Abstract
Objectives
To examine the association of cardiovascular disease (CVD) and its risk factors with age-associated hearing loss, in a cohort of older black and white adults.
Study Design
Cross-sectional cohort study
Setting
The Health, Aging, and Body Composition (Health ABC) study; A community-based cohort study of older adults from Pittsburgh, PA and Memphis TN.
Participants
2,049 well-functioning adults (mean age: 77.5 years; 37% black)
Measurements
Pure-tone audiometry and history of clinical CVD were obtained at the 4th annual follow-up visit. Pure-tone averages in decibels reflecting low frequencies (250, 500, and 1000 Hz) middle frequencies (500, 1000, and 2000 Hz) and high frequencies (2000, 4000, and 8000Hz) were calculated for each ear. CVD risk factors, aortic pulse-wave velocity, and ankle-arm index were obtained at the study baseline.
Results
In gender-stratified models, after adjustment for age, race, study site and occupational noise exposure, risk factors associated with poorer hearing sensitivity among men included higher triglyceride levels, higher resting heart rate and history of smoking. Among women, poorer hearing sensitivity was associated with higher BMI, higher resting heart rate, faster pulse-wave velocity, and low ankle-arm index.
Conclusion
Modifiable risk factors for CVD may play a role in the development of age-related hearing loss.
Keywords: hearing, presbycusis, race, cardiovascular disease, pulse wave velocity
INTRODUCTION
Approximately 33 to 50% of Americans aged 70 years or greater perceive themselves as having a hearing loss [1] although higher rates are found upon direct testing of hearing sensitivity. With hearing loss defined as the occurrence of a pure-tone average (PTA) >25 dB HL for hearing thresholds obtained with 500, 1000, 2000, and 4000 Hz tones, the Epidemiology of Hearing Loss Study found that 66% of adults aged 70–79 years, and 90% of the adults aged 80–92 years had a hearing loss. In adults, the typical hearing loss configuration is a bilateral high-frequency sensorineural loss with preservation of hearing in the low frequencies [1-3]. Hearing loss is one of the most commonly reported chronic conditions in adults over 65 years and so ubiquitous that it is often seen as a normal part of aging [4-6]. Presbycusis (age-related hearing loss), while not life-threatening, negatively influences the quality of life, physical function, and psychosocial well-being of older adults [7-11]. The search for preventable risk factors is thus an important public health goal.
The precise etiology of presbycusis is unknown and multiple factors are likely involved. Cardiovascular disease has been associated with hearing loss in previous studies [12-15]. However, not all studies have found a link between CVD and hearing loss [16-18], nor has a consistent pattern of cardiovascular risk factors for hearing loss emerged across studies [12, 14, 19]
Given the rich capillary supply to the stria vascularis with in the cochlea and its sensitivity to disruptions in arterial blood supply, there is reason to believe that CVD could influence hearing. Studies of cardiovascular disease biomarkers such as lipids, glucose, and clotting factors revealed few if any associations with hearing sensitivity [20, 21]. However in the Framingham study, high systolic blood pressure (men and women) and high glucose and low HDL-C (women only) were associated with poorer hearing sensitivity, particularly in the lower frequencies [14]. Prevalent clinical CVD was also associated with higher odds of low-frequency hearing loss, suggesting that the CVD process itself may influence hearing sensitivity in older adults. Diabetes, which shares many risk factors with CVD, has also been associated with hearing loss, with microvascular damage postulated as a cause [19, 22, 23].
There are gender differences in the progression of both cardiovascular disease and hearing loss. Hypertension and CVD are more common among men up until middle age. However after middle age, there is a faster age-related increase in cardiovascular risk among women, resulting in similar CVD rates in both genders in old age [24]. Regarding hearing sensitivity, a relatively constant rate of decline in the mid- and high-frequency range has been observed across the lifespan with men [2]. Among women, some researchers found smaller decreases in hearing initially but faster decline than men in older age ranges [2, 25], and others found faster declines in hearing sensitivity among women up until age 69, with comparable declines for both genders thereafter [26].
Thus, there is accumulating evidence suggesting that either CVD itself or its precursors mighty play a role in presbycusis. The purpose of this study was to re-examine the relationship between hearing sensitivity and CVD and its risk factors, separately in men and women, in the community-based Health, Aging and Body Composition (Health ABC) Study, a longitudinal cohort study of older adults that included black as well as white participants. We were particularly interested in whether subclinical measures of CVD, including ankle-arm blood pressure index, a surrogate measure of peripheral occlusion, and arterial pulse-wave velocity, an indicator of arterial stiffness, were associated with hearing, in an attempt to discover what part of the CVD process might have the most influence on hearing sensitivity.
METHODS
Study Population
The Health, Aging, and Body Composition (Health ABC) study is a population-based, prospective cohort study designed to examine the impact of changes in weight and body composition on age-related physiological and functional changes[27]. The cohort was established between March 1997 and July 1998. Participants aged 70-79 years of age were drawn from a random sample of Medicare beneficiaries residing in ZIP codes surrounding two metropolitan centers, Pittsburgh, PA and Memphis, TN. Eligibility criteria consisted of reporting twice before enrollment being able to walk a quarter of a mile without difficulty, being able to walk up a flight of stairs without difficulty, and having the ability to perform basic activities of daily living such as getting in and out of a bed or chair, bathing, dressing, and eating. In addition, participants were required to have no life threatening illness and no intention to move from their respective metropolitan area for 3 or more years.
The Health ABC baseline cohort consisted of 3,075 individuals (48% men; 42% classified as Black). Of these, 2,203 members of the cohort (83% of survivors) had complete audiometric data collected at the 4th annual follow-up visit (2001-2002). Participants with both ears completely obstructed were excluded from the analysis (n=154). Thus, the analytic sample consisted of 2,049 participants (52% women; 37% black). Surviving participants who did not have the hearing test were more likely to be older, homebound, black, and from Memphis (all differences p<0.001). Compared to the analytic sample, those without the hearing test had a higher prevalence of subclinical CVD (higher pulse wave velocity and low ankle-arm index; both p<0.05), higher HDL-C (p<0.01) and higher systolic blood pressure (p<0.01). There was no difference in the presence of clinical CVD, and other lipid levels did not vary by hearing study participation. Study protocol and informed consent forms were approved by the institutional review boards of the University of Pittsburgh and University of Tennessee, and were signed by all participants.
Audiometric Assessment
Audiometric evaluations were conducted in a sound-treated booth within a quiet examination room. Information on ear infections, tinnitus, ear surgery, use of hearing aids, limitations associated with hearing, and prior noise exposure was obtained. Participants were classified as having been exposed to occupational noise if they worked for at least a year in a job requiring a raised voice for communication. Otoscopy was used to identify obstructions of the external auditory canals.
Air conduction pure-tone thresholds were obtained for audiometric frequencies between 250-8,000 Hz with a Maico MA 40 audiometer with TDH 39 supra-aural earphones (Maico Diagnostics, Eden Prairie, MN). The audiometer was calibrated according to American National Standards Institute standards (ANSI S3.6-2004, ANSI, 2004a), and biological quality-control checks for sound intensity and quality were conducted each morning before the day’s first test. Hearing thresholds, measured in hearing level in decibels (dB HL) were obtained using current standard methods for manual audiometry (ANSI, 2004b). From the thresholds, three pure-tone averages were calculated: Low frequency (PTAlow) (average of hearing thresholds at 250, 500, and 1000 Hz), Mid frequency (PTAmid) (500, 1000, and 2000 Hz), and high frequency (PTAhi) (2000, 4000, and 8000 Hz). Pure-tone averages for both the better- and worse-hearing ear were calculated. Analysis of the worse-hearing ear is typically presented in the presbycusis literature as it captures all instances of hearing loss. However, using the better-hearing may better capture bilateral hearing loss that might be expected to result from systemic disease.
Pulse-Wave Velocity
Aortic pulse wave velocity was measured using nondirectional transcutaneous Doppler flow probes by Parks Medical Electronics Inc. (model 810A, 9.0 – 10.0 MHz probes) during the baseline (1997-1998) visit. All study personnel involved in the collection of PWV data were trained and certified by the National Institute on Aging, Laboratory of Cardiovascular Science, Gerontology Research Center (Baltimore, MD). Pulsatile flow was measured from simultaneous flow signals obtained from the right carotid and femoral arteries. Data in the form of 10-second waveforms were recorded by customized programming for later analysis. Three separate pulsatile flow runs were ascertained for each participant. All usable runs were then averaged. Distance between the carotid and femoral probes was measured above the surface of the body with a metal tape measure. Pulse wave velocity (PWV) was calculated as the time in seconds between the foot of the velocity signal at the carotid and femoral sites divided by the associated carotid-to-femoral distance in centimeters. A faster PWV is associated with a stiffer vessel.
Ankle-Arm Index and Blood Pressure
Two seated resting blood pressures were taken at each clinic visit and were averaged. Ankle-arm blood pressure was collected at the baseline clinic visit. Blood pressures were measured on the right arm and both ankles using a hand-held 8 MHz Doppler probe with built-in speaker and mercury column sphygmomanometers. Ankle-arm index (AAI) was subsequently calculated as the average of two ratios between the tibial and brachial arteries. The lower of the left and right ankle-arm indices was used to classify the individual presence of lower extremity arterial disease, defined as an AAI of less than 0.9 on the baseline assessment.
Laboratory and Additional Clinic Data
Medical history was ascertained by questionnaire at each annual visit. Participants were asked whether a physician had informed them of specific health conditions, including stroke, myocardial infarction, or coronary heart disease. A participant was classified as having prevalent CVD if they reported any of the above outcomes at any of the five annual examinations, if they were taking medications specific to the disease at the baseline exam, or if they were hospitalized for a CVD event during follow-up. Diabetes status was based on self-report of a physician diagnosis. Smoking status and alcohol use were measured based on self-report at the baseline visit. Medication history was assessed at each clinic visit with the exception of the third annual follow-up visit. We collected information on the use of antilipidemic and antihypertensive medications. Participants who took these drugs at each of the measured clinic visits (baseline, year 2, year 4, year 5) were considered regular users.
Blood chemistries were analyzed using samples collected in the baseline clinic visit. Glucose, triglyceride, and HDL-C levels were determined using a colorimetric technique on a Johnson and Johnson Vitros 950 analyzer HDL-C was determined after magnetic precipitation of LDL-C, VLDL-C, and chylomicrons. The Friedwald equation was used to estimate LDL-C values [28]. Fasting insulin was assayed with a microparticle enzyme immunoassay.
Anthropometric measurements for height, weight, and girth were assessed at the baseline visit. Standing height was measured using a stadiometer and weight was measured with a standard balance-beam scale with the participant wearing lightweight clothing. For consistency, all risk factor measurements used in the analysis, including those obtained from blood chemistry, were from the baseline visit, four years prior to the audiometric evaluation.
Statistical Methods
Gender differences between risk factors were assessed with a t-tests or Wilcoxon signed-rank test for continuous variables, or Chi-square tests for categorical variables. The outcome measures for the analysis were the three pure tone averages: PTAlow , PTAmid and PTAhi , evaluated separately in the worse and better ear. PTAlow and PTAmid were log transformed in order to normalize their distributions. All analyses were stratified by gender due to the presence of significant gender/risk factor interactions. Further stratification by race was deemed unnecessary since only one race/risk factor interaction was discovered (weight in men).
Possible associations between PTA and cardiovascular risk factors were assessed in separate linear regression models that adjusted for age, race, site and occupational noise exposure. Models including components of the metabolic syndrome (BMI, HDL-C, LDL-C, cholesterol) were further adjusted for diabetes status. Models of the effect of fasting insulin and fasting glucose on hearing were stratified based on diabetes status. Models including lipid or blood pressure measurements were run with and without controlling for the regular use of antilipidemic or antihypertensive medication.
Risk factors significant in the models described above, at the p<0.15 level, were then included in fully adjusted regression models to test for independent associations with PTA. SPSS Statistical Software v17.0 (Chicago, IL) was used for all analyses.
RESULTS
Baseline risk factor data are presented in Table 1. Significant gender differences were seen for all variables with the exception of systolic blood pressure, fasting insulin, triglycerides, and lower extremity arterial disease (AAI <0.9). After stratification by race within gender groups, differences between black and white women were noted for all risk factors except height, heart rate, and total cholesterol. Compared to white women, black women were heavier and had higher blood pressure, glucose, insulin and HDL-C levels, and lower levels of triglycerides. Black women also had higher pulse wave velocities. Compared to white men, black men had significantly higher blood pressure, higher HDL-C levels and lower triglycerides.
Table 1.
Baseline Characteristics by Gender
| Men N=968 | Women N=1081 | p-value | |
|---|---|---|---|
| Age (years) mean ± SD | 73.6 ± 2.8 | 73.3 ± 2.8 | 0.01 |
| Race (black) n (%) | 310 (32.0) | 451 (41.7) | <0.001 |
| BMI kg/m2 | 27.1 (3.9) | 27.6 (5.4) | 0.02 |
| Systolic Blood Pressure (mm Hg) mean ± SD | 134.7 ± 19.8 | 134.9 ± 20.0 | 0.78 |
| Diastolic Blood Pressure (mm Hg) mean ± SD | 72.6 ± 11.3 | 69.8 ± 11.7 | <0.001 |
| Heart Rate (beats/minute) median (IQR) | 62.0 (56.0 – 70.0) | 65.0 (58.0 – 72.0) | 0.01 |
| Fasting Glucose (mg/dL) median (IQR) | 96.0 (89.0 – 108.0) | 92.0 (86.0 – 102.0) | <0.001 |
| Fasting Insulin (IU/mL) median (IQR) | 6.7 (4.9 – 9.9) | 7.0 (4.9 – 10.3) | 0.46 |
| Cholesterol (mg/dL) mean ± SD | 192.5 ± 34.9 | 213.5 ± 38.1 | <0.001 |
| LDL-C (mg/dL) mean ± SD | 118.6 ± 32.3 | 125.2 ± 35.6 | <0.001 |
| HDL-C (mg/dL) median (IQR) | 45.0 (38.0 – 54.0) | 58.0 (48.0-69.0) | <0.001 |
| Triglycerides (mg/dL) median (IQR) | 119.0 (88.0 – 163.0) | 120.0 (92.0 – 168.0) | 0.30 |
| Pulse-Wave Velocity (cm/s)* median (IQR) | 831.0 (644.0 – 1083.3) | 772.5 (608.0 – 986.0) | 0.001 |
| AAI < 0.9 | 110 (11.9) | 128 (12.4) | 0.78 |
| Current Smoker n (%) | 83 (8.6) | 84 (7.8) | <0.001 |
| Past Smoker n (%) | 578 (59.8) | 364 (33.7) | <0.001 |
| 12 years education or above n (%) | 736 (76.0) | 864 (79.9) | 0.04 |
| History of Occupational Noise Exposure n (%) | 484 (50.2) | 133 (12.4) | <0.001 |
| Characteristics at fourth annual follow-up visit | |||
| History of CVD‡ n (%) | 411 (42.5) | 318 (29.4) | <0.001 |
| History of coronary heart disease n(%) | 350 (36.2) | 245 (22.7) | <0.001 |
| History of myocardial infarction n (%) | 289 (29.9) | 189 (17.5) | <0.001 |
| History of stroke n (%) | 132 (13.6) | 121 (11.2) | 0.09 |
N=784 and N=911
IQR: inter-quartile range; SD: standard deviation AAI: Ankle-Arm Index CVD: Cardiovascular Disease BMI: Body Mass Index HDL-C: High Density Lipoprotein Cholesterol; LDL-C: Low Density Lipoprotein Cholesterol
Means presented with standard deviation and t-test p-values
Medians presented with interquartile range and Wilcoxon test p-values
Frequencies presented with N and chi-square p-values
Includes history of coronary heart disease, myocardial infarction, or stroke
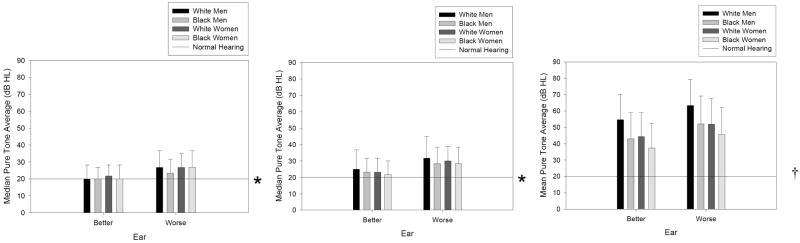
Median low- and mid-frequency pure tone averages, and mean high-frequency pure tone averages, are presented in Figure 1. Since PTAlow and PTAmid were not normally distributed, median values and interquartile ranges are presented.
Figure 1.
Median low-frequency (250, 500, and 1000 Hz), mid-frequency (500, 1000 and 2000 Hz), and high-frequency (2000, 4000 and 8000 Hz) by gender and race.
* indicates the upper hearing level for normal hearing in young adults; error bars reflect the upper range of the inter-quartile ranges
† indicates the upper hearing level for normal hearing in young adults; error bars reflect the standard deviations.
The prevalence of CVD events (history of stroke, myocardial infarction, or coronary heart disease) was 42.5% and 29.4% among men and women, respectively. Age-, race-, and site-adjusted mean hearing thresholds for low-, mid- and high-range PTAs were calculated for participants with and without a history of CVD-related event (data not shown). No differences in PTAs were found by clinical CVD status (any cardiovascular disease, myocardial infarction, stroke, coronary heart disease) were seen, regardless of gender. However after further stratification by race, black women with a history of stroke had higher high-frequency hearing thresholds compared to black women without a history (p<0.01).
As expected, low-, mid- and high-frequency PTAs were correlated with age (r low =0.137, rmid=0.197, rhigh=0.198; all p<0.0001). Age, race and occupational noise exposure were included as covariates in all models given their consistent association with hearing sensitivity in the literature. For a summary of CVD variables associated with poorer hearing sensitivity, see Table 2. Models of the effects of individual CVD risk factors on hearing sensitivity in the worse and better ear are presented in Table 3.
Table 2.
Summary of CVD Risk Factors Associated With Hearing Sensitivity in the Health, Aging and Body Composition Study
| Men | Women |
|---|---|
| Faster resting heart rate*†‡ | Fasting resting heart rate*†‡§¶ |
| Higher triglyceride level*‡§ | Higher BMI†‡§ |
| History of smoking*†¶ | Faster pulse wave velocity†¶ |
| Low ankle-arm index†‡§¶ | |
| History of congestive heart failure†¶ |
BMI: Body Mass Index
significant association (p<0.05) with hearing in worse ear
significant association (p<0.05) with hearing in better ear
associated with low frequency hearing sensitivity
associated with mid-frequency hearing sensitivity
associated with high-frequency hearing sensitivity
Table 3.
Vascular Risk Factors Associated With Hearing Thresholds: Adjusted* Standardized Regression Coefficients
| Worse Ear | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | Women | |||||||||||
| PTAlow | PTAmid | PTAhi | PTAlow | PTAmid | PTAhi | |||||||
| β | p- value | B | p- value | β | p- value | β | p- value | β | p-value | β | p- value | |
| BMI kg/m2 | 0.033 | 0.30 | 0.052 | 0.09 | 0.038 | 0.20 | 0.055 | 0.09 | 0.058 | 0.07 | 0.031 | 0.33 |
| Heart Rate (beats/minute) | 0.065 | 0.04 | 0.049 | 0.11 | 0.874 | 0.38 | 0.086 | 0.004 | 0.089 | 0.003 | 0.075 | 0.01 |
| Triglycerides (mg/dL)† | 0.077 | 0.02 | 0.085 | 0.01 | .050 | 0.10 | -0.028 | 0.38 | -0.005 | 0.87 | 0.000 | 0.99 |
| LDL-C (mg/dL)† | 0.025 | 0.44 | 0.018 | 0.56 | 0.008 | 0.79 | -0.058 | 0.06 | -0.051 | 0.09 | -0.053 | 0.07 |
| Pulse-Wave Velocity (25 cm/s) ‡ | 0.015 | 0.66 | 0.014 | 0.70 | -0.005 | 0.89 | 0.052 | 0.11 | 0.048 | 0.16 | 0.058 | 0.07 |
| Past Smoker | 0.035 | 0.26 | 0.049 | 0.11 | 0.068 | 0.02 | -0.022 | 0.47 | 0.000 | 0.99 | 0.026 | 0.038 |
| Better Ear | ||||||||||||
| BMI kg/m2 | 0.025 | 0.44 | 0.049 | 0.12 | 0.023 | 0.43 | 0.102 | 0.002 | 0.085 | 0.007 | 0.018 | 0.55 |
| Heart Rate (beats/minute) | 0.051 | 0.10 | 0.051 | 0.10 | 0.041 | 0.16 | 0.123 | <0.001 | 0.119 | <0.001 | 0.099 | 0.001 |
| Triglycerides (mg/dL)† | 0.054 | 0.10 | 0.065 | 0.04 | 0.048 | 0.11 | -0.006 | 0.85 | 0.013 | 0.67 | 0.022 | 0.46 |
| Pulse-Wave Velocity (25 cm/s) ‡ | -0.014 | 0.70 | 0.048 | 0.17 | 0.008 | 0.081 | 0.051 | 0.13 | 0.056 | 0.10 | 0.069 | 0.03 |
| AAI < 0.9 | 0.007 | 0.83 | 0.019 | 0.56 | 0.001 | 0.97 | 0.065 | 0.04 | 0.077 | 0.01 | 0.062 | 0.03 |
| Past Smoker | 0.019 | 0.54 | 0.022 | 0.49 | 0.073 | 0.01 | 0.001 | 0.81 | 0.017 | 0.57 | 0.024 | 0.40 |
| History of Congestive Heart Failure | 0.000 | 0.98 | 0.028 | 0.36 | 0.039 | 0.18 | 0.038 | 0.20 | 0.042 | 0.016 | 0.060 | 0.04 |
BMI: Body Mass Index; LDL-C: Low Density Lipoprotein Cholesterol
Adjusted for age, race, study site and occupational noise exposure -- denotes factors not associated with hearing thresholds (p > 0.15)
Also adjusts for history of diabetes
Also adjusts for systolic blood pressure
BMI: Body Mass Index; AAI: Ankle-Arm Index
Bold type highlights statistical significance at p<0.05
PTAlow: Log-transformed pure tone average of 250, 500 and 1000 Hz, hearing level in dB; measured in the better ear
PTAmid: Log-transformed pure tone average of 500, 1000, and 2000 Hz, hearing level in dB; measured in the better ear
PTAhi: Pure tone average of 2000, 4000 and 8000 Hz, hearing level in dB; measured in the better ear
In models of hearing sensitivity in the worse-hearing ear, among men, faster resting heart rate was associated with poorer low-frequency hearing sensitivity. Higher triglyceride levels were associated with poorer low- and mid-frequency hearing sensitivity. Associations between hearing sensitivity and triglyceride levels persisted after controlling for the regular use of antihyperlipidemics. History of smoking was associated with poorer high frequency hearing sensitivity among men. Among women, faster resting heart rate was the only risk factor associated with poorer hearing sensitivity, with associations noted for all three PTAs.
The above mentioned analyses were repeated using pure-tone averages of the better ear as an outcome. Among men, higher triglyceride levels were associated with poorer mid-frequency hearing sensitivity (with and without adjustment for antihyperlipidemic therapy), and smoking history was associated with poorer high-frequency hearing sensitivity. Among women, Higher BMI was associated with poorer low- and mid-frequency hearing sensitivity, faster resting heart rate and low ankle-arm index were associated with poorer hearing in all frequency ranges, and faster pulse-wave velocity was associated with poorer high-frequency hearing sensitivity.
Hearing sensitivity was not associated with cholesterol (total, LDL-C, HDL-C) or blood pressure (systolic or diastolic) (data not shown). Adjustment for regular antihyperlipidemic or antihypertensive use did not alter findings. Insulin and glucose were not associated with hearing loss, regardless of diabetes status (data not shown).
Clinical CVD variables (history of coronary heart disease, myocardial infarction, stroke or any CVD event) were not associated with hearing sensitivity in either men or women (data not shown), with the exception of history of congestive heart failure, which was associated with poorer better-ear high frequency hearing sensitivity among women (β=0.06 p=0.04).
Finally, fully-adjusted gender-stratified models were constructed. Separate models were constructed for each frequency range. These models were adjusted for age, race, study site and occupational noise exposure and included all variables significant at p<0.15 in the minimally adjusted models. Among men, higher triglyceride levels were independently associated with poorer low-frequency hearing sensitivity in the worse ear (β=0.077 p=0.02), and history of smoking was associated with poorer high frequency hearing sensitivity in both ears (worse ear β=0.068 p=0.02; better ear β=0.073 p=0.021). Among women, faster resting heart rate was independently associated with poorer hearing sensitivity in most frequency ranges, using both the worse and better ears as the dependent variable (worse ear PTAmid β=0.074 p=0.02; better ear: PTAlo β=0.11 p=0.001, PTAmid β=0.10 p=0.004, PTAhi β=0.086 p=0.01). Additionally, higher BMI was independently associated with poorer low frequency hearing sensitivity in the better ear (β=0.078 p=0.03), and low ankle-arm index was independently associated with poorer mid-frequency hearing sensitivity in the better ear (β=0.070 p=0.04).
DISCUSSION
Cardiovascular disease and hearing loss are both highly prevalent among older adults. In this community-based study of older black and white adults, certain CVD risk factors, including higher levels of triglycerides and history of smoking (among men), higher BMI (among women) and higher resting heart rate (both genders), were related to poorer hearing, whereas clinical CVD generally was not. We found that subclinical CVD, characterized by faster pulse-wave velocity and low ankle arm index, was associated with poorer hearing among women. Our findings may offer insight into possible etiological mechanisms related to the development of presbycusis.
It has been suggested that cardiovascular disease may result in hearing loss by causing reduced vascular supply to the cochlea [14]. Insufficient nutrient supply due to capillary constriction within the stria vascularis, a rich bed of membrane capillaries that supplies nutrients to cochlear components, can lead to death of stereocilia and result in reduced hearing sensitivity, particularly in the lower frequencies [12-14, 29, 30]. Atherosclerosis has been hypothesized to cause reduced blood flow to the cochlea via stiffening or constriction of the internal auditory artery. Regardless of whether caused by micro- or macro-vascular pathology, insufficient cochlear blood supply can disrupt the chemical balance of the inner ear fluid, endolymph. This in turn affects the electrical activity of the hair cells and subsequently activation of the auditory nerve.
Diabetes, a known cause of microvascular disease, is believed to contribute to hearing loss by causing cochlear microvascular dysfunction [22, 31-36]. A recent study using retinal microvascular abnormalities as a marker for systemic microvascular damage found that older women with retinopathy were twice as likely to have hearing loss in the lower frequencies as those without, after adjustment for other hearing loss risk factors [13]. In our previous work, we found that diabetes was associated with hearing impairment [11]. Some of the factors associated with poorer hearing sensitivity in the current study (e.g. higher triglyceride levels [men], higher BMI [women]) are components of the metabolic syndrome and may be markers of a pre-diabetic state.
Unlike findings from the Framingham study [14], we found no associations between cholesterol levels and hearing, however we did find that higher triglyceride levels were associated with poorer hearing among men, and the use of antilipidemic drugs was associated with better mid-frequency hearing among men. The Framingham cohort was established when participants were less than 35 years of age, and CVD risk factors in mid-life were associated with hearing loss in later life. The Health ABC cohort on the other hand recruited healthy individuals at an older age (70-79). Thus it is possible that differences in the duration of exposure to CVD risk factors may underlie disparate findings, and that selection and survival bias may be operational within our cohort. Absence of a hearing sensitivity/cholesterol relationship in our study may also reflect our cohort’s better lipid profile.
Like others [1, 37, 38], we found that history of smoking was associated with poorer hearing sensitivity. Cigarette smoking may contribute to hearing loss by affecting antioxidative mechanisms in the auditory system or by affecting the vasculature of the inner ear [1, 39]. In our study this association was seen with the high frequencies among men, but was not seen among women. Previous studies suggest that there is a synergistic relationship between noise exposure and exposure to cigarette smoke, wherein smokers with higher noise exposure had much worse hearing than smokers with limited noise exposure [40]. Thus it is possible that the women in our sample, who were less exposed to occupational noise, may have been at lesser risk of smoking-related hearing damage.
Consistent with a recent multinational European study [37], we found that higher BMI was associated with poorer low- and mid-frequency hearing sensitivity, although in our case among women only. Along the same lines, a recent study of Taiwanese adults found that higher waist circumference was independently associated with age-related hearing loss [41]. Obesity is a risk factor for both diabetes and CVD and thus may contribute to hearing loss via both macro- and micro-angiopathy.
Unlike several previous studies finding associations between hearing, myocardial infarction and coronary artery disease [12, 14, 30], we found little correlation between hearing sensitivity and clinical CVD. In the present study, history of coronary artery disease was associated with poorer hearing among women, although only in the high frequencies. However, measures of subclinical CVD, including pulse-wave velocity, a measure of arterial stiffness, and peripheral arterial disease as assessed by ankle-arm index, were associated with poorer hearing sensitivity in women. Faster resting heart rate was associated with poorer hearing in both genders, although more consistently so among women. In pathophysiological studies, faster resting heart rate has been associated with myocardial ischemia, left ventricular dysfunction, arterial rigidity among hypertensives, and faster progression of coronary atherosclerosis [42]. Thus, elevated resting heart rate might also be considered a sign of subclinical cardiovascular disease.
We found associations between hearing sensitivity and subclinical CVD among women but not among men. Women are generally less exposed to occupational noise and thus demonstrate less noise-induced hearing loss, perhaps making associations with subclinical CVD easier to observe. Alternatively, different pathophysiologic mechanisms (possibly influenced by hormones) may be in place [43].
After middle age, compared to men, women experience more marked age-related changes in autonomic mechanisms tied to vascular and heart rate regulation. This may partially explain increases in overall CVD risk among women in late life [24]. Interestingly, in a recent study of changes in hearing sensitivity over time, between the ages of 48-69 years, women demonstrated more rapid declines in hearing sensitivity than men at certain mid-range frequencies [26]. The gender-specific associations we found between hearing sensitivity and intermediary indicators of the CVD pathophysiologic process suggests that macrovascular disease, perhaps promoted by CVD risk factors such as higher BMI and diabetes, may play an especially important role in age-related hearing loss among women.
We examined better- and worse-ear hearing levels for low, middle, and high-frequency PTAs. Our results were somewhat inconsistent depending on whether the better- or worse-hearing ear was used as the outcome variable. Notably, subclinical CVD (faster pulse-wave velocity and low ankle-arm index) was associated with poorer hearing sensitivity among women in the better hearing ear, but not in the worse hearing ear. It is possible that using the better hearing ear better captures hearing loss associated with macrovascular disease; nonetheless we did not find associations between clinical CVD and hearing sensitivity in either ear. Regarding frequency ranges, poorer low frequency hearing was associated with fasting resting heart rate (men and women), higher BMI (women) and higher triglyceride levels (men); poorer mid-frequency hearing was associated with triglycerides (men), resting heart rate and low ankle-arm index (women); and poorer high frequency hearing was associated with smoking (men), resting heart rate (women), and subclinical CVD variables (women). Thus, unlike the findings of some previous studies [14], we found that CVD risk factors were not predominantly associated with low frequency hearing.
Confidence in our findings is strengthened by several factors. The Health ABC Study is a large, community-based study that included both black and white participants, making our findings more generalizable than previous studies that included only white participants. Also, our measurements of hearing sensitivity and cardiovascular factors were of high quality. This study expanded upon previous research by including measures of subclinical CVD. Nonetheless, this study has limitations. As mentioned earlier, the Health ABC recruited healthy individuals at an older age (70-79). A recent study measured hearing sensitivity and CVD risk factors closer to mid-life, and found stronger associations with risk factors such as smoking and BMI [37]. Thus it is possible that survival bias affected our findings, especially with regards to the lack of association between hearing sensitivity and clinical CVD. It is also possible that cumulative noise exposure masked relationships between hearing and some of the CVD risk factors. We had incomplete information on lifetime noise exposure and thus could not completely account for this important risk factor in our analyses. There were some differences in cardiovascular factor prevalence between participants who had the hearing assessment and those who did not, however these differences were inconsistent. For example, those who did not undergo hearing testing were more likely to have subclinical CVD, higher systolic blood pressure and higher BMI, but were less likely than the analysis sample to have clinical CVD.. This may have biased our findings. Finally, the cross-sectional nature of our study precluded investigation of the influence of cardiovascular risk factors on change in hearing over time, which will be an important subject for future study. Future research is also needed to explore the interplay between vascular disease factors and noise exposure in the progression of presbycusis, particularly whether the presence of CVD and its risk factors predispose older adults to noise-induced hearing damage.
In conclusion, we found that certain CVD risk factors (women: higher BMI, higher fasting resting heart rate; men: faster resting heart rate, triglyceride levels, and history of smoking) were associated with poorer hearing sensitivity. Subclinical CVD measures were associated with hearing sensitivity among women. Clinicians should urge patients to address cardiovascular risk factors, not only to prevent heart disease but also potentially to aid in the preservation of hearing in later life. The prevention of CVD and its contributing factors has the potential to slow the progression of age-related hearing loss
Acknowledgments
This work was supported through the National Institute on Aging contract numbers N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106.
| Elements of Financial/Personal Conflicts | *Author 1 EPH | Author 2 AP | Author 3 SP | Author 4 KST | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | *Author 5 JC | Author 6 ET | Author 7 EK | Author 8 TH | ||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | *Author 9 SS | Author 10 JD | Author 11 AN | Author 12 | ||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | |||||
| Grants/Funds | x | x | x | |||||
| Honoraria | x | x | x | |||||
| Speaker Forum | x | x | x | |||||
| Consultant | x | x | x | |||||
| Stocks | x | x | x | |||||
| Royalties | x | x | x | |||||
| Expert Testimony | x | x | x | |||||
| Board Member | x | x | x | |||||
| Patents | x | x | x | |||||
| Personal Relationship | x | x | x | |||||
Footnotes
Author Contributions: Indicate authors’ role in study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
EPH: Study concept and design, analysis and interpretation of data, preparation of manuscript
AP: Study concept and design, analysis and interpretation of data, preparation of manuscript
SP: Analysis and interpretation of data, preparation of manuscript
KST: Study concept and design, preparation of manuscript
JC: Study concept and design, acquisition of subjects, preparation of manuscript
ET: Study concept and design, preparation of manuscript
EK: interpretation of data, preparation of manuscript
TH: acquisition of subjects, preparation of manuscript
SS: acquisition of subjects, preparation of manuscript
JD: preparation of manuscript
AN: Study concept and design, acquisition of subjects, preparation of manuscript
Conflict of Interest Checklist: Below is the table for all authors to complete and attach to their papers during submission.
References
- 1.Cruickshanks KJ, Klein R, Klein BEK, et al. Cigarette Smoking and Hearing Loss: The Epidemiology of Hearing Loss Study. JAMA. 1998;279:1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 2.Morrell CH, Gordon-Salant S, Pearson JD, et al. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. J Acoust Soc Amer. 1996;100:1949–1967. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- 3.Moscicki EK, Elkins EF, Baurn HM, et al. Hearing Loss in the Elderly: An Epidemiologic Study of the Framingham Heart Study Cohort. Ear Hear. 1985;6:184–190. [PubMed] [Google Scholar]
- 4.Pleis JR, Coles R. Vital Health Stat 2003. National Center for Health Statistics; Hyattsville, MD: Summary health statistics for U.S. adults: National Health Interview Survey, 1999; pp. 1–137. [PubMed] [Google Scholar]
- 5.Cruickshanks KJ, Wiley TL, Tweed TS, et al. Prevalence of Hearing Loss in Older Adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- 6.Ries PW. Data from the National Health Survey, in Vital and Health Statistics 1982. National Center for Halth Statistics; Hyattsville, MD: Hearing ability of persons by sociodemographic and health characteristics: United States. [PubMed] [Google Scholar]
- 7.Lee P, Smith JP, Kington R. The relationship of self-rated vision and hearing to functional status and well-being among seniors 70 years and older - a multivariate analysis. Amer J Ophthalmol. 1999;127:447–452. doi: 10.1016/s0002-9394(98)00418-8. [DOI] [PubMed] [Google Scholar]
- 8.Cacciatore F, Napoli C, Abete P, et al. Quality of life determinants and hearing function in an elderly population: Osservatorio Geriatrico Campano Study Group. Gerontology. 1999;45:323–328. doi: 10.1159/000022113. [DOI] [PubMed] [Google Scholar]
- 9.Carabellese C, Appollonio I, Rozzini R, et al. Sensory impairment and quality of life in a community elderly population. J Amer Geriatr Soc. 1993;41:401–407. doi: 10.1111/j.1532-5415.1993.tb06948.x. [DOI] [PubMed] [Google Scholar]
- 10.Mulrow CD, Aquilar C, Endicott JE, et al. Association between hearing impairment and the quality of life of elderly individuals. J Amer Geriatr Soc. 1990;38:45–50. doi: 10.1111/j.1532-5415.1990.tb01595.x. [DOI] [PubMed] [Google Scholar]
- 11.Heine C, Browning CJ. Communication and psychosocial consequences of sensory loss in older adults: overview and rehabilitation directions. Disabil Rehabil. 2002;24:763–773. doi: 10.1080/09638280210129162. [DOI] [PubMed] [Google Scholar]
- 12.Torre P, III, Cruickshanks KJ, Klein BEK, et al. The Association Between Cardiovascular Disease and Cochlear Function in Older Adults. J Speech Lang Hear Res. 2005;48:473–481. doi: 10.1044/1092-4388(2005/032). [DOI] [PubMed] [Google Scholar]
- 13.Liew G, Wong TY, Mitchell P, et al. Retinal Microvascular Abnormalities and Age-Related Hearing Loss: The Blue Mountains Hearing Study. Ear Hear. 2007;28:394–401. doi: 10.1097/AUD.0b013e3180479388. [DOI] [PubMed] [Google Scholar]
- 14.Gates GA, Cobb JL, D’Agostino RB, et al. The Relation of Hearing in the Elderly to the Presence of Cardiovascular Disease and Cardiovascular Risk Factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 15.Rosen S, Olin P. Hearing Loss and Coronary Heart Disease. Int J Audiol. 1966;5:156–158. [Google Scholar]
- 16.Dimitrios GK, Nicholas GK, George MZ, et al. Brainstem Auditory Evoked Potentials in Patients With Ischemic Heart Disease. Laryngoscope. 1996;106:54–57. doi: 10.1097/00005537-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Parving A, Hein HO, Suadicani P, et al. Epidemiology of Hearing Disorders: Some Factors Affecting Hearing. The Copenhagen Male Study. Scand Audiol. 1993;22:101–107. doi: 10.3109/01050399309046025. [DOI] [PubMed] [Google Scholar]
- 18.Pratt SR, Kuller L, Talbott EO, et al. Prevalence of Hearing Loss in Black and White Elders: Results of the Cardiovascular Health Study. J Speech Lang Hear Res. 2009;52:973–989. doi: 10.1044/1092-4388(2009/08-0026). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helzner EP, Cauley JA, Pratt SR, et al. Race and Sex Differences in Age-Related Hearing Loss: The Health, Aging and Body Composition Study. J Amer Geriatr Soc. 2005;53:2119–2127. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee F-S, Matthews LJ, Mills JH, et al. Analysis of Blood Chemistry and Hearing Levels in a Sample of Older Persons. Ear Hear. 1998;19:180–190. doi: 10.1097/00003446-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Drettner B, Hedstrand H, Klockhoff I. Cardiovascular Risk Factors and Hearing Loss: A Study of 1000 Fifty-Year-Old Men. Acta Oto-laryngol. 1975;79:366–371. doi: 10.3109/00016487509124698. [DOI] [PubMed] [Google Scholar]
- 22.Dalton DS, Cruickshanks KJ, Klein R, et al. Association of NIDDM and hearing loss. Diabetes Care. 1998;21:1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- 23.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and Hearing Impairment in the United States: Audiometric Evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Int Med. 2008;149:1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narkiewicz K, Kjeldsen SE, Hedner T. Hypertension and cardiovascular disease in women: Progress towards better understanding of gender-specific differences? Blood Pressure. 2006;15:68–70. doi: 10.1080/08037050600750165. [DOI] [PubMed] [Google Scholar]
- 25.Lee F-S, Matthews LJ, Dubno JR, et al. Longitudinal Study of Pure-Tone Thresholds in Older Persons. Ear Hear. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Wiley TL, Chappell R, Carmichael L, et al. Changes in hearing thresholds over 10 years in older adults. J Amer Acad Audiol. 2008;19:281–292. doi: 10.3766/jaaa.19.4.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes Is Associated With Subclinical Functional Limitation in Nondisabled Older Individuals. Diabetes Care. 2003;26:3257–3263. doi: 10.2337/diacare.26.12.3257. [DOI] [PubMed] [Google Scholar]
- 28.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, Without Use of the Preparative Ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 29.Seidman MD, Quirk WS, Shirwany NA. Mechanisms of Alterations in the Microcirculation of the Cochlea. Ann NY Acad Sci. 1999;884:226–232. doi: 10.1111/j.1749-6632.1999.tb08644.x. [DOI] [PubMed] [Google Scholar]
- 30.Friedland DR, Cederberg C, Tarima S. Audiometric pattern as a predictor of cardiovascular status: Development of a model for assessment of risk. Laryngoscope. 2009;119:473–486. doi: 10.1002/lary.20130. [DOI] [PubMed] [Google Scholar]
- 31.Kakarlapudi V, Sawyer R, Staecker H. The Effect of Diabetes on Sensorineural Hearing Loss. Otol Neurotol. 2003;24:382–386. doi: 10.1097/00129492-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol. 1993;107:179–182. doi: 10.1017/s0022215100122571. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Pan C, Gu R, et al. Hearing impairment in diabetics. Chin Med J. 1992;105:44–48. [PubMed] [Google Scholar]
- 34.Duck SW, Prazma J, Bennett PS, et al. Interaction Between Hypertension and Diabetes Mellitus in the Pathogenesis of Sensorineural Hearing Loss. Laryngoscope. 1997;107:1596–1605. doi: 10.1097/00005537-199712000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Wackym PA, Linthicum FHJ. Diabetes Mellitus and Hearing Loss: Clinical and Histopathologic Relationships. Otol Neurotol. 1986;7:176–182. [PubMed] [Google Scholar]
- 36.Smith T, Raynor E, Prazma J, et al. Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope. 1995;105:236–240. doi: 10.1288/00005537-199503000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Fransen E, Topsakal V, Hendrickx J-J, et al. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a european population-based multicenter study. J Assoc Res Otolaryngol. 2008;9:264–276. doi: 10.1007/s10162-008-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gopinath B, Flood VM, McMahon CM, et al. The Effects of Smoking and Alcohol Consumption on Age-Related Hearing Loss: The Blue Mountains Hearing Study. Ear Hear. 2010;31:277–282. doi: 10.1097/AUD.0b013e3181c8e902. [DOI] [PubMed] [Google Scholar]
- 39.Maffei G, Miani P. Experimental Tobacco Poisoning: Resultant Structural Modifications of the Cochlea and Tuba Acustica. Arch Otolaryngol. 1962;75:386–396. doi: 10.1001/archotol.1962.00740040397002. [DOI] [PubMed] [Google Scholar]
- 40.Ferrite S, Santana V. Joint effects of smoking, noise exposure and age on hearing loss. Occupational Medicine-Oxford. 2005;55:48–53. doi: 10.1093/occmed/kqi002. [DOI] [PubMed] [Google Scholar]
- 41.Hwang J-H, Wu C-C, Hsu C-J, et al. Association of Central Obesity With the Severity and Audiometric Configurations of Age-related Hearing Impairment. Obesity. 2009;17:1796–1801. doi: 10.1038/oby.2009.66. [DOI] [PubMed] [Google Scholar]
- 42.Fox K, Borer JS, Camm AJ, et al. Resting Heart Rate in Cardiovascular Disease. J Am Coll Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- 43.Kim SH, Kang BM, Chae HD, et al. The Association Between Serum Estradiol Level and Hearing Sensitivity in Postmenopausal Women. Obstetrics & Gynecology. 2002;99:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]