) also completely stopped the VT and appears to be more effective than VK-II-86 (1.7 mg kg−1) probably due to its beta-blocking activity, which would reduce the strength of the original epinephrine trigger.
Efficacy of combined SOICR inhibition and beta-blockade in preventing CPVT
We used metoprolol or bisoprolol as a selective beta-blocker and VK-II-86 as a SOICR inhibitor to test the premise that higher anti-arrhythmic efficacy can be achieved by both SOICR inhibition and beta-blockade. We found that treating R4496C+/− mice with VK-II-86 plus metoprolol or with VK-II-86 plus bisoprolol was much more effective in preventing CPVT than VK-II-86 (P<0.01), metoprolol (P<0.01), bisoporlol (P<0.05) (Fig.5h, Fig.S8), or propranolol (P<0.05) (Fig.S9) alone. These data demonstrate that the combination of SOICR inhibition and beta-blockade increases the anti-arrhythmic efficacy.
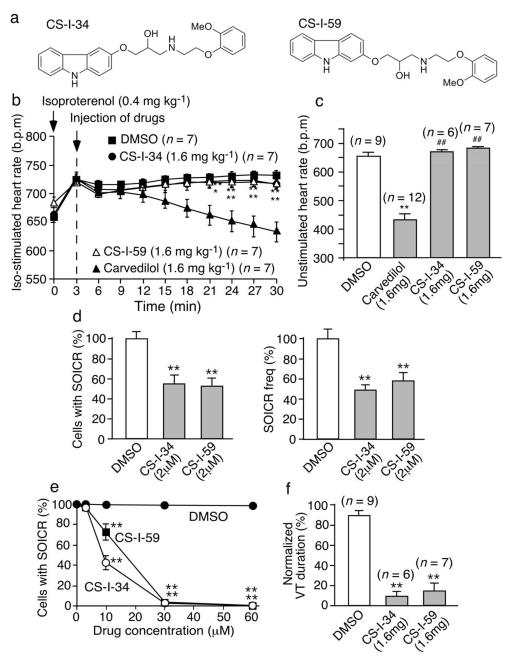
We also generated two additional carvedilol analogues (CS-I-34 and CS-I-59) (Fig.6a). Like VK-II-86, CS-I-34 and CS-I-59 had no effect on the Iso-enhanced heart rate (Fig.6b) or the non-stimulated heart rate (Fig.6c). CS-I-34 and CS-I-59 reduced the occurrence and frequency of SOICR in cardiomyocytes (P<0.01) (Fig.6d) and suppressed SOICR in HEK293 cells (Fig.6e). CS-I-34 and CS-I-59 also significantly reduced the duration of VT in R4496C+/− mice (Fig.6f; P<0.01). We also found that flecainide inhibited SOICR frequency and suppressed CPVT in RyR2- R4496C+/− mice (Fig. S10), consistent with those reported previously36.
Fig. 6. Effect of CS-I-34 and CS-I-59 on heart rate, SOICR and CPVT.
(a) Chemical structures of CS-I-34 and CS-I-59. (b) Iso-stimulated heart rate and (c) unstimulated heart rates in R4496C+/− mice treated with drugs (mg kg−1 or mg kg−1 d−1 for 5 days). (d) Single cell epifluorescence imaging of SOICR in R4496C+/− ventricular myocytes (141-144) treated with drugs for 30 min (n=4). (e) The % HEK293 RyR2-R4496C cells (137-457) with SOICR in the presence of drugs (n=5-9). (f) VT duration (%) in R4496C+/− mice treated with drugs (mg kg−1 d−1 for 5 days) (*P < 0.05; ** P < 0.01; vs DMSO or control: ## P < 0.01 vs carvedilol).
DISCUSSION
Carvedilol is the beta-blocker of choice in preventing VTs and cardiovascular mortality in patients with HF5-7. Yet the mechanism underlying carvedilol’s anti-arrhythmic benefit is not well defined. In the present study, we tested the action of a number of known beta-blockers on SOICR, and surprisingly found that only carvedilol effectively inhibited SOICR. Since SOICR and SOICR-induced DADs are frequent in failing hearts12, 13, 30, 31, carvedilol’s unique anti-SOICR activity among beta-blockers likely contributes to its favorable anti-arrhythmic benefit.
An important question is how does carvedilol suppress arrhythmogenic SOICR. Using single channel recordings in lipid bilayers, we demonstrate that carvedilol directly modifies the gating properties of RyR2 by reducing the duration and increasing the frequency of channel openings. Interestingly, reducing the duration of RyR2 channel openings has also been shown to be the underlying mechanism by which flecainide, a Na channel blocker, suppresses SOICR and prevents SOICR-evoked CPVT in both mice and humans36-38. Thus, it appears that reducing RyR2 open duration is not only common to both drugs (carvedilol and flecainide) but also an effective means to suppress SOICR and SOICR-evoked VTs. However, flecainide is contraindicated in patients with previous myocardial infarction or significant left ventricular dysfunction because of the risk of VTs39. Carvedilol has also been reported to suppress SR ‘Ca2+ leak’ by reducing the levels of RyR2 phosphorylation or oxidation via its beta-blocking and anti-oxidant activities 40, 41. These modulation pathways of SR Ca2+ leak, however, are not carvedilol specific. In contrast, we show here that carvedilol’s unique anti-SOICR activity arises from a direct action on the RyR2 channel and is independent of the drug’s beta-blocking and anti-oxidant activities. Thus, it is mechanistically different from those described by Reiken et al. and Mochizuki et al.40, 41.
The peak plasma concentration of carvedilol at therapeutic doses is ~100 ng/mL with more than 98% of this bound to plasma proteins34, 35. Despite its high plasma protein association, carvedilol has a large volume of distribution (132L)34, indicating that it accumulates (over time) in extravascular tissues like cardiac muscle42. This is likely due to its high degree of lipophilicity8, 43. In line with this view, the concentrations of carvedilol found in the heart are 5-7 fold higher than that in the plasma44, 45. We also found that lower carvedilol concentrations were needed to suppress SOICR at longer drug exposure times. Thus, carvedilol at therapeutic doses may accumulate in cardiac cells to a level that is able to modify the function of RyR2.
Since carvedilol possesses both the beta-blocking and anti-SOICR activities, it would suppress both the stress-induced SR Ca2+ overload that triggers SOICR and the SOICR itself. This dual action of carvedilol likely contributes to its favorable anti-arrhythmic benefits. However, the concentrations of carvedilol required to suppress SOICR (0.3-1 μM) are much higher than those for beta-blockade (~1 nM)46. Therefore, strong SOICR inhibition would require high doses of carvedilol, which could produce excessive beta-blockade and the accompanying adverse effects such as bradycardia47. In other words, carvedilol’s anti-SOICR benefit is dose limited by the drugs beta-blocking activity. We were successful at pharmacologically separate carvedilol’s beta-blocking and anti-SOICR activities, so that the benefits of beta-blocking and SOICR inhibition could be individually and optimally titrated. We synthesized a number of carvedilol analogues with structural changes in regions thought to be essential for beta-blockade. Three carvedilol analogues, VK-II-86, CS-I-34, and CS-I-59 exhibited minimal beta-blocking activity, but retained the ability to suppress SOICR. These novel agents prevented CPVT in mice without causing bradycardia. Further, we showed that VK-II-86 when combined with selective beta-blockers (metoprolol or bisoprolol) is more effective than VK-II-86 or metoprolol or bisoprolol alone. Thus, the combination of agents (our new SOICR inhibitor and a selective beta-blocker) represents a novel and more effective approach for preventing SOICR-evoked VTs.
Carvedilol is a pleiotropic agent with diverse actions5, 6, 48-51. Thus, it is important to note that our novel carvedilol analogues, even though they lack beta-blocking activity, may have other actions besides the anti-SOICR activity demonstrated here. Given the significant role of SOICR in arrhythmogenesis, however, it is very likely that their anti-SOICR activity will play a key role in their anti-arrhythmic action.
METHODS
Single-cell Ca2+ imaging of HEK293 cells
Stable, inducible HEK293 cells expressing a CPVT-causing RyR2 mutant, R4496C, display robust SOICR, but parental HEK293 cells do not15, 16. These RyR2-R4496C cells were used to assess the impact of beta-blockers and other reagents on SCR using single-cell Ca2+ imaging as described previously15, 16.
Generation of knock-in mouse model harboring the CPVT RyR2 mutation, R4496C
Detailed experimental procedures for the generation of the RyR2-R4496C mutant mice are available in the Supplementary Methods.
Single-cell Ca2+ imaging of mouse ventricular myocytes
Ca2+ waves in freshly isolated ventricular myocytes were measured using single-cell Ca2+ imaging as described previously52.
Confocal Imaging
Laser scanning confocal imaging of Ca2+ dynamics was performed as described previously53.
Synthesis of carvedilol analogues
Detailed experimental procedures and characterization data of novel compounds are described in the Supplementary Methods.
Animal studies
All animal studies were approved by the Institutional Animal Care and Use Committees at the University of Calgary, and performed in accordance with NIH guidelines. Adult R4496C mutant mice and wild type control littermates (2-4 months old) were used for all experiments.
ECG recordings in anesthetized mice
The effects of drugs on ventricular tachycardias provoked by epinephrine and caffeine were studied in anesthetized R4496C mutant mice. Briefly, mice were lightly anesthetized with isoflurane vapor (0.5-1%). Anesthetized mice were placed on a heating pad (28 °C) and subcutaneous needle electrodes were applied to the left, right upper limb and right lower limb for ECG recording (BIOPAC MP System, Goleta, CA). Baseline ECG was recorded for 5 minutes, followed by the intraperitoneal injection of epinephrine (1.6 mg/kg) and caffeine (120 mg/kg). ECG was then continuously recorded for another 30 minutes. ECG recordings were analyzed using the AcqKnowledge 3.9.1 program (BIOPAC, Goleta, CA).
Drug tests in RyR2-R4496C mutant mice
Epinephrine (1.6 mg/kg)/caffeine (120 mg/kg) challenge was performed twice in the same R4496C+/− mutant mouse at an interval of 7 days. All animals went through first ECG test before any drug treatments to obtain the baseline. These animals were then randomized into different groups and treated with various drugs by intraperitoneal injection for 5 days. The final dose of drug was administered 30 minutes before the second ECG test. The percentage of time that the mice were in ventricular tachycardia within the 30 min recording period in both the first and second ECG tests was determined and compared. To evaluate the acute effect of different drugs on CPVT, the R4496C+/+ mutant mice, continuously monitored with ECG recordings, were intraperitoneally injected with epinephrine (1.6 mg/kg) and caffeine (120 mg/kg) to induce ventricular tachycardia. One dose of each drug tested was then intraperitoneally injected into the mice 12 minutes after the injection of epinephrine and caffeine. ECG was continuously recorded for additional 21 minutes.
Statistical analysis
All values shown are mean ± SEM unless indicated otherwise. To test for differences between groups, we used Student’s t test (2-tailed) or one-way ANOVA with post hoc test. Statistical analyses were performed using the SPSS V.15.0 (SPSS, Chicago, IL). A P value <0.05 was considered to be statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
Supported by grants from the Canadian Institutes of Health Research to S.R.W.C., US National Institutes of Health to S.R.W.C., L-S.S., M.F., and A.M.F., the Heart and Stroke Foundation of Alberta to A.M.G. and H.J.D., the Intramural Research Program of the National Institute on Aging to D.Y. and H.C.. D.J., W.C., X.T. had Alberta Innovates-Health Solutions (AIHS) Studentship Awards, C.D.S.: AIHS Fellowship Awards, P.P.J.: AIHS and Heart and Stroke Foundation Fellowship Awards, H.J.D. and S.R.W.C.: AIHS Scientist Awards. We want to thank the King family and the Libin Cardiovascular Institute of Alberta for their generous donations and Drs. S. Pogwizd and S. Priori for critical reading of the manuscript.
Footnotes
Extended, detailed experimental procedures are available in the Supplementary Information.
AUTHOR CONTRIBUTIONS Q.Z., J.X., D.J., R.W., K.V., A.W., C.S., W.Z., D.Y., J.C., A.M.G., H.J.D., H.C., A.M.F., L-S.S., M.F., T.G.B., S.R.W.C., designed research; Q.Z., J.X., D.J., R.W., K.V., A.W., C.S., C.X., W.C., J.Z., W.Z., X.T., P.J., X.Z., A.G., H.C., L.Z., D.Y., X.L., performed research; Q.Z., J.X., D.J., C.X., W.C., J.Z., W.Z., X.T., P.J., X.Z., A.G., H.Y., L.Z., D.Y., analyzed data; and Q.Z., R.W., C.S., W.Z., M.F., T.G.B., S.R.W.C., wrote the paper.
The authors declare no competing financial interests.
REFERENCES
- 1.Waldo AL, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 2.Miller L. Limitations of current medical therapies for the treatment of heart failure. Rev Cardiovasc Med. 2003;4(Suppl 2):S21–9. [PubMed] [Google Scholar]
- 3.Kamath GS, Mittal S. The role of antiarrhythmic drug therapy for the prevention of sudden cardiac death. Prog. Cardiovasc. Dis. 2008;50:439–448. doi: 10.1016/j.pcad.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Poole-Wilson PA, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 5.Stroe AF, Gheorghiade M. Carvedilol: beta-blockade and beyond. Rev. Cardiovasc. Med. 2004;5(Suppl 1):S18–27. [PubMed] [Google Scholar]
- 6.Greenberg B. Nonselective versus selective beta-blockers in the management of chronic heart failure: clinical implications of the carvedilol or Metoprolol European Trial. Rev. Cardiovasc. Med. 2004;5(Suppl 1):S10–7. [PubMed] [Google Scholar]
- 7.Remme WJ. Which beta-blocker is most effective in heart failure? Cardiovasc. Drugs Ther. 2010;24:351–358. doi: 10.1007/s10557-010-6247-7. [DOI] [PubMed] [Google Scholar]
- 8.Hjalmarson A. Cardioprotection with beta-adrenoceptor blockers. Does lipophilicity matter? Basic Res. Cardiol. 2000;95(Suppl 1):I41–5. doi: 10.1007/s003950070008. [DOI] [PubMed] [Google Scholar]
- 9.Foody JM, Farrell MH, Krumholz HM. {beta}-Blocker Therapy in Heart Failure: Scientific Review. JAMA. 2002;287:883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 10.Kukin ML, et al. Combined alpha-beta blockade (doxazosin plus metoprolol) compared with beta blockade alone in chronic congestive heart failure. Am. J. Cardiol. 1996;77:486–491. doi: 10.1016/s0002-9149(97)89342-3. [DOI] [PubMed] [Google Scholar]
- 11.Thomson MJ, Puntmann V, Kaski JC. Atherosclerosis and oxidant stress: the end of the road for antioxidant vitamin treatment? Cardiovasc. Drugs Ther. 2007;21:195–210. doi: 10.1007/s10557-007-6027-1. [DOI] [PubMed] [Google Scholar]
- 12.Bers DM. Calcium and cardiac rhythms: physiological and pathophysiological. Circ. Res. 2002;90:14–7. [PubMed] [Google Scholar]
- 13.Pogwizd SM, Bers DM. Cellular Basis of Triggered Arrhythmias in Heart Failure. Trends Cardiovasc. Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt S, et al. Altered calcium handling is critically involved in the cardiotoxic effects of chronic beta-adrenergic stimulation. Circulation. 2004;109:1154–1160. doi: 10.1161/01.CIR.0000117254.68497.39. [DOI] [PubMed] [Google Scholar]
- 15.Jiang D, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc.Natl.Acad.Sci.U.S.A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, et al. Enhanced Store Overload-Induced Ca2+ Release and Channel Sensitivity to Luminal Ca2+ Activation Are Common Defects of RyR2 Mutations Linked to Ventricular Tachycardia and Sudden Death. Circ. Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 17.Kass RS, Tsien RW. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys. J. 1982;38:259–69. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orchard C, Eisner D, Allen D. Oscillations of intracellular Ca2+ in mammalian cardiac muscle. Nature. 1983;304:735–738. doi: 10.1038/304735a0. [DOI] [PubMed] [Google Scholar]
- 19.Stern M, Kort A, Bhatnagar G, Lakatta E. Scattered-light intensity fluctuations in diastolic rat cardiac muscle caused by spontaneous Ca++-dependent cellular mechanical oscillations. J. Gen. Physiol. 1983;82:119–153. doi: 10.1085/jgp.82.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wier W, Kort A, Stern M, Lakatta E, Marban E. Cellular calcium fluctuations in mammalian heart: direct evidence from noise analysis of aequorin signals in Purkinje fibers. Proc. Natl. Acad. Sci. U. S. A. 1983;80:7367–7371. doi: 10.1073/pnas.80.23.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marban E, Robinson SW, Wier WG. Mechanisms of arrhythmogenic delayed and early afterdepolarizations in ferret ventricular muscle. J. Clin. Invest. 1986;78:1185–192. doi: 10.1172/JCI112701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlotthauer K, Bers DM. Sarcoplasmic reticulum Ca(2+) release causes myocyte depolarization. Underlying mechanism and threshold for triggered action potentials. Circ. Res. 2000;87:774–80. doi: 10.1161/01.res.87.9.774. [DOI] [PubMed] [Google Scholar]
- 23.Xie LH, Weiss JN. Arrhythmogenic consequences of intracellular calcium waves. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H997–H1002. doi: 10.1152/ajpheart.00390.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerrone M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ. Res. 2005;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 25.Liu N, et al. Arrhythmogenesis in Catecholaminergic Polymorphic Ventricular Tachycardia: Insights From a RyR2 R4496C Knock-In Mouse Model. Circ. Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 26.Cerrone M, et al. Arrhythmogenic mechanisms in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circ. Res. 2007;101:1039–1048. doi: 10.1161/CIRCRESAHA.107.148064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paavola J, et al. Mutant ryanodine receptors in catecholaminergic polymorphic ventricular tachycardia generate delayed afterdepolarizations due to increased propensity to Ca2+ waves. Eur. Heart J. 2007;28:1135–1142. doi: 10.1093/eurheartj/ehl543. [DOI] [PubMed] [Google Scholar]
- 28.Kannankeril PJ, et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12179–12184. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uchinoumi H, et al. Catecholaminergic Polymorphic Ventricular Tachycardia Is Caused by Mutation-Linked Defective Conformational Regulation of the Ryanodine Receptor. Circ. Res. 2010 doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen J, et al. Triggered activity and automaticity in ventricular trabeculae of failing human and rabbit hearts. Cardiovasc. Res. 1994;28:1547–1554. doi: 10.1093/cvr/28.10.1547. [DOI] [PubMed] [Google Scholar]
- 31.Shannon TR, Pogwizd SM, Bers DM. Elevated Sarcoplasmic Reticulum Ca2+ Leak in Intact Ventricular Myocytes From Rabbits in Heart Failure. Circ. Res. 2003;93:592–594. doi: 10.1161/01.RES.0000093399.11734.B3. [DOI] [PubMed] [Google Scholar]
- 32.Priori SG, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi M, et al. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation. 2009;119:2426–2434. doi: 10.1161/CIRCULATIONAHA.108.829267. [DOI] [PubMed] [Google Scholar]
- 34.Neugebauer G, Akpan W, von Mollendorff E, Neubert P, Reiff K. Pharmacokinetics and disposition of carvedilol in humans. J. Cardiovasc. Pharmacol. 1987;10(Suppl 11):S85–8. [PubMed] [Google Scholar]
- 35.Tenero D, et al. Steady-state pharmacokinetics of carvedilol and its enantiomers in patients with congestive heart failure. J. Clin. Pharmacol. 2000;40:844–853. doi: 10.1177/00912700022009576. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe H, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat. Med. 2009;15:380–383. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hilliard FA, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J. Mol. Cell. Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang HS, et al. Inhibition of Cardiac Ca2+ Release Channels (RyR2) Determines Efficacy of Class I Antiarrhythmic Drugs in Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Arrhythm Electrophysiol. 2011 doi: 10.1161/CIRCEP.110.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Echt DS, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 40.Reiken S, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459–2466. doi: 10.1161/01.CIR.0000068316.53218.49. [DOI] [PubMed] [Google Scholar]
- 41.Mochizuki M, et al. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J. Am. Coll. Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 42.Doze P, et al. Synthesis and evaluation of radiolabeled antagonists for imaging of beta-adrenoceptors in the brain with PET. Neurochem. Int. 2002;40:145–155. doi: 10.1016/s0197-0186(01)00081-x. [DOI] [PubMed] [Google Scholar]
- 43.Caron G, et al. Structure-lipophilicity relationships of neutral and protonated beta-blockers. Helvetica Chimica ACTA. 1999;82:1211–1222. [Google Scholar]
- 44.Fujimaki M. Stereoselective disposition and tissue distribution of carvedilol enantiomers in rats. Chirality. 1992;4:148–154. doi: 10.1002/chir.530040304. [DOI] [PubMed] [Google Scholar]
- 45.Stahl E, Mutschler E, Baumgartner U, Spahn-Langguth H. Carvedilol stereopharmacokinetics in rats: affinities to blood constituents and tissues. Arch. Pharm. (Weinheim) 1993;326:529–533. doi: 10.1002/ardp.19933260907. [DOI] [PubMed] [Google Scholar]
- 46.Yao A, et al. Characteristic effects of alpha1-beta1,2-adrenergic blocking agent, carvedilol, on [Ca2+]i in ventricular myocytes compared with those of timolol and atenolol. Circ J. 2003;67:83–90. doi: 10.1253/circj.67.83. [DOI] [PubMed] [Google Scholar]
- 47.Ko DT, et al. Adverse Effects of {beta}-Blocker Therapy for Patients With Heart Failure: A Quantitative Overview of Randomized Trials. Arch. Intern. Med. 2004;164:1389–1394. doi: 10.1001/archinte.164.13.1389. [DOI] [PubMed] [Google Scholar]
- 48.McMurray J, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J. Am. Coll. Cardiol. 2005;45:525–530. doi: 10.1016/j.jacc.2004.09.076. [DOI] [PubMed] [Google Scholar]
- 49.Yue TL, et al. Carvedilol, a new vasodilator and beta adrenoceptor antagonist, is an antioxidant and free radical scavenger. J. Pharmacol. Exp. Ther. 1992;263:92–98. [PubMed] [Google Scholar]
- 50.Ohlstein EH, et al. Carvedilol, a cardiovascular drug, prevents vascular smooth muscle cell proliferation, migration, and neointimal formation following vascular injury. Proc. Natl. Acad. Sci. U. S. A. 1993;90:6189–6193. doi: 10.1073/pnas.90.13.6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okafor C, et al. Chronic treatment with carvedilol improves ventricular function and reduces myocyte apoptosis in an animal model of heart failure. BMC Physiol. 2003;3:6. doi: 10.1186/1472-6793-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hunt DJ, et al. K201 (JTV519) suppresses spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association. Biochem. J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen B, Wu Y, Mohler PJ, Anderson ME, Song LS. Local control of Ca2+-induced Ca2+ release in mouse sinoatrial node cells. J. Mol. Cell. Cardiol. 2009;47:706–715. doi: 10.1016/j.yjmcc.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.