Abstract
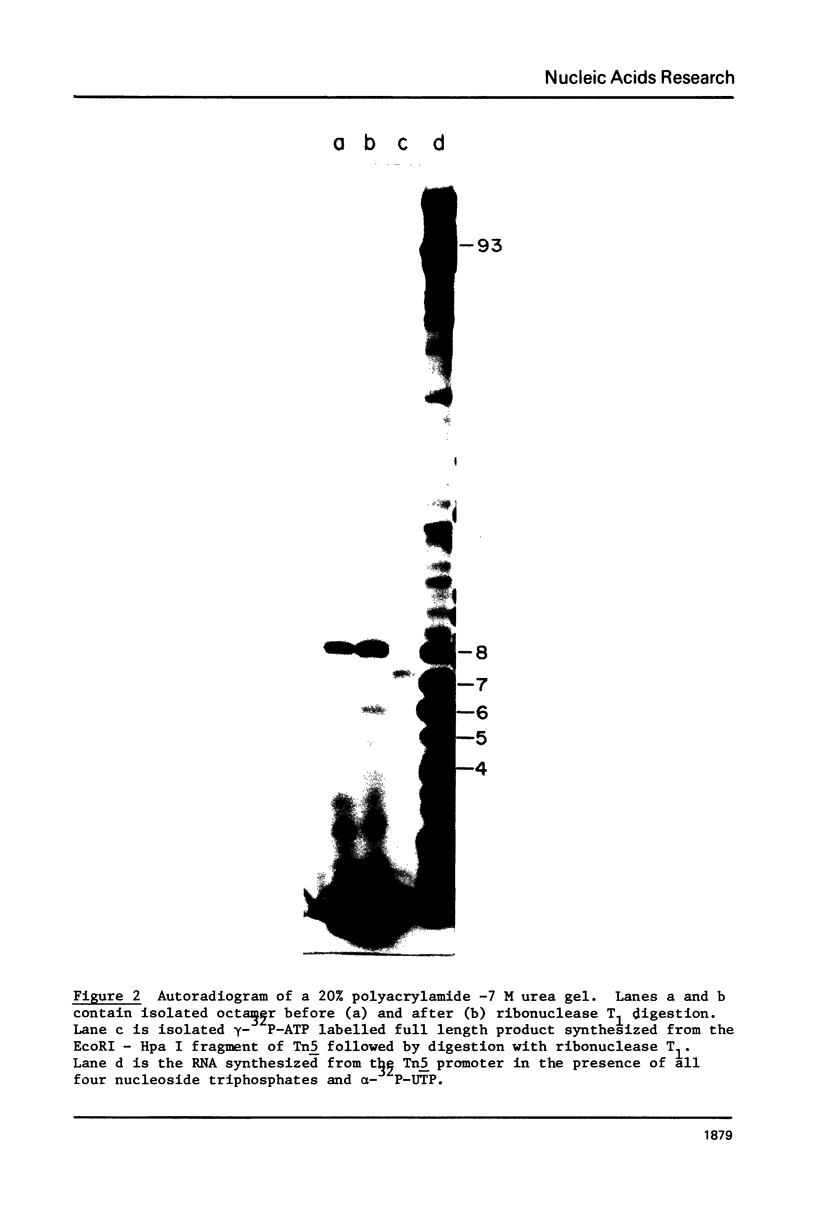
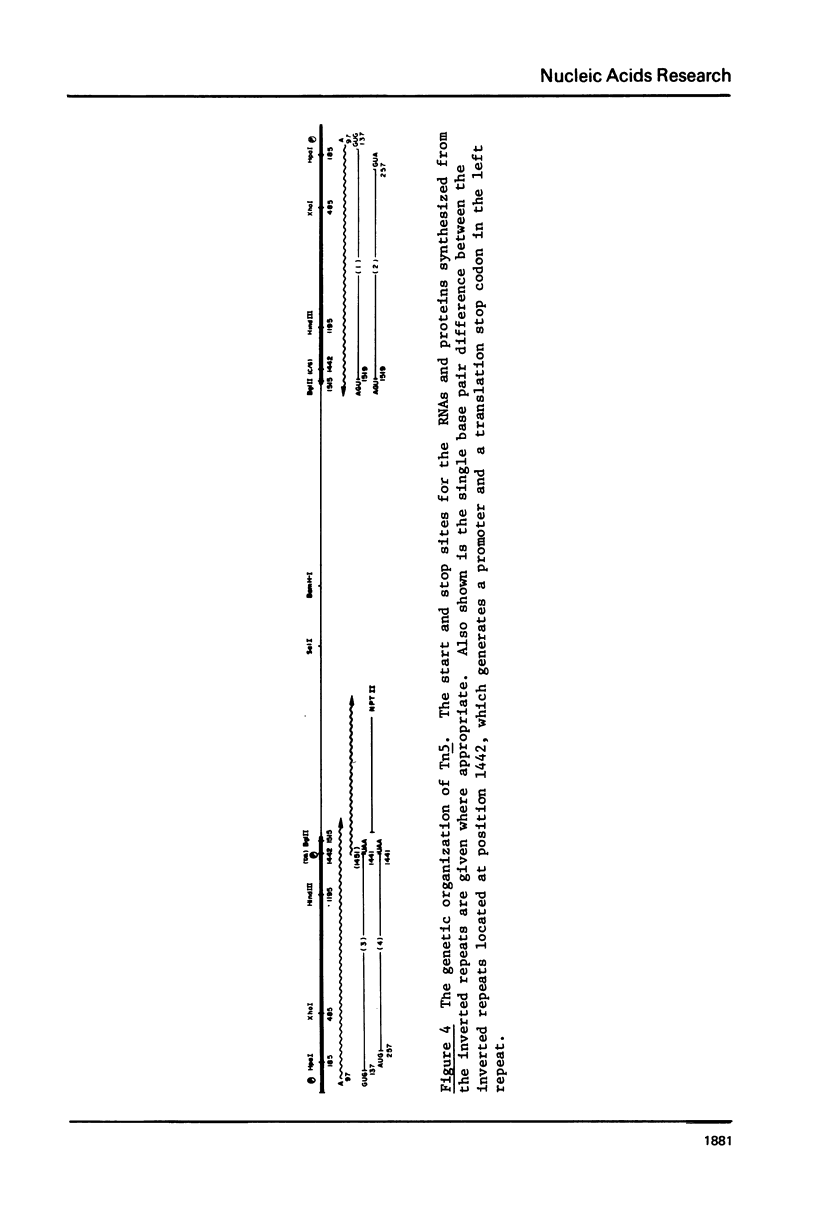
RNA polymerase is known to undergo a cycling process during initiation of transcription in vitro in which large amounts of small oligonucleotides are released. We have used this cycling reaction to determine the 5' end of the RNA synthesized from the outer ends of the Tn5 inverted repeats. By analyzing the size of the radiolabeled oligonucleotides synthesized using different labeled nucleoside triphosphates and in reactions deficient for a particular nucleoside triphosphate, the partial 5' sequence was obtained. This sequence was correlated with the DNA sequence of the region and an unambiguous origin for the mRNA was determined. The start site for the RNA, which is located at 97 base pairs from the outer ends of the inverted repeats, was confirmed by digesting gamma-32 P-ATP labeled full length (run off) transcripts with ribonuclease T1. The resulting gamma-labeled T1 generated oligonucleotide corresponded to the predicted size determined using the cycling reaction. With the knowledge of the RNA start site, the probable translation start sites for the protein(s) known to be required for Tn5 transposition can be predicted. Possible implications of the DNA sequence with respect to the regulation of the transposase are also discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biek D., Roth J. R. Regulation of Tn5 transposition in Salmonella typhimurium. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6047–6051. doi: 10.1073/pnas.77.10.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Cech C. L., Lichy J., McClure W. R. Characterization of promoter containing DNA fragments based on the abortive initiation reaction of Escherichia coli RNA polymerase. J Biol Chem. 1980 Mar 10;255(5):1763–1766. [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Maizels N. M. The nucleotide sequence of the lactose messenger ribonucleic acid transcribed from the UV5 promoter mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3585–3589. doi: 10.1073/pnas.70.12.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkley E. G., Pribnow D. Transcription of the early region of bacteriophage T7: selective initiation with dinucleotides. J Mol Biol. 1973 Jun 25;77(2):255–277. doi: 10.1016/0022-2836(73)90335-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]





